Abstract
Previously, published studies have reported mixed results regarding the role of the TRPM5 cation channel in signaling sweet taste by taste sensory cells. Some studies have reported a complete loss of sweet taste preference in TRPM5 knockout (KO) mice, whereas others have reported only a partial loss of sweet taste preference. This study reports the results of conditioned aversion studies designed to motivate wild-type (WT) and KO mice to respond to sweet substances. In conditioned taste aversion experiments, WT mice showed nearly complete LiCl-induced response suppression to sucrose and SC45647. In contrast, TRPM5 KO mice showed a much smaller conditioned aversion to either sweet substance, suggesting a compromised, but not absent, ability to detect sweet taste. A subsequent conditioned flavor aversion experiment was conducted to determine if TRPM5 KO mice were impaired in their ability to learn a conditioned aversion. In this experiment, KO and WT mice were conditioned to a mixture of SC45647 and amyl acetate (an odor cue). Although WT mice avoided both components of the stimulus mixture, they avoided SC45647 more than the odor cue. The KO mice also avoided both stimuli, but they avoided the odor component more than SC45647, suggesting that while the KO mice are capable of learning an aversion, to them the odor cue was more salient than the taste cue. Collectively, these findings suggest the TRPM5 KO mice have some residual ability to detect SC45647 and sucrose, and, like bitter, there may be a TRPM5-independent transduction pathway for detecting these substances.
Keywords: animal psychophysics, conditioned flavor aversion, conditioned taste aversion, olfaction
Introduction
The capability to taste is one of our most crucial senses, enabling an animal to identify and select what they ingest. For example, umami tastes may signal the presence of protein, and sweet may signal the presence of carbohydrates, whereas bitter often indicates the presence of toxic substances. Understanding the mechanisms through which these tastes are perceived is fundamental to understanding how food choice may be influenced and has important implications for clinical populations with dietary challenges, such as diabetics and the elderly.
Substances that humans perceive as sweet, bitter, or umami are generally detected by Type II taste sensory cells via G-protein–coupled receptors (GPCRs) (Roper 2007). Activation of taste GPCRs causes activation of the coupled G-protein gustducin (McLaughlin et al. 1992), and, in turn, gustducin’s βγ subunit activates phospholipase Cß2, which cleaves phosphatidylinositol 4,5-bisphosphate into inositol trisphosphate and diacylglycerol (Rossler et al. 1998; Liu and Liman 2003; Zhang et al. 2003). Inositol triphosphate then binds to the inositol triphosphate receptor on the membrane of the endoplasmic reticulum, resulting in the release of internal Ca2+ stores (Bernhardt et al. 1996; Spielman et al. 1996; Clapp et al. 2001). Ca2+ binds to transient receptor potential channels, subfamily M, member 5, or TRPM5 channels. These are nonselective monovalent cation channels that, when open, allow the influx of Na+ to depolarize the cell (Liu and Liman 2003; Perez et al. 2003; Damak et al. 2006). Depolarization likely releases adenosine triphosphate to bind to receptors on the afferent gustatory nerve or to Type III or presynaptic cells (Finger et al. 2005; Kinnamon 2009).
Much of the initial interest in TRPM5 channels focused on their role in bitter taste. Early studies on TRPM5 knockout (KO) mice developed by Zhang et al. (2003) in San Diego found that these mice lacked all responses (behavioral and nerve responses) to bitter substances. However, Damak et al. (2006), using KO mice they developed independently at Mount Sinai, showed that these mice displayed behavioral and nerve responses to bitter substances, although these responses were smaller than those of wild-type (WT) mice. Oliveira-Maia et al. (2009) also demonstrated the importance of TRPM5 in bitter taste detection, in this case using nicotine. They found that TRPM5 KO mice still showed chorda tympani nerve responses to nicotine, but they were greatly reduced compared with WT mice. Moreover, TRPM5 KO mice are able to develop a preference for and regulate the dietary intake of sucrose and glucose (Ren et al. 2010). Collectively, these studies suggest that at least some bitter tastes and possibly other substances may involve TRPM5-dependent as well as TRPM5-independent pathways.
The importance of the TRPM5 channel in signaling the presence of sweet stimuli is still not clear. TRPM5 channels appear to have a role for signaling sweet substances but studies, employing 2-bottle or brief-access behavioral methods and nerve recordings, have yielded somewhat mixed results for KO mice. Like bitter, Zhang et al. (2003) did not detect a response to sucrose or SC45647 (an artificial sweetener) with their KO mice, nor did they see a response by the chorda tympani nerve for either substance. Their data suggest that signaling for these 2 substances is solely dependent upon a TRPM5 pathway. On the other hand, Damak et al. (2006) using 2-bottle preference tests reported only a reduced response to sucrose and to SC45647 by their TRPM5 KO mice. However, in their procedures, the mice were tested first with sucrose and then with SC45647. Sclafani et al. (2007, 2010) found that KO mice can show a preference for sucrose after having experienced the postingestive effects of sucrose. Given the order of testing, it is possible that the preferences for both taste substances might have been affected by the exposure of these mice to the postingestive effects of sucrose. Integrated chorda tympani nerve recordings of Damak et al. (2006) showed a reduced response to sucrose, to several other natural sweeteners, and to saccharin but no detectable response to SC45647. In addition, recordings of the glossopharyngeal nerve were unable to detect a significant response to either sucrose or SC45647. Ohkuri et al. (2009) also reported chorda tympani nerve responses to sucrose and glucose in TRPM5 mice, although they were weaker than WT mice. In sum, it is not clear from these reports whether mice with the TRPM5 channel genetically deleted are still capable of detecting substances that elicit a sweet taste in humans.
Behavioral studies of the role of TRPM5 in signaling sweet substances have been limited mostly to brief-access testing. These methods are capable of detecting the hedonic value of a taste stimulus, but they are not necessarily sensitive to other attributes of a taste stimulus. To further explore the capacity of TRPM5 KO mice to detect sweet substances, TRPM5 KO mice developed at Mount Sinai were obtained from R.F.M. for testing with conditioned taste aversion (CTA) methods. CTA methods were chosen because the precise effect of the KO on taste is unknown. That is, a genetic KO of a taste receptor or downstream signaling channel such as TRPM5 may alter how the taste is perceived or abolish signaling for the taste all together. For example, it is possible that a taste signal is still being transmitted, but the KO eliminates some component of a taste quality without eliminating all of the quality or it weakens or eliminates any hedonic information (i.e., there is a taste sensation, but it is not necessarily attractive or aversive). CTA methods motivate the mouse to assign a negative hedonic value to the taste substance serving as the conditioned stimulus (CS), even when the substance might not be capable of eliciting an innately positive hedonic response or has only a weak hedonic value for the mouse. To assess the role of the TRPM5 channel in sweet taste, we examined both a natural sweetener (sucrose) and an artificial sweetener (SC45647) using CTA methods with concentrations at the lower end of the range from which Damak et al. (2006) found responses. The advantage of using SC45647 is that it has no known postingestive effect on sweetener preference over a wide range of concentrations. Postingestive effects must be considered in these types of experiments because, as Sclafani and Glendinning (2003, 2005) and Sclafani et al. (2007, 2010) have shown with 24-h 2-bottle tests, sugars in particular can cause physiological changes after consumption that affect the preference for the taste solution and may alter the effectiveness of conditioning. Because the KO mice exhibited a weak CTA to each sweet stimulus compared with WT mice, KO mice were also tested with a conditioned flavor aversion (CFA) to determine if they had deficits in their ability to learn a conditioned aversion. The terms sweet and bitter are based on human perceptional experiences and are used for convenience to describe the substances used to test taste sensations of mice.
Materials and methods
Subjects
Breeding pairs of TRPM5−/− KO mice, developed from C57BL/6J embryonic stem cells and maintained in that background, were obtained from R.F.M. and maintained in a separate breeding colony from the WT mice. The targeting construct, described by Damak et al. (2006), had the entire Trpm5 gene removed, including the promoter region, exons 1–4, and the translation start site within exon 2. Polymerase chain reaction verification of the KO was conducted on randomly selected mice to ensure the genetic status of the mice. Once the pups were weaned, they were moved to the colony used to maintain all mice involved in behavioral experiments. WT C57BL/6J mice were obtained at 40–50 days of age from Taconic Farms and maintained in the same colony room with the KO mice. None of the mice were put on water deprivation until they were at least 70 days of age. All mice were acclimatized to a 22-h water-deprivation schedule for at least 10 days before the first experiment. Throughout the experiment, all mice were allowed 1 h of water access in the home cage each day beginning 30 min after the end of each 15-min session. The mice were maintained on a 12:12 h light:dark cycle. All testing occurred at the same time each day during the light portion of the cycle. Food (Purina Lab Chow) was available ad libitum on the home cage. All procedures were approved by the University of Vermont Institutional Animal Care and Use Committee.
Apparatus
All CTA training and testing took place in a computer-controlled lickometer (Davis MS160, DiLog Instruments). The apparatus consisted of a chamber with clear Plexiglas on the sidewalls and a stainless steel wall with an opening at one end. A shutter covered the opening until the computer opened it to allow brief access to the sipper tube of a bottle. These bottles, containing stimulus solutions, were placed in a tray, which can be moved laterally by the computer. Once the computer positioned the assigned bottle, it opened the shutter to give the mouse access to the sipper tube and then counted each contact with the sipper tube. The diameter of the opening of the sipper tube was 2.5 mm.
Procedure
CTA experiments
These aversion experiments were designed to determine if TRPM5 KO mice were able to detect 2 substances that elicit a sweet sensation in humans. In one experiment, WT and KO mice were tested after conditioning with 300 mM sucrose as the CS and as the results reported below suggest, KO mice showed some ability to avoid sucrose when paired with LiCl. In view of the potential for postingestive effects of sucrose to act as a CS (Sclafani and Glendinning 2003, 2005; Sclafani et al. 2007), a second experiment was conducted using 0.5 mM SC45647, an artificial sweetener which does not appear to have postingestive metabolic effects at this concentration, as a CS. The methods used in each experiment were the same except for the CS presented on conditioning day. Therefore, the procedures for these 2 experiments are presented together.
Throughout each experiment, each session lasted until 34 stimuli had been presented or 15 min elapsed, whichever occurred first. For each experiment, 32 water-deprived mice (16 WT and 16 KO) were trained to lick water for 5 days to ensure consistent licking. On day 6, mice were presented with the stimulus assigned as the CS. During these sessions, an equal number of CS solutions were randomly intermixed with water presentations. Immediately following exposure to the CS, the mice were randomly selected to receive an IP injection of either LiCl (225 mM; 0.1 ml/10 g body weight) to induce gastric distress or 150 mM saline (control). The next day was a recovery day in which the mice were presented only water in the test apparatus. On day 8, conditioning procedures were repeated. All mice licked during at least 10 of the CS trials during the first session and at least 7 trials during the second conditioning session. The next 2 days were recovery days in which water-only trials were presented to extinguish contextual conditioning and stabilize the motivational states of the mice. On day 11, the mice were tested with an array of 6 test solutions. The mice were then given one more day with water-only presentations. The next day, a second test session was conducted using the same array of solutions.
Preliminary CTA data indicated that WT mice were capable of responding to SC45647 at concentrations of 0.01 mM or less. These data also suggested that KO mice could detect SC45647 at concentrations between 0.01 and 0.05 mM and sucrose at 25 mM or possibly lower. For the CTA experiments, 2 concentrations of each substance were selected such that one should be well above aversion threshold and a second should be near the aversion threshold. Consequently, 6 solutions were tested during each test session: 1) water, 2) 0.05 and 0.5 mM SC45647, 3) 30 and 300 mM sucrose, and 4) 75 mM NaCl. This selection enabled an evaluation of the strength of aversive conditioning to the CS and the degree to which the aversion generalized to the opposite sweet substance. NaCl, a taste stimulus signaled by different transduction mechanisms (Roper 2007), was used to determine if the aversion was specific for the CS or a neophobic response (Spector and Grill 1988). Once the shutter opened to expose the sipper tube, the mouse had 60 s to lick or the shutter closed, a score of zero was registered, and the computer initiated the next intertrial interval. If the mouse began to lick the sipper tube, the computer counted all contacts with the sipper tube for 5 s before closing the shutter. During each test session, the first 3 stimulus presentations were always water trials before each of 2 consecutive blocks of trials were presented. Each stimulus solution was tested twice, once in each block. The order of stimulus presentations was randomized within a block for each mouse using a modified Latin square procedure. Each stimulus was separated by 1–2 water rinse trials. Because water was presented frequently, 3 of the 8 tubes contained water to minimize the possibility of the mouse associating a single tube with water. Intertrial intervals were 5 s. All solutions were mixed fresh in deionized water (Millipore) on conditioning and test days.
CFA experiment
The TRPM5 KO mice showed a weakened ability to form an aversion to either sucrose or SC45647. This raises a question about whether TRPM5 KO mice have a learning impairment, which could interfere with their ability to learn a conditioned aversion. To examine this issue, WT and KO mice from the CTA experiments were tested in a CFA experiment to determine whether the TRPM5 KO mice can form a conditioned aversion to a CS that included nontaste cues. At least 3 weeks after the completion of the initial CTA experiments, 4 mice from each group in the CTA experiments were randomly selected for the CFA experiment. Training, conditioning, recovery, and testing procedures followed the same daily cycle as the first experiment, except that the mice were conditioned using a mixture of 0.1 mM SC45647 and 0.001% amyl acetate (a “banana” odor) as the CS. This mixture was used successfully as a flavor mixture to assess taste functions of P2X2/P2X3Dbl−/− mice in a previous study (Eddy et al. 2009). SC45647 was used as the taste stimulus to minimize any potential postingestive effects and other oral sensations that might accompany other taste stimuli, for example, aversive properties of citric acid (Finger et al. 2005) and osmotic effects of NaCl. Because all the mice in this experiment had already been conditioned with either SC45647 or sucrose, lower concentrations of SC45647 were selected to minimize the potential of the taste stimulus to be much more salient than the odor component of the mixture. The concentrations of amyl acetate selected for conditioning and testing are well above absolute thresholds in WT mice (Song et al. 2008; Van Houten et al. 2008) and have little effect on taste detection (Slotnick et al. 1997). Those KO and WT mice conditioned with LiCl in the first experiment were also conditioned using LiCl in the CFA experiment. Likewise, mice initially conditioned using saline in the first experiment were conditioned with saline in this experiment. During each test session, the lick rates of the WT and TRPM5 KO mice were measured when presented with 1) water (0 mM), 2) SC45647 (0.02 and 0.1 mM), 3) amyl acetate (0.0005% and 0.001%), and 4) the stimulus mixture (0.1 mM SC45647 + 0.001% amyl acetate).
Data analysis
To minimize a bias resulting from shifting motivational states during the session and to help minimize postingestive cues, scores were included for analysis only as long as the mouse continued to lick during the water rinse trials. More specifically, if the mean lick rate of 3 consecutive water trials dropped below 40% of the mean lick rate of the first 3 water trials or if 10 min had elapsed after the beginning of the first trial (whichever came first), then the data for the trials which followed were excluded from any data analyses. In the CTA experiments, any trial without a lick response was not included in the data set for statistical analyses, but they were counted separately to determine their frequency. Because the mice were trained to avoid an odor cue combined with a taste cue in the CFA experiment, any trial with zero licks within the set of trials meeting the criteria above was given a score of 0.5. Before analyzing the data for the CTA or CFA experiments, the lick rates for each subject were normalized to minimize the impact of differences in basal lick rates or differences in initial motivational states. This was accomplished by dividing the mean lick rates for each taste stimulus, including 2 water trials (each preceded by at least one water rinse trial) treated as taste stimuli, by the mean lick rate during the water rinse trials of that subject, then multiplying by 100. These normalized lick rates were initially examined with a 3-factor analysis of variance (ANOVA) procedure for mixed designs treating the injection condition (LiCl or saline) and mouse type (WT or TRPM5 KO mice) as between-subject variables and test solution (6 levels) as a within-subject variable. For significant interactions, simple effects tests and t-tests with Bonferroni corrections were then used as needed to partition the data to determine where differences existed between WT and KO mice (Howell 2007). A conditioned aversion was revealed when the LiCl-injected mice showed significantly lower lick rates compared with the saline-injected mice, indicating that this aversion was learned and was not due to a naturally occurring (unconditioned) aversive quality.
Results
CTA experiments
In general, the results of the CTA experiments described below show that the WT mice developed strong aversions for either sweet substance that generalized well to the opposite sweet substance. As expected, these aversions were concentration dependent and were not the result of neophobia. TRPM5 KO mice were able to learn and generalize an aversion to either sweet substance in a concentration-dependent manner, an effect that also was not due to neophobia. However, their aversion response was much weaker than the response of the WT mice, indicating a weakened ability to detect each substance.
Sucrose experiment
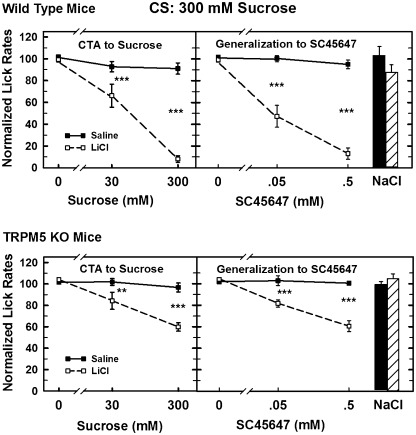
The 3-way ANOVA indicated significant main effects of mouse type, injection conditions, and all interactions (all P < 0.001), including the 3-way interaction (F5,156 = 3.73, P < 0.005; see Figure 1). An initial 2-way ANOVA for mixed designs indicated that the lick rates of the saline-injected WT and KO mice did not differ significantly from each other nor were there any differences between the lick rates for any of the test solutions. The 2-way ANOVA for mixed designs used to examine the lick data of the saline- and LiCl-injected WT mice indicated significant effects for injection (F1,12 = 105.44, P < 0.001), test solution (F5,70 = 34.85, P < 0.001), and the interaction between the 2 variables (F5,70 = 21.38, P < 0.001). Simple effects tests indicated that the LiCl-injected WT mice licked significantly less (all P < 0.001) for both concentrations of sucrose and both concentrations of SC45647 than the saline-injected WT mice. The 2-way ANOVA of the data for the TRPM5 KO mice also found a significant main effect for injection condition (F1,12 = 25.71, P < 0.001) and for the test solutions (F5,70 = 13.42, P < 0.001). It also indicated that there was a significant interaction between the 2 variables (F5,70 = 12.02, P < 0.001). Simple effects tests indicated that the LiCl-injected KO mice licked significantly less than the saline-injected KO mice (P < 0.01 or less) for both concentrations of SC45647 and both concentrations of sucrose. These tests also showed that the normalized lick rates of LiCl-injected mice were not different from saline-injected mice when presented with 75 mM NaCl. A 2-way ANOVA for mixed designs, used to compare the data for the LiCl-injected WT mice with the data for the LiCl-injected KO mice, indicated the KO mice licked significantly more frequently than the WT mice (F5,70 = 40.43, P < 0.001). It also detected a significant effect of solution (F5,70 = 52.50, P < 0.001) and a significant interaction between mouse type and solution (F5,70 = 5.76, P < 0.001). The simple effects tests indicated that the KO mice licked more frequently for both concentrations of SC45647 and 300 mM sucrose than the WT mice (P < 0.001). The groups did not differ in their lick rates during water trials, 30 mM sucrose or 75 mM NaCl. Finally, 1-way ANOVAs were conducted on the data of each of the LiCl-injected groups to determine if there was a concentration-dependent decrease in lick rates for sucrose. Similar ANOVAs were also conducted on the SC45647 data for each group. Each of these ANOVAs identified a significant concentration-dependent decreases in lick rates for each substance of these substance (F2,14 ≥ 17.42, P < 0.01). Post hoc comparisons indicated that WT and KO mice had significantly different (P < 0.025 or less) normalized lick rates for all 3 concentrations (0, 30, and 300 mM), except for KO mice at 0 and 30 mM, which only approached significance (P < 0.06). For generalization to SC45647, lick rates of both mouse types decreased significantly for all concentrations as concentration increased from 0 to 0.05 mM and from 0.05 to 0.5 mM (all P < 0.005).
Figure 1.
Comparison of water-deprived WT (top panels) and TRPM5 KO (bottom panels) mice on a CTA test in which 300 mM sucrose was the CS and the generalization test was to SC45647. Lick rates for each taste solution were normalized by dividing the mean lick rate for each taste solution by the mean lick rate for water, then multiplying by 100. The mean (±standard error of the mean) normalized lick rates (ordinate) are plotted against the corresponding taste stimulus (abscissa). Scores for 0 mM were derived from water trials selected from those preceded by at least one other rinse trial. LiCl-injected WT and KO mice showed significant aversions to sucrose and generalization of those aversions to SC45647 compared with saline-injected mice. However, the lick rates of the KO mice were not suppressed nearly as much as those of the WT mice (P < 0.001). Lick rates for 75 mM NaCl were not diminished by LiCl (Saline-injected: Solid bar; LiCl-injected: Striped bar), indicating that this was not a neophobic response. **P < 0.01, ***P < 0.001.
SC45647 experiment
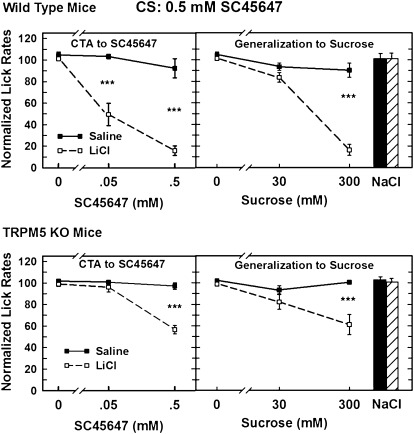
The data for the groups conditioned with 0.5 mM SC45647 as the CS were analyzed in a similar manner (see Figure 2). The 3-way ANOVA comparing the data of the 2 mouse types in each of the 2 injection conditions tested with all 6 solutions indicated that all main effects and all interactions, except Mouse Type × Injection, were significant (all P < 0.025 or less). The 2-way mixed ANOVA comparing the 2 saline-injected groups did not detect any significant differences between the normalized lick rates of the 2 groups. The ANOVA comparing the saline-injected WT mice with the LiCl-injected WT mice indicated that the LiCl mice licked less than the saline-injected mice (F1,12 = 62.54, P < 0.001). It also indicated that the main effect for solution (F5,70 = 40.04, P < 0.001) and the Injection × Solution interaction (F5,70 = 26.63, P < 0.001) were significant. The simple effects test showed that LiCl-injected WT mice licked significantly less for the 0.05 and 0.5 mM SC45647 and the 300 mM sucrose solutions than the saline-injected mice (P < 0.001). The 2-way ANOVA comparing the lick rates of the 2 groups of TRPM5 KO mice also showed an effect of LiCl injections on normalized lick rates that depended upon the solution (Injection: F1,12 = 15.88, P < 0.002; Solution: F5,70 = 8.69, P < 0.001; and Injection × Solution: F5,70 = 5.62, P < 0.001). The corresponding simple effect tests indicated that the LiCl-injected KO mice licked significantly less for the 0.5 mM SC45647 and the 300 mM sucrose solutions than saline-injected KO mice (P < 0.001). The 2-way ANOVA that compared the normalized lick rates of LiCl-injected WT mice with the lick rates of the LiCl-injected TRPM5 KO mice indicated that WT mice licked more frequently, although the magnitude of the difference was dependent upon the solution (Mouse Type: F1,12 = 22.86, P < 0.001; Solution: F5,70 = 49.83, P < 0.001; and Type × Solution: F5,70 = 11.87, P < 0.001). This was clarified by simple effects tests that compared the lick rates of WT and KO mice injected with LiCl for each solution. These tests revealed that the WT mice licked significantly less for the 0.05 and 0.5 mM SC45647 and for 300 mM sucrose than the KO mice (all P < 0.001). Additional analyses of the lick rates for each concentration of SC45647 showed that both types of mice significantly decreased their lick rates for 0.05 mM SC45647 and again at 0.5 mM (all P < 0.008). During generalization testing, neither WT nor KO mice showed a significant decrease in lick rates for 30 mM sucrose compared with 0 mM, but both mouse types licked significantly less for 300 mM sucrose than for 30 mM sucrose.
Figure 2.
Comparison of water-deprived WT (top panels) and TRPM5 KO (bottom panels) mice on a CTA test in which 0.5 mM SC45647 was the CS and the generalization test was to sucrose. Lick rates for each taste solution were normalized by dividing the mean lick rate for each taste solution by the mean lick rate for water, then multiplying by 100. The mean (±standard error of the mean) normalized lick rates (ordinate) are plotted against the corresponding taste stimulus (abscissa). Scores for 0 mM were derived from water trials selected from those preceded by at least one other rinse trial. LiCl-injected WT and KO mice showed significant aversions to SC45647 and generalization of those aversions to sucrose compared with saline-injected mice. However, the lick rates of the KO mice were not suppressed nearly as much as those of the WT mice (P < 0.001). Lick rates for 75 mM NaCl were not altered by LiCl (Saline-injected: solid bar; LiCl-injected: striped bar), indicating that this was not a neophobic response. ***P < 0.001.
In summary, WT mice showed strong conditioning to their respective CS and generalization to the opposite sweet stimulus after 2 days of conditioning. Lick rates of WT mice decreased to below 20% of water lick rates for the high concentration of both sweet substances. On the other hand, the lick rates of LiCl-injected TRPM5 KO mice decreased to about 60–68% of water lick rates for both substances, indicating that the aversion exhibited by the KO mice was much weaker than the amount of aversion exhibited by the WT mice, even with 2 conditioning sessions. Neither mouse type showed any detectible evidence of neophobia. Together, these data suggest that the TRPM5 KO mice are capable of detecting sucrose and SC45647, but the sensory signal is compromised by the genetic deletion.
CFA experiment
In brief, the results of the CFA experiments indicate that the WT and KO mice showed a strong and comparable aversion to the stimulus compound. However, WT mice appeared to be responding more to the sweet stimulus than to the odor cue, whereas the KO mice appeared to be responding more to the odor cue rather than to the taste cue.
SC45647 + amyl acetate CS compound
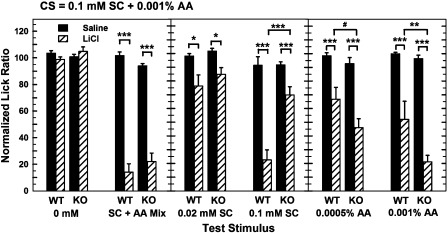
The 3-way ANOVA revealed significant main effects for the injection and solution variables, and significant interactions including the 3-way interaction between Mouse Type × Injection × Solution (F5,156 = 7.65, P < 0.001). The 2-way ANOVA comparing the saline-injected WT and KO mice did not detect any differences in their lick rates. The 2-way ANOVA used to examine the responses of the 2 groups of WT mice indicated that the LiCl-injected mice licked significantly less than the saline-injected mice (F1,12 = 64.05, P < 0.001). Normalized lick rates were also dependent upon the solution variable (F5,70 = 18.56, P < 0.001) and the interaction between the injection condition and solution (F5,70 = 14.70, P < 0.001). The simple effects tests showed that the LiCl-injected WT mice licked significantly less than control WT mice for all solutions except water (P < 0.01 or less). Paired t-tests with Bonferroni corrections were used to compare the lick rates for the taste/odor mixture with solutions of either SC45647 or amyl acetate (see Figure 3). LiCl-injected WT mice licked the SC45647/amyl acetate mixture significantly less than they licked for either solution of amyl acetate alone or for 0.02 mM SC45647 (P < 0.025 or less). Their normalized lick rates for the mixture did not differ from the lick rates for 0.1 mM SC45647. This suggests that the aversion seen with the WT mice was based more on the SC45647 component of the mixture than amyl acetate or the mixture of the 2 stimuli.
Figure 3.
Comparison of water-deprived WT and TRPM5 KO mice on a CTA test after conditioning with a stimulus mixture (CS compound) of 0.1 mM SC45647 + 0.001% amyl acetate. Lick rates (ordinate) were normalized as a ratio in the same manner as described for Figures 1 and 2. Mean (±standard error of the mean) normalized rates (ordinate) are plotted for each test solution (abscissa). Scores for 0 mM were derived from water trials preceded by at least one other rinse trial. Saline-injected (solid bars) WT and KO mice did not avoid any stimulus. The LiCl-injected (striped bars) WT mice reduced their lick rates for solutions containing only SC45647, but the amount of response suppression seen for 0.1 mM SC45647 was equivalent to the amount of suppression elicited by the stimulus mixture. LiCL-injected WT mice suppressed their licking of the amyl acetate solutions more than did the saline-injected WT mice. However, they showed significantly less suppression than the LiCl-injected KO mice. The LiCl-injected TRPM5 KO mice exhibited comparable avoidance of the stimulus mixture and 0.001% amyl acetate. Their lick rates for 0.02 and 0.1 mM SC45647 were significantly lower than those of saline-injected KO mice, although their rates for 0.1 mM SC45647 were significantly greater than those of the WT mice. These results show that the TRPM5 KO mice are capable of learning the association between an odor cue and gastric distress but have a weakened ability to identify the taste components of the stimulus mixture. #P < 0.025, *P < 0.01, **P < 0.005, and ***P < 0.001.
The ANOVA analysis of the 2 groups of TRPM5 KO mice lick data also found significant effects of injection (F1,12 = 143.70, P < 0.001), solution (F5,70 = 40.36, P < 0.001), and the Injection × Solution interaction (F5,70 = 30.37, P < 0.001). Simple effects testing indicated that the LiCl-injected KO mice licked significantly less (P < 0.001) of the 3 solutions containing amyl acetate. They also licked significantly less for 0.02 mM (P < 0.01) and 0.1 mM (P < 0.001) SC45647 than their saline-injected KO counterparts. Bonferroni-corrected paired t-tests applied to the data of the LiCl-injected KO mice showed that their lick rates for water were significantly greater than their lick rates for 0.02 mM (P < 0.015) and 0.1 mM SC45647 (P < 0.002). The difference between 0.02 and 0.1 mM SC45647 began to approach but did not reach significance (P < 0.15). Together, these analyses indicate that the KO mice had an aversion to both concentrations of this sweet substance when the odor cue was not present. TRPM5 KO mice licked the mixture significantly less than they did for 0.0005% amyl acetate or either concentration of SC45647 (P < 0.015 or less). However, their lick rates for the mixture did not differ from their lick rates for 0.001% amyl acetate, suggesting that the learned aversion for KO mice was based more on the odor component of the mixture than on the taste component or an association based on the combination of the 2 stimuli.
The ANOVA comparing the lick rates of the LiCl-injected WT and KO mice uncovered significant effects for solution (F5,70 = 45.67, P < 0.001) and for the interaction between solutions and mouse type (F5,70 = 9.26, P < 0.001). Simple effects testing indicated that the WT mice licked significantly less for 0.1 mM SC45647 (P < 0.001) and significantly more for 0.001% (P < 0.005) amyl acetate solutions than the KO mice. The normalized lick rates of WT and KO mice did not differ for the other solutions, including the compound of 0.001% amyl acetate mixed with 0.1 mM SC45647.
Because rats and mice are able to detect many substances by olfactory cues, it is often assumed that if a mouse can smell a solution in a sipper tube for which the mouse has an aversion, it will avoid the solution without drinking from the tube. To assess the prevalence of this occurring in the CFA experiment, the data that met the criteria for analyses were examined to count the number of trials in which the mice did not lick. Because the frequency of nonlick trials was generally low, the counters were pooled over concentrations for each substance. Of the NaCl-injected mice, the WT mice had only 1 nonlick trial, and KO mice had 0 nonlick trials for SC45647 solutions. In contrast, the LiCl-injected WT mice had 5 nonlick trials of the 49 SC45647 trials (10.2%), 12 nonlick trials of 52 amyl acetate trials (23.1%), and 9 nonlick trials of the 25 amyl acetate + SC45647 trials (35.5%) in the CFA experiment. The LiCl-injected KO mice did not have any nonlick trials when presented SC45647 alone but did not drink during 15 of 60 amyl acetate trials (25%) and 9 of 31 mixtures (27.4%). This analysis was similarly applied to both CTA experiments and, because the data for the NaCl- and LiCl-injected mice were very similar, the data for the 2 CTA experiments were pooled. For the KO mice, there was only one nonlick trial across both experiments. For the WT mice in both CTA experiments, there were only 6 nonlick trials in the 143 SC45647 trials (4.2%), 7 nonlick trials in the 132 sucrose trials (5.3%), and 1 nonlick trial in the 70 NaCl trials (1.4%). Although these data are only an indirect measure of how odor cues might affect licking of a conditioned aversive stimulus, they suggest that when a detectable odor cue is salient part of the CS, mice may avoid completely the solution as much as a quarter to a third of the time. Complete avoidance of SC45647 and sucrose occurred infrequently, especially by the KO mice. It should be emphasized, however, that these data do not rule out the possibility that odor cues were present or that they might have influenced the number of licks in a trial.
Discussion
Previous work has not shown conclusively how much, if any, sweet taste capabilities remain intact in TRPM5 KO mice. Other behavioral studies of the TRPM5 channel have focused largely on bitter taste capabilities using substance that have inherent negative hedonic qualities (e.g., Devantier et al. 2008; Oliveira-Maia et al. 2009), divalent salts (Riera et al. 2009), or sweet–bitter interactions (Talavera et al. 2008). These studies used either 2-bottle or brief-access preference tests. Here, we focused on sweet taste using sucrose, a natural sweetener, and SC45647, an artificial sweetener. CTA methods were chosen because they motivate mice to assign a negative hedonic value to a taste substance and to respond to the taste qualities of a substance, even if the natural hedonic value of stimulus is weak or the taste stimulus is only weakly detectable. A hallmark feature of CTA conditioning is that an aversion to one substance will generalize to other substances with similar taste qualities, a form of stimulus generalization that is well documented (Spector and Grill 1988; Spector 2003; Bouton 2007). That is, in general, the greater the similarities between the taste qualities elicited by 2 substances, the greater the strength of generalization of the aversion from the CS to the second substance. Typically, a control substance that elicits different taste qualities (NaCl in these experiments) is also included during generalization testing to ensure that the animal is not simply avoiding any taste stimulus (neophobia) but rather only those that elicit a similar sensation (no evidence of neophobia in any condition). The WT mice exhibited strong CTAs to sucrose and to SC45647, which generalized to the opposite sweet substance. The LiCl-injected KO mice showed a significant reduction in licking for the assigned CS and comparable generalization to the opposite substance, although the strength of these effects was much smaller than that seen in the WT mice. Notably, the magnitude of the reduction by the KO mice was similar for both sucrose and SC45647 after 2 pairings of the assigned CS with LiCl. It is possible that the size of the effect might be smaller or undetectable after only one pairing or larger after more pairings. The strength of the CTA is also influenced by the intensity of the stimulus (Spector and Grill 1988; Spector 2003; Bouton 2007). For example, a low intensity CS solution will not generally elicit as much avoidance or response suppression as the concentration of the solution initially paired with LiCl. The magnitude of the response suppression for the CS substance and the generalization substance were concentration dependent for both strains. The inability by both strains of mice to detect generalization to 30 mM sucrose after conditioning to SC45647 likely indicates either that this concentration elicited a sensation relatively weak compared with 300 mM sucrose and/or was ignored because of the water-deprivation state of the animals. The results of the CTA and CFA experiments presented here indicate that TRPM5 KO mice have a reduced, but still present, ability to detect both sucrose and the artificial sweetener, SC4547. There are several possible factors that might explain these findings.
One such possibility is a difference in the nature of the genetic methods used to create the 2 different TRPM5 KO strains. Zhang et al. (2003) found that their TRPM5 KO mice lacked all responses to bitter, sweet, and umami. The construct used by Zhang et al. (2003) for generating the San Diego KO strain resulted in the sparing of 1 of 6 transmembrane domains and the C terminus of TRPM5. If this incomplete TRPM5 KO generates protein, it may act as a dominant negative to interfere with taste responses. In contrast, the construct used by Damak et al. (2006) to generate the Mount Sinai TRPM5 KO strain led to the deletion of the entire Trpm5 region such that no partial protein would be present. These authors detected evidence of nerve and behavioral responses by their TRPM5 KO mice to some bitter, sweet, and umami substances, although these responses were diminished compared with WT mice. Another potentially important difference between the 2 different strains of TRPM5 KO mice is that the Mount Sinai mouse was developed in C57BL/6J embryonic stem cells and maintained in that background, whereas the Zhang et al. (2003) mouse was generated in R1 129 embryonic stem cells and backcrossed for 2 generations with C57BL/6J mice, followed by intercrossing. The contribution of 129 traits (e.g., sweet-insensitive “nontaster”) or founder effects could contribute to the lack of sweet responses detected. A direct comparison of the 2 strains of mice with the same behavioral measures is needed to assess any potential differences in the taste responses between these 2 independently derived TRPM5 mice strains.
Another possible explanation is that the suppression of licking by LiCl-injected mice is the result of postingestive factors. Sclafani et al. (2007, 2010) found that extensive experience with carbohydrates in 24-h 2-bottle tests can induce postingestive effects that influence subsequent taste preferences in WT mice. In contrast, this experience generally has only minimal effects on taste preferences of TRPM5 KO mice, although these mice appeared capable of responding to postingestive effects of Polycose and lipids. de Araujo et al. (2008) reported that TRPM5 mice can be conditioned to select a specific sipper tube associated with sucrose but not sucralose which is a noncaloric sweetener. Also, Ren et al. (2010) found that TRPM5 KO mice can learn to regulate dietary intake of sucrose and glucose on the basis of caloric intake. Both studies reported evidence that the regulation of sucrose intake was the result of central mechanisms responding to changes in blood glucose levels following sucrose ingestion. de Araujo et al. (2008) found that blood glucose levels were already increasing by 10 min after the start of ingestion, which might generate the necessary signaling to influence the lick rates late in the test sessions of this study. Ren et al. (2010) also found that WT mice began to show increases in respiration, an indirect measure of the metabolic effects of glucose levels, about 10 min after beginning to ingest glucose and these effects peaked within a few minutes. However, TRPM5 KO mice did not show a change in respiration until 20 min after glucose ingestion and were much slower to reach a peak effect. In the current experiments, the conditioning sessions were limited to a maximum of 15 min to minimize any overlap of oral sensations with the postingestive effects of sucrose intake during conditioning. Only the data for no more than the first 10 min of each test session were evaluated to minimize the opportunity for the mice to change their lick rates on the basis of potentially detectable changes in internal states during the test session. More importantly, SC45647, an artificial sweetener, was also used as a CS and for generalization testing because it has no known postingestive effects reported in the literature for the concentrations used in these experiments. One related study showed that activation of T1R3 receptors in the intestine can influence hormonal signals of the gut (Margolskee et al. 2007). Because SC45647 presumably is able to activate these receptors in the gut, it might be possible for SC45647 at the concentrations used in this study to also influence these signals, but this has not been tested to the authors’ knowledge. However, another noncaloric, highly preferred sweetener, sucralose, infused directly into the stomach did not affect preference learning in the same manner as sucrose (Sclafani et al. 2010). Even so, our analyses detected evidence of CTA for each substance, which generalized equally between sucrose and SC45647 for both mouse strains. WT mice responded to each sweetener in a similar manner and showed comparable degrees of generalization to the opposite substance. TRPM5 KO mice also responded similarly to sucrose and SC45647, albeit with a much weaker aversion to the conditioning sweetener and weaker generalization to the opposite substance than seen with the WT mice. Postingestive effects cannot be ruled out but, when combined with the time course of postingestive effects reported in other studies, the similarities of the respective CTA response functions of WT and KO mice to sucrose and SC45647 suggest that the lick rates seen in these experiments are likely influenced by oral rather than postingestive cues.
Because the TRPM5 KO mice showed only modest aversive response to either CS under the same learning conditions as the WT mice, we conducted the CFA experiment to determine if the lack of avoidance was due to some change in taste signaling and not due to a compromised ability to learn the task or associate the LiCl-induced malaise to an aversive CS. In the CFA experiment, we presented the mice with a stimulus mixture consisting of SC45647 and amyl acetate, which elicits a banana-like odor. This experiment revealed that TRPM5 KO mice are indeed capable of forming a conditioned aversion under these conditions. When presented with the taste/odor mixture, the KO mice suppressed their lick rates to a level equivalent to the response of LiCl-injected WT mice. Thus, the weak aversion to sucrose or SC45647 was likely to be the result of a weak taste or other oral signal during conditioning and testing and not due to a deficit in ability to learn.
The results of the flavor CFA experiment are also very interesting because they suggest that the KO mice are quite capable of detecting amyl acetate. A recent report had raised the question of whether the TRPM5 channel might play an important role in olfactory transduction. The TRPM5 channel is found widely throughout the olfactory epithelium and vomeronasal organ (Kaske et al. 2007; Lin et al. 2008). The function of these channels has yet to be elucidated, but morphological and immunocytochemical evidence suggest that the TRPM5 channel may not be directly involved in olfactory signaling pathways because these channels are found in microvillous cells rather than chemosensory cells of the olfactory epithelium (Hansen and Finger 2008). In the present study, the functionality of the olfactory system of the TRPM5 KO mice was tested with just one odor. Nevertheless, these KO mice appeared to detect the odor easily, transmit the signal centrally, and associate the odor with the effects of LiCl. This leads to a question of whether the KO mice might have detected sucrose and SC45647 through olfactory cues. This cannot be ruled out. Zukerman et al. (2009) have shown that T1R3 KO mice can learn a taste preference for sucrose when they experience high concentrations of sucrose at least in part through olfaction. In our experience (Eddy et al. 2009), when mice learn a CFA, they will often not lick at all during a trial in which the odor is present. This was verified in part in the CFA experiment by the nonlick data for the amyl acetate and taste–odor mixture stimuli. The frequency of nonlicking for either sucrose or SC45647 alone was very low for WT and even less for KO mice, suggesting that the odor of these solutions may have been only weakly salient in the CTA experiments.
A closer look at the pattern of responses to the individual components of the taste/odor mixture also supports the notion that the KO mice were able to detect a taste quality. When presented with SC45647 or amyl acetate alone, WT mice clearly showed a strong avoidance of the taste stimulus, similar to that seen with the stimulus mixture, whereas the aversion to the odor cue was significantly less than to the mixture. These data suggest that both CSs, after 2 pairings with LiCl to induce aversive conditioning, were capable of suppressing lick rates when tested either individually or together, but the suppression elicited by the taste stimulus was greater than the suppression elicited by the odor stimulus. The greater impact of the taste stimulus may have been in part due to a bias generated by the previous conditioning with the taste stimulus because the concentration of the odor stimulus used in this experiment is clearly salient to mice (Song et al. 2008; Van Houten et al. 2008; Eddy et al. 2009). These training conditions and the responses of the WT mice to the individual stimuli and to the mixture are similar to the Kamin blocking effect that is well characterized in the learning literature (Bouton 2007). This effect can be seen when the subject is first conditioned with a single stimulus (CS1), then subsequently is conditioned again but this time with CS1 paired with a second stimulus (CS2) forming what is called a stimulus compound in the learning literature (Bouton 2007). Subsequent testing of the stimulus compound can show strong conditioning by its control over behavior. When testing the individual stimuli, CS1 is also able to exert a strong effect on behavior alone, but CS2 is only able to exert a weak effect on behavior; that is, CS1 blocks in part any conditioning to CS2. In the CFA experiment, CS1 would be the taste stimulus, and CS2 would be the odor stimulus of the mixture, which appeared to have a weaker effect on lick rates than the sweetener. In contrast, the genetic deletion appeared to reduce the Kamin effect. TRPM5 KO mice exhibited a strong aversion for the stimulus mixture and a near equivalent aversion to the 0.001% amyl acetate stimulus presented alone, but only a weak aversion to SC45647 alone. This suggests that the new odor cue was more salient than SC45647 for the KO mice and thus was more easily associated with the gastric distress induced by LiCl. Importantly, however, these mice also showed some suppression of licking to SC45647, even though the CS concentration was lower than that used in the first experiment and the TRPM5 channel was absent from their taste transduction pathway. This also opens the possibility that the previous conditioning to the taste of SC45647 might have actually potentiated the conditioning for amyl acetate (Batsell et al. 2001). However, an additional control condition using naïve KO mice would be needed to determine if SC45647 potentiated odor learning. In either case, the results of this experiment show that the TRPM5 KO mice are capable of learning a conditioned aversion and that even with amyl acetate present, the KO mice responded to SC45647.
The findings of these experiments are congruous with other evidence of TRPM5-dependent and TRPM5-independent pathways for detecting bitter (Talavera et al. 2008; Oliveira-Maia et al. 2009) and sweet tastes (Ohkuri et al. 2009; Oliveira-Maia et al. 2009). It has been known for a long time that quinine is capable of inhibiting sweet tastes, and recent evidence suggests that the TRPM5 channel is key to that interaction. Talavera et al. (2005) studied single fiber and whole-nerve responses to sucrose, quinine and quinidine (quinine stereoisomer), and denatonium. They found that quinine and quinidine inhibited sucrose responses in WT mice but not in TRPM5 KO mice. They hypothesized that quinine is able to interact directly with the sweet transduction pathway by inhibiting the TRPM5 channel. Because quinine only partially inhibited the sweet response and because quinine had no effect on the response of TRPM5 KO mice to sucrose and glucose, they argued that there were 2 TRPM5-independent pathways, one that carried signals for bitter and another for sweet. Additional physiological evidence for a TRMP5-independent pathway for sweet was reported by Ohkuri et al. (2009). These investigators examined the effects of temperature and the sweet-inhibiting effects of gurmarin on sweet responses of the chorda tympani nerve. They found the nerve responses of TRPM5 KO mice to sucrose and glucose considerably smaller than those of WT mice. More importantly, the nerve response of TRPM5 KO mice to sucrose and glucose were reduced, but not completely abolished by gurmarin, an effect that was unaltered by temperature. A TRPM5-independent transduction pathway within taste sensory cells might be activated by a different sweet receptor (Zukerman et al. 2009) or a second messenger pathway other than IP3, the pathway normally associated with activation of TRPM5 channels. A number of investigators have reported evidence showing that sucrose can increase cyclic adenosine monophosphate (cAMP) levels in taste sensory cells as well as IP3. Increases in cAMP levels are believed to activate a nucleotide-gated channel or activate protein kinase A to phosphorylate a K+ channel in taste cells (Cummings et al. 1993, 1996; Bernhardt et al. 1996; Misaka et al. 1997; Nakashima and Ninomiya 1999; Lindemann 2001; Trubey et al. 2006). The present study provides behavioral evidence that supports a TRPM5-independent signal transduction pathway for sweet taste, possibly involving a cAMP transduction pathway. Further studies are required to verify and elucidate the nature of a TRPM5-independent pathway.
In summary, the experiments presented in this study challenged TRPM5 KO mice to develop a conditioned aversion to sucrose and the artificial sweetener, SC45647, to determine if these KO mice can taste sweet qualities and how much of a loss of function is induced by the genetic deletion. Collectively, the results of the CTA and CFA experiments suggest a residual ability to detect sucrose and SC45647 in TRPM5 KO mice and the existence of a TRPM5-independent pathway for sweet signaling. These findings are consistent with previous studies indicating alternative signaling pathways for sweet taste may be present in these KO mice.
Funding
This work was supported by National Science Foundation [grant IOS-0951016] awarded to E.R.D. and National Institutes of Health [grant R01 DC003155] awarded to R.F.M.
Acknowledgments
Competing interest statement: Dr. Margolskee has a personal financial interest in the form of stock ownership in the Redpoint Bio company and is an inventor on patents and patent applications, which have been licensed to the Redpoint Bio company.
References
- Batsell WP, Jr., Paschall GY, Gleason DI, Batson JD. Taste preconditioning augments odor-aversion learning. J Exp Psychol Anim Behav Process. 2001;21:30–47. [PubMed] [Google Scholar]
- Bernhardt SJ, Naim M, Zehavi U, Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol. 1996;490:325–336. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Learning and behavior: a contemporary synthesis. Sunderland (MA): Sinauer Associates, Inc; 2007. [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings TA, Daniels C, Kinnamon SC. Sweet taste transduction in hamster: sweeteners and cyclic nucleotides depolarize taste cells by reducing a K+ current. J Neurophysiol. 1996;75:1256–1263. doi: 10.1152/jn.1996.75.3.1256. [DOI] [PubMed] [Google Scholar]
- Cummings TA, Powell J, Kinnamon SC. Sweet taste transduction in hamster taste cells: evidence for the role of cyclic nucleotides. J Neurophysiol. 1993;70:2326–2336. doi: 10.1152/jn.1993.70.6.2326. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr., Glendinning JI, Ninomiya Y, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Devantier HR, Long DJ, Brennan FX, Carlucci SA, Hendrix C, Bryant RW, Salemme FR, Palmer RK. Quantitative assessment of TRPM5-dependent oral aversiveness of pharmaceuticals using a mouse brief-access taste aversion assay. Behav Pharmacol. 2008;19:673–682. doi: 10.1097/FBP.0b013e3283123cd6. [DOI] [PubMed] [Google Scholar]
- Eddy MC, Eschle BK, Barrows J, Hallock RM, Finger TE, Delay ER. Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem Senses. 2009;34:789–797. doi: 10.1093/chemse/bjp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Hansen A, Finger TE. Is TrpM5 a reliable marker for chemosensory cells? Multiple types of microvillous cells in the main olfactory epithelium of mice. BMC Neurosci. 2008;9:115. doi: 10.1186/1471-2202-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Statistical methods for psychology. 6th ed. Belmont (CA): Duxbury Press; 2007. [Google Scholar]
- Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon SC. Umami taste transduction mechanisms. Am J Clin Nutr. 2009;90(Suppl):753S–755S. doi: 10.3945/ajcn.2009.27462K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Ezekwe EAD, Jr., Zhen Z, Liman ER, Restrepo D. TRPM5-expressing microvillous cells in the main olfactory epithelium. BMC Neurosci. 2008;9:114. doi: 10.1186/1471-2202-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. PNAS. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- Misaka T, Kusakabe Y, Emori Y, Gonoi T, Arai S, Abe T. Taste buds have a cyclic nucleotide-activated channel, CNGgust. J Biol Chem. 1997;272:22623–22629. doi: 10.1074/jbc.272.36.22623. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Ninomiya Y. Transduction for sweet taste of saccharin may involve both inositol 1,4,5-triphosphate and cAMP pathways in the fungiform taste buds in C57BL mice. Cell Physiol Biochem. 1999;9:90–98. doi: 10.1159/000016305. [DOI] [PubMed] [Google Scholar]
- Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R960–R971. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan T-HT, Mummalaneni S, Melone P, DeSimone JA, Nicolelis MAL, Simon SA. Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci U S A. 2009;106:1596–1601. doi: 10.1073/pnas.0810184106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CA, Margolskee RF, Kinnamon SC, Ogura T. Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium. 2003;33:541–549. doi: 10.1016/s0143-4160(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera CE, Vogel H, Simon SA, Damak S, le Coutre J. Sensory attributes of complex tasting divalent salts are mediated by TRPM5 and TRPV1 channels. J Neurosci. 2009;29:2654–2662. doi: 10.1523/JNEUROSCI.4694-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD. Signal transduction and information processing in mammalian taste buds. Pflügers Arch. 2007;454:759–776. doi: 10.1007/s00424-007-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–788. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1504–R1513. doi: 10.1152/ajpregu.00364.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM, Westbrook F, Darling FMC. What the rat's nose tells the rat's mouth: long delay aversion conditioning with aqueous odors and potentiation of taste by odors. Anim Learn Behav. 1997;25:357–369. [Google Scholar]
- Song Y, Cygnar KD, Sagdullaev B, Valley M, Hirsh S, Stephan A, Reisert J, Zhao H. Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation. Neuron. 2008;58:374–386. doi: 10.1016/j.neuron.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC. Psychophysical evaluation of taste function in nonhuman mammals. In RL Doty editor. Handbook of Olfaction and Gustation. 2nd ed. New York (NY): Marcel Dekker, Inc. 2003:861–879. [Google Scholar]
- Spector AC, Grill HJ. Differences in the taste quality of maltose and sucrose in rats: issues involving the generalization of conditioned taste aversions. Chem Senses. 1988;13:95–113. [Google Scholar]
- Spielman AI, Nagai H, Sunavala G, Dasso M, Breer H, Boekhoff I, Huque T, Whitney G, Brand JG. Rapid kinetics of second messenger production in bitter taste. Am J Physiol. 1996;270:C926–C931. doi: 10.1152/ajpcell.1996.270.3.C926. [DOI] [PubMed] [Google Scholar]
- Talvera K, Yasumatsu K, Yoshida R, Margolskee RF, Voets T, Ninomiya Y, Nilius B. The taste transduction channel TRPM5 is a locus for bitter-sweet taste interactions. FASEB J. 2008;22:1343–1355. doi: 10.1096/fj.07-9591com. [DOI] [PubMed] [Google Scholar]
- Trubey KR, Culpepper S, Maruyama Y, Kinnamon SC, Chaudhari N. Tastants evoke cAMP signal in taste buds that is independent of calcium signaling. Am J Physiol Cell Physiol. 2006;291:C237–C244. doi: 10.1152/ajpcell.00303.2005. [DOI] [PubMed] [Google Scholar]
- Van Houten JL, Ponissery-Saidu S, Ghatak A, Valentine MS, Weeraratne SD, Falls W, Delay E, Delay R. Plasma membrane calcium ATPase 2 knock out shows slower calcium clearance from olfactory sensory neurons and deficits in olfactory driven behavior. Program No. 117.7. Abstract Viewer/Itinerary Planner. Washington (DC): Society for Neuroscience; 2008. Online. [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJP. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]