Abstract
Chemesthetic sensations elicited by ibuprofen, extra-virgin olive oil, and capsaicin were compared to quantify perceptual differences between known agonists of TRPA1 and TRPV1. Extra virgin olive oil contains a phenolic compound, oleocanthal, which is thought to share unique chemesthetic qualities with the nonsteroidal anti-inflammatory drug, ibuprofen. Pilot work suggested participants had difficulty distinguishing between multiple chemesthetic subqualities (e.g., burn, sting, itch, tickle, etc.) in a multiattribute rating task. Here, we assessed overall irritation via direct scaling, and a check all that apply task was used to collect information about chemesthetic subqualities over time. Replicated ratings were collected at discrete intervals using the generalized labeled magnitude scale to generate time-intensity curves; maximum intensity (Imax) and area under the curve were extracted for each participant. Intensity responses varied substantially across participants, and within a participant, the relationship was strongest between ibuprofen and olive oil. However, there were also positive, albeit weaker, correlations between capsaicin and ibuprofen and capsaicin and olive oil. The correlation found between olive oil and capsaicin may suggest the presence of unknown TRPV1 agonists in olive oil. This view was also supported by the qualitative data: Capsaicin was described most often as burning and warm/hot, whereas ibuprofen was numbing and tickling. Olive oil shared characteristics with both capsaicin (warm/hot) and ibuprofen (tickle).
Keywords: check all that apply, chemesthesis, direct scaling, individual differences, psychophysics
Introduction
The quality of extra-virgin olive oils is primarily assessed using a standardized procedure developed by the International Olive Council. This method uses trained panelists to detect and rate bitter, pungent, and fruity characteristics of olive oils to establish their commercial grade (Delgado and Guinard 2011). Interestingly, pungency and bitterness are considered positive qualities by experts (but not consumers; Delgado and Guinard 2011), as they speak to the composition and concentration of the phenolic fraction in the oil. This desirable pungency is attributed to the phenolic compound oleocanthal (“oleo” for olive, “canth” for sting, and “al” for aldehyde) (Andrewes et al. 2003; Beauchamp et al. 2005). Despite structural dissimilarity, oleocanthal has been shown to have similar sensory and pharmacological properties to the nonsteroidal anti-inflammatory drug ibuprofen (Figure 1). Both compounds cause a distinct pungency that is restricted to the throat (Beauchamp et al. 2005). This is peculiar because most chemesthetic stimuli elicit irritation throughout the oral cavity and other mucosal tissues, although the intensity of sensation may differ by site (Lawless and Stevens 1988; Green and Schullery 2003).
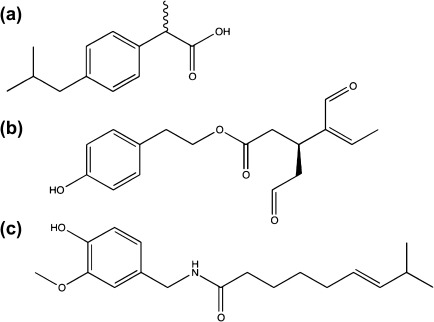
Figure 1.
Chemical structures of (a) the nonsteroidal anti-inflammatory drug ibuprofen (b) (−)-deacetoxy-dialdehydic ligstroside aglycone [oleocanthal], and (c) capsaicin, a known agonist of the TRPV1 channel.
Because of the cardioprotective effects of olive oil in the Mediterranean diet, oleocanthal bioactivity has received substantial attention as an alternative causative mechanism, as cardioprotection might arise from beneficial anti-inflammatory action rather than a favorable fatty acid profile (Cicerale et al. 2008). This is because oleocanthal and ibuprofen have similar pharmacological effects. Both are COX-1 and COX-2 inhibitors, and oleocanthal's IC50 value for COX-2 inhibition is nearly 10 times lower than that of ibuprofen (Beauchamp et al. 2005), suggesting that oleocanthal may be more potent at equivalent concentrations. Due to these potential health benefits, efforts have been made to determine how individuals differ in their perception of oleocanthal (Cicerale et al. 2009) and to explore the extent of its protective effects (e.g., Pitt et al. 2009). However, no published work to date has compared the sensory properties of oleocanthal and ibuprofen behaviorally in the same population.
Here, we quantified the overall intensity of oropharyngeal irritation elicited by extra virgin olive oil and ibuprofen as well as their predominant and secondary irritation subqualities; capsaicin was also included as a control stimulus. We reasoned that if oleocanthal and ibuprofen share a common receptor, they should be described by the same subqualities, and the intensity of the irritancy elicited by these compounds should be correlated with each other across individuals. Indeed, the localized irritation from oleocanthal and ibuprofen has been attributed to the specificity of these compounds for the TRPA1 receptor (Peyrot des Gachons, Uchida, et al. 2011). Moreover, if that mechanism is independent of TRPV1, the irritancy from olive oil and ibuprofen should not correlate with the irritancy elicited by the prototypical TRPV1 agonist capsaicin, similar to the independence of bitterness observed for tastants that act via different receptors (e.g., Horne et al. 2002).
The applied sensory science literature is replete with reliable and consistent methods that can be used to collect quantitative data on the qualitative differences between stimuli. Such methods often involve calibrating a panel of trained judges and using these judges as analytical sensors. Unfortunately, these techniques are not well suited to chemesthetic stimuli. These limitations have been described in detail elsewhere (Green 2002). Briefly, the training of panelists is extremely difficult due to issues of sensitization, desensitization, and the time course of sensation that limit the number of samples that can be presented in a given session (e.g., Lawless 1984; Karrer and Bartoshuk 1991). Also, with tastants, reference compounds that are strictly sweet, sour, bitter, salty, or umami exist and are easy to prepare for training. Many chemesthetic stimuli are hydrophobic and so require preparation with ethanol and surfactants, which may or may not affect the integrity of the stimulus. Additionally, we and others have found that the diffuse nature of chemesthetic sensations makes them hard to characterize (Cliff and Heymann 1992). Even with verbal or written descriptions of irritation subqualities (e.g., Cliff and Green 1996), it seems that panelists find it difficult to associate a description with an actual oral sensation. Thus, it becomes a circular problem, meaningful qualitative data are difficult to collect without training, and training is difficult to perform due to the nature of the stimuli and lack of training standards (references compounds). Nonetheless, behavioral work is critical to determine how our perceptions are ultimately related to recently elucidated molecular mechanisms, as it provides a context for the study of natural ligands found in foods. Because a trained panel approach is not feasible, novel approaches for collecting qualitative or semiqualitative data on irritants are required to move forward in this area.
In a pilot study that aimed to quantify and correlate multiple oral irritation subqualities from capsaicin, olive oil, and ibuprofen over time, we found the magnitude of irritancy from olive oil was correlated with ibuprofen within individuals. However, there was also a positive, albeit weaker relationship between olive oil and capsaicin irritancy, implying that olive oil may also contain an unknown TRPV1 agonist. Additionally, scatter matrices of individual responses across the subqualities suggested the task might have been overly complex for participants. Contrary to the dumping effects that are well documented in time-intensity studies for classical tastants and odorants (e.g., Frank et al. 1993; Clark and Lawless 1994), we found the opposite: a “smearing” bias. We suspect that when untrained participants struggle to distinguish between different types of irritancy (e.g., burning, tickle, itch, etc.), they spread their ratings across multiple attribute scales. Whether this resulted from providing too many different response options (7) or the unfamiliar nature of the chemesthetic sensation was unclear. It was also evident that there could be a response bias (e.g., Green et al. 2010), as the quality ratings occurred in a fixed order, with “burn” always occurring first. Thus, more work was needed to further explore the qualitative aspects of the irritancy from olive oil, ibuprofen, and capsaicin. In the simplified task described here, participants rated overall irritation intensity and then indicated the predominant sensation and levels of each of the subqualities used in the pilot study. Also, the order in which the subqualities were presented was pseudorandomized to avoid any order bias for the first and last positions.
Our primary goal was to compare the irritation (i.e., chemesthetic subqualities) of capsaicin, ibuprofen, and olive oil within the same group of individuals. A secondary goal was to explore individual differences in irritation intensity. It was not the purpose of this experiment to elucidate the receptor mechanisms of ibuprofen and oleocanthal, but instead to supplement the current molecular data, by providing behavioral evidence that the chemesthetic nature of olive oil and ibuprofen is distinctive from other more commonly studied irritants.
Materials and methods
Subjects
Reportedly healthy nonsmoking adults (n = 37; 10 men; aged 18–45 years) were recruited from the Penn State community. Procedures were approved by the Institutional Review Board, informed consent was obtained, and participants were paid for their time. All data were collected in a one-on-one setting at the Sensory Evaluation Center at Penn State.
Stimuli
The test stimuli were 5 mL samples of 2.5% (w/v) (121.2 mM) USP grade ibuprofen (Spectrum, CAS# 15687-27-1), 75 mg/L (∼0.246 mM) natural capsaicin (65% capsaicin/35% dihydrocapsaicin; Sigma Aldrich, CAS# 404-86-4), and commercially, available extra virgin olive oil (Fruttato Colavita) held at 35 °C and presented in 30 mL plastic medicine cups. Solutions of ibuprofen and capsaicin were prepared in canola oil (Wegmans), as canola more closely mimics the fatty acid composition of olive oil (compared with corn oil). Stimuli concentrations were selected from previously published reports (Lawless et al. 2000; Breslin et al. 2001) and revised based on intensity data from the pilot study to ensure approximately equal irritation intensity. All samples were presented in randomized order and labeled with random 3-digit blinding codes.
Procedure
Participants were asked to refrain from eating and the use of chemesthetic agents (i.e., toothpaste, mouthwash, spicy food) for at least 2 h prior to their session. Before beginning the test, participants were oriented to the generalized labeled magnitude scale (Snyder et al. 2004) using a list of 15 imagined or remembered sensations that included both oral and nonoral items (Hayes JE, Bennett SM, Allen AL, under review). Scale instructions encouraged participants to make ratings in a generalized context by indicating that the top of the scale should reflect their “strongest sensation of any kind.” (The modifier “imaginable” is not needed to generalize the scale; see discussion in Snyder et al. 2004). During training, participants were also introduced to a list of 7 irritation subqualities and their definitions (Table 1). The list of subqualities and definitions was visible to participants throughout the entire test.
Table 1.
List of irritation subqualities and definitions provided to participants during task orientation
| Burning | The sensation that commonly results from exposure to very high temperatures (i.e., thermal burns), skin abrasions (e.g., run or floor burns), or chemical irritants (e.g., alcohol) may or may not be accompanied by a thermal sensation |
| Stinging/Pricking | Sharp sensations similar to those produced by an insect bite—other than itching—or a pinprick may be constant (stinging) or brief (pricking) |
| Itching | The sensation that provokes the desire to scratch |
| Tingling | A lively pins and needles sensation |
| Warm/Hot | Sensations of mild (warm) or extreme (hot) heating |
| Numbness | The diffuse (e.g., fuzzy) sensation produced during the onset of an anesthetic (e.g., novocaine); it is “not” the complete absence of sensation |
| Tickle | The sensation in the back of the mouth or throat that when weak causes the urge to clear the throat and when strong causes coughing |
Adapted from Cliff and Green (1996) and Breslin et al. (2001).
To evaluate irritation localized to the throat, the stimulus delivery method was based on 2 previously published reports (Beauchamp et al. 2005; Cicerale et al. 2009). Briefly, participants were instructed to place the 5 mL oil sample in their mouth and tilt their head back to allow the oil to reach the throat. Then, they were instructed to allow the oil to sit at the back of the throat for 5 s before swallowing in 2 stages (swallowing, then immediately swallowing again). Swallowing in 2 stages purportedly ensures that the stimulus is distributed to the whole surface of the throat. This method is designed specifically to localize the stimulus exposure to the throat and minimize contact in the rest of the oral cavity. The participant's first rating was made immediately after the second swallow. Discrete-interval time-intensity ratings for “overall irritation in your throat” were collected every 30 s for 180 s using Compusense five (Guelph). Participants were asked to keep the subqualities of irritation in mind while they rated. Then, before rinsing with water, participants indicated their “predominant sensation” from the list of 7 subqualities and endorsed each subquality as “no sensation,” “low,” or “high.” After rating, participants were allowed to rinse with 35 °C reverse osmosis water ad libitum but were asked to sit quietly with their mouth closed to maintain a constant temperature in the mouth. A minimum interstimulus interval (ISI) of an additional 180 s was enforced between each sample. If a participant had any residual irritation at the end of the 3 min ISI (6 min after initial sample presentation), they were given more water and asked to wait until all irritation had subsided before proceeding to the next sample. A total of 6 stimuli (3 samples × 2 replicates) were presented within a single session (∼45 min). When designing the experiment, we considered issues of participant fatigue and desensitization/sensitization that would arise from presenting 6 samples within a session. However, we decided against splitting testing across days, both because we were worried about the increased variability that would arise across multiple days and because prior reports indicate ibuprofen does not sensitize or desensitize across trials (Breslin et al. 2001); since they act on the same receptor, we presume oleocanthal similarly does not desensitize. Moreover, even if some small degree of sensitization or desensitization occurred for capsaicin, the presentation order was counterbalanced, so this would only introduce noise and not a systematic bias into our data.
Data analysis
All analyses were conducted in SAS 9.2. To characterize the qualitative aspects of each stimulus, the number of endorsements for predominant quality for all replicates were summed and expressed as a percentage of total possible responses (i.e., 2 replicates × 37 people = 74 total responses for a descriptor). Because our secondary hypothesis involved comparing individual differences between participants across compounds, we extracted 2 scaffolding parameters from the discrete interval time-intensity functions: the maximum intensity (Imax) and the total area under the curve (AUC). The AUCs were calculated using the trapezoid rule (Matthews et al. 1990): the sum of a series of isosceles trapezoids, each with an area equal to 1/2*[(height1 + height2) × width]. These summary parameters (Imax and AUC) were compared across stimuli using linear regression. Additionally, replicate means at each time point were analyzed via repeated measures mixed model analyses of variance (ANOVAs).
Results
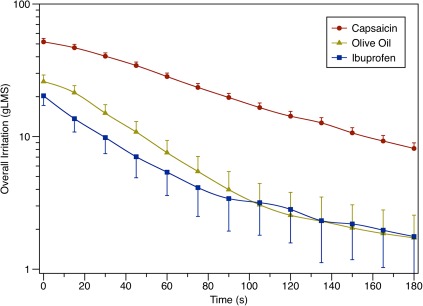
Individual differences in perception were seen for responses to capsaicin, olive oil, and ibuprofen irritation (not shown), consistent with previous reports (Green 1996; Breslin et al. 2001; Cicerale et al. 2009) and our pilot work. Group means of overall irritation were used to evaluate the relationships between overall temporal patterns of each stimulus across the group. These results are shown in Figure 2. As expected, in 2-way (stimulus by time) repeated measures ANOVA, there was a significant main effect of time (F12,1296 = 193.8, P < 0.0001). There was also a significant interaction of stimulus by time (F24,1296 = 19.1, P < 0.0001); pairwise comparisons (Tukey–Kramer) revealed capsaicin ratings were higher than ibuprofen and olive oil at all time points (all Ps < 0.0001), whereas olive oil and ibuprofen ratings never differed from each other (all Ps > 0.1). Thus, although the olive oil and ibuprofen irritation levels were successfully brought into the same range, the capsaicin concentration was still a little high, resulting in an Imax that was closer to “very strong” than just above “moderate” for the other 2 stimuli. Visual inspection of the curves also suggests the irritation from olive oil and ibuprofen decays at a faster rate than the capsaicin irritation, particularly in the first 110 s.
Figure 2.
Intensity ratings (group means with standard errors) for “overall irritation” in the throat for each stimulus. Capsaicin was significantly different from olive oil and ibuprofen at all time points, whereas olive oil and ibuprofen did not differ significantly at any time point. This figure appears in color in the online version of Chemical Senses.
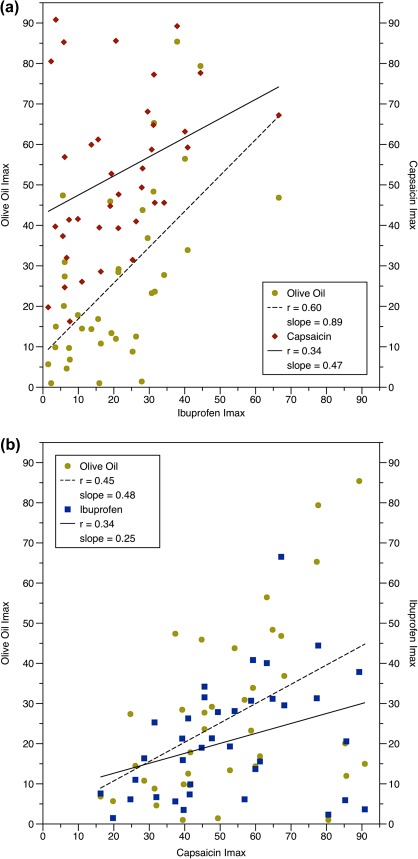
Linear regression was used to compare individual Imax and AUC values for each stimulus (Figure 3). Unexpectedly, significant correlations were seen between the Imax ratings of all 3 stimuli. Nonetheless, the effect size for the ibuprofen–olive oil relationship (r = +0.60, P < 0.0001) was larger than the ibuprofen–capsaicin relationship (r = +0.34, P = 0.038). The size of the capsaicin–olive oil relationship fell in between these values (r = +0.45, P = 0.005), suggesting olive oil may contain an unknown TRPV1 agonist. Analysis of the AUC values (not shown) revealed similar results: ibuprofen–olive oil (r = 0.72; P < 0.001), ibuprofen–capsaicin (r = 0.37; P = 0.025), and capsaicin–olive oil (r = 0.49; P = 0.002).
Figure 3.
(a and b) Correlations between Imax values for overall irritation for each stimulus. (A) Ibuprofen correlated with both olive oil (P < 0.0001) and capsaicin (P = 0.038). (B) Capsaicin was also correlated with olive oil (P = 0.005). Similar results were seen for the AUC values (see text). This figure appears in color in the online version of Chemical Senses.
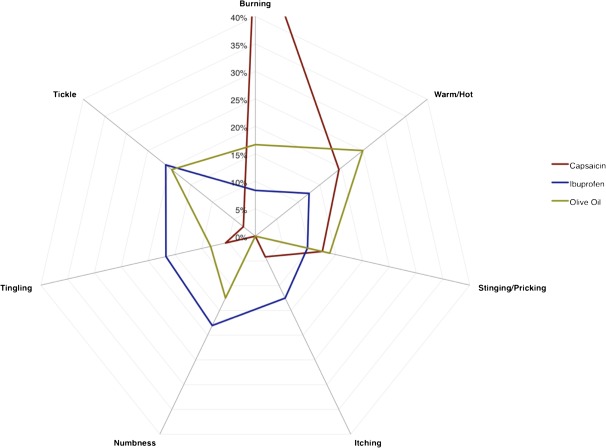
Frequency counts (expressed as percentages of total responses) were plotted to show the relative relationship of the subqualities described as the predominant sensation for each stimulus (Figure 4). Although it is clear from this plot that the simplified CATA task was successful in pulling apart the irritation profiles of the 3 stimuli, the plot also suggests capsaicin is a much cleaner stimulus than ibuprofen or olive oil. By “cleaner,” we mean that that there was general agreement that the predominant subquality of capsaicin was captured by 2 perceptually similar subqualities. Figure 4 shows the tendency of capsaicin to be described predominantly by “burning” and “warm/hot” and ibuprofen as evoking “numbness” and “tickle.” The qualitative aspects of extra virgin olive oil show similarities to both capsaicin (warm/hot) and ibuprofen (tickle), as would be expected if olive oil contains both TRPA1 and TRPV1 agonists.
Figure 4.
Percentages for the number of times each of the subqualities was described as the “predominant sensation” for the irritation from a particular stimulus. Note that this measure does not directly reflect the intensity of the sensations. Capsaicin was rated as burning 56% of the time, but a disjointed scale is used to highlight differences between the other subqualities. This figure appears in color in the online version of Chemical Senses.
Discussion
General findings
In this laboratory-based study of adults, we confirm that olive oil and ibuprofen irritancy vary across individuals. We extend prior work by demonstrating that response to both covaries in vivo—individuals who show reduced sensory response to ibuprofen also tend to show reduced response to olive oil. Consistent with the idea that oleocanthal and ibuprofen act via a capsaicin independent mechanism, we found that the strength of the relationship between ibuprofen and olive oil was stronger than the relationships between these stimuli and capsaicin. Unexpectedly, however, we did find a positive relationship between olive oil response and capsaicin, suggesting olive oil contains an unknown TRPV1 agonist. This is also supported by qualitative data on the perceptual subqualities of each stimulus, as olive oil shared attributes of both capsaicin and ibuprofen.
The anticipated predominant irritation subquality for ibuprofen was sting, itch, or tickle (Breslin et al. 2001). In contrast, capsaicin is typically associated with burning and warming (e.g., Lawless and Stevens 1988), although side tastes like bitterness have also been reported (Lawless and Stevens 1988; Green and Hayes 2003). These qualitative differences in vivo are consistent with in vitro data showing capsaicin and ibuprofen activate TRPV1 and TRPA1, respectively (Caterina et al. 1997; Peyrot des Gachons, Uchida, et al. 2011). We anticipated that olive oil would therefore show a response pattern similar to ibuprofen as the irritancy of olive oil has been attributed to oleocanthal, a known TRPA1 agonist. Instead, we found that the qualitative aspects of olive oil were intermediate between ibuprofen (predominantly tickling and tingling and numbing) and capsaicin (burning, hot/warming). This suggests that although oleocanthal may be the major source of irritancy in olive oil (Andrewes et al. 2003; Cicerale et al. 2009), it may not be the only one. Indeed, homovanillic and vanillic acids, as well as a number of structurally similar compounds, have been identified in the minor phenolic fraction of extra virgin olive oils (Harwood and Aparicio 1999). The mere presence of these vanilloids in olive oil is not sufficient to conclude that they actively contribute to the pungency of olive oil, as they may be present at levels well below human detection thresholds (i.e., below the “window of perception”), but it is not unreasonable to imagine that they may play a secondary role considering how little oleocanthal or capsaicin is required to initiate a sensory response.
As such, the presence of vanilloids in olive oil likely accounts for the correlation between the irritation elicited by capsaicin and olive oil observed here. This relationship was initially unexpected, as we had hypothesized, there would be no association between capsaicin response and the other 2 stimuli, due to the ubiquity of TRPV1-mediated irritation in contrast to the locus-specific nature of oleocanthal and ibuprofen irritancy. Previous human work had shown olive oil irritancy did not covary with the irritancy from carbon dioxide (Cicerale et al. 2009), a known TRPA1 agonist (Wang et al. 2010). Subsequently, oleocanthal was shown to activate TRPA1, at least in rat trigeminal ganglion (Peyrot des Gachons, Uchida, et al. 2011). The lack of a correlation between carbon dioxide and olive oil in humans in spite of a common receptor can be explained in 1 of 3 ways. It may be that carbon dioxide is promiscuous, activating more than one TRP receptor. Alternatively, it may have a mechanism that is more complicated than a single agonist–receptor relationship (Komai and Bryant 1993). Finally, it may reflect the idea that oleocanthal acts on TRPA1 via a unique mechanism that is not shared with other known TRPA1 agonists like allyl isothiocyanate or cinnamaldehyde and is somehow specific to channels present in the throat (Peyrot des Gachons, Uchida, et al. 2011). The trigeminal nerve appears to capable of expressing TRPA1, as pure oleocanthal burns in the nose (Peyrot des Gachons, Uchida, et al. 2011), so the reason for the lack of burn in the mouth is unclear.
Although the most parsimonious explanation for the capsaicin–olive oil correlation is the presence of vanilloids in olive oil, another potential explanation would be coexpression of TRPA1 and TRPV1 in vivo. In rodent trigeminal neurons, coexpression of TRPV1 has been reported in 100% of TRPA1-expressing neurons (Bautista et al. 2005, 2006). Thus, differences in the number of neurons that dually express TRPV1 and TRPA1 across people could also account for the correlation between capsaicin and the other 2 stimuli. Additionally, TRPV1 exclusive neurons may be thermospecific labeled lines that transduce sensations associated with heat pain, whereas TRPA1 acts as a more diffuse generalized chemo-sensor system intended to notify the body of chemical toxins. This later role is usually attributed to bitterness (Glendinning 1994), although evolutionarily functional similarities between bitterness and chemesthesis have been discussed previously (Lim and Green 2007). This would also help explain the difficultly our participants had in characterizing a specific percept (subquality) from the ibuprofen and olive oil.
Using time-intensity data alone to explore agonist–receptor relationships can be complicated by biophysical factors. In contrast to single time point ratings, generation of time-intensity curves is more influenced by tissue access and retention of the compounds as well as cellular adaptation. Each of these becomes even more complicated when more than one stimulus is presented within a session. Differential access and retention can result from the compound's lipophilicity; the degree of lipophilicity is often expressed as log P. Reported log P values for capsaicin, ibuprofen, and oleocanthal are 3.8 (Iida et al. 2003), 3.5 (Stuer-Lauridsen et al. 2000), and 1.5 (Peyrot Des Gachons, Sperry, et al. 2011), respectively. Log P values greater than 1 indicate that a compound is more lipophilic than hydrophilic. In this experiment, stimuli were presented in oil, so highly lipophilic molecules may be less likely to partition out of the lipid matrix and into the aqueous salivary environment. This may influence how and, more critically, when these molecules reach their receptors.
Limitations and conclusions
Here, a natural product, extra virgin olive oil, was used instead of pure oleocanthal in solution. This limits our ability to speak directly to the nature of percepts arising from oleocanthal in humans. However, this also increases our generalizability toward real foods and thus dietary habits and ingestive behavior.
This work also provides qualitative perceptual data that can only be obtained behaviorally. In contrast to dumping that typically occurs when response options are overly restricted, pilot work revealed evidence of a smearing bias where participants struggled somewhat to characterize the subqualities arising from ibuprofen. Whether this might be improved with some sort of panelist training or reflects the diffuse nature of the ibuprofen/oleocanthal percept is unclear. Previously, reduced intensity ratings (compression) have been observed in rating tasks when too many response options are provided to participants (van der Klaauw and Frank 1996). We do not anticipate this is the case with the check all that apply task used here, but more work is needed to confirm that CATA approaches are more robust in this respect. Here, capsaicin was found to be predominantly but not exclusively burning and warming, and ibuprofen was numbing and tickling; olive oil was intermediate, sharing qualities with each.
In summary, both qualitative and quantitative data suggest that olive oil contains vanilloid compounds that actively contribute to the perceived irritancy of olive oil. We confirm that olive oil and ibuprofen irritancy each vary across individuals and demonstrate that this variable response covaries in vivo.
Funding
Portions of this work were supported by the National Institute of Health Institute of Deafness and Communication Disorders [grant number DC010904 to J.E.H.].
Acknowledgments
This manuscript was completed in partial fulfillment of the requirements for a Master of Science degree at the Pennsylvania State University by S.M.B. The authors warmly thank Meghan Kane and Laura Boone for their assistance with data collection and our study participants for their time and participation.
References
- Andrewes P, Busch JL, de Joode T, Groenewegen A, Alexandre H. Sensory properties of virgin olive oil polyphenols: identification of deacetoxy-ligstroside aglycon as a key contributor to pungency. J Agric Food Chem. 2003;51(5):1415–1420. doi: 10.1021/jf026042j. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102(34):12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Keast RSJ, Morel D, Lin JM, Pika J, Han Q, Lee CH, Smith AB, Breslin PAS. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437(7055):45–46. doi: 10.1038/437045a. [DOI] [PubMed] [Google Scholar]
- Breslin PAS, Gingrich TN, Green BG. Ibuprofen as a chemesthetic stimulus: evidence of a novel mechanism of throat irritation. Chem Senses. 2001;26(1):55–65. doi: 10.1093/chemse/26.1.55. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cicerale S, Breslin PAS, Beauchamp GK, Keast RSJ. Sensory characterization of the irritant properties of oleocanthal, a natural anti-inflammatory agent in extra virgin olive oils. Chem Senses. 2009;34(4):333–339. doi: 10.1093/chemse/bjp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerale S, Conlan XA, Sinclair AJ, Keast RSJ. Chemistry and health of olive oil phenolics. Crit Rev Food Sci Nutr. 2008;49(3):218–236. doi: 10.1080/10408390701856223. [DOI] [PubMed] [Google Scholar]
- Clark CC, Lawless HT. Limiting response alternatives in time-intensity scaling: an examination of the halo-dumping effect. Chem Senses. 1994;19(6):583–594. doi: 10.1093/chemse/19.6.583. [DOI] [PubMed] [Google Scholar]
- Cliff M, Heymann H. Descriptive analysis of oral pungency. J Sens Stud. 1992;7(4):279–290. [Google Scholar]
- Cliff MA, Green BG. Sensitization and desensitization to capsaicin and menthol in the oral cavity: interactions and individual differences. Physiol Behav. 1996;59(3):487–494. doi: 10.1016/0031-9384(95)02089-6. [DOI] [PubMed] [Google Scholar]
- Delgado C, Guinard J-X. How do consumer hedonic ratings for extra virgin olive oil relate to quality ratings by experts and descriptive analysis ratings? Food Qual Prefer. 2011;22(2):213–225. [Google Scholar]
- Frank R, van der Klaauw N, Schifferstein HNJ. Both perceptual and conceptual factors influence taste-odor and taste-taste interactions. Percept Psychophys. 1993;54(3):343–354. doi: 10.3758/bf03205269. [DOI] [PubMed] [Google Scholar]
- Glendinning JI. Is the bitter rejection response always adaptive? Physiol Behav. 1994;56(6):1217–1227. doi: 10.1016/0031-9384(94)90369-7. [DOI] [PubMed] [Google Scholar]
- Green BG. Regional and individual differences in cutaneous sensitivity to chemical irritants: capsaicin and menthol. Cutan Ocul Toxicol. 1996;15(3):277–295. [Google Scholar]
- Green BG. Psychophysical measurement of oral Chemesthesis. In: Simon SA, Nicolelis MAL, editors. Methods in chemosensory research. Boca Raton (FL): CRC Press; 2002. pp. 3–19. [Google Scholar]
- Green BG, Hayes JE. Capsaicin as a probe of the relationship between bitter taste and chemesthesis. Physiol Behav. 2003;79(4–5):811–821. doi: 10.1016/s0031-9384(03)00213-0. [DOI] [PubMed] [Google Scholar]
- Green BG, Lim J, Osterhoff F, Blacher K, Nachtigal D. Taste mixture interactions: suppression, additivity, and the predominance of sweetness. Physiol Behav. 2010;101(5):731–737. doi: 10.1016/j.physbeh.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Schullery MT. Stimulation of bitterness by capsaicin and menthol: differences between lingual areas innervated by the glossopharyngeal and chorda tympani nerves. Chem Senses. 2003;28(1):45–55. doi: 10.1093/chemse/28.1.45. [DOI] [PubMed] [Google Scholar]
- Harwood JL, Aparicio R. The handbook of olive oil. Gaithersburg (MD): Aspen Publishers; 1999. [Google Scholar]
- Horne J, Lawless HT, et al. Bitter taste of saccharin and acesulfame-K. Chem Senses. 2002;27(1):31–38. doi: 10.1093/chemse/27.1.31. [DOI] [PubMed] [Google Scholar]
- Iida T, Moriyama T, Kobata K, Morita A, Murayama N, Hashizume S, Fushiki T, Yazawa S, Watanabe T, Tominaga M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology. 2003;44(7):958–967. doi: 10.1016/s0028-3908(03)00100-x. [DOI] [PubMed] [Google Scholar]
- Karrer T, Bartoshuk L. Capsaicin desensitization and recovery on the human tongue. Physiol Behav. 1991;49(4):757–764. doi: 10.1016/0031-9384(91)90315-f. [DOI] [PubMed] [Google Scholar]
- Komai M, Bryant BP. Acetazolamide specifically inhibits lingual trigeminal nerve responses to carbon dioxide. Brain Res. 1993;612(1–2):122–129. doi: 10.1016/0006-8993(93)91652-9. [DOI] [PubMed] [Google Scholar]
- Lawless H. Oral chemical irritation: psychophysical properties. Chem Senses. 1984;9(2):143–155. [Google Scholar]
- Lawless HT, Hartono C, Hernandez S. Thresholds and suprathreshold intensity functions for capsaicin in oil and aqueous based carriers. J Sens Stud. 2000;15(4):437–477. [Google Scholar]
- Lawless HT, Stevens DA. Responses by humans to oral chemical irritants as a function of locus of stimulation. Percept Psychophys. 1988;43(1):72–78. doi: 10.3758/bf03208975. [DOI] [PubMed] [Google Scholar]
- Lim J, Green BG. The psychophysical relationship between bitter taste and burning sensation: evidence of qualitative similarity. Chem Senses. 2007;32(1):31–39. doi: 10.1093/chemse/bjl033. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot Des Gachons C, Sperry JB, Bryant B, Breslin PAS, Smith AB, Beauchamp GK. Use of the irritating principal oleocanthal in olive oil, as well as structurally and functionally similar compounds. 2011. US Patent US 2011/0020424. 2011 Jan 27. [Google Scholar]
- Peyrot des Gachons C, Uchida K, Bryant B, Shima A, Sperry JB, Dankulich-Nagrudny L, Tominaga M, Smith AB, Beauchamp GK, Breslin PAS. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J Neurosci. 2011;31(3):999–1009. doi: 10.1523/JNEUROSCI.1374-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J, Roth W, Lacor P, Smith AB, Blankenship M, Velasco P, De Felice F, Breslin PAS, Klein WL. Alzheimer's-associated Abeta oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol Appl Pharmacol. 2009;240(2):189–197. doi: 10.1016/j.taap.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D, Fast K, Bartoshuk LM. Valid comparisons of suprathreshold sensations. J Conscious Stud. 2004;11:96–112. [Google Scholar]
- Stuer-Lauridsen F, Birkved M, Hansen LP, Lutzhoft HCH, Halling-Sorensen B. Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere. 2000;40(7):783–793. doi: 10.1016/s0045-6535(99)00453-1. [DOI] [PubMed] [Google Scholar]
- van der Klaauw NJ, Frank RA. Scaling component intensities of complex stimuli: the influence of response alternatives. Environ Int. 1996;22(1):21–31. [Google Scholar]
- Wang YY, Chang RB, Liman ER. TRPA1 is a component of the nociceptive response to CO2. J Neurosci. 2010;30(39):12958–12963. doi: 10.1523/JNEUROSCI.2715-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]