Abstract
Objective
To determine the extent of fluctuation in circadian intraocular pressure (IOP) and the efficacy of topical dorzolamide 2% q 8h in lowering IOP and blunting circadian fluctuation in IOP in glaucomatous cats.
Animals Studied
7 adult cats with primary congenital glaucoma (PCG).
Procedures
Measurements of IOP and pupil diameter were obtained for both eyes (OU) of each cat q 4h for 12 days. Cats were housed in a laboratory animal facility with a 12 hour light:dark cycle. Baseline values were established for 2 days. For the next 5 days, placebo (1.4% polyvinyl alcohol) was administered OU q 8h. Dorzolamide 2% (Trusopt, Merck and Co., Inc., West Point, PA) was then administered OU q 8h for a further 5 days. A multivariate mixed linear model was fitted to the data, with parameters estimated from a Bayesian perspective. The 4am time point was selected as the reference for the purposes of comparisons.
Results
Estimated mean IOP for the reference time point pre-treatment was symmetric (about 33mmHg OU). In all cats, IOP was significantly lower during the diurnal phase, relative to the 4 am measurements, with highest IOP observed 2-6h after the onset of the dark-phase. Circadian fluctuations in IOP were dampened during the treatment period. There was a significant decrease in IOP in all cats during the dorzolamide treatment period (estimated mean for the treatment period reference =17.9 mmHg OU).
Conclusion
Topical dorzolamide 2% q 8 h is effective in reducing IOP and IOP fluctuation in cats with PCG.
Keywords: glaucoma, feline, intraocular pressure, pupil diameter, carbonic-anhydrase, inhibitor, circadian
Introduction
The glaucomas are a group of disorders associated with increases in intraocular pressure (IOP) that ultimately result in optic nerve atrophy, retinal ganglion cell death and visual field loss.(1) Glaucoma is generally classified as either primary, without an antecedent cause, or secondary to other ocular disease. Feline glaucoma occurs most commonly as a sequela to uveitis, neoplasia or trauma,(2-3) although primary glaucoma has been reported in the Siamese,(4-5) Persian,(6) Burmese,(7) and European Shorthair breeds.(6, 8) Both open-angle glaucoma,(9) and glaucoma secondary to a syndrome of aqueous humor misdirection have been reported in older cats.(10) Primary congenital glaucoma is inherited as an autosomal recessive condition in the Siamese cat and is associated with bilateral IOP elevation and glaucomatous optic nerve cupping and loss of retinal ganglion cell axons.(McLellan, G.J. et al, manuscript in process)
In veterinary ophthalmology, the medical management of glaucoma is problematic and often only transiently effective, with marked species differences noted in drug efficacy. Elevation in IOP represents a major risk factor for the progression of vision loss, thus medical management has primarily focused on controlling IOP. Topical medications are often preferred, decreasing the frequency and severity of systemic side effects relative to their systemically administered counterparts. Topical adrenergic agents, such as the non-selective beta blocker timolol and the alpha-2 agonist apraclonidine, have been investigated in normal cats.(11-12) Topical timolol administration may cause decreases in heart rate and blood pressure as well as bronchoconstriction, while only resulting in modest reduction in intraocular pressure in cats.(11, 13)Timolol administration is therefore contraindicated in cats with cardiac and pulmonary disorders.(13)Although mildly effective at lowering intraocular pressure in normal cats, decreases in resting heart rate and vomiting were noted with apraclonidine administration.(12)
Topical prostaglandin analogues have demonstrated conflicting results when used to control intraocular pressure in cats.(14) Commercially available topical prostaglandin analogues optimized for human use, with enhanced FP receptor specificity, are generally ineffective at lowering IOP in normal cats.(15-16) In contrast, the administration of PGF2α lowered IOP in normal cats possibly due to non-selective binding to EP1 receptors in the ciliary body.(17-19)
As a result of the questionable IOP-lowering efficacy of other drug classes, medical management of glaucoma in cats has focused on the use of topical carbonic anhydrase inhibitors(CAIs). The active secretion of aqueous humor is dependent on carbonic anhydrase, a metalloenzyme that catalyzes the formation of bicarbonate from carbon dioxide and carbonic acid. As bicarbonate is formed, water enters into the posterior chamber of the eye, contributing to the volume of aqueous humor.(20)Aqueous humor production is decreased and intraocular pressure is lowered when carbonic anhydrase isoenzyme II in the pigmented and nonpigmented ciliary epithelium is inhibited.(20)Carbonic anhydrase is also found in high concentrations in the nephrons of the kidney, in erythrocytes, and in the respiratory tract. Since CAIs will inhibit all carbonic anhydrase isoenzymes to some degree, the oral CAIs initially used in the treatment of glaucoma resulted in diuresis, potassium depletion and metabolic acidosis.(21)Topical CAIs were developed in order to decrease the undesirable systemic effects associated with oral administration.(20)
Dorzolamide hydrochloride (Trusopt, Merck and Co., Inc., West Point, PA) was the first CAI approved for the topical treatment of glaucoma in humans.(22)Dorzolamide effectively penetrates the cornea and sclera, achieving an effective concentration of 2-10 μM which is comparable to that achieved with the administration of systemic CAIs. (23)In contrast, plasma concentrations are about 100 times lower than with systemic CAIs and are 1/200 of that needed to induce systemic side effects. (23)Topical CAIs have documented -IOP-lowering efficacy in humans, (22)rabbits and monkeys,(24)and dogs.(25-27)
The clinical effect of topical CAIs on normal cats has been equivocal. In one study, topical dorzolamide administered twice a day significantly lowered IOP in normal cats,(28) but another study found no significant decrease when brinzolamide was administered topically twice daily.(29) Previous studies in dogs have documented that topical CAIs will lower IOP to a much larger degree in glaucomatous compared to normal animals.(25-27) To our knowledge, there have been no published studies that document the effect of topical anti-glaucoma medications in cats with glaucoma.
The aims of this study were to: 1) determine the degree of circadian variation in IOP and its relationship with pupil diameter in cats with primary congenital glaucoma; and 2) determine if topical dorzolamide 2% is effective at decreasing IOP and blunting circadian fluctuation in IOP in cats with primary congenital glaucoma.
Materials and Methods
All procedures were conducted in accordance with the Association for Research in Vision and Ophthalmology’s Statement for the Use of Animals in Ophthalmic and Vision Research and with the approval of the institutional animal care and use committee.
Seven adult Siamese cats (mean age = 2.4 years) with recessively inherited primary congenital glaucoma were included in the study. Two cats were intact males (2 years and 4 years old) and 5 cats were intact females (ages 1-4 years old). All cats belonged to a research breeding colony established in 2004 at Iowa State University and were housed in a laboratory animal facility with a 12 hour light (6am-6pm) and dark cycle. Cats were fully conditioned and were accustomed to measurement of IOP on a regular basis. A full ophthalmic exam, including slit-lamp biomicroscopy, indirect ophthalmoscopy, gonioscopy and applanation tonometry, was performed on each cat prior to the study. Diagnosis of primary congenital glaucoma was based on the presence of characteristic clinical signs of globe enlargement, elongation of the ciliary processes, subtle optic disc cupping and IOP elevation. With the exception of these characteristic features of primary congenital glaucoma, no other evidence of significant ocular disease was evident at the time of the study. No topical medications were administered for at least one month prior to the study, with the exception of proparacaine HCl 0.5% prior to applanation tonometry.
Horizontal pupil diameter and intraocular pressure measurements were obtained by one trained observer (KJS) every 4 hours (8 am, 12 pm, 4 pm, 8 pm, 12 am, 4 am) for 12 days in both eyes. The maximal horizontal pupil diameter was measured using Jameson calipers held approximately 8-10mm from the corneal surface and IOP was measured by applanation tonometry (Tonopen XL, Mentor, Norwell, MA) following the administration of 50 μl of proparacaine HCl 0.5% solution. All cats were monitored for blepharospasm and conjunctival hyperemia. Light-phase measurements (8 am, 12 pm and 4 pm) were obtained under consistent ambient light conditions in the same rooms of the animal laboratory animal facility. During the dark phase, measurements were obtained under dim red light.
During the first two days of the study, baseline values were established. For the next 5 days, a single drop of placebo (1.4 % polyvinyl alcohol) was administered to both eyes every 8 hours (7 am, 3 pm, 11 pm) and one hour prior to pupil and IOP measurements. For the next 5 days, a single drop (30μl) of dorzolamide 2% (Trusopt, Merck and Co., Inc., West Point, PA) was administered to both eyes every 8 hours (7am, 3 pm, 11 pm) and one hour prior to subsequent pupil diameter and IOP measurements. All cats were observed grossly for several minutes following application of placebo or drug, and at each ensuing time point thereafter, for clinical signs of ocular discomfort such as squinting, rubbing or tearing and for conjunctival hyperemia or other adverse effects.
Statistical Analyses
A linear mixed model with a fixed effect for treatment and measurement time and a random effect for cat was fitted to the two-dimensional measurements obtained for each cat at each time point. The correlation between the measurements from the two eyes of the same cat was accommodated by jointly modeling both eyes. The correlation between measurements over time in the same cat was accommodated via the inclusion of the cat-specific random effect in the model. Bayesian methods were used to estimate model parameters and WinBUGS version 1.4.1 was used for computations. The Bayesian methodology permits fitting a complex model, such as the one required in this study, even when the sample size is relatively small. Ninety five percent credible sets (roughly equivalent to classical confidence intervals) can be used to decide whether the effect of treatment is significantly different from zero and whether the differences in outcome between measurement times are also significant. As in the classical approach, we can conclude that the effect of treatment is significant if the credible set (CS) for the difference between two treatments does not include the value 0. For all tests we selected a significance level equal to 95% and based our conclusions on credible sets as described above.
Results
No adverse effects, such as ocular irritation, were noted in any cat at any time point as a result of the administration of either the placebo or topical dorzolamide 2%.
Circadian Fluctuations in IOP
During the two day acclimation phase of the study and during the 5 day placebo period a circadian variation in IOP was evident in all cats, with marked elevations in IOP occurring during the dark phase. (Fig. 1). Light-phase measurements (8 am, 12 pm and 4 pm) were not significantly different from each other and dark-phase measurements (8 pm, 12 am and 4 am) were not significantly different from each other at the 5% level. The degree of fluctuation in IOP was significantly decreased in each cat during the treatment phase relative to both the acclimation and the placebo phases of the study (Fig. 2). The average fluctuation in IOP during the acclimation phase was 20.3 mmHg OD and 18 mmHg OS; in the placebo phase was 18.3 mmHg OD and 18.1 mmHg OS, and during the treatment phase was 7.3 mmHg OD and 8.5 mmHg OS.
Figure 1.
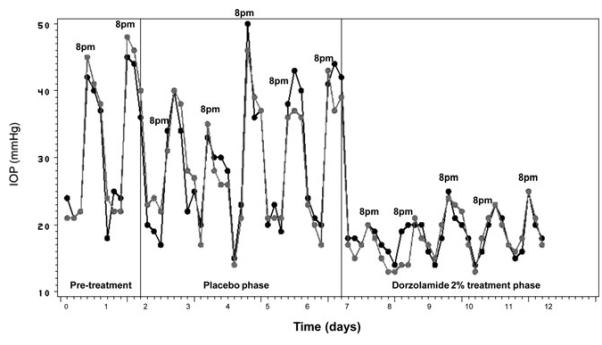
Plot of circadian intraocular pressure (IOP) in a representative glaucomatous cat. Dramatic dark-phase increases in IOP are reduced by dorzolamide treatment. Data for right eye is represented in black and for left eye is represented in gray. (Each tick mark on the abscissa represents 4 hours).
Figure 2.
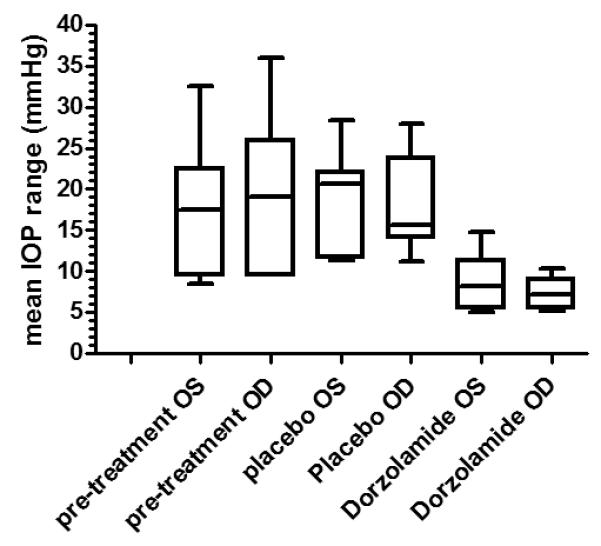
Box and Whiskers plot illustrating significant reduction in circadian intraocular pressure (IOP) fluctuation in glaucomatous cats (n=7) during treatment with dorzolamide 2%, relative to pre-treatment and placebo phases of the study. Data are shown for left eyes (OS) and right eyes (OD). Lines within boxes represent median values, whiskers represent 5-95% confidence intervals.
Mean Intraocular Pressures
Estimated mean IOPs for the placebo reference time period (4 am) were 32.8 mmHg OD (CS: 30.3-35.4 mmHg) and 33.2 mmHg OS (CS: 30.6-35.7 mmHg). Estimated mean IOPs for the treatment reference time period (4 am) were 17.9 mmHg OU (CS: 16.5-19.2 mmHg). The differences between IOPs prior to and during dorzolamide treatment were significant (p < 0.05), and represented a decrease in mean IOP of 14.9 mmHg OD (CS: 12.1-17.9 mmHg) and 15.3 mmHg OS (CS: 12.4-18.2 mmHg). This corresponded to an approximately 45% decrease in IOP at the 4am reference time in both eyes.
Estimated mean IOPs for all cats for all time points during the placebo period were 28.0 mmHg OD (CS: 27.2-28.9 mmHg) and 29.2 mmHg OS (CS: 28.3-30.1 mmHg) while in the treatment period these values were 17.5 mmHg OD (CS: 17.0-18.0 mmHg) and 17.7 mmHg OS (CS: 17.3-18.1 mmHg). The mean differences between the estimated mean IOPs for all time points for all cats were 10.6 mmHg OD (CS: 9.6-11.5 mmHg) and 11.5 mmHg OS (CS: 10.5-12.5 mmHg); corresponding to a 38% decrease in estimated mean IOP OU.
Pupil Diameter
Estimated mean pupil diameters for the reference time point (4 am) were 11.7 mm OD (CS: 11.0-12.3 mm) and 11.6 mm OS (CS: 11.0-12.2 mm) for the placebo phase and 12.8 mm OD (CS: 12.2-13.3 mm) and 12.7 mm OS (CS: 12.2-13.3 mm) for the treatment phase. Estimated mean pupil diameters for all cats over all time points were 10.9 mm OD (CS: 10.7-11.1 mm) and 10.8 mm OS (CS: 10.6-11.0 mm) during the placebo phase and 11.0 mm OD (CS: 10.8-11.2 mm) and 10.8 mm OS (CS: 10.7-11.0 mm) during the treatment phase. There was no significant difference in pupil diameter during the treatment versus the placebo phase of the study for either the 4am reference time point or for the average of all time periods. Pupil diameters were not significantly correlated (p > 0.05) with IOP. A degree of circadian variation was noted in pupillary diameter, with diameters being larger during the dark phase than during the light phase.
Discussion
A marked circadian variation in IOP was noted in the glaucomatous cats in our study, with pressures lowest during the light phase and highest early in the dark phase. Our findings are in agreement with previous reports in normal cats that documented lower IOP in the morning, although IOP was only measured for a 12 hour period and no overnight measurements were obtained in those studies.(11, 30) More recently, a study of circadian rhythm in IOP of normal cats documented peak IOP values soon after the onset of the dark phase,(31) consistent with our findings in glaucomatous cats. This circadian pattern is similar to normal rabbits,(32-33) but is in contrast to normal and glaucomatous dogs, in which IOP is highest in the morning.(34-35)
In the present study, pupil diameter was poorly correlated with IOP and there was no significant difference noted between the treatment and placebo phases of the study. The cats in this study however had relatively advanced glaucoma. All subjects were buphthalmic, and had blue irides with varying degrees of iris stromal hypoplasia. As such, the extent of pupil constriction, regardless of IOP, may have been limited by their pre-existing disease. Their relative degree of pupil dilation may have limited our ability to establish a clear relationship between pupil diameter and IOP, as may our protocol which dictated collection of dark-phase measurements under lower levels of ambient light in order to limit disruption of the normal light-dark cycle.
The physiologic basis for circadian fluctuations in IOP in both normal and glaucomatous animals remains unclear. Circadian fluctuations in IOP have been attributed to cyclic changes in the volume of aqueous humor produced, changes in outflow facility and episcleral venous pressure, and alterations in systemic arterial and venous blood pressure.(36-38) It has been speculated that changes in cortisol levels may be involved in circadian fluctuations in IOP.(39-40) Measurement of serum cortisol levels was beyond the scope of the current study. Sympathetic (adrenergic) tone also plays a role in circadian IOP rhythms, as demonstrated in rabbits.(41) A study conducted in humans found that IOP measurements obtained immediately upon waking were initially relatively high but fell to baseline within a 15 minute period, indicating that a transient “waking spike” may occur. In the present study, no cat was awakened in order to obtain an IOP since they were all observed to be awake at the front of their cages when the investigator entered the room. In cats, it seems unlikely that the circadian variation in IOP is related to a sleep cycle. Cats, unlike humans, are a crepuscular species that nap throughout the day and do not sleep continuously at night.
The magnitude of circadian fluctuation in IOP observed in glaucomatous cats (mean 19.14 mmHg, with a range of 8.5-32.5 mmHg) was dramatic in comparison to that reported for normal cats (which demonstrate circadian IOP fluctuations in IOP in the order of 4mmHg).(31) The actual magnitude of circadian fluctuation in glaucomatous cats is likely substantially higher than reported in the present study, as the Tono-pen XL applanation tonometer that was used to measure IOP in this study has been shown to underestimate IOP above about 30mmHg in both normal and glaucomatous cats.(42-44) Rebound tonometry has recently been shown to provide a more accurate means of measuring IOP in normal and glaucomatous cats, but was not available at the time that the present study was conducted. (44-45) The range of circadian variation in glaucomatous dogs and humans is 1.5 to 3 times that of normal individuals.(34, 46) Large diurnal fluctuations in IOP are thought to be more damaging to the eye.(47-48) A retrospective analysis of humans with open-angle glaucoma and a normal IOP during office visits found that the range of the initial daily IOP variation was more predictive of the progression of visual field loss than was the mean or peak IOP.(47) As such, medications that decrease the magnitude of daily fluctuations in IOP should be selected in an effort to slow the progression of glaucoma. In our study, dorzolamide effectively “blunted” circadian fluctuations in IOP in glaucomatous cats.
The topical application of dorzolamide 2% three times daily to glaucomatous cats resulted in a statistically significant decrease in IOP, representing an approximately 45% reduction in mean IOP. In previously published studies, topical dorzolamide administration in normal cats, applied at the lower frequency of twice daily, decreased IOP by 18-20% in one study,(28) and by 8.8% in another.(49) Since the current study was completed, other investigators have reported a significant reduction in both aqueous humor flow rate and IOP in normal cats treated three times daily with topical dorzolamide.(50) It has been shown in a number of species, including cats, dogs, rabbits and monkeys, that carbonic anhydrase inhibitors demonstrate a greater IOP-lowering effect in glaucomatous versus normal animals.(27, 51-53)
One of the limitations of our study is that all of the subjects were affected by a single form of primary glaucoma that is infrequently encountered in clinical practice. Caution should be exercised in extrapolating data regarding response to glaucoma therapies between subjects, without giving consideration to underlying mechanisms of aqueous outflow obstruction. However, CAIs target aqueous production rather than aqueous humor outflow. Thus, dorzolamide should have broad applicability in feline glaucoma patients.
In humans, the most common side effects associated with the administration of topical dorzolamide include a burning and stinging sensation, conjunctival hyperemia and punctuate epithelial erosions.(22) Although the assessment of tolerability of the topical formulation was not a specific aim of the current study, we noted no ocular adverse effects following topical administration of dorzolamide in the relatively small number of glaucomatous cats evaluated. This observation is in agreement with previously published studies in normal cats and dogs.(25, 28, 49)
In summary, topical dorzolamide 2% was effective at lowering IOP, and blunting circadian fluctuations in IOP, in glaucomatous cats in our study. A pronounced circadian fluctuation in IOP was identified in all cats, with IOPs significantly higher during the dark phase. The range of IOP fluctuation observed was extreme and suggests that caution should be exercised when interpreting a single daytime measurement of IOP in a glaucomatous cat, or feline glaucoma suspect. A 24 hour IOP curve may be necessary to establish glaucoma status in some cats and to determine if medical control of IOP is adequate, particularly in cats with evidence of disease progression despite apparently “successful” IOP-lowering therapy.
Acknowledgements
The authors thank Chimene Peterson and Angel Leon for technical assistance and Dr N. Matthew Ellinwood for his assistance in establishing a viable breeding colony of cats affected by primary congenital glaucoma.
Funding: Supported by the Glaucoma Research Foundation and NIH grant K08EY018609
Footnotes
Dr Sigle’s current address is: Carolina Veterinary Specialists 501 Nicholas Rd Greensboro, NC 27409
References
- 1.Gelatt KN, Brooks DE. The Canine Glaucomas. In: Gelatt KN, editor. Veterinary Ophthalmology. 3rd ed Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 701–754. [Google Scholar]
- 2.Blocker T, van der Woerdt A. The feline glaucomas: 82 cases (1995-1999) Veterinary Ophthalmology. 2001 Jun 01;4(2):81–85. doi: 10.1046/j.1463-5224.2001.00169.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilcock BP, Peiffer RL, Jr., Davidson MG. The causes of glaucoma in cats. Veterinary Pathology. 1990 Jan;27(1):35–40. doi: 10.1177/030098589002700105. [DOI] [PubMed] [Google Scholar]
- 4.Brown A, Munger R, Peiffer RL., Jr Congenital glaucoma and iridoschisis in a Siamese cat. Veterinary and Comparative Ophthalmology. 1994;4(3):121–124. [Google Scholar]
- 5.McLellan GJ, Betts D, Sigle K, Grozdanic S. Congenital glaucoma in the Siamese cat- a new spontaneously occurring animal model for glaucoma research. (abstract). 35th Annual Meeting of the American College of Veterinary Ophthalmologists; Washington, DC. 2004. [Google Scholar]
- 6.Brooks DE. Glaucoma in the dog and cat. Veterinary Clinics of North America Small Animal Practice. 1990 May;20(3):775–797. doi: 10.1016/s0195-5616(90)50062-5. [DOI] [PubMed] [Google Scholar]
- 7.Hampson EC, Smith RI, Bernays ME. Primary glaucoma in Burmese cats. Australian Veterinary Journal. 2002 Nov;80(11):672–680. doi: 10.1111/j.1751-0813.2002.tb11292.x. [DOI] [PubMed] [Google Scholar]
- 8.Trost K, Peiffer RL, Jr., Nell B. Goniodysgenesis associated with primary glaucoma in an adult European Short-haired cat. Veterinary Ophthalmology. 2007 Nov-Dec;10(Suppl 1):3–7. doi: 10.1111/j.1463-5224.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 9.Jacobi S, Dubielzig RR. Feline primary open angle glaucoma. Veterinary Ophthalmology. 2008 May-Jun;11(3):162–165. doi: 10.1111/j.1463-5224.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 10.Czederpiltz JM, La Croix NC, van der Woerdt A, Bentley E, Dubielzig RR, Murphy CJ, et al. Putative aqueous humor misdirection syndrome as a cause of glaucoma in cats: 32 cases (1997-2003) Journal of the American Veterinary Medical Association. 2005 Nov 1;227(9):1434–1441. doi: 10.2460/javma.2005.227.1434. [DOI] [PubMed] [Google Scholar]
- 11.Wilkie DA, Latimer CA. Effects of topical administration of timolol maleate on intraocular pressure and pupil size in cats. American Journal of Veterinary Research. 1991 Mar;52(3):436–440. [PubMed] [Google Scholar]
- 12.Miller PE, Rhaesa SL. Effects of topical administration of 0.5% apraclonidine on intraocular pressure, pupil size, and heart rate in clinically normal cats. American Journal of Veterinary Research. 1996 Jan;57(1):83–86. [PubMed] [Google Scholar]
- 13.Plumb DC. Veterinary Drug Handbook. 3rd Edn Iowa State University Press; Ames: 1999. [Google Scholar]
- 14.Willis AM, Diehl KA, Robbin TE. Advances in topical glaucoma therapy. Veterinary Ophthalmology. 2002 Mar 01;5(1):9–17. doi: 10.1046/j.1463-5216.2001.00202.x. 2002. [DOI] [PubMed] [Google Scholar]
- 15.Studer ME, Martin CL, Stiles J. Effects of 0.005% latanoprost solution on intraocular pressure in healthy dogs and cats. American Journal of Veterinary Research. 2000 Oct;61(10):1220–1224. doi: 10.2460/ajvr.2000.61.1220. [DOI] [PubMed] [Google Scholar]
- 16.Bartoe JT, Davidson HJ, Horton MT, Jung Y, Brightman AH. The effects of bimatoprost and unoprostone isopropyl on the intraocular pressure in normal cats. Veterinary Ophthalmology. 2005;8(4):247–252. doi: 10.1111/j.1463-5224.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacherjee P, Williams BS, Paterson CA. Responses of intraocular pressure and the pupil of feline eyes to prostaglandin EP1 and FP receptor agonists. Investigative Ophthalmology and Visual Science. 1999 Nov;40(12):3047–3053. [PubMed] [Google Scholar]
- 18.Woodward DF, Krauss AH, Wang JW, Protzman CE, Nieves AL, Liang Y, et al. Identification of an antagonist that selectively blocks the activity of prostamides (prostaglandin-ethanolamides) in the feline iris. British Journal of Pharmacology. 2007 Feb;150(3):342–352. doi: 10.1038/sj.bjp.0706989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharif NA, Kaddour-Djebbar I, Abdel-Latif AA. Cat iris sphincter smooth-muscle contraction: comparison of FP-class prostaglandin analog agonist activities. Journal of Ocular Pharmacology and Therapeutics. 2008 Apr;24(2):152–163. doi: 10.1089/jop.2007.0076. [DOI] [PubMed] [Google Scholar]
- 20.Sharir M. Topical Carbonic Anhydrase Inhibitors. In: Zimmerman TJ, editor. Textbook of Ocular Pharmacology. Lippincott-Raven; Philadelphia: 1997. pp. 287–290. [Google Scholar]
- 21.Piper J. Oral Carbonic Anhydrase Inhibitors. In: Zimmerman TJ, editor. Textbook of Ocular Pharmacology. Lippincott-Raven; Philadelphia: 1997. pp. 277–285. [Google Scholar]
- 22.Wilkerson M, Cyrlin M, Lippa EA, Esposito D, Deasy D, Panebianco D, Fazio R, Yablonski M, Shields MB. Four-week safety and efficacy study of dorzolamide, a novel, active topical carbonic anhydrase inhibitor. Archives of Ophthalmology. 1993 Oct;111(10):1343–1350. doi: 10.1001/archopht.1993.01090100051026. [DOI] [PubMed] [Google Scholar]
- 23.Maren TH, Conroy CW, Wynns GC, Levy NS. Ocular absorption, blood levels, and excretion of dorzolamide, a topically active carbonic anhydrase inhibitor. Journal of Ocular Pharmacology and Therapeutics. 1997 Feb;13(1):23–30. doi: 10.1089/jop.1997.13.23. [DOI] [PubMed] [Google Scholar]
- 24.Toris CB, Zhan GL, McLaughlin MA. Effects of brinzolamide on aqueous humor dynamics in monkeys and rabbits. Journal of Ocular Pharmacology and Therapeutics. 2003 Oct;19(5):397–404. doi: 10.1089/108076803322472962. [DOI] [PubMed] [Google Scholar]
- 25.Cawrse MA, Ward DA, Hendrix DV. Effects of topical application of a 2% solution of dorzolamide on intraocular pressure and aqueous humor flow rate in clinically normal dogs. American Journal of Veterinary Research. 2001 Jun;62(6):859–863. doi: 10.2460/ajvr.2001.62.859. [DOI] [PubMed] [Google Scholar]
- 26.Gelatt KN, MacKay EO. Changes in intraocular pressure associated with topical dorzolamide and oral methazolamide in glaucomatous dogs. Veterinary Ophthalmology. 2001 Mar;4(1):61–67. doi: 10.1046/j.1463-5224.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 27.King TC, Gum GG, Gelatt KN. Evaluation of a topically administered carbonic anhydrase inhibitor (MK-927) in normotensive and glaucomatous beagles. American Journal of Veterinary Research. 1991 Dec;52(12):2067–2070. [PubMed] [Google Scholar]
- 28.Rainbow ME, Dziezyc J. Effects of twice daily application of 2% dorzolamide on intraocular pressure in normal cats. Veterinary Ophthalmology. 2003 Jun 01;6(2):147–150. doi: 10.1046/j.1463-5224.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 29.Gray HE, Willis AM, Morgan RV. Effects of topical administration of 1% brinzolamide on normal cat eyes. Veterinary Ophthalmology. 2003 Dec 01;6(4):285–290. doi: 10.1111/j.1463-5224.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 30.Wilkie DA, Latimer CA. Effects of topical administration of 2.0% pilocarpine on intraocular pressure and pupil size in cats. American Journal of Veterinary Research. 1991 Mar;52(3):441–444. [PubMed] [Google Scholar]
- 31.Del Sole MJ, Sande PH, Bernades JM, Aba MA, Rosenstein RE. Circadian rhythm of intraocular pressure in cats. Veterinary Ophthalmology. 2007 May-Jun;10(3):155–161. doi: 10.1111/j.1463-5224.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu JHK. Circadian rhythm of intraocular pressure. Journal of Glaucoma. 1998;7(2):141–147. [PubMed] [Google Scholar]
- 33.Akaishi T, Ishida N, Shimazaki A, Hara H, Kuwayama Y. Continuous monitoring of circadian variations in intraocular pressure by telemetry system throughout a 12-week treatment with timolol maleate in rabbits. Journal of Ocular Pharmacology and Therapeutics. 2005 Dec;21(6):436–444. doi: 10.1089/jop.2005.21.436. [DOI] [PubMed] [Google Scholar]
- 34.Gelatt KN, Gum GG, Barrie KP, Williams LH. Diurnal variations in intraocular pressure in normotensive and glaucomatous beagles. Glaucoma. 1981;3(2):121–124. [Google Scholar]
- 35.Giannetto C, Piccione G, Giudice E. Daytime profile of the intraocular pressure and tear production in normal dog. Vet Ophthalmol. 2009 Sep-Oct;12(5):302–305. doi: 10.1111/j.1463-5224.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 36.Blaise P, Guillaume S. Circadian variations in intraocular pressure and their clinical implications. Journal Français d’Ophtalmologie. 2005 Mar;28(3):317–325. doi: 10.1016/s0181-5512(05)81061-7. [DOI] [PubMed] [Google Scholar]
- 37.Chiquet C, Denis P. The neuroanatomical and physiological bases of variations in intraocular pressure. Journal Français d’Ophtalmologie. 2004 Sep;27(Spec No 2):2S11–2S18. [PubMed] [Google Scholar]
- 38.Zhao M, Hejkal JJ, Camras CB, Toris CB. Aqueous humor dynamics during the day and night in juvenile and adult rabbits. Investigative Ophthalmology and Visual Science. 2010 Jun;51(6):3145–3151. doi: 10.1167/iovs.09-4415. [DOI] [PubMed] [Google Scholar]
- 39.Weitzman ED, Henkind P, Leitman M, Hellman L. Correlative 24-hour relationships between intraocular pressure and plasma cortisol in normal subjects and patients with glaucoma. British Journal of Ophthalmology. 1975 Oct;59(10):566–572. doi: 10.1136/bjo.59.10.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu JH, Dacus AC. Endogenous hormonal changes and circadian elevation of intraocular pressure. Investigative Ophthalmology and Visual Science. 1991 Mar;32(3):496–500. [PubMed] [Google Scholar]
- 41.Liu JH, Dacus AC, Bartels SP. Adrenergic mechanism in circadian elevation of intraocular pressure in rabbits. Investigative Ophthalmology and Visual Science. 1991 Jul;32(8):2178–2183. [PubMed] [Google Scholar]
- 42.Passaglia CL, Guo X, Chen J, Troy JB. Tonopen XL calibration curves for cats, cows and sheep. Veterinary Ophthalmology. 2004;7(4):261–264. doi: 10.1111/j.1463-5224.2004.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoiber J, Fernandez V, Lamar PD, Hitzl W, Fantes F, Parel JM. Ex vivo evaluation of Tono-Pen and pneumotonometry in cat eyes. Ophthalmic Research. 2006;38(1):13–18. doi: 10.1159/000088492. [DOI] [PubMed] [Google Scholar]
- 44.McLellan GJ, Kemmerling JP, Kiland JA. Evaluation Of Rebound And Applanation Tonometry In Normal And Chronically Glaucomatous Cats (abstract). 40th Annual Meeting American College of Veterinary Ophthalmologists; Chicago,IL. 2009. [Google Scholar]
- 45.Rusanen E, Florin M, Hassig M, Spiess BM. Evaluation of a rebound tonometer (Tonovet) in clinically normal cat eyes. Veterinary Ophthalmology. 2010 Jan;13(1):31–36. doi: 10.1111/j.1463-5224.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 46.Drance SM. Diurnal Variation of Intraocular Pressure in Treated Glaucoma. Significance in Patients with Chronic Simple Glaucoma. Archives of Ophthalmology. 1963 Sep;70:302–311. doi: 10.1001/archopht.1963.00960050304004. [DOI] [PubMed] [Google Scholar]
- 47.Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. Journal of Glaucoma. 2000 Apr;9(2):134–142. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Wilensky JT. The role of diurnal pressure measurements in the management of open angle glaucoma. Current Opinions in Ophthalmology. 2004 Apr;15(2):90–92. doi: 10.1097/00055735-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Dietrich UM, Chandler MJ, Cooper T, Vidyashankar A, Chen G. Effects of topical 2% dorzolamide hydrochloride alone and in combination with 0.5% timolol maleate on intraocular pressure in normal feline eyes. Veterinary Ophthalmology. 2007 Nov-Dec;10(Suppl 1):95–100. doi: 10.1111/j.1463-5224.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 50.Crumley WR, Rankin AJ. The effect of topical 2% dorzolamide solution on aqueous humor flow rate and intraocular pressure in normal cats. (abstract). 41st Annual Conference of the American College of Veterinary Ophthalmologists; San Diego, CA. 2010. [Google Scholar]
- 51.Othman GM, Samy MT. Can carbonic anhydrase inhibitors relieve intraocular pressure in cats with glaucoma? Veterinary Medicine. 1987;82(5):498–509. [Google Scholar]
- 52.Sugrue MF. The preclinical pharmacology of dorzolamide hydrochloride, a topical carbonic anhydrase inhibitor. Journal of Ocular Pharmacology and Therapeutics. 1996 Fall;12(3):363–376. doi: 10.1089/jop.1996.12.363. [DOI] [PubMed] [Google Scholar]
- 53.Gelatt KN, Gum GG, Williams LW. Ocular hypotensive effects of carbonic anhydrase inhibitors in normotensive and glaucomatous Beagles. American Journal of Veterinary Research. 1979;40:334–345. [PubMed] [Google Scholar]


