Abstract
Objective
Plasma interleukin-8 (IL-8) levels of < 220 pg/ml have an excellent negative predictive value (94–95%) for death at 28 days in children with septic shock and thus may be useful for risk stratification in clinical trial enrollment in this population. Whether plasma IL-8 would have similar utility in adults with septic shock is unknown.
Design, Setting, and Patients
Analysis of plasma IL-8 levels in 192 adults with vasopressor-dependent septic shock enrolled in clinical trials of acute lung injury conducted by the Acute Respiratory Distress Syndrome Network
Measurements and Main Results
Plasma IL-8 levels ≥ 220 pg/ml were significantly associated with death at 28 days in this cohort (odds ratio 2.92, 95% CI 1.42–5.99; p=0.001). However, in contrast to the findings in pediatric septic shock, a plasma IL-8 cutoff below 220 pg/ml had a negative predictive value for death of only 74% (95% CI 66–81%) in adults with septic shock. Receiver-operating characteristic analysis found an area under the curve of 0.59 for plasma IL-8, indicating that plasma IL-8 is a poor predictor of mortality in this group. In adults under age 40, a plasma IL-8 cutoff < 220 pg/ml had a negative predictive value of 92%.
Conclusions
In contrast to similar pediatric patients, plasma IL-8 levels are not an effective risk stratification tool in older adults with septic shock. Future studies of biomarkers for risk stratification in critically ill subjects will need to be replicated in multiple different populations before being applied in screening for clinical trials.
Keywords: interleukin-8, acute lung injury, sensitivity, specificity, negative predictive value, risk stratification
INTRODUCTION
Appropriate patient selection is a key step in the performance of efficient and ethical clinical trials.[1, 2] As expenditures required to perform clinical trials increase and such trials become ever more complex,[3] the need to minimize required sample size becomes ever more pressing. Enrolling patients at low risk of death in a clinical trial using mortality as an endpoint results in an increased sample size required to detect a beneficial effect for novel therapies.[2] In addition, the responsibilities of performing human research dictate that patients who are unlikely to benefit from a given therapeutic intervention should not be offered enrollment in a clinical trial, particularly when that trial involves more than minimal risk. At the present time, for clinical syndromes such as sepsis, we have limited ability to accurately predict mortality at ICU admission. Clinical prediction systems may be helpful on a population basis;[4] however, for individual patients, clinical predictive scoring systems often fall short.[5] Thus, novel means of risk stratification for patients prior to enrollment in clinical trials of novel therapies in critical illness are needed.
Plasma biomarkers provide a potentially useful approach to risk stratification of patients prior to enrollment in clinical trials.[6, 7] For instance, a biomarker that excludes subjects at low risk of mortality could be useful in selecting a higher risk sub-group for enrollment in clinical trials, thus improving the risk-benefit ratio and reducing the necessary sample size. Although the use of biologic markers to stratify risk for patients for enrollment in clinical trials is not currently common in critical care, in other fields such as oncology, biologic markers are routinely used to triage patients for enrollment in trials of novel therapies.[8] Such an approach may be particularly useful in the setting of a syndrome like sepsis or acute lung injury that is defined by clinical criteria and therefore somewhat heterogenous.[6, 7] Many plasma biomarkers can be measured relatively quickly and using a small amount of plasma (as little as 20 microliters for some assays) and thus may be practical tools for screening and risk stratification.[9]
Interleukin-8 (IL-8) is the main neutrophil chemokine and activator in humans and has been previously associated with both severity of disease and poor clinical outcomes in adults and children with sepsis and septic shock.[10–13] In children with septic shock, a plasma IL-8 cut-off of less than 220 picograms per milliliter identified subjects with a low risk of death, with a negative predictive value of 94–95% for 28-day mortality in separate derivation and validation cohorts.[14] We hypothesized that plasma IL-8 levels would have similar utility in risk stratification in adults with septic shock and tested this hypothesis in patients with septic shock enrolled in large randomized clinical trials of acute lung injury (ALI).
MATERIALS AND METHODS
Clinical data and biological samples were obtained from patients enrolled in the NHLBI Acute Respiratory Distress Syndrome (ARDS) Network’s randomized controlled trials of lower tidal volume ventilation[15] and higher vs. lower positive end-expiratory pressure (PEEP).[16] Details of the original trials have been previously published in full.
Briefly, the first study enrolled 861 patients to test the hypothesis that ventilation with a low tidal volume, plateau pressure-limited strategy would reduce mortality in patients with ALI.[15] The trial demonstrated a significant reduction in 180-day mortality in the low tidal volume treatment group (31% vs 40%, p=0.007); once the benefit of the lower tidal volume strategy had been demonstrated, an additional 41 patients were assigned to the lower tidal volume to complete a factorialized trial of lisofylline versus placebo,[17] and these patients are also included in this analysis. Patients treated with the higher tidal volume strategy were excluded from the primary analysis due to the effect of higher tidal volume on mortality. Clinical data and plasma samples for biomarker measurements were collected at baseline prior to randomization; all patients were followed until death, 180 days or until discharge home with unassisted breathing, whichever occurred first.
The second study enrolled 549 patients to test the hypothesis that higher levels of PEEP would reduce mortality in patients with ALI.[16] Clinical data and plasma samples for biomarker measurements were collected at baseline prior to randomization; patients were followed until death, day 90, or discharge home with unassisted breathing. The data safety and monitoring board terminated the trial after enrollment of 549 patients on the basis of futility. The trial found no difference in 90-day mortality and no modulation of circulating interleukin-6 (IL-6), surfactant protein D (SP-D), or ICAM-1 with higher versus lower levels of PEEP.
The etiology of ALI was determined in both trials by the site investigator; in cases in which more than one potential cause of ALI was present, the site investigator then determined the principal cause. For this analysis, patients were defined as having septic shock if both of the following criteria were met: (1) the primary etiology of ALI was classified as either sepsis or pneumonia, and (2) the patient required vasopressor therapy in the 24 hours prior to randomization. Subjects missing data on vasopressor use over the prior 24 hours were considered not to have vasopressor-dependent shock. In a separate sensitivity analysis, we used an alternative time window to define septic shock, as follows: (1) the primary etiology of ALI was classified as either sepsis or pneumonia, and (2) the patient required vasopressor therapy at any point from the time of randomization until midnight the same day. Ventilator-free days were defined as the number of days between 0 and 28 that the patient was free from mechanical ventilation, if the patient tolerated at least 48 hours without mechanical ventilation.
Biological Assay
Plasma IL-8 levels were measured on baseline, pre-intervention samples using a commercially available enzyme-linked immunoassay (R&D Systems, Minneapolis, MN). Plasma measurements were made on the basis of plasma availability and have been previously described in other analyses.[18, 19]
Statistical Methods
Statistical analysis was performed with Stata 10.0 (College Station, TX). We used 28-day mortality as the primary endpoint of the study, as in prior studies of IL-8 in pediatric sepsis.[14] Pre-hoc power analysis demonstrated a power of 91% to detect an odds ratio for mortality of 4.0 or greater for an IL-8 cutoff of 220 pg/ml (as was demonstrated in the original pediatric study). Data from the two original ARDS Network studies was combined for this analysis. Categorical data was analyzed using the chi-squared test or Fisher’s exact test. Normally distributed continuous variables were analyzed using the t-test or analysis of variance. Non-parametric continuous variables were analyzed using the Kruskal-Wallis test or Wilcoxon ranksum. Pairwise comparisons after chi-squared, ANOVA, or Kruskal-Wallis tests were adjusted using the Bonferroni correction for multiple comparisons. Alpha was set at 0.05 for all analyses, and two-tailed tests of hypothesis were used throughout.
RESULTS
From the original cohort of 1451 subjects, 297 had evidence of vasopressor-dependent septic shock. After excluding patients treated with a higher tidal volume strategy (n=92), patients in whom plasma quantities were insufficient to measure IL-8 (n=22, 10 of whom were also treated with higher tidal volumes), and patients under age 18 (n=1), 192 subjects with vasopressor-dependent septic shock were included in this analysis. The clinical characteristics of these patients are compared with those of other trial participants, and with patients with vasopressor-dependent shock who were excluded due to missing IL-8 data or the use of higher tidal volumes, in Table 1. As compared with the remainder of the cohort, included subjects with vasopressor-dependent septic shock had significantly higher severity of illness scores and fewer ventilator-free days, as well as higher IL-8 levels. There were no significant differences between included and excluded patients with vasopressor-dependent septic shock. As in prior studies, plasma IL-8 was associated with severity of illness scores (APACHE III, r=0.23; p=0.002) and the number of organ failure free days (r=−0.23; p=0.002) in included subjects with septic shock.
Table 1.
Differences Between Included Subjects with Vasopressor-Dependent Septic Shock, Excluded Subjects with Vasopressor-Dependent Septic Shock, and Overall Cohort
| Characteristic | Included Patients with Vasopressor-Dependent Septic Shock (n=192) | Excluded Patients with Vasopressor-Dependent Septic Shock (n= 105) | Remainder of cohort (n=1154) | p-value |
|---|---|---|---|---|
| Age, years, mean±SD | 52±16 | 53±18 | 51±17 | 0.37 |
| Male gender | 93 (48) | 49 (47) | 470 (41) | 0.08 |
| Race/Ethnicity | ||||
| White, non-Hispanic | 133 (69) | 70 (67) | 870 (75) | |
| Black | 35 (18) | 22 (21) | 175 (15) | |
| Hispanic | 15 (8) | 8 (8) | 63 (6) | 0.07 |
| Asian/PI | 6 (3) | 5 (5) | 33 (3) | |
| Native American | 0 | 0 | 10 (1) | |
| Other | 3 (2) | 0 | 3 (0.3) | |
| AIDS | 18 (10) | 4 (4) | 62 (5) | 0.08 |
| Leukemia | 4 (2) | 4 (4) | 19 (2) | 0.19 |
| Lymphoma | 3 (2) | 1 (1) | 12 (1) | 0.71 |
| Metastatic cancer | 3 (2) | 4 (4) | 17 (2) | 0.18 |
| Immunosuppression | 22 (12) | 14 (14) | 146 (13) | 0.88 |
| Cirrhosis | 7 (4) | 5 (5) | 33 (3) | 0.38 |
| Diabetes | 38 (20) | 11 (11) | 160 (14) | 0.06 |
| Dialysis-dependent renal failure | 8 (4) | 2 (2) | 28 (3) | 0.37 |
| Pa02:Fi02 ratio, mean±SD | 119 ± 58* | 121 ± 51 | 131 ± 58 | 0.007 |
| APACHE III, mean | 105 ± 33** | 99 ± 27 | 83 ± 29 | <0.0001 |
| Mortality at 28 days | 63 (33)† | 46 (44) | 287 (25) | <0.001 |
| Ventilator-Free Days, median (IQR) | 8 (0, 21)†† | 3 (0, 20) | 16 (0, 24) | 0.0001 |
| Plasma IL-8, pg/ml, median (IQR) | 81 (30, 227)# | 83 (40, 234) | 35 (20, 79) | 0.0001 |
All values represent n (%) unless otherwise specified. Data was missing on 13 subjects for chronic health information, 1 subject for P:F ratio, 9 subjects for ventilator-free days, and 142 subjects for IL-8. Subjects with vasopressor-dependent septic shock were excluded for the following reasons: Use of high tidal volume ventilation, age < 18, or lack of IL-8 data. All p-values below are corrected for multiple comparisons.
Comparison with remainder of cohort: p=0.01; comparison with excluded patients with vasopressor-dependent septic shock: p=1.0
Comparison with remainder of cohort: p<0.001; comparison with excluded patients with vasopressor-dependent septic shock: p=0.39
Comparison with both remainder of cohort and excluded patients non-significant
Comparison with remainder of cohort: p=0.003; comparison with excluded patients with vasopressor-dependent septic shock: p=0.17
Comparison with remainder of cohort: p<0.0001; comparison with excluded patients with vasopressor-dependent septic shock: p=0.4
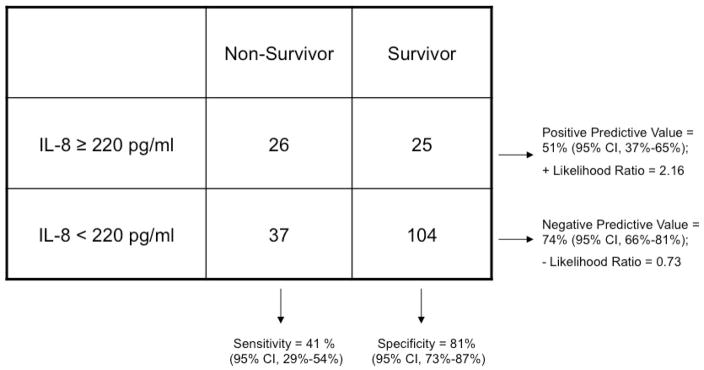
Given the demonstrated utility of a cutpoint of 220 pg/ml in subjects with pediatric septic shock, we dichotomized baseline plasma IL-8 levels as greater than/equal to or less than 220 pg/ml and tested the value of this cutoff for mortality prediction in this cohort (Table 2). Although the association between IL-8 levels ≥ 220 pg/ml and death was statistically significant (odds ratio 2.92, 95% CI 1.42–5.99; p=0.001), the sensitivity and negative predictive value compared unfavorably to the results in pediatric septic shock (Table 2). In particular, the negative predictive value of the plasma IL-8 cutoff, which was 94–95% in pediatric septic shock, was 74% (95% CI 66–81%) in this population.
Table 2.
Contingency Table Demonstrating Association Between High Plasma IL-8 Level (≥220 pg/ml) and Mortality at 28 days
 |
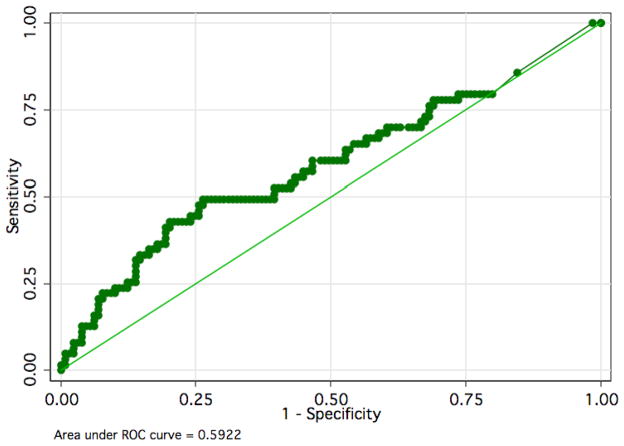
To determine whether the optimal cutpoint for plasma IL-8 levels might differ in adult subjects compared with pediatric subjects, we used receiver-operating characteristic analysis (Figure 1). This analysis demonstrated that plasma IL-8 provides poor discrimination in this population regardless of the exact cutoff used, with an area under the curve of 0.59 (95% CI 0.52–0.66).
Figure 1.
As a sensitivity analysis, we sought to determine whether vasopressor use nearer the time of plasma sampling would more accurately define the population of patients with septic shock. For this analysis, subjects were defined as having septic shock if they required vasopressors at any point from the time of randomization (and plasma sampling) until midnight the same day AND had ALI due to either sepsis or pneumonia. This definition generated a total of 206 patients with vasopressor-dependent septic shock. The use of this alternative definition for septic shock did not substantially affect the sensitivity, specificity, positive or negative predictive value of the IL-8 cutoff of 220 pg/ml. Likewise, the inclusion of patients ventilated with higher tidal volumes did not substantively affect the test performance characteristics (data not shown).
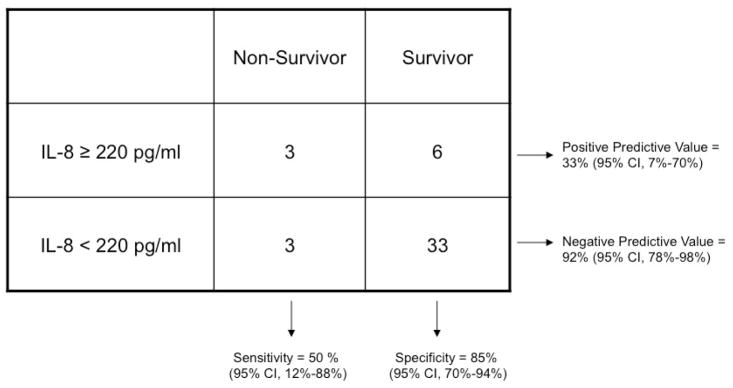
Since the prior data supporting an IL-8 cutoff of 220 pg/ml came from a pediatric population, we separately analyzed the value of this cutoff in subjects in our dataset between 18 and 40 years of age (Table 3). In this subgroup (n=45) with mortality of 13%, an IL-8 cutoff of 220 pg/ml had a negative predictive value of 92% (95% CI 78–98%). Likewise, the area under the curve in receiver operator characteristic analysis was 0.83 (95% CI 0.68–0.92), indicating fairly good discrimination in this subset of patients.
Table 3.
Contingency Table Demonstrating Association Between High Plasma IL-8 Level (≥220 pg/ml) and Mortality at 28 days in Subjects Under 40 Years of Age
 |
DISCUSSION
In contrast to prior studies in pediatric sepsis patients, we found that a plasma IL-8 of less than 220 pg/ml was not an effective tool for detecting older adult patients with septic shock at low risk of death at 28 days. This result contrasts sharply with the previously reported finding that this IL-8 cutoff had a high negative predictive value for mortality in pediatric septic shock patients.[14] While plasma IL-8 levels higher than 220 pg/ml were significantly associated with death at 28 days in this cohort, the association was much weaker than in the previous pediatric study, with a much smaller area under the receiver operator curve (0.59 compared with 0.86). In the subgroup of patients under age 40, an IL-8 cutoff of 220 pg/ml had a comparable negative predictive value to that previously reported in pediatric patients, though the size of this group was relatively small.
Why might the results of our study of plasma IL-8 in adult septic shock patients differ so considerably from those of the prior pediatric study? Several explanations are possible. First, the pathophysiology and predictors of mortality in older adult and pediatric septic shock may differ considerably. The finding that plasma IL-8 performed well in subjects under 40 years of age in our cohort supports this interpretation of the data. Mortality in adults with septic shock is strongly driven by the presence of underlying comorbidities such as AIDS, cancer, liver disease, and immunosuppression.[4] In contrast, in children with sepsis, acute severity of illness seems to be a stronger predictor of mortality, with many children previously healthy before developing septic shock.[20, 21] Likewise, the majority of adult patients with severe sepsis are 65 years of age or older, with increasing age a risk factor for death in adults but not in children.[22, 23] Also, decisions regarding end-of-life care and withdrawal of support likely differ substantially in adults and children, with major implications for mortality since 90% of deaths in adult ICUs are preceded by withdrawal of life support.[24, 25] These differences in the determinants of severity of illness and aggressiveness of support may significantly affect the performance of IL-8 as a predictor of 28 day mortality in older adult patients. Interestingly, among included patients with vasopressor-dependent septic shock, plasma IL-8 was as strongly associated with organ failure free days in adults under 40 as in older adults (data not shown), providing further suggestion that underlying co-morbidities and/or end of life decisions may be partially responsible for differences in IL-8’s prognostic value in these age groups.
Second, septic shock was not prospectively defined in the ARDS Network studies; rather, we used clinical data prospectively collected on the presence of sepsis or pneumonia, concurrent with the requirement for vasopressors, to define septic shock. This approach may result in a slightly different population than a prospective assessment for septic shock would have: in particular, the patients selected by our method may have more severe disease, thus affecting the prevalence of mortality in the sample and the negative predictive value as a result, as further detailed below.
Third, the timing of sampling relative to the onset of septic shock may differ in the two populations studied. In the original pediatric sepsis paper by Wong and colleagues, plasma IL-8 levels were drawn within 24 hours of ICU admission, an ideal time frame for the development of biomarker-based stratification tools.[14] In contrast, in the current study population, plasma IL-8 samples were drawn within 36 hours of the onset of acute lung injury; however, data on the timing of the onset of sepsis and/or pneumonia was unavailable. Although relatively little is known about the clearance and kinetics of plasma IL-8, levels may have declined rapidly after the onset of sepsis, making even small differences in timing of sampling relevant to the predictive value of IL-8.[26, 27]
Fourth, changes in the prevalence of a condition (in this case, an outcome) may affect the positive and negative predictive values of a diagnostic test; the more common a disease is in the population studied, the less likely a positive test result is to be a “false positive.” Put differently, increased prevalence of a condition is expected to lead to an increase in the positive predictive value of a diagnostic test and a similar decrease in the negative predictive value of the test.[28] While the mortality rate in pediatric severe sepsis is 10%,[22] mortality in adults with septic shock is 18–20% in general, and mortality in the patients we studied with septic shock was 33%.[29] Thus, our findings of a higher positive predictive value and lower negative predictive value of an IL-8 cutoff of 220 pg/ml in adults as compared to children align with the expected effect of the higher prevalence of mortality in this group. This result emphasizes the issues with generalizability that may arise when attempting to apply diagnostic test results to populations with differing prevalence of disease.
What implications does this “negative” result have for future study of biomarkers as risk stratification tools in clinical trials in the ICU? While plasma IL-8 certainly cannot be applied on the basis of these results as a screening tool for clinical trials in adult septic shock patients, the theoretical rationale for using a biomarker in this manner remains strong.[7] In oncology [8] and cardiovascular medicine,[30] biomarkers are routinely used as entry criteria for clinical trials with excellent results; thus, the search for similar markers in critical illness should not be abandoned. At the same time, our results point out the importance of studying diverse populations to insure the generalizability of a result before markers are employed in either clinical practice or clinical trials.
The strengths of our study include its multicenter nature, including a geographically diverse set of intensive care units across the US, the diversity of racial and ethnic groups and gender balance of the subjects, and the strength of the clinical data, which was collected within the framework of a randomized controlled trial and as such should be of high fidelity. This study has some limitations as well. First, as mentioned above, septic shock was not prospectively defined in the database we used. Both sepsis and vasopressor use were prospectively defined; moreover, sensitivity analyses varying the timing of vasopressor requirements yielded similar results to our primary analysis. Nevertheless, this retrospective definition applied to prospectively collected data may have altered the spectrum of disease we were able to capture. Second, by definition, all the patients we studied with septic shock also had acute lung injury, which may have affected the predictive value of IL-8 levels in this cohort. Although we excluded patients randomized to the higher tidal volume arm of the original ARDS Network trial, it is possible that baseline IL-8 levels could have been affected by pre-randomization ventilator strategy or other biologic processes unique to ALI, thus diluting their predictive value for septic shock-related mortality. Finally, the assay used to measure IL-8 in plasma differed between this study and the prior pediatric publication. The previous pediatric IL-8 study used an IL-8 ELISA kit from Biosource in some patients and a multiplexing platform (Flowmetrix) in others. Our study used an IL-8 ELISA purchased from R&D Systems. These different assays may have contributed to differences in absolute biomarker values between the two populations.
In conclusion, a plasma IL-8 cutoff of 220 pg/ml in adult patients with septic shock did not adequately identify patients at low risk of death at 28 days, in stark contrast to prior results in the pediatric septic shock population. While the future of using biomarkers as screening tools for clinical trials in the ICU remains bright, these results highlight the importance of replicating intriguing results in related but different patient populations.
Acknowledgments
NATIONAL INSTITUTES OF HEALTH
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE ARDS NETWORK
Network Participants: Cleveland Clinic Foundation, Herbert P. Wiedemann, M.D.,* Alejandro C. Arroliga, M.D., Charles J. Fisher, Jr., M.D., John J Komara, Jr., B.A., R.R.T., Patricia Periz-Trepichio, B.S., R.R.T.; Denver Health Medical Center, Polly E. Parsons, M.D., Denver VA Medical Center, Carolyn Welsh, M.D.; Duke University Medical Center, William J. Fulkerson, Jr., M.D.,* Neil MacIntyre, M.D., Lee Mallatratt, R.N., Mark Sebastian, M.D., John Davies, R.R.T., Elizabeth Van Dyne, R.N., Joseph Govert, M.D.; Johns Hopkins Bayview Medical Center, Jonathan Sevransky, M.D., Stacey Murray, R.R.T.; Johns Hopkins Hospital, Roy G. Brower, M.D., David Thompson, M.S., R.N., Henry E. Fessler, M.D.; LDS Hospital, Alan H. Morris, M.D.,* Terry Clemmer, M.D., Robin Davis, R.R.T., James Orme, Jr., M.D., Lindell Weaver, M.D., Colin Grissom, M.D., Frank Thomas, M.D., Martin Gleich, M.D. (posthumous); McKay-Dee Hospital, Charles Lawton, M.D., Janice D’Hulst, R.R.T.; MetroHealth Medical Center of Cleveland, Joel R. Peerless, M.D., Carolyn Smith, R.N.; San Francisco General Hospital Medical Center, Richard Kallet, M.S., R.R.T., John M. Luce, M.D.; Thomas Jefferson University Hospital, Jonathan Gottlieb, M.D., Pauline Park, M.D., Aimee Girod, R.N., B.S.N., Lisa Yannarell, R.N., B.S.N.; University of California, San Francisco, Michael A. Matthay, M.D.,* Mark D. Eisner, M.D., M.P.H., Brian Daniel, R.C.P., R.R.T.; University of Colorado Health Sciences Center, Edward Abraham, M.D.,* Fran Piedalue, R.R.T., Rebecca Jagusch, R.N., Paul Miller, M.D., Robert McIntyre, M.D., Kelley E. Greene, M.D.; University of Maryland, Henry J. Silverman, M.D.,* Carl Shanholtz, M.D., Wanda Corral, B.S.N., R.N., University of Michigan, Galen B. Toews, M.D.,* Deborah Arnoldi, M.H.S.A., Robert H. Bartlett, M.D., Ron Dechert, R.R.T., Charles Watts, M.D.; University of Pennsylvania, Paul N. Lanken, M.D.,* Harry Anderson, III, M.D., Barbara Finkel, M.S.N., R.N., C. William Hanson, III, M.D.; University of Utah Hospital, Richard Barton, M.D., Mary Mone, R.N.; University of Washington/Harborview Medical Center, Leonard D. Hudson, M.D.,* Greg Carter, R.R.T., Claudette Lee Cooper, R.N., Annemieke Hiemstra, R.N., Ronald V. Maier, M.D., Kenneth P. Steinberg, M.D.; Utah Valley Regional Medical Center, Tracy Hill, M.D., Phil Thaut, R.R.T.; Vanderbilt University, Arthur P. Wheeler, M.D.,* Gordon Bernard, M.D.,* Brian Christman, M.D., Susan Bozeman, R.N., Linda Collins, Teresa Swope, R.N., Lorraine B. Ware, M.D.
Clinical Coordinating Center: Massachusetts General Hospital, Harvard Medical School, David A. Schoenfeld, Ph.D.,* B. Taylor Thompson, M.D., Marek Ancukiewicz, Ph.D., Douglas Hayden, M.A., Francine Molay, M.S.W., Nancy Ringwood, B.S.N., R.N., Gail Wenzlow, M.S.W., M.P.H., Ali S. Kazeroonin, B.S.
NHLBI Staff: Dorothy B. Gail, Ph.D., Andrea Harabin, Ph.D.,*Pamela Lew, Myron Waclawiw, Ph.D.
*Steering Committee: Gordon R. Bernard, M.D., Chair; Principal Investigator from each center as indicated by an asterisk.
Data and Safety Monitoring Board: Roger G. Spragg, M.D., Chair, James Boyett, Ph.D., Jason Kelley, M.D., Kenneth Leeper, M.D., Marion Gray Secundy, Ph.D., Arthur Slutsky, M.D.
Protocol Review Committee: Joe G. N. Garcia, M.D., Chair, Scott S. Emerson, M.D., Ph.D., Susan K. Pingleton, M.D., Michael D. Shasby, M.D., William J. Sibbald, M.D.
Support: This work was supported by contracts (NO1-HR 46054, 46055, 46056, 46057, 46058, 46059, 46060, 46061, 46062, 46063, and 46064) with the National Heart, Lung, and Blood Institute (NHLBI). Dr. Calfee was supported by HL090833 and by the Flight Attendant Medical Research Institute, and by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130. Dr. Matthay was supported by HL 51856. Dr. Ware was supported by HL081332. Dr. Wong was supported by R01 GM064619
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- 1.Altman DG, Schulz KF, Moher D, Egger M, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 2.Altman DG. Statistics and ethics in medical research: III How large a sample? Br Med J. 1980;281:1336–1338. doi: 10.1136/bmj.281.6251.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glickman SW, McHutchison JG, Peterson ED, Cairns CB, et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360:816–823. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- 4.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 5.Lemeshow S, Klar J, Teres D. Outcome prediction for individual intensive care patients: useful, misused, or abused? Intensive Care Med. 1995;21:770–776. doi: 10.1007/BF01704747. [DOI] [PubMed] [Google Scholar]
- 6.Marshall JC, Reinhart K. Biomarkers of sepsis. Crit Care Med. 2009;37:2290–2298. doi: 10.1097/CCM.0b013e3181a02afc. [DOI] [PubMed] [Google Scholar]
- 7.Marshall JC. Biomarkers of sepsis. Curr Infect Dis Rep. 2006;8:351–357. doi: 10.1007/s11908-006-0045-1. [DOI] [PubMed] [Google Scholar]
- 8.Dowsett M, Dunbier AK. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res. 2008;14:8019–8026. doi: 10.1158/1078-0432.CCR-08-0974. [DOI] [PubMed] [Google Scholar]
- 9.Levitt JE, Gould MK, Ware LB, Matthay MA. The pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med. 2009;24:151–167. doi: 10.1177/0885066609332603. [DOI] [PubMed] [Google Scholar]
- 10.Van der Kaay DC, De Kleijn ED, De Rijke YB, Hop WC, et al. Procalcitonin as a prognostic marker in meningococcal disease. Intensive Care Med. 2002;28:1606–1612. doi: 10.1007/s00134-002-1505-1. [DOI] [PubMed] [Google Scholar]
- 11.Vermont CL, Hazelzet JA, de Kleijn ED, van den Dobbelsteen GP, et al. CC and CXC chemokine levels in children with meningococcal sepsis accurately predict mortality and disease severity. Crit Care. 2006;10:R33. doi: 10.1186/cc4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damas P, Canivet JL, de Groote D, Vrindts Y, et al. Sepsis and serum cytokine concentrations. Crit Care Med. 1997;25:405–412. doi: 10.1097/00003246-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bozza FA, Salluh JI, Japiassu AM, Soares M, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong HR, Cvijanovich N, Wheeler DS, Bigham MT, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med. 2008;178:276–282. doi: 10.1164/rccm.200801-131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 16.The Acute Respiratory Distress Syndrome Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 17.The Acute Respiratory Distress Syndrome Network. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Parsons PE, Eisner MD, Thompson BT, Matthay MA, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 19.Calfee CS, Eisner MD, Ware LB, Thompson BT, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35:2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009;124:87–95. doi: 10.1542/peds.2007-2462. [DOI] [PubMed] [Google Scholar]
- 22.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 23.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med. 1997;155:15–20. doi: 10.1164/ajrccm.155.1.9001282. [DOI] [PubMed] [Google Scholar]
- 25.Cook D, Rocker G, Marshall J, Sjokvist P, et al. Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. N Engl J Med. 2003;349:1123–1132. doi: 10.1056/NEJMoa030083. [DOI] [PubMed] [Google Scholar]
- 26.Darbonne WC, Rice GC, Mohler MA, Apple T, et al. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest. 1991;88:1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cepkova M, Brady S, Sapru A, Matthay MA, et al. Biological markers of lung injury before and after the institution of positive pressure ventilation in patients with acute lung injury. Crit Care. 2006;10:R126. doi: 10.1186/cc5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldessarini RJ, Finklestein S, Arana GW. The predictive power of diagnostic tests and the effect of prevalence of illness. Arch Gen Psychiatry. 1983;40:569–573. doi: 10.1001/archpsyc.1983.01790050095011. [DOI] [PubMed] [Google Scholar]
- 29.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Danielson E, Fonseca FA, Genest J, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]