Abstract
Although social withdrawal is a prominent symptom of sickness, the mechanisms associated with this behavioral change remain unclear. In animals, the amygdala is a key neural region involved in sickness-induced social withdrawal. Consistent with this, in humans, heightened amygdala activity to negative social cues is associated with social avoidance tendencies. Based on these findings, we investigated whether an experimental inflammatory challenge selectively increased amygdala activity to socially threatening images as well as whether this activity related to feelings of social disconnection. Thirty-nine participants were randomly assigned to receive either placebo or low-dose endotoxin, which increases inflammatory activity. Pro-inflammatory cytokines were assessed at 7 hourly time points via blood draws; self-reported feelings of social disconnection and physical sickness symptoms were assessed hourly as well. Two hours post-injection, participants underwent an fMRI procedure to assess amygdala reactivity during the presentation of socially threatening images (fear faces) as well as non-socially threatening images (guns), socially non-threatening images (happy faces), and non-social, non-threatening images (household objects). Endotoxin led to greater amygdala activity in response to socially threatening vs. all other types of images. No such differences were found for placebo participants. Additionally, increased amygdala activity in endotoxin participants during the viewing of socially vs. non-socially threatening images was associated with increased feelings of social disconnection. These findings highlight the amygdala as a neural region that may be important for sickness-induced social withdrawal. The implications of amygdalar involvement in sickness-induced social withdrawal are discussed.
Keywords: amygdala, inflammation, social, functional magnetic resonance imaging, cytokines, social withdrawal
Introduction
In response to illness or infection, organisms display a coordinated motivational response, termed “sickness behavior,” which includes symptoms such as fatigue, loss of appetite, hyperalgesia, and social withdrawal (Dantzer et al., 2008; Hart, 1988). Sickness behavior occurs in response to the release of proinflammatory cytokines, such as interleukin-6 (IL-6), which act directly on the brain to change behavior (Dantzer et al., 2008). These behavioral changes are considered to be a survival adaptation that motivates rest and recovery in order to cope with infectious pathogens (Dantzer et al., 2008; Hart, 1988). In addition, evolutionary analyses suggest that social withdrawal might play a role in limiting the spread of infectious diseases (Cole, 2006).
Although social withdrawal is commonly observed in response to inflammation, little is known about the neural mechanisms that lead people to withdraw socially when ill. Based on previous research, the amygdala is one neural region that may relate to sickness-induced sensitivity to social cues and social withdrawal. In animals, lipopolysaccharide (LPS)-induced inflammation increases activity in the amygdala (Frenois et al, 2007), which is associated with a reduction of certain social behaviors, such as grooming, sniffing, and close following with an interaction partner (Marvel et al., 2004). Furthermore, blocking activity in the amygdala by using a reversible lesion eliminates this inflammation-induced social withdrawal behavior (Marvel et al, 2004). In humans, the contribution of amygdala activity to sickness-induced social withdrawal has not been investigated, but substantial work has shown that social withdrawal tendencies, such as those observed in social phobia and inhibited temperament, are linked with heightened amygdala activity to negative and/or novel social cues (Blackford et al., 2010; Blair et al., 2008; Phan et al., 2006; Schwartz et al., 2003). Taken together, these findings suggest that the amygdala contributes to sickness-induced social withdrawal in humans.
The current study examined the role of the amygdala in inflammatory-induced sensitivity to socially threatening cues and social withdrawal in humans. Specifically, this study examined the effect of endotoxin, an inflammatory challenge safe for use in humans (Andreasen et al., 2008; Suffredini & O’Grady, 1999), vs. placebo on amygdala activity to socially threatening images (e.g., fearful facial expressions), which are known to elicit amygdala activity (Morris et al., 1996; Whalen et al., 2001). Amygdala activity to socially threatening images was compared with amygdala activity to: non-socially threatening images (e.g., spiders, weapons), socially non-threatening images (e.g. happy facial expressions), and non-social, non-threatening images (e.g., household objects). To the extent that inflammatory activity increases social withdrawal by heightening amygdala activity to socially threatening images, we hypothesized that endotoxin vs. placebo would lead to greater amygdala activity in response to the socially threatening images vs. all the other image types. Finally, amygdala activity to socially vs. non-socially threatening images and their relation to feelings of social disconnection was also examined. We hypothesized that greater amygdala activity in response to socially vs. non-socially threatening images would be associated with greater feelings of social disconnection.
Methods and Materials
Participants
Thirty-nine participants (mean age = 21.8+3.4 years; range: 18-36 years) were randomly assigned to receive either endotoxin (n=23, 12 females) or placebo (n=16, 8 females). All participants were confirmed to be in good health and scanner ready (metal-free, right-handed, not claustrophobic) during an initial telephone interview. The procedures outlined below have been described previously (Eisenberger et al., 2009, 2010a, 2010b), but are summarized below. Informed consent and procedures were carried out under the approval of UCLA’s Institutional Review Board.
After the telephone interview, participants were scheduled for an in-person interview for further screening. During this session, a trained interviewer administered the Structured Clinical Interview for DSM Disorders (SCID), then took height, weight, vitals, and a urine sample to test for drug use. Finally, blood was drawn for lab screening and, if female, to screen for pregnancy. Participants were excluded if they 1) had a BMI greater than 30, 2) reported physical health problems or medication use, 3) evidenced an Axis I psychiatric disorder based on the SCID assessment, 4) showed evidence of drug use from a positive urine test, 5) had a positive pregnancy test, if female, or 6) showed any abnormalities on their screening laboratory tests. Participants received $20 for completing this screening session. The final sample was 39% European-American, 18% Asian, 18% Hispanic, 7% African-American, and 18% “other”.
Procedure
The study used a randomized, double-blind, placebo-controlled design. Once participants arrived at the UCLA General Clinical Research Center (GCRC) a nurse inserted a catheter into the dominant forearm (right) for blood draws and one into the non-dominant forearm (left) for a continuous saline flush (to keep participants hydrated throughout the study) and for drug administration. Throughout the day, vital signs were assessed every half hour (except during the neuroimaging session) and blood draws were collected at baseline and then approximately every hour after that for the next six hours. In addition, participants completed self-report measures of physical sickness symptoms (e.g., muscle pain, nausea) and feelings of social disconnection (measures described below) with every blood draw.
Ninety minutes after arrival at the GCRC, each participant was randomly assigned to receive either endotoxin (0.8 ng/kg of body weight) or placebo (same volume of 0.9% saline). The endotoxin used in this study was derived from Escherichia coli (E. coli group O:113) and was provided by the National Institutes of Health Clinical Center as a reference endotoxin for studies of experimental inflammation in humans (Suffredini et al., 1999). No significant differences in age, years of education, or body weight were found between the two groups.
Approximately two hours post-injection, when proinflammatory cytokines have been shown to peak in previous studies (Krabbe et al., 2005; Reichenberg et al., 2001; Suffredini et al., 1999; Wright et al., 2005), participants completed a neuriomaging session. Thirty-six of the 39 participants completed this neuroimaging session (the first 2 participants did not complete the imaging session to ensure that all other procedures were running smoothly prior to the addition of the imaging component; one participant did not complete the imaging session because the scanner was non-operational). Participants were escorted to the UCLA Brain Mapping Center where they completed a task to assess amygdala reactivity to social and non-social images that were either threatening or non-threatening, among other tasks (Eisenberger et al., 2009; 2010b). Upon completion of the neuroimaging session, participants returned to the GCRC to complete the rest of the study procedures. Participants were discharged from the GCRC following the last blood draw upon approval from the study’s physician (M. I.). At the end of the study, participants were thanked, debriefed, and paid for their participation ($200).
Behavioral Assessments
Physical sickness symptoms
Subjects reported their physical sickness symptoms (headache, muscle pain, shivering, nausea, breathing difficulties, fatigue) at baseline and then hourly following the endotoxin or placebo administration for six hours. Participants rated the extent to which they felt the symptoms listed on a scale from 0 (no symptoms) to 4 (very severe symptoms).
Feelings of social disconnection
Feelings of social disconnection were also assessed hourly. Participants rated the extent to which they were feeling the “following feelings right now” on a 5-point Likert scale (1-not at all, to 5-very much so): (1) “I feel like being around other people,” (2) “I feel like being alone,” (3) “I feel overly sensitive around others (e.g., my feelings are easily hurt),” (4) “I feel connected to others,” and (5) “I feel disconnected from others.” Items 1 and 4 were reverse-coded, and scores were averaged at each time point to create a measure of self-reported social disconnection. The reliability of the scale (assessed at the time of peak response) was high (α=.84).
fMRI Paradigm
Amygdala responses were assessed as participants viewed four different block types: (1) socially threatening images, (2) non-socially threatening images, (3) socially non-threatening images, and (4) non-social, non-threatening images. Participants were instructed to view the images and to press a button with their index finger each time a new image appeared on the screen. Button pressing was used to ensure that participants were attending to the task. Social images were taken from a set of standardized facial expressions (Tottenham et al., 2009) and included pictures of people making fearful facial expressions (socially threatening images) and people making mildly happy expressions (close-mouthed smiles; socially non-threatening images). Non-social images included threatening scenes such as pictures of snakes, spiders, and guns (non-socially threatening images) and more neutral images of fish, household items, and cars (non-social, non-threatening images). The non-social images were selected from the International Affective Picture System (IAPS; Lang et al., 1999).
Participants viewed a total of 8 30-second blocks, 2 blocks of each of the four image types. Participants saw 20 images per block for 1.5 secs each followed by 18 seconds of rest in which they viewed a fixation crosshair. Each participant saw one of four scripts; scripts were counterbalanced across participants. Each script presented the blocks in a different pseudorandom order. For the purposes of this study, all conditions were compared to blocks of fixation crosshair in order to have a single control condition for each condition of interest.
fMRI Data Acquisition and Data Analysis
Data were acquired on a Siemens Allegra 3T head-only scanner. Head movements were restrained with foam padding and surgical tape placed across the forehead. For each participant, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo; TR=5000ms; TE=33ms; matrix size 128x128; 36 axial slices; FOV=20-cm; 3-mm thick, skip 1-mm) was acquired coplanar with the functional scans. One functional scan, lasting 6 minutes and 34 seconds, was acquired (echo planar T2*-weighted gradient-echo, TR=2000ms, TE=25ms, flip angle=90°, matrix size 64x64, 36 axial slices, FOV=20-cm; 3-mm thick, skip 1-mm).
The imaging data were analyzed using SPM5 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Images for each subject were realigned to correct for head motion, normalized into a standard stereotactic space, and smoothed with an 8mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio. The design was modeled as a block design using a boxcar function convolved with a canonical hemodynamic response function. For each participant, each block type was modeled separately. After the task was modeled for each participant, planned comparisons were computed as linear contrasts to investigate neural activity separately during each of the four types of imagescompared to baseline (fixation crosshairs). Random effects analyses of the group were computed using the contrast images generated for each participant.
Based on a-priori predictions regarding the amygdala’s sensitivity to socially threatening images, region-of-interest (ROI) analyses focusing on the left and right amygdala were performed. ROIs were structurally defined using the Automated Anatomical Labeling (AAL; Tzourio-Mazoyer et al., 2002) Atlas. Parameter estimates were extracted from the left and right amygdala ROIs using MarsBar (Brett et al., 2002). To examine the effect of inflammation on neural activity, parameter estimates from these ROIs were submitted to a 2 (condition: endotoxin vs. placebo) x 2 (social: social vs. non-social) x 2 (threat: threatening vs. non-threatening ) x 2 (hemisphere: left vs. right amygdala) mixed analysis of variance (ANOVA) in SPSS. Gender was included as an additional variable in a separate model; however, because there were no gender differences in amygdala activity and no interactions between gender and any other variables, all further analyses collapsed across gender. Based on the prediction that inflammation would selectively increase amygdala activity to socially threatening images, we predicted a 3-way interaction (condition x social x threat), such that the greatest amygdala activity would be observed to the socially threatening images in the endotoxin participants. Significant 3-way interactions were followed up with repeated measures ANOVAs for each condition (endotoxin, placebo) separately followed by simple t-tests to further examine the direction of these effects. Because no specific directional hypotheses for hemisphere or gender were made, p-values reported below are based on two-tailed tests.
We also examined whether greater amygdala activity in response to socially vs. non-socially threatening images was associated with greater increases in self-reported feelings of social disconnection (taken from baseline to two hours post-injection, immediately prior to the scanning session). To examine this, correlational analyses were run for each group separately (endotoxin, placebo) as well as for both groups together (p< .05, one-tailed for specific directional hypotheses). Follow-up analyses examined whether these effects remained after controlling for increases in self-reported sickness symptoms (across the same time interval).
In addition, there was one outlier in left and right amygdala activity in response to viewing socially threatening images (vs. baseline) in the endotoxin group (> 3SDs above the mean) and one outlier in left and right amygdala activity in response to viewing non-social, non-threatening images (vs. baseline) in the placebo group (>3SDs below the mean). To limit the influence of these outliers while still maximizing sample size, these data points were winsorised (moved to 3 SDs from the sample mean without the outlier included) and included in the final sample. It should be noted, however that none of the reported results change significantly when these outliers were removed.
Results
Behavioral Analyses
As reported previously (Eisenberger et al., 2009, 2010a, 2010b), endotoxin (vs. placebo) led to significant increases over time in proinflammatory cytokines (IL-6, TNF-α), vital signs (body temperature, pulse), physical sickness symptoms, and feelings of social disconnection.
Effect of Condition and Stimulus Type on Amygdala Activity
To test whether inflammation selectively increased amygdala activity (in anatomically defined ROIs) to socially threatening images, a 2 (condition: endotoxin vs. placebo) x 2 (social: social vs. non-social) x 2 (threat: threatening vs. non-threatening) x 2 (hemisphere: right vs. left amygdala) repeated measures ANOVA was conducted. There was a marginal main effect of condition (F(1, 34)=3.72, p=.06) with a greater amygdala response in the endotoxin than the placebo participants and a main effect of hemisphere (F(1,34)=10.46, p=.003) with greater left than right amygdala activity. Additionally, participants displayed more amygdala activity to the social than non-social images (F(1, 34)=6.97, p = .01) and the threatening than non-threatening images (F(1,34)=13.82, p = .001). Most importantly and consistent with hypotheses, a three-way interaction (condition x social x threat) was found (F(1,32)=7.30, p = .01).
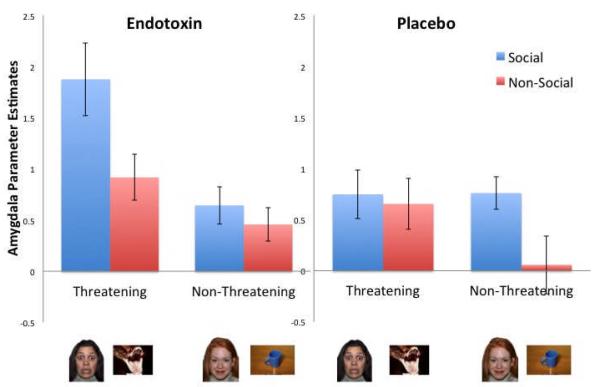
To further examine the pattern of this three-way interaction, a 2 (social: social vs. non-social) x 2 (threat: threatening vs. non-threatening) repeated-measures ANOVA was performed separately for the endotoxin and placebo groups. (Because there were no significant interactions between these variables and hemisphere (left vs. right), analyses collapsed across hemisphere.) As expected, there was a significant interaction between the social and threat factors in the endotoxin group (F(1, 18)=10.63, p =.004), but not in the placebo group (p=.20) (Figure 1). Further analysis of the interaction in the endotoxin group revealed that amygdala activity to the socially threatening stimuli was greater than amygdala activity to the other three conditions (p’s<.005). However, for the placebo group, amygdala activity to the socially threatening stimuli did not differ from the other three conditions (p’s>.10).
Figure 1.
Neural activity from the average of left and right amygdala regions of interest (ROIs) during the viewing of social and non-social, threatening and non-threatening images for endotoxin and placebo participants.
Thus, inflammatory activity appears to specifically increase amygdala responsivity to socially threatening images. As another way of illustrating this, when comparing neural responses between the endotoxin and placebo groups, the only difference in amygdala activity occurred in response to the socially threatening images (t(34) = 2.5, p = .02). Thus, endotoxin vs. placebo subjects showed significantly more amygdala activity during the socially threatening images. There were no other differences between the two groups in amygdala activity to any of the other conditions (p’s > .21).
Correlation between Amygdala Activity and Self-Reported Social Disconnection
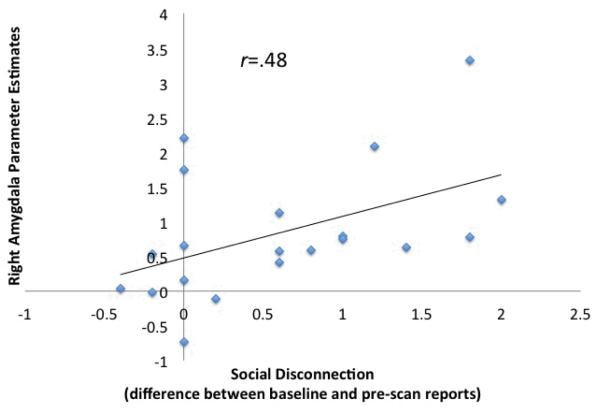
To examine whether amygdala activity in response to socially threatening vs. non-socially threatening images was related to feelings of social disconnection in endotoxin subjects, correlations tested the association between amygdala activity and changes in feelings of social disconnection (from baseline to two hours post-injection, immediately prior to the imaging session). As hypothesized, right amygdala activity during viewing of socially vs. non-socially threatening images correlated positively with increased feelings of social disconnection, such that those who showed the greatest right amygdala activity also showed the greatest increase in feelings of social disconnection (r=.48, p=.02; Fig. 2). This correlation remained after controlling for self-reported physical sickness symptoms (r =.37, p=.05). A similar pattern emerged when investigating the full sample (both endotoxin and placebo subjects together) (r=.44, p=.004), and these effects remained marginally significant after controlling for physical sickness symptoms (r=.23, p=.06). Correlations with the left amygdala, though in the right direction were not significant after controlling for sickness symptoms (p’s > .14). Finally, when just looking at the placebo subjects alone, feelings of social disconnection were not associated with either left or right amygdala activity (p’s>.2), suggesting that these effects may be specific to inflammation-related processes.
Figure 2.
Correlation between self-reported feelings of social disconnection and right amygdala activity during socially threatening compared to non-social threatening images for the endotoxin subjects.
Discussion
In line with our hypothesis, subjects exposed to an inflammatory challenge, compared to placebo participants, showed a selective increase in amygdala activity to socially threatening images (relative to all other image types). Moreover, among endotoxin-exposed participants, greater amygdala activity in response to socially vs. non-socially threatening images was associated with greater increases in self-reported feelings of social disconnection. Together, these results highlight a possible role for the amygdala in social withdrawal during sickness and are largely consistent with previous animal work on the role of the amygdala in sickness-induced social withdrawal.
These results are also consistent with work linking heightened amygdala activity in response to negative social cues with social withdrawal tendencies. For instance, relative to healthy controls, individuals with social phobia–who tend to fear and avoid certain social situations–display heightened amygdala activity to negative faces (Bruhl et al., 2011; Evans et al., 2008; Yoon et al., 2007; Phan et al., 2006). Moreover, the extent of amygdala activity to these faces correlates positively with the severity of the social anxiety symptoms (Bruhl et al., 2011; Phan et al., 2006). Taken together, these findings highlight the role of amygdala reactivity in social withdrawal tendencies more generally and in inflammatory-induced social withdrawal more specifically.
The results from the current study also shed light on another possible function of social withdrawal behavior. The most commonly described function of social withdrawal is to promote recovery from illness or infection. However, increased amygdala activation in response to inflammation runs counter to a purely rest-facilitating motive, as amygdala activity is often associated with an activated response to fearful, threatening, or high arousal stimuli (Adolphs et al., 1999; Feinstein et al., 2011; Hariri et al., 2002; Whalen et al., 2001). Thus, inflammatory-induced amygdala activity to negative social cues may be more directly related to a second proposed function of sickness-induced social withdrawal, namely to prevent the spread of infection by minimizing contact with others (Cole, 2006). Specifically, increased amygdala activity to socially threatening stimuli may lead to avoidance of those stimuli and social withdrawal, thus preventing the spread of infection.
Indeed, according to an epidemiologic simulation of disease transmission within typically structured human social networks, reducing contact with just 10% of one’s social network can increase the survival rate of the larger population by more than half (Cole, 2006). In addition, these protective effects are amplified when sick individuals selectively withdraw contact from their most socially distant, low-frequency interaction partners, but not from their closer network members (Cole, 2006). This may occur for two reasons. First, restricting social withdrawal to distant, but not close, interaction partners prevents large jumps of disease through social space. Second, restricting withdrawal to socially distant, rather than close, individuals may increase the likelihood that the sick individual will receive care and help from their close social network members. Thus, there may also be a survival advantage associated with not withdrawing from close others while sick in order to elicit care and help from them. Although the current study did not specifically examine neural responses to images of socially distant vs. close network members, it is possible that threatening faces were interpreted as socially distant whereas smiling faces (the socially non-threatening stimuli) were interpreted as potential sources of care and help, similar to socially close individuals. Additional research that includes neutral facial expressions may help disentangle the effect of withdrawal from socially threatening vs. socially inviting faces, as neutral faces–if interpreted as socially distant–would be expected to elicit amygdala activation, similar to that seen in response to fearful facial expressions. Future work, however, will be needed to more directly examine whether increased amygdala activity to socially threatening vs. socially inviting faces does indeed increase social withdrawal behavior thereby reducing the spread of infection in a social network.
In sum, findings from the current study highlight a neural mechanism by which social withdrawal, a major but understudied feature of sickness behavior, increases following inflammation. In addition, the findings may highlight another possible function of social withdrawal behaviors (Cole, 2006)–namely to prevent the spread of infection to others thereby increasing the survival chances of social groups; however, more work will need to directly test this hypothesis.
Acknowledgments
Research was funded by a NARSAD Young Investigator Award, a Dana Foundation grant, a UCLA Faculty Senate Grant, and a postdoctoral research fellowship (T32-MH19925) to N.I.E. The authors wish to thank the staff and support of the UCLA General Clinical Research Center, Anthony Suffredini, M. D. and George Grimes, R. P. at the National Institute of Health, Warren Grant Magnuson Clinical Center, for providing standard reference endotoxin and Thanh Luu and Elizabeth Breen for completing the cytokine assays. Additionally, the authors acknowledge Grants HL-079955, AG-026364, CA-10014152, CA-116778, P30-AG028748, M01-RR00865, and the UCLA Cousins Center at the Semel Institute for Neurosciences, the UCLA Claude D. Pepper Older Americans Independence Center Inflammatory Biology Core, and the General Clinical Research Centers Program (M01-RR00865).
Footnotes
Financial Disclosures The authors report no financial gain or conflicts of interest.
References
- Adolphs R, Russell JA, Tranel D. A role for the human amygdala in recognizing emotional arousal from unpleasant stimuli. Psychol. Sci. 1999;10:167–171. [Google Scholar]
- Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Møller K. Human endotoxemia as a model of systemic inflammation. Curr. Med. Chem. 2008;15:1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Soc. Cogn. Affect. Neurosci. 2010;5:1–9. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney DS, Blair RJ, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am. J. Psychiatry. 2008;165:1193–202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2-6, 2002; 2002. Available on CD-ROM in NeuroImage. [Google Scholar]
- Brühl AB, Rufer M, Delsignore A, Kaffenberger T, Jäncke L, Herwig U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- Cole SW. The complexity of dynamic host networks. In: Deisboeck TS, Kresh JY, editors. Complex Systems Science in BioMedicine. Kluwer Academic; New York: 2006. pp. 605–629. [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry. 2010;68:748–54. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection. Brain Beh. Imm. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress. Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Curr. Biol. 2011;21:34–8. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–31. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–23. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Reichenberg A, Yirmiya R, Smed A, Pedersen BK, Bruunsgaard H. Low-does endotoxemia and human neuropsychological functions. Brain Behav. Immun. 2005;19:453–60. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. University of Florida, The Center for Research in Psychophysiology; Gainsville: 1999. [Google Scholar]
- Marvel FA, Chen CC, Badr N, Gaykema RP, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav. Immun. 2004;18:123–34. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response to the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol. Psychiatry. 2006;59:424–9. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–3. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, Hochstein HD, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J. Infect. Dis. 1999;179:1278–1282. doi: 10.1086/314717. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, O’Grady NP. Pathophysiological responses to endotoxin in humans. In: Morrison D, editor. Endotoxin in Health Diseases. Marcel Dekker Inc.; New York: 1999. pp. 817–830. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot B. Automated anatomical labeling of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear vs. anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon I, Steptoe A. Acute inflammation and negative mood: Mediation by cytokine activation. Brain Behav. Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Res. 2007;154:93–8. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]