Abstract
Cats with glaucoma typically present late in the course of disease. It is likely that glaucoma in cats is under-diagnosed due to its insidious onset and gradual progression, as well as limitations of some commonly used tonometers in this species. Treatment of glaucoma in feline patients presents a clinical challenge, particularly as glaucoma is often secondary to other disease processes in cats. In this review, we consider the clinical features, patho-physiology and classification of the feline glaucomas and provide current evidence to direct selection of appropriate treatment strategies for feline glaucoma patients.
Keywords: Feline, Cat, Glaucoma, Intraocular pressure, patho-physiology, therapy
What is Glaucoma?
Glaucoma is not a single disease entity. Rather, “the glaucomas” should be considered to represent a large, diverse group of disorders. In all species, this group of disorders is unified in their final common pathway of characteristic optic nerve and retinal pathology resulting in loss of vision. Glaucoma is therefore widely considered a neuro-degenerative disease.
The single most important risk factor for the development of glaucoma in humans and animals is intraocular pressure (IOP). However, caution should be exercised in diagnosing glaucoma based solely on a single, elevated IOP reading. Elevated IOP in the absence of clinical evidence of glaucomatous optic nerve and retinal damage is termed “ocular hypertension”, to distinguish it from overt glaucoma. Both ocular hypertension and glaucoma should be distinguished from falsely increased IOP measurements due to improper tonometric technique, inappropriate restraint, patient stress and corneal factors that may render the tonometer inaccurate. Conversely, a diagnosis of glaucoma cannot be excluded based on a single low or normal IOP reading. In glaucomatous cats, as in other species including glaucomatous dogs and humans,(1-2) IOP may fluctuate considerably, both within and between days. The magnitude of circadian fluctuation in IOP observed in glaucomatous cats in one recent study that utilized a Tono-pen XL,(3) was 2 to 8 times that reported for normal cats, which demonstrated circadian fluctuations in IOP in the order of 4 mmHg.(4) It is clear that a single measurement of IOP obtained during an office visit may not accurately reflect the cumulative IOP to which the eye has been exposed. Furthermore, in advanced glaucoma, degeneration of the ciliary body may limit aqueous production, ultimately lowering IOP.(5-6) In such cases, a diagnosis of glaucoma relies on the identification of characteristic posterior segment changes, supported by other secondary abnormalities, as described later in this review.
What is normal IOP in cats and how do we measure it?
As elevations in IOP contribute to the progression of glaucoma, an accurate and reliable means of measuring IOP is crucial to effective diagnosis and monitoring of this disease. Normal feline IOP varies with the time of day, age and reproductive status.(4, 7-8) Normal cats exhibit a pronounced circadian rhythm in IOP, with highest values at night (by about 4- 5 mmHg) and a gradual decline in IOP occurring during the day (with IOP in the mid-to late afternoon about 1-1.5 mmHg lower than morning values). Intraocular pressure in cats is also influenced by the age of the subject, being considerably lower in geriatric cats than in young cats; higher in adolescent than in adult cats, and lower in young kittens within the first few weeks of life than in adolescent cats. In one large study involving 538 cats 7 years of age and older, normal mean IOP as measured by Tono-pen XL was 12.3 ±4.0 mmHg.(7) In those cats in which IOP was measured on multiple occasions over time, IOP decreased progressively and it was not unusual for aged cats to have very low IOPs (≤ 7 mmHg) in the absence of any signs of anterior uveitis. This reduction in IOP may reflect reduction in active secretion of aqueous humor associated with declining systemic health.(7) Marked asymmetry in IOPs, with a difference of 12mmHg or more between eyes, or an IOP of 25mmHg or more in older cats (measured with a Tono-pen XL), should prompt a thorough evaluation for ocular abnormalities including glaucoma.(7) While reproductive status has been shown to influence IOP in cats, with significantly higher IOP observed in estrus females,(8) this influence may be of limited clinical significance in veterinary practice, as relatively few feline patients are sexually intact. In clinical settings, it is also important to be aware that prior application of mydriatic may lead to substantial increases in IOP in both normal and glaucomatous cats.(9-11)
Differences in measurements obtained by different tonometer types are also clinically significant. It is therefore important that a consistent tonometer type and model is used for clinical monitoring and that the tonometer type used to measure IOP is specified in the medical record. Veterinary clinicians should also be aware of the common limitations of the tonometer that is used. A number of tonometers have been investigated in cats (Table 1), including the Schiøtz indentation tonometer;(12) pneumotonometer;(13) Mackay-Marg type applanation tonometers (such as the Mackay-Marg, Tono-pen, Tonopen XL and TonoPen Vet);(13-16) Perkins applanation tonometer, (17) and, most recently an induction/impact or rebound tonometer (TonoVet®).(16,18) Although all these tonometers demonstrated acceptable accuracy within the normal physiological range in cats, it is noteworthy that most applanation tonometers dramatically underestimated IOP above about 30mm Hg when compared to manometry. Despite their widespread application in clinical veterinary practice, the systematic underestimation of IOP in glaucomatous cats by most applanation tonometers may have contributed to an underestimation of the true prevalence of glaucoma in the feline population. The TonoVet® rebound tonometer is more accurate than the Tono-Pen, particularly at IOPs > 30mmHg; does not require application of topical anesthetic; is well-tolerated by cats, and may be considered most suitable among current, commercially-available tonometers, for diagnosis and monitoring of glaucoma in cats.(16,18)
Table 1.
Summary of published studies evaluating different tonometer types in cats.
| Tonometer Type | Tonometric Principle | IOP in normal cats (mmHg) | Limitations/Comments | Reference |
|---|---|---|---|---|
| Schiotz tonometer | Indentation | 21.6±5 (n=37) | 5.5gm weight;1955 human conversion table | (12) |
| Pneumotonometer (Mentor O & O) | Applanation | Not established | Underestimates IOP >20mmHg, overestimates IOP <15mmHg | (13) |
| Tono-Pen | Applanation | 19.7±5.6 (n=41) | Underestimates higher IOPs | (14) |
| Mackay-Marg | Applanation | 22.6±4 (n=37) | Underestimates higher IOPs | (14) |
| Tono-Pen XL (also Tono-Pen Vet§) | Applanation | 18.4 ±0.6 (SEM) (n=33) | Markedly underestimates higher IOPs | (13, 15-16, 18)§ |
| TonoVet | Induction-Impact (rebound) | 20.74±0.5 (n= 76) | Accurate but less precise at high IOPs. | (16, 18) |
| Perkins | Applanation | 15.1 +/- 1.7 (n=20)* | Upper maximal scale measurement limits IOP readings to approx. = 50mmHg. *Not tested outside normal physiologic range | (17) |
Mean IOP is presented ± standard deviation unless indicated otherwise;
SEM = standard error of mean; n = number of animals used to determine reference range where applicable.
Asterisk indicates data derived after calibration curve correction
Normal Feline Aqueous Humor Dynamics
As is typical for mammalian species, maintenance of normal IOP in cats relies on the equilibrium between aqueous outflow and the production of aqueous humor, by active and passive processes at the level of the non-pigmented epithelium of the ciliary processes. In cats, the bulk (>97%) of aqueous humor outflow occurs by the conventional route through the pupil from the posterior to anterior chamber, exiting the eye via the trabecular meshwork and angular aqueous plexus,(19) into the intrascleral venous plexus then ultimately the general circulation.(Fig. 1) The uveoscleral route (via the iris and ciliary body stroma to the suprachoroidal circulation and vortex veins, and through the sclera to the episcleral tissues) accounts for a very small percentage of aqueous humor outflow (<3%) in this species.(20)
Figure 1.
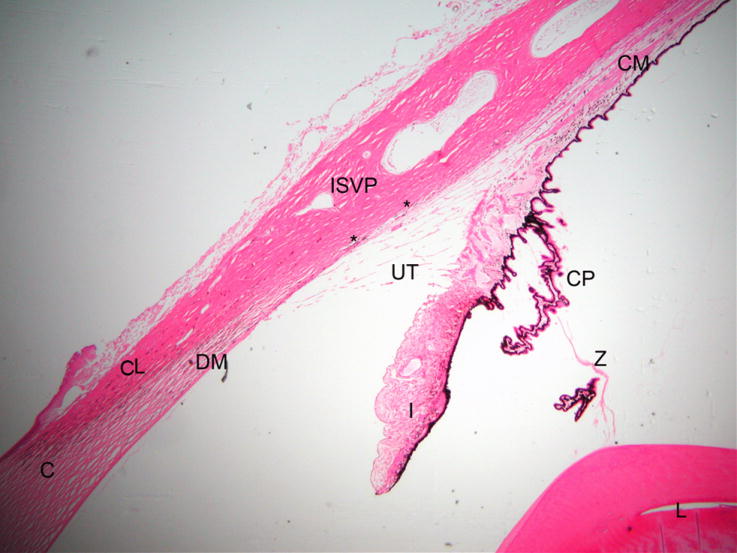
Photomicrograph illustrating the morphology of the normal feline outflow pathway. (C = cornea, DM = Descemet’s membrane, CL= corneoscleral limbus, I = iris, L= lens, CP = ciliary processes, ISVP = intrascleral venous plexus, asterisks mark angular aqueous plexus, UT = uveal trabecular meshwork)
In normal neonatal kittens, neither aqueous humor production nor outflow have reached adult levels and the anterior chamber is flattened, with apposition between the iris and cornea. Over the first few months of life, a process of expansion and rarefraction leads to an adult conformation of the aqueous outflow pathways, with a deep anterior chamber, widely spaced trabecular beams and well defined uveal meshwork and corneoscleral meshwork, the latter lying adjacent to the angular aqueous plexus that is situated well posterior to the termination of Descemet’s membrane.(21) In contrast to humans, the trabecular meshwork of the adult cat is located within a long and relatively wide ciliary cleft (Fig.1). The anterior chamber is deeper and the opening of the iridocorneal angle in cats is considerably wider than in humans and dogs. The individual fibers of the pectinate ligament, that span the anterior opening of the ciliary cleft, are very fine and relatively sparse in cats (Fig. 2), in comparison to the typical appearance of the canine drainage angle. Although the feline iridocorneal angle and opening of the ciliary cleft are best evaluated by gonioscopy utilizing a goniolens, it is possible to evaluate a significant portion of the feline drainage angle by direct observation using focal illumination and magnification, or even using an indirect ophthalmoscope and condensing lens held at an extreme angle to view the pectinate ligament and opening of the ciliary cleft.
Figure 2.
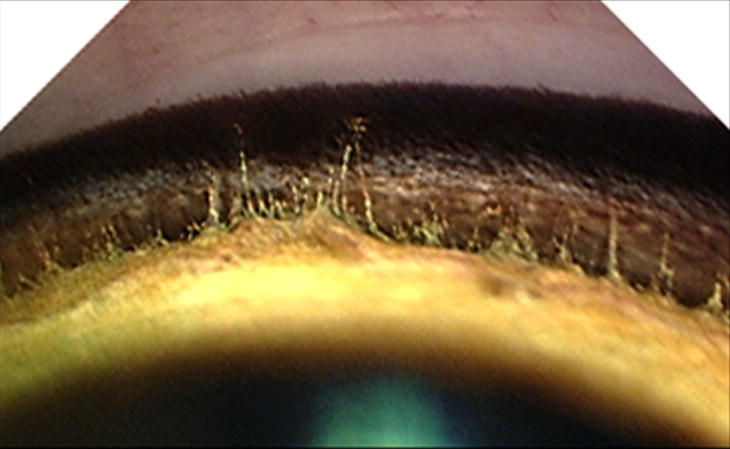
Gonioscopic appearance of a normal cat irido-corneal angle. Compared to the appearance of a normal canine irido-corneal angle, the opening to the feline ciliary cleft is wide and the pectinate ligament fibers are delicate and widely spaced.
Epidemiology and Clinical Features of Glaucoma in Cats
Glaucoma is a relatively uncommon clinical diagnosis in the cat, compared to the dog, although it is likely that many feline cases go unrecognized. According to data collected by the Veterinary Medical Data Base, 1 in 367 cats presenting to University Teaching Hospitals had a diagnosis of glaucoma. This referral population estimate, however, may be low as a prospective study found that 0.9% of older cats “screened” (1 in 108) had abnormally high IOP.(7) Glaucoma is also a relatively common indication for enucleation in cats, accounting for 29% of submissions to the Comparative Ocular Pathology Laboratory of Wisconsin (COPLOW).(22) In contrast to the situation in dogs, feline glaucoma is generally an insidious, gradually progressive disease. Moderate elevations in IOP are associated with few overt clinical signs and cats are frequently not presented for evaluation until late in the disease process.(5-6, 23-24) In one retrospective study, 73% of glaucomatous cats were blind at the time of initial presentation.(6) However, some vision may be preserved in chronically glaucomatous cats despite gross buphthalmos.(25-26)
The degree of discomfort, or at least overt clinical signs of discomfort, is highly variablein affected cats, and there are seldom signs of persistent, severe ocular discomfort unless IOP is markedly elevated. In some animals with antecedent ocular disease, particularly uveitis and/or intraocular neoplasia, a degree of ocular discomfort consistent with the presence of these disorders may be observed. However, in cats with primary glaucoma profound elevation of IOP may be associated with surprisingly little clinical evidence of ocular discomfort, and chronically affected cats generally maintain a normal appetite and relatively normal activity level. The acutely painful, fulminant presentation of glaucoma that is typical of the disease in dogs is seldom recognized in cats.
The feline eye is remarkably resilient in terms of susceptibility to glaucomatous damage – with relative preservation of ganglion cells in the retina of cats who have sustained acute IOP elevations, and relatively minor corneal changes observed. Dense, diffuse corneal edema that characterizes glaucoma in dogs is less often seen in cats at comparable levels of IOP. With sustained elevations in IOP, and ensuing buphthalmos, tears in Descemet’s membrane (Haab’s striae) can be observed in some patients but are not common (Fig. 3). Globe enlargement can be particularly dramatic in young animals and may be associated with exposure keratopathy. Ophthalmoscopic evidence of optic disc cupping is very difficult to discern in cats, due to the lack of myelination of the normal feline optic nerve head (ONH), and its normal depression relative to the plane of the neurosensory retina. Subtle changes that may be observed include a peri-papillary pigmented or hyper-reflective halo, with increased prominence of the laminar pores, referred to as the “laminar dot sign”. However, similar ophthalmoscopic features may be observed in some normal cats and ophthalmoscopic diagnosis of optic disc cupping can prove particularly challenging in cats with bilateral disease.(Fig. 4) Advanced imaging techniques such as Optical Coherence Tomography (OCT) facilitate the detection of ONH changes and thinning of the retinal nerve fiber layer in glaucomatous cats (Fig. 4C and D) but are currently limited to a few research institutions and require general anesthesia.(27) Pan-retinal degeneration, a common feature of canine glaucoma, is seldom observed even in long-standing glaucoma in cats. Functional vision may be relatively preserved, even in animals with readily appreciable buphthalmos. However, in uncontrolled, chronic glaucoma, progressive vision loss and extensive loss of retinal ganglion cell (RGC) bodies from the inner retina are observed (Fig. 5). In cats, the histological phenomenon that involves relatively sparing of RGCs within the superior versus inferior retina, does not appear to be as consistently observed as in dogs. (22)
Figure 3.
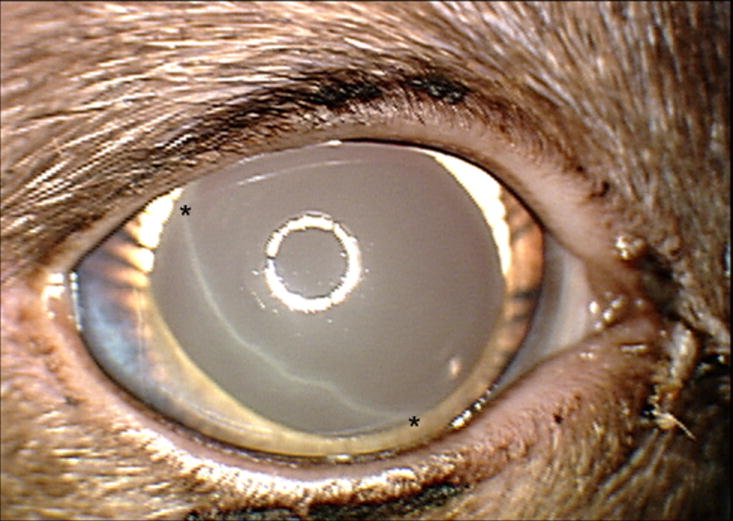
Haab’s stria (between asterisks), which reflects a tear in Descemet’s membrane due to globe stretching in an adult Siamese cat with primary congenital glaucoma. Note the very limited degree of corneal edema in this subject. In addition to globe enlargement, other clinical features depicted in this photograph, including iris hypoplasia, prominent, elongated ciliary processes and spherophakia are characteristic of this recessively inherited disease.
Figure 4.
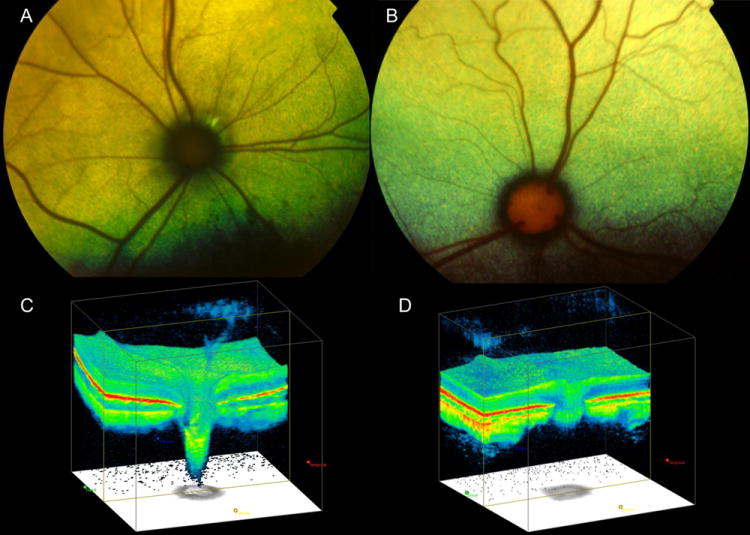
Fundus photographs illustrating (A) the optic nerve head (ONH) appearance of a cat with advanced primary congenital glaucoma, with ONH cupping and optic nerve degenerationand (B) a normal cat, which demonstrates normal prominence of the laminar pores. Note that in the glaucomatous cat (A) the ONH is small and dark and surrounded by a dark ring and by focal peri-papillary hyper-reflectivity. Optic nerve cube scans acquired by spectral-domain Optical Coherence Tomography (OCT; Cirrus, Carl Zeiss Meditec Inc., Dublin, CA) in a cat with glaucoma that demonstrates dramatic posterior displacement of the lamina cribrosa (C) compared to a normal cat (D).
Figure 5.
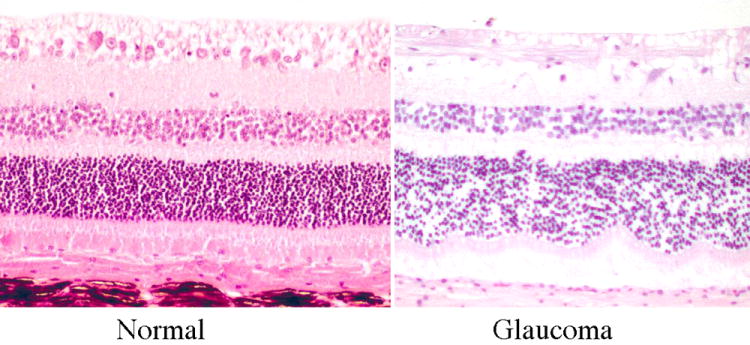
Photomicrographs illustrating illustrating the histological appearance of superior retina from normal and glaucomatous cats. Note the virtual absence of retinal ganglion cell bodies from the inner retina of a cat with chronic glaucoma compared with a normal cat (image courtesy of R.R. Dubielzig, Comparative Ophthalmic Pathology Laboratory of Wisconsin)
The pathophysiology and classification of the feline glaucomas
A number of systems for classifying the glaucomas have been used. In a clinical setting, glaucoma is often classified according to whether the inciting cause of aqueous outflow obstruction is “primary” or “secondary”. As a further subdivision of these two categories, the status of the iridocorneal angle (or more accurately the opening of the ciliary cleft) as either “open” or “closed”, as well as by the duration of the disease as “acute” or “chronic” may be recorded. While these classifications have some utility in establishing prognosis for vision, or even the life of the animal, or may implicate a genetic basis for the disease, they are overly simplistic and give little insight into underlying pathogenic mechanisms responsible for IOP elevation. Such classification schemes are arguably of limited value for clinicians seeking the most appropriate treatment choices for an individual patient.
Glaucoma in cats is considered to be almost invariably due to impaired aqueous humor outflow. One of the most clinically useful ways of classifying glaucoma in individual patients relies on determining the location of the “block” or impediment to aqueous humor outflow. The spectrum of causal mechanisms for aqueous outflow impairment is wide (as summarized in Table 2). Cats with long-standing glaucoma often have pathology at multiple locations that contribute to outflow impairment, which at least partly accounts for the difficulty in treating glaucoma in this species. (28-30) Understanding the location of these blocks and appreciating the complex patho-physiological mechanisms contributing to glaucoma in each affected cat, forms the basis for creating a rational treatment plan, tailored for each individual. These blocks in aqueous humor outflow may act to impede flow of aqueous humor from the ciliary processes into the posterior chamber; through the pupil to the anterior chamber; into the irido-corneal angle / opening of the ciliary cleft, to trabecular meshwork, and thence to the angular aqueous plexus and scleral venous plexus. In some patients, an increase in episcleral and scleral venous pressure counters aqueous outflow.(28-30) (31)
Table 2.
Potential locations of obstruction to aqueous humor uutflow, with examples of pathologic mechanisms.
| Block at the level of the ciliary Body/ posterior chamber/ Vitreous |
|---|
“Crowding” of posterior chamber by:
|
Vitreous expanded or pushed forward (so-called “malignant” glaucoma):
|
|
Pupil block |
Obstruction to flow of aqueous through pupil by:
|
|
Block at the level of the irido-corneal angle |
Primary angle-closure
|
Secondary angle closure
|
|
Trabecular Meshwork obstructions |
Primary malformation of the ciliary cleft
|
Secondary to obstruction of a conformationally “open angle” by
|
|
Post-trabecular block / Increased episcleral and/or scleral venous pressure |
Innapropriate restraint
|
Feline primary open-angle glaucoma
|
Feline primary congenital glaucoma
|
Orbital space occupying lesions
|
Infiltration of the episclera and / or sclera
|
Note that aqueous humor outflow is frequently obstructed at multiple locations within individual patients and that the proposed mechanisms should not be considered mutually exclusive. (Adapted from : Miller PE. “Feline Glaucoma”. In: Bonagura JD and KirkRW (eds). Kirk’s Current Veterinary Therapy XIV: SmallAnimal Practice. Philadelphia, Pennsylvania, WB Saunders Co. 2009.)
Feline Primary Glaucomas
In the primary glaucomas there is no other readily identifiable antecedent ocular or systemic disorder responsible for reduction in aqueous outflow. Although apparently relatively rare in cats compared to dogs,(29) feline cases of spontaneous primary glaucoma are likely under-diagnosed and may be encountered sporadically. Primary glaucoma in cats may present as either a unilateral or bilateral disease, has a fairly strong breed predisposition (although domestic short- and long-haired cats are most frequently affected) and may or may not be heritable. Breeds that may be at increased risk of primary glaucoma include the Siamese, Burmese and Persian.(5,22,23,25)
Primary glaucoma may be congenital, with onset of clinical signs recognized early in life, but may also be first recognized later in life, in middle-aged and older cats. Open-angle glaucoma, has been reported in adult, domestic short- and long-haired cats and in defined breeds including the Siamese and Burmese.(23, 32-33) A small series of Burmese cats with primary “narrow to closed” angle glaucoma has also been reported.(25) However, primary angle closure glaucoma, as is commonly diagnosed in dogs, is seldom, if ever, recognized in cats. Rare cases of glaucoma associated with pectinate ligament dysplasia, have also been reported in adult cats.(23,24)
Although not commonly encountered in clinical practice, a form of “open angle, open cleft” glaucoma has been recognized in feline eyes submitted for histological evaluation.(33) The disease is characterized as an insidious, gradually progressive glaucoma of older cats that, at least as documented in submissions to COPLOW, appears to be unilateral in presentation (although asymmetric presentation cannot be excluded due to insufficient follow-up in most cases). Burmese cats appear to be over-represented. In affected cats there is histopathological evidence of retinal ganglion cell loss and subtle myxomatous change within the vascular channels associated with aqueous outflow, including the scleral venous plexus and the vortex veins (Fig. 6). No pathology is recognizable within the iridocorneal angle or trabecular meshwork, thus it is proposed that resistance to aqueous outflow occurs at the level of the scleral venous plexus and vortex veins.(33) However, obstruction to outflow at the level of the trabecular meshwork cannot be excluded based on histomorphological appearance alone. At this time clinical correlates to accompany these pathological descriptions of primary open-angle glaucoma remain sparse, and the clinical course of this particular disease in affected animals remains unclear.
Figure 6.
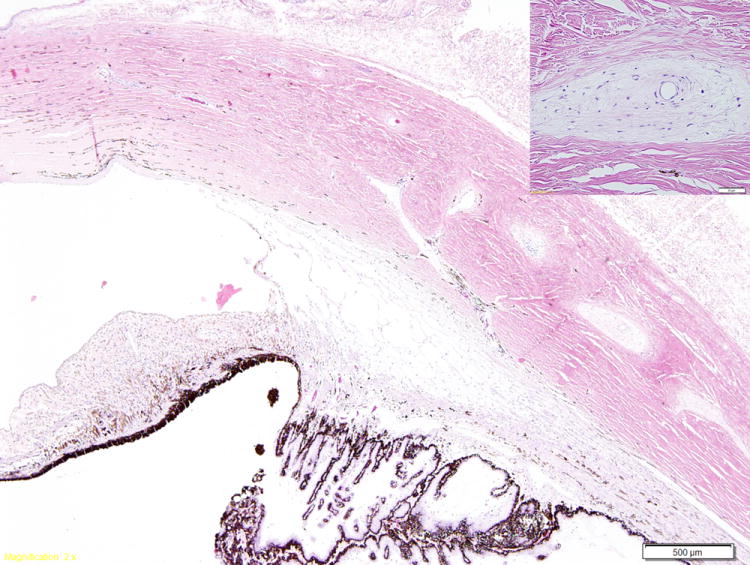
Photomicrograph illustrating myxomatous changes surrounding the intrascleral veins (inset) in a cat with primary open angle glaucoma. The ciliary cleft is open and the trabecular meshwork appears normal. (Image courtesy of Dr. R.R. Dubielzig, Comparative Ophthalmic Pathology Laboratory of Wisconsin)
Sporadic cases of feline congenital or early-onset glaucoma associated with various ocular malformations, including microphakia, ectopia lentis, iridoschisis, pectinate ligament dysplasia, multiple iridociliary cysts and persistent pupillary membranes have been reported in the veterinary literature.(23,35) A research colony founded by breeding Siamese cats has been established in the United States. These cats have relatively symmetric, slowly progressive glaucoma characterized by elongated ciliary processes, globe enlargement and spherophakia (Fig. 3).(26) In affected kittens, postnatal development of the structures of the ciliary cleft is arrested, and affected cats maintain the immature conformation of their aqueous outflow pathways.(36) The mutation responsible for this form of primary congenital glaucoma has now been identified (Kuehn, M.H. et al, manuscript in process). It would appear from sporadic reports in the veterinary literature of glaucoma occurring secondary to uveitis,(37) and of primary microphakia with lens luxation,(23, 38-39) in Siamese cats, that this disease may actually have existed in the Siamese cat breed in the USA and Europe for more than 50 years.
Feline Secondary Glaucomas
The secondary glaucomas, constituting 95-98% of feline glaucoma cases, are associated with antecedent ocular or systemic disease processes, such as uveitis, neoplasia, trauma and intra-ocular hemorrhage that alter aqueous humor dynamics by a range of mechanisms.(5-7, 23-24, 40) Depending on the underlying pathogenesis, they may be unilateral in presentation or, less often, bilateral, and are most often seen in adult cats.
Intraocular inflammation, particularly chronic lympho-plasmacytic uveitis, is the most frequently reported cause of glaucoma in cats.(41) Lymphoplasmacytic uveitis with glaucoma accounted for 10% of all feline submissions to COPLOW.(40) Uveitis may lead to elevation of IOP through a number of different pathogenic mechanisms.(5-6, 23, 32, 42) While obliteration of the aqueous outflow pathway by inflammatory infiltrates is often proposed as a mechanism for IOP elevation (Fig. 7), in reality the extent of the inflammatory infiltrate observed histologically in enucleated globes may be minimal. Chronic lympho-plasmacytic uveitis may also lead to lens luxation; condensation, degeneration and prolapse of vitreous; formation of pre-iridal fibrovascular membranes and synechiae.(32, 40, 43) Angle recession may also contribute to aqueous outflow obstruction.(40) Although identified histologically in glaucomatous feline eyes,(40) the role played by angle recession in the development of feline glaucoma remains unclear and it is possible that this histomorphological phenomenon may be secondary to buphthalmia, and associated changes in anterior segment anatomic relationships. The proposed pathogenesis for angle recession in humans is that blunt force trauma deforms the globe, pushing the cornea anteriorly and that the subsequent rebound effect on the lens exerts traction on the ciliary body causing cyclodialysis. Angle recession is most often seen histologically in feline eyes with concommitant lympho-plasmacytic uveitis, and a history of trauma is generally lacking, perhaps due to the protracted course of clinical disease prior to enucleation in many glaucomatous cats. (40)
Figure 7.
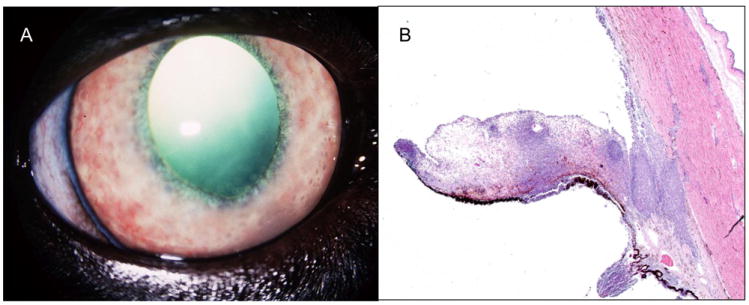
Clinical photograph (A) illustrating features of chronic lympho-plasmacytic uveitis, including rubeosis iridis and keratic precipates, in a cat with secondary glaucoma. (B) Photomicrograph of inflammatory infiltrate which has obliterated the structures of the ciliary cleft and iridocorneal angle in a cat with lympho-plasmacytic uveitis and secondary glaucoma. (Image courtesy of Dr. R.R. Dubielzig, Comparative Ophthalmic Pathology Laboratory of Wisconsin)
Intra-ocular neoplasia is another major cause of glaucoma in cats.(6, 23, 32, 40) In order of frequency, anterior uveal melanoma (Fig.8), lymphoma (Fig.9), post-traumatic ocular sarcoma,(44-45) and iridociliary epithelial tumors,(46) may all be associated with secondary glaucoma. Intraocular neoplasia may be associated with diffuse infiltration and obliteration of the trabecular meshwork and ciliary cleft, or the development of neovascular glaucoma with angle closure.(40) Diffuse iris melanoma is a relatively common cause of feline glaucoma. About half of all feline submissions to COPLOW were diagnosed with diffuse iris melanoma, and just over 10% of all feline submissions were diagnosed with glaucoma secondary to melanoma. (40)
Figure 8.
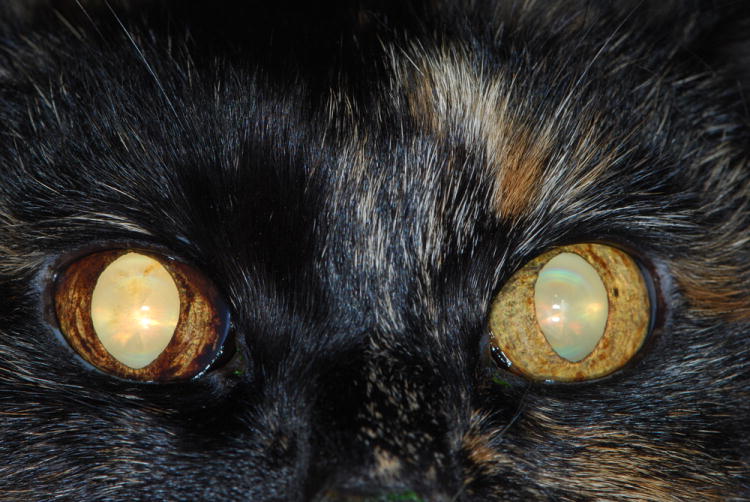
Clinical photograph of an elderly cat with glaucoma secondary to diffuse anterior uveal melanoma in the right eye. In addition to the diffuse iris hyperpigmentation, note subtle anisocoria due to mydriasis in the right eye.
Figure 9.
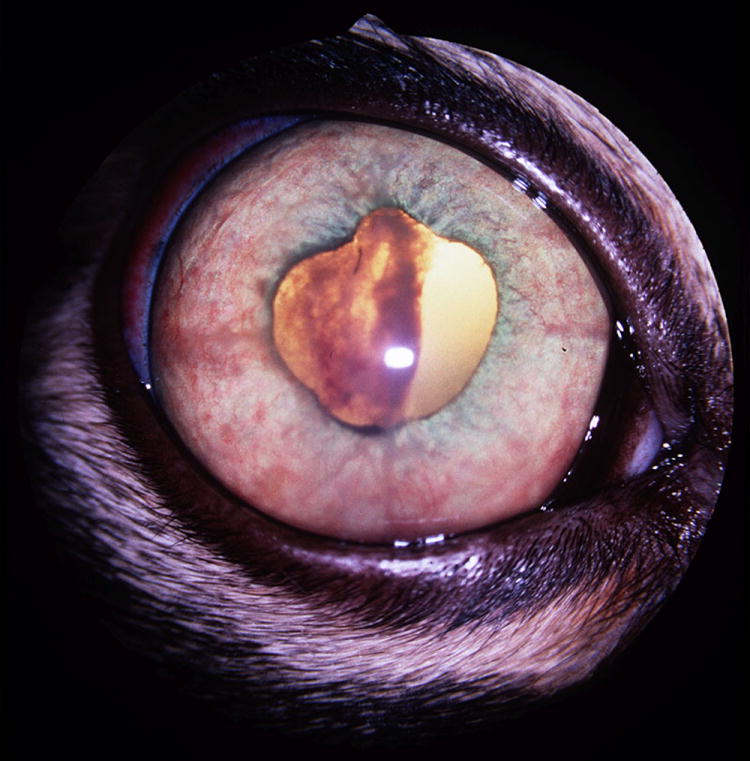
Clinical photograph of the right eye of a cat with lymphoma and secondary glaucoma. The iris is congested, distorted and expanded by the infiltrate. A large clot of fibrin and hemorrhage within the pupil has also contributed to dyscoria. A cytologic diagnosis of lymphoma made following aqueous humor paracentesis. Glaucoma responded to symptomatic therapy with topical administration of carbonic anhydrase inhibitor and corticosteroid, combined with systemic chemotherapy.
Intraocular hemorrhage, particularly related to systemic hypertension in older cats, may also result in secondary glaucoma. Development of glaucoma secondary to intraocular hemorrhage is of particular concern in animals with “eight-ball hyphema” caused by episodes of re-bleeding. To the authors’ knowledge, glaucoma secondary to retinal detachment in cats has not been specifically reported in the veterinary literature, and may be less of a concern than in dogs.
Lens-associated glaucoma in cats may occur secondary to phacoclastic uveitis or septic implantation syndrome resulting from lens trauma,(32, 42, 47) or, in the long-term, lens trauma may be associated with the development of post-traumatic ocular sarcoma that may present as glaucoma.(48) In contrast to dogs, lens luxation in cats is seldom primary and most often occurs secondary to glaucoma or uveitis. Even if lens luxation may be considered a “primary” event, as may occur in elderly cats with senile zonular degeneration, or in animals with hypermature cataract, lens luxation in cats seldom directly results in glaucoma.(49) Intumescent cataract and phacomorphic glaucoma are also rarely encountered in cats.
Aqueous Humor Misdirection Syndrome is an unusual form of insidious glaucoma, characterized by a uniformly shallow anterior chamber, that has been recognized in older cats.(6, 50-51) In addition to a uniformly shallow anterior chamber, affected eyes often exhibit dilated pupils. (Fig. 10) This mydriasis frequently results in anisocoria as the glaucoma is often unilateral, or at least asymmetric, at the time of initial presentation. The degree of IOP elevation in affected eyes ranges from mild to severe, with modest elevations being most typical and in the early stages IOP may actually be normal. In severely affected eyes in one series, a pronounced myopia (as much as -16.5D) was identified, and was attributed to the anteriorly positioned lens. (51) Ocular ultrasonography and histopathology in affected cats reveal thickening of the anterior vitreous face, anterior displacement of the iris and lens, and clear spaces in the vitreous cavity.(Fig. 11) The disease has been termed “Feline Aqueous Humor Misdirection Syndrome” (FAHMS), as it has been postulated that aqueous humor is misdirected posteriorly through breaks in the anterior vitreous face that act as one-way valves, resulting in it becoming progressively trapped within “pools” in the vitreous cavity. Subsequently, anteriorly displaced vitreal elements become condensed, compressed and juxtaposed against the lens and ciliary processes. This juxtaposition, so-called “cilio-vitreal-lenticular block”, impedes flow of aqueous humor produced at the ciliary processes to the posterior chamber, further diverting aqueous humor into the vitreous cavity. Obstruction of aqueous flow from posterior to anterior chambers (pupil block) is intensified by the anterior displacement of the lens, enhancing iris-lens contact. With progressive increase in IOP and forward displacement of the iris-lens diaphragm, collapse of the ciliary cleft and narrowing of the iridocorneal angle ensues. (51) Although surgical intervention has been proposed, including phaco-emulsification and anterior vitrectomy to relieve pupil block, results of surgery are frequently disappointing, perhaps because surgery is often performed late in the disease process, in medically unresponsive cases. In severely affected animals, impediments to outflow at the level of the iridocorneal angle and ciliary cleft are likely to have developed and persist after surgery. Given the slowly progressive nature of disease, and its tendency to affect mainly elderly animals, conservative management, with application of topical carbonic anhydrase inhibitors when tolerated, may be associated with preservation of limited vision in at least one eye for periods of up to several years.(51) The authors have also observed cats in which the anterior chamber spontaneously deepens and IOP normalizes, perhaps due to to the formation of a more well-developed break in the vitreous face, thereby eliminating the “one-way valve effect”. In contrast, acute pronounced increases in IOP have also been identified in cats with FAHMS following treatment with systemic corticosteroids for other disorders such as inflammatory bowel disease.
Figure 10.
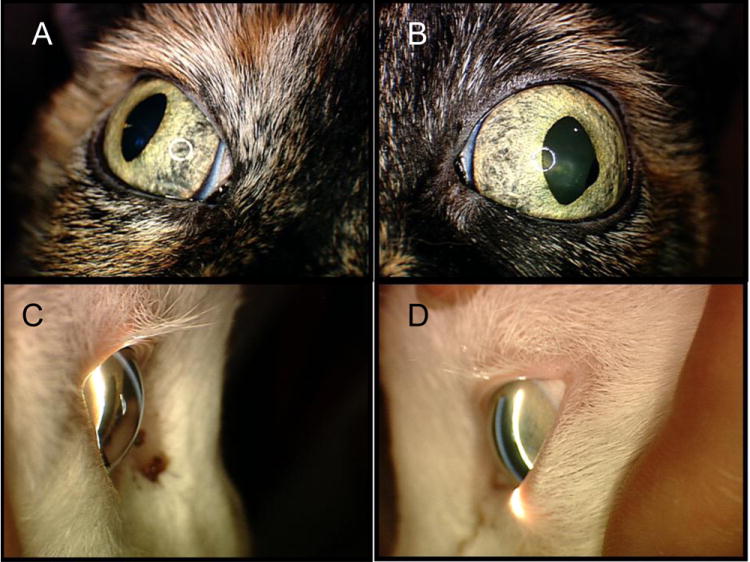
Aqueous humor misdirection syndrome in two elderly cats. Although both the right (A,C) and left (B,D) eyes are affected in both cats, glaucoma is often asymmetric in presentation and in these cats the left eye is more severely affected, manifest as mydriasis (B) and more pronounced, diffuse narrowing of the anterior chamber on slit-lamp biomicroscopy (D) compared to the contralateral eyes. An iridociliary cyst is also visible at the pupillary margin (B)
Figure 11.
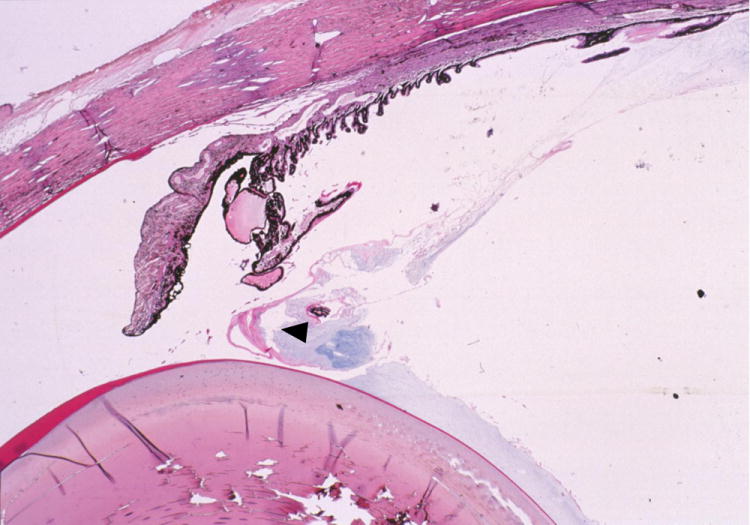
Aqueous humor misdirection syndrome. Photomicrograph illustrating condensation of the anterior vitreous face, which is pushed anteriorly (arrow head) by posteriorly misdirected aqueous humor (Image courtesy of R.R. Dubielzig, Comparative Ophthalmic Pathology Laboratory of Wisconsin)
Clinical Management of Glaucoma in Cats
When selecting an appropriate management strategy for feline glaucoma patients, there are a number of important clinical considerations that should be taken into account.(Table 3)
Table 3.
Important clinical considerations when formulating treatment strategies for feline glaucoma
| Is the glaucoma primary or secondary? |
|---|
|
|
Has the underlying etiology been determined? |
|
|
|
What are the specific mechanisms for IOP elevation? |
|
|
Are the pathogenic mechanisms reversible or irreversible? |
|
|
What is the likely potential for vision in the affected eye and is the contralateral eye at risk?? |
|
|
What is the likelihood of owner and patient compliance? |
|
Medical Therapy for Feline Glaucoma
When interpreting the findings of published studies, anatomical, physiological and pharmacological differences between species must be taken into account as should the heterogeneity of response within a given species or even individual patient. For example, it is not uncommon for certain individual animals to be unresponsive to a specific anti-glaucoma drug even when most cats will exhibit reduced IOP with its administration. Additionally, one should exercise caution in extrapolating results of studies conducted in normal subjects, as the response of glaucomatous subjects to the same drug or procedure may be very different. Ideally, experimental study designs should take diurnal and nocturnal fluctuations in IOP into account. Clinical trials should involve appropriate controls and, where possible, observers should be “masked” as to treatment in order to reduce or eliminate bias. Unfortunately, there is currently a paucity of published, randomized, prospective clinical trials that meet these criteria.
Ocular Hypotensive Drugs
(See Table 4 for summary)
Table 4.
summary of currently available anti-glaucoma drugs of potential value in cats
| Drug | Class | Route | Frequency | Mechanism | Contraindications / Adverse effects | References |
|---|---|---|---|---|---|---|
| Dorzolamide 2% | CAI | Topical | Q 8 hour | ↓ production | May be transient salivation. Innappetance in some cats. Sterile conjunctivitis | (3,54-56) |
| Brinzolamide 1% | CAI | Topical | Q 8 hours | ↓ production | May be less effective but cause less ocular irritation than dorzolamide. Does not affect IOP in normal cats. | (57-58) |
| Methazolamide, Diclorphenamide | CAI | Oral | 0.5-2 mg/kg q8-24 hours | ↓ production | Cats very susceptible to adverse effects (anorexia, GI disturbances, increased respiratory rate due to metabolic acidosis) and should be monitored closely | (52-53, 64, 93) |
| Acetazolamide | 10-25mg/kg PO; 5-10mg/kg IV | |||||
| Timolol 0.5% | Beta-blocker | Topical | Q 12 hrs | ↓ production | Avoid in animals with feline asthma, cardiovascular disease or pupil block. Less effective during sleep. | (53, 59-60) |
| Betaxolol 0.5% | Beta-blocker | Topical | Q 12 hrs | ↓ production | More Beta 2 –selective, may be safer in cats with respiratory or cardiovascular disease. Questionable efficacy in cats due to predominance of β-1 receptors in feline anterior segment | (61);Not studied |
| Epinephrine 1-2% | Adrenergic agonist | Topical | Q 6-12 hours | ↑ outflow | Not reported but local irritation might be anticipated | (67) |
| ↓ production | ||||||
| Dipivefrin 0.1% | Beta-adrenergic agonist | Topical | Q 6-12 hours | ↑ outflow | Do not use with cholinesterase inhibitors | Not described in cats |
| ↓ production | ||||||
| Pilocarpine 2% | Cholinergic | Topical | Q 6-12 hours | ↑ outflow | Contra-indicated where pre-existing uveitis, aqueous humor misdirection, pupil block and/or phacomorphic glaucoma. Systemic toxicity may be observed | (53, 65-66) |
Carbonic Anhydrase Inhibitors
Carbonic anhydrase inhibitors (CAIs) reduce IOP in cats by lowering rates of active aqueous humor production.(52-54) Topical dorzolamide 2% has been shown to significantly lower IOP in both normal and glaucomatous cats when administered three times daily.(3, 55-56) Brinzolamide 1% did not significantly lower IOP in normal cats when applied twice daily,(57) but did significantly lower IOP and blunt circadian fluctuation in IOP in glaucomatous cats when administered three times daily, albeit to a lesser degree than dorzolamide.(58) Systemic CAIs such as acetazolamide, methazolamide and dichlorphenamide are effective in lowering IOP in cats but their use is limited by their systemic toxicity. Cats are particularly sensitive to systemic adverse effects that include panting (associated with metabolic acidosis) and changes in mentation, inappetance and gastrointestinal upset (related, at least in part, to hypokalemia). Topical CAIs are therefore preferred over systemic CAIs in this species. Topical CAI application is associated with conjunctival irritation, hypersalivation and inappetance in some cats. However, this class of drug probably remains the first-line therapy of choice for glaucoma in cats, regardless of the underlying pathogenesis of glaucoma, as CAIs do not contribute to pupil block or intensify any pre-existing uveitis.
Beta-blockers
Timolol, a non-selective beta-adrenergic blocking agent, reduces IOP in cats by lowering the rate of aqueous humor production.(53, 59) In normal cats, topical application of a single dose of timolol maleate 0.5% led to a mean IOP reduction of about 22% in the treated eye, first observed at 6 hours after treatment, and a 16% reduction in the contralateral, untreated eye. Miosis was observed in the treated eye only, and persisted for up to a week post-treatment.(60) Timolol therapy may therefore intensify some forms of pupil block glaucoma, and may be contraindicated in patients with uveitis. No significant additive ocular hypotensive effect was observed in normal cats treated with a combination of the CAI, dorzolamide 2%, and timolol 0.5%, relative to the effect of treatment with dorzolamide 2% alone.(56) This data indicates that a CAI alone can maximally suppress aqueous humor flow in cats. As beta-2 receptors predominate in the feline anterior segment, beta-1 selective blockers (such as betaxolol) may have lower efficacy in this species.(61) Beta blockers also are ineffective at lowering IOP during sleep,(62) due to lower sympathetic tone,(61, 63) which may reduce their efficacy in felines that nap frequently during the day, and exhibit peak IOP during the nocturnal phase. Timolol may cause bradycardia and bronchoconstriction and is probably contraindicated in cats with feline asthma or cardiac disease.(64)
Cholinergics
These miotic drugs are believed to reduce IOP in cats by increasing aqueous outflow.(53) Topical administration of a single dose of the direct-acting cholinergic, pilocarpine 2% reduced IOP by about 15% in the treated eye and caused miosis in both the treated and untreated eye of normal cats.(65) This contralateral effect observed following unilateral application, indicates that systemic, adverse cholinergic effects might be anticipated during long-term treatment. Due to their miotic effect and ability to destabilize the blood-ocular-barrier,(66) these drugs are generally contra-indicated in animals with pre-existing intraocular inflammation or a tendency to pupil block. Demecarium bromide 0.125%, a cholinesterase inhibitor, is a more potent and longer lasting miotic, but to our knowledge its’ effects have not been specifically evaluated in cats.
Adrenergic Agonists
The non-specific adrenergic agonist, epinephrine when applied twice daily reduced IOP in normal cats by about 27% in treated eyes, related to a reduction in both aqueous production, as determined by fluorophotometry, and an increase in outflow facility.(67) Although this study did not specifically evaluate or report ocular tolerability, ocular surface irritation might be expected, based on reported adverse effects in human patients. Dipivefrin, a pro-drug of epinephrine, might be expected to exert similar effects to epinephrine but its use has not been specifically investigated in cats. Dipivefrin requires corneal esterases to convert the pro-drug to its active form, and probably should not be combined with cholinesterase inhibitors.(68) Topical adrenergic agonists are associated with mild pupillary dilation, and do not exacerbate uveitis.
Adrenergic Alpha-2 Agonists
Alpha-2 agonists such as apraclonidine and brimonidine may reduce IOP by increasing conventional outflow, decreasing aqueous humor production and reducing episcleral venous pressure.(69) Apraclonidine reduced IOP in normal cats by an average of 24% within 6 hours of treatment but also resulted in miosis that persisted for up to 24 hours. Evidence of systemic toxicity following topical application of 0.5% apraclonidine, consisting of a mean reduction in heart-rate of about 12%, and vomiting that was noted in 8/9 cats treated with the drug, precludes use of the commercially available formulation in cats.(70)
Prostaglandin Analogs
Species differences in prostanoid receptor distribution within different ocular tissues have major implications for the effects, and efficacy of topical prostaglandin analog therapy for glaucoma. For example, the intense miosis observed in cats treated with prostaglandin analogs such as latanoprost, travaprost, unoprostone and bimatoprost, (71-73) is attributable to the presence of exquisitely well-coupled FP receptors in the iris sphincter muscle of cats, compared to humans.(74-75) Although FP receptors are largely responsible for the effects of prostaglandin analogs on the canine and human ciliary body, these receptors are lacking in the ciliary body of cats and EP receptors are predominantly responsible for relaxation of feline ciliary muscle.(76-77) Significant IOP-lowering effects have been observed in cats treated with PGA-2 derivatives but these have been associated with unacceptable ocular side-effects in humans and non-human primates and are not commercially available.(78) Unfortunately, with the refinement of prostaglandin analogs to maximize their specificity for FP receptors on the human ciliary body, in order to minimize adverse effects such as ocular inflammation, commercially available prostaglandin analogs are less likely to have any ocular hypotensive effect in cats.
The FP receptor agonists, including latanoprost, travoprost and bimatoprost, have no significant IOP-lowering effect in normal cats.(71-73) A recent study (McDonald, J.E. et al, manuscript in process) indicates that latanoprost 0.005% transiently lowers IOP in glaucomatous cats following a single topical application, but this effect is diminished following three weeks of twice daily administration of the drug. In this recent study, twice daily application actually led to a tendency towards an increase in cumulative IOP exposure relative to the pre-treatment period, over the course of a three-week treatment period in both normal and glaucomatous cats. These results do not support the use of latanoprost in the treatment of feline glaucoma, at least at the application frequency tested. Although the proposed mechanism for IOP reduction in humans and non-human primates is enhanced uveoscleral outflow as a result of alteration in extracellular matrix of the ciliary body,(79) this effect does not account for the rapid IOP lowering response observed in dogs, that was also observed following a single application in glaucomatous cats. In dogs, reduction in aqueous humor flow rates has been proposed as a mechanism for this rapid IOP reduction,(80) however no significant effect on aqueous humor flow rate was observed in latanoprost-treated normal cats by fluorophotometry, (McDonald, J.E. et al, manuscript in process) or in human subjects or non-human primates.(81)
Topical Corticosteroids: Indications and Contraindications
Topical corticosteroid therapy, most commonly with formulations containing either 0.1% dexamethasone or 1% prednisolone, is generally considered to be indicated for the management of chronic lympho-plasmacytic uveitis in cats. However, steroid induced ocular hypertension is an important consideration both in the management of glaucomatous cats that have evidence of ocular inflammation, and in the monitoring of cats with chronic uveitis that may receive long-term topical corticosteroid therapy. As is the case in a subset of the human population, normal cats treated with either topical dexamethasone or 1% prednisolone exhibit a significant increase in IOP after about 2-3 weeks of treatment two or three times daily. The magnitude of the increase documented in normal cats has ranged from about 5mmHg, up to about 10mmHg.(82-83) Should steroid-induced ocular hypertension prove to be a concern in feline patients, then a change in the route of administration, or selection of a different topical anti-inflammatory drug may be warranted. Following withdrawal of topical corticosteroid therapy in normal cats, IOP was found to return to pre-treatment values within 6-7 days.(82) Although species-specific data is lacking, studies in human “steroid responders” indicate that systemic corticosteroids may result in IOP increases that are only about 60% of those observed in response to topical corticosteroids.(84) The authors have clinically observed increased difficulty in managing glaucoma in cats when systemic corticosteroids are administered for other disorders, such as atopy or inflammatory bowel disease. Studies in humans indicate that other corticosteroids, such as rimexolone, may have less ocular hypertensive effects,(85) but to our knowledge these have not been specifically evaluated in cats.
Surgical Management of Feline Glaucoma
Gonio-implantation
Gonio-implantation surgery in glaucomatous cats also presents challenges. Due to the association between chronic uveitis and the development of secondary glaucoma, pre-existing inflammation is common in glaucomatous feline eyes and may contribute to increased risk of shunt failure due to obstruction of the anterior chamber tube. The degree of buphthalmos that is also common in cats, even at the time of first presentation, combined with relatively “tight” eyelid and orbital conformation in this species, may also complicate surgical placement of conventional drainage implants and limit the formation of an adequate sub-conjunctival filtering bleb.
Cyclodestructive Procedures
The authors’ experience, as well as outcomes presented in published case reports,(5,25,34) suggest that success rates following laser cyclophotocoagulation or cyclocryotherapy are considerably lower in glaucomatous cats than in dogs and often repeated treatments are required. Disappointing responses to cyclophotocoagulation may reflect relatively sparse pigmentation within the ciliary body stroma of some cats, since the ocular hypotensive response to cyclophotocoagulation may in part result from reduced perfusion of the ciliary body stroma and processes, as much as specific destruction of the ciliary epithelium. In addition, there appears to be marked inter-individual variation in the location of the ciliary processes relative to the limbus in glaucomatous cats and bupthalmia may further enhance this variation. In one experimental study, cyclophotocoagulation with Nd:YAG laser led to a mean reduction in IOP of about 30% in normal cats.(86) However, the chronic nature of IOP elevation in most glaucomatous cats and variable degree of associated globe enlargement makes it difficult to determine the most appropriate site for trans-scleral laser or cryosurgical application based solely on knowledge of the anatomy of normal cats. Pre-operative ultrasound biometry, as an aid to determining the location of the ciliary body in individual glaucomatous cats, could feasibly increase the success rate of surgical, cyclodestructive procedures. Both cyclophotocoagulation and cyclocryotherapy incite considerable inflammation and are contraindicated in cats with glaucoma secondary to uveitis or neoplasia.
Intravitreal gentamicin
Intravitreal injection of gentamicin for pharmacologic ablation of the ciliary epithelium is widely considered to be contraindicated in cats,(24) due to the potential for malignant transformation of the feline lens epithelium when damaged, which may contribute to the development of life-threatening feline post-traumatic ocular sarcoma.(48) Although this is a valid concern, in reality a causal relationship between intravitreal gentamicin injections and ocular sarcoma in cats has not been definitively established. In a recent survey of the COPLOW archive, only 5 feline cases of malignant intraocular neoplasia (three sarcomas and two uveal melanomas) were identified in cats that had a history of prior intravitreal gentamicin injections. In those cases, it was not possible to determine whether intravitreal gentamicin injections had actually been administered to cats with glaucoma secondary to an occult intraocular neoplasm; neoplasia arose independently of the injection, or whether the injection incited neoplastic transformation.(Strong, T. and Dubielzig, R.R., personal communication) Nevertheless, pharmacologic cycloablation cannot be considered a treatment of choice for feline glaucoma, given that the procedure carries a relatively low rate of success, about 66% in one limited study.(87) Furthermore, malignant intraocular neoplasia is a common cause of secondary glaucoma in this species, that may be unsuspected prior to, and more difficult to detect following intravitreal gentamicin injection.
Enucleation and Evisceration in cats
For eyes that are irreversibly blind, or in which the possibility of intraocular neoplasia is suspected or cannot be excluded on the basis of clinical findings, enucleation warrants strong consideration. Enucleation is generally preferred over evisceration with intrascleral prosthesis placement as the success rate of the latter procedure may be lower in cats than in dogs.(88-89) In addition, the cosmetic result achieved by implantation of dark-colored spheres in cats is often sub-optimal. This has led some to use colored spheres and to tattoo a slit pupil onto the cornea in an effort to improve the eye’s final post-operative cosmetic appearance (Dr Dennis Hacker, personal communication). In the COPLOW archive, the ratio of corneo-scleral shell failures to evisceration samples indicates that the failure rate for this procedure in cats approaches 15%.(40) In a recent series, recurrence of malignant intraocular neoplasia, with life-threatening potential, was a major reason for corneoscleral shell failure, and was diagnosed in over 70% of cats that had subsequent enucleation following intra-scleral prosthesis placement.(90)
The risk of late complications associated with placement of intra-orbital prostheses following enucleation may also be greater in cats than in dogs, and may necessitate removal of the orbital prosthesis in some cats.(91-92) In the authors’ experience, irreversibly blind glaucomatous cats tolerate even bilateral enucleation extremely well. Given the possibility of subsequent bilateral involvement and/ or malignant intraocular neoplasia in cats presenting with unilateral glaucoma, it is important to remember that valuable lessons can be learned from the histomorphological features of those eyes that may be considered by clinicians as our “failures”,(40) thus all enucleated globes and all evisceration specimens should be submitted for histopathological evaluation.
In summary, glaucoma in cats is typically an insidious and gradually progressive disease that is most often secondary to other ocular and/or systemic disease processes. Treatment of glaucoma is undoubtedly challenging in cats. Adverse systemic and local side-effects are commonly encountered and, coupled with a relatively poor tolerance for the frequent application of topical medication by most cats, frequently lead to poor patient and owner compliance with prescribed therapy. In some cases, the signs associated with topical or systemic therapy for glaucoma may be more severe than minimal signs of ocular discomfort or illness observed prior to therapy. Careful consideration of the “cost-to-benefit ratio” for individual cats may favor the adoption of a more conservative approach of temporization and/or palliative therapy rather than aggressive vision-sparing medical or surgical therapy, particularly in older patients. In cats with irreversibly blind, painful eyes, and animals in which intra-ocular neoplasia is suspected or cannot be excluded on the basis of clinical findings, enucleation is generally the most appropriate treatment provided that the animal’s general health status permits surgical intervention.
Acknowledgments
The authors are indebted to Dr Richard R. Dubielzig for providing photographs illustrating ocular pathology in feline glaucoma
Funding: GJM is supported by NIH grant K08EY018609
References
- 1.Drance SM. Diurnal Variation of Intraocular Pressure in Treated Glaucoma. Significance in Patients with Chronic Simple Glaucoma. Archives of Ophthalmology. 1963 Sep;70:302–311. doi: 10.1001/archopht.1963.00960050304004. [DOI] [PubMed] [Google Scholar]
- 2.Gelatt KN, Gum GG, Gwin RM, Bromberg NM, Merideth RE, Samuelson DA. Primary open angle glaucoma: inherited primary open angle glaucoma in the beagle. American Journal of Pathology. 1981 Feb;102(2):292–295. [PMC free article] [PubMed] [Google Scholar]
- 3.Sigle KJ, Camaño-Garcia G, Carriquiry AL, Betts DM, Kuehn MH, McLellan GJ. The Effect of Dorzolamide 2% on Circadian Intraocular Pressure in Cats with Primary Congenital Glaucoma. Veterinary Ophthalmology. 2011 doi: 10.1111/j.1463-5224.2011.00913.x. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Sole MJ, Sande PH, Bernades JM, Aba MA, Rosenstein RE. Circadian rhythm of intraocular pressure in cats. Veterinary Ophthalmology. 2007 May-Jun;10(3):155–161. doi: 10.1111/j.1463-5224.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 5.Ridgway MD, Brightman AH. Feline glaucoma: a retrospective study of 29 clinical cases. Journal of the American Animal Hospital Association. 1989;25:485–490. [Google Scholar]
- 6.Blocker T, van der Woerdt A. The feline glaucomas: 82 cases (1995-1999) Veterinary Ophthalmology. 2001 Jun 01;4(2):81–85. doi: 10.1046/j.1463-5224.2001.00169.x. [DOI] [PubMed] [Google Scholar]
- 7.Kroll MM, Miller PE, Rodan I. Intraocular pressure measurements obtained as part of a comprehensive geriatric health examination from cats seven years of age or older. Journal of the American Veterinary Medical Association. 2001 Nov 15;219(10):1406–1410. doi: 10.2460/javma.2001.219.1406. [DOI] [PubMed] [Google Scholar]
- 8.Ofri R, Shub N, Galin Z, Shemesh M, Shore LS. Effect of reproductive status on intraocular pressure in cats. American Journal of Veterinary Research. 2002 Feb;63(2):159–162. doi: 10.2460/ajvr.2002.63.159. [DOI] [PubMed] [Google Scholar]
- 9.Stadtbaumer K, Kostlin RG, Zahn KJ. Effects of topical 0.5% tropicamide on intraocular pressure in normal cats. Veterinary Ophthalmology. 2002 Jun;5(2):107–112. doi: 10.1046/j.1463-5224.2002.00226.x. [DOI] [PubMed] [Google Scholar]
- 10.Stadtbaumer K, Frommlet F, Nell B. Effects of mydriatics on intraocular pressure and pupil size in the normal feline eye. Veterinary Ophthalmology. 2006;9(4):233–237. doi: 10.1111/j.1463-5224.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 11.Espinheira Gomes F, Bentley E, Lin T-L, McLellan GJ. Effects of unilateral topical administration of 0.5% tropicamide on anterior segment morphology and intraocular pressure in normal cats and cats with primary congenital glaucoma. Veterinary Ophthalmology. 2011 doi: 10.1111/j.1463-5224.2011.00927.x. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller PE, Pickett JP. Comparison of the human and canine Schiotz tonometry conversion tables in clinically normal cats. Journal of the American Veterinary Medical Association. 1992 Oct 1;201(7):1017–1020. [PubMed] [Google Scholar]
- 13.Stoiber J, Fernandez V, Lamar PD, Hitzl W, Fantes F, Parel JM. Ex vivo evaluation of Tono-Pen and pneumotonometry in cat eyes. Ophthalmic Research. 2006;38(1):13–18. doi: 10.1159/000088492. [DOI] [PubMed] [Google Scholar]
- 14.Miller PE, Pickett JP, Majors LJ, Kurzman ID. Evaluation of two applanation tonometers in cats. American Journal of Veterinary Research. 1991 Nov;52(11):1917–1921. [PubMed] [Google Scholar]
- 15.Passaglia CL, Guo X, Chen J, Troy JB. Tono-Pen XL calibration curves for cats, cows and sheep. Veterinary Ophthalmology. 2004 Jul-Aug;7(4):261–264. doi: 10.1111/j.1463-5224.2004.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rusanen E, Florin M, Hassig M, Spiess BM. Evaluation of a rebound tonometer (Tonovet) in clinically normal cat eyes. Veterinary Ophthalmology. 2010 Jan;13(1):31–36. doi: 10.1111/j.1463-5224.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 17.Andrade SF, Cremonezi T, Zachi CA, Lonchiati CF, Amatuzzi JD, Sakamoto KP, et al. Evaluation of the Perkins handheld applanation tonometer in the measurement of intraocular pressure in dogs and cats. Veterinary Ophthalmology. 2009 Sep-Oct;12(5):277–284. doi: 10.1111/j.1463-5224.2009.00702.x. [DOI] [PubMed] [Google Scholar]
- 18.McLellan GJ, Kemmerling JP, Kiland JA. Evaluation Of Rebound And Applanation Tonometry In Normal And Chronically Glaucomatous Cats. 40th Annual Meeting of the American College of Veterinary Ophthalmologists; Chicago, IL. 2009. abstract. [Google Scholar]
- 19.Tripathi RC. Ultrastructure of the exit pathway of the aqueous in lower mammals (a preliminary report on the “angular aqueous plexus”) Experimental Eye Research. 1971;12:311–314. doi: 10.1016/0014-4835(71)90155-2. [DOI] [PubMed] [Google Scholar]
- 20.Bill A. Formation and drainage of aqueous humour in cats. Experimental Eye Research. 1966;5:185–190. doi: 10.1016/s0014-4835(66)80020-9. [DOI] [PubMed] [Google Scholar]
- 21.Richardson TM, Marks MS, Ausprunk DH, Miller M. A morphologic and morphometric analysis of the aqueous outflow system of the developing cat eye. Experimental Eye Research. 1985 Jul;41(1):31–51. doi: 10.1016/0014-4835(85)90092-2. [DOI] [PubMed] [Google Scholar]
- 22.Dubielzig RR, Ketring KL, McLellan GJ, Albert DM. Veterinary Ocular Pathology A Comparative Review. Oxford: Saunders Elsevier; 2010. The Glaucomas. [Google Scholar]
- 23.Walde I, Rapp E. Feline glaucoma. Clinical and morphological aspects (a retrospective study of 38 cases) European Journal of Companion Animal Practice. 1993;4:87–105. [Google Scholar]
- 24.Dietrich U. Feline Glaucomas. Clinical Techniques in Small Animal Practice. 2005;20:108–116. doi: 10.1053/j.ctsap.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Hampson EC, Smith RI, Bernays ME. Primary glaucoma in Burmese cats. Australian Veterinary Journal. 2002 Nov;80(11):672–680. doi: 10.1111/j.1751-0813.2002.tb11292.x. [DOI] [PubMed] [Google Scholar]
- 26.McLellan GJ, Betts D, Sigle K, Grozdanic S. Congenital glaucoma in the Siamese cat- a new spontaneously occurring animal model for glaucoma research. 35th Annual Meeting of the American College of Veterinary Ophthalmologists; Washington, DC. 2004. abstract. [Google Scholar]
- 27.McLellan GJ, Seo K, Finch A, Xiong K, Rasmussen CA. 41st Annual Conference of the American College of Veterinary Ophthalmologists. San Diego, CA: ACVO; 2010. SD-OCT Imaging of the Retina and Optic Nerve in Normal and Glaucomatous Cats. abstract. [Google Scholar]
- 28.Bedford PG. The aetiology of canine glaucoma. Veterinary Record. 1980 Jul 26;107(4):76–82. doi: 10.1136/vr.107.4.76. [DOI] [PubMed] [Google Scholar]
- 29.Gelatt KN, Brooks DE, Samuelson DA. Comparative glaucomatology. I: The spontaneous glaucomas. Journal of Glaucoma. 1998 Jun;7(3):187–201. [PubMed] [Google Scholar]
- 30.Gelatt KN, Brooks DE. The Canine Glaucomas. In: Gelatt KN, editor. Veterinary Ophthalmology. 3. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 701–754. [Google Scholar]
- 31.Pauli AM, Bentley E, Diehl KA, Miller PE. Effects of the application of neck pressure by a collar or harness on intraocular pressure in dogs. Journal of the American Animal Hospital Association. 2006 May-Jun;42(3):207–211. doi: 10.5326/0420207. [DOI] [PubMed] [Google Scholar]
- 32.Wilcock BP, Peiffer RL, Jr, Davidson MG. The causes of glaucoma in cats. Veterinary Pathology. 1990 Jan;27(1):35–40. doi: 10.1177/030098589002700105. [DOI] [PubMed] [Google Scholar]
- 33.Jacobi S, Dubielzig RR. Feline primary open angle glaucoma. Veterinary Ophthalmology. 2008 May-Jun;11(3):162–165. doi: 10.1111/j.1463-5224.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 34.Trost K, Peiffer RL, Jr, Nell B. Goniodysgenesis associated with primary glaucoma in an adult European Short-haired cat. Veterinary Ophthalmology. 2007 Nov-Dec;10(Suppl 1):3–7. doi: 10.1111/j.1463-5224.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 35.Brown A, Munger R, Peiffer RL., Jr Congenital glaucoma and iridoschisis in a Siamese cat. Veterinary and Comparative Ophthalmology. 1994;4(3):121–124. [Google Scholar]
- 36.McLellan GJ, Kuehn MH, Ellinwood NM, Kim CY, Jens J, Sigle KJ, et al. A feline model of primary congenital glaucoma- histopathological and genetic characterization. Association for Research in Vision and Ophthalmology Annual Meeting; Fort Lauderdale, FL. 2006. abstract. [Google Scholar]
- 37.Coop MC, Thomas JR. Bilateral glaucoma in the cat. Journal of the American Veterinary Medical Association. 1958;133:369–370. [PubMed] [Google Scholar]
- 38.Aguirre GD, Bistner SI. Microphakia with lenticular luxation and subluxation in cats. Veterinary Medicine: Small Animal Clinician. 1973;68:498–500. [PubMed] [Google Scholar]
- 39.Molleda JM, Martin E, Ginel PJ, Novales M, Moreno P, Lopez R. Microphakia associated with lens luxation in the cat. Journal of the American Animal Hospital Association. 1995;31:209–212. doi: 10.5326/15473317-31-3-209. [DOI] [PubMed] [Google Scholar]
- 40.Dubielzig RR, Ketring KL, McLellan GJ, Albert DM. Veterinary Ocular Pathology: a comparative review. Saunders Elsevier; 2010. The Glaucomas; pp. 419–448. [Google Scholar]
- 41.Peiffer RL, Jr, Wilcock BP. Histopathologic study of uveitis in cats: 139 cases (1978-1988) Journal of the American Veterinary Medical Association. 1991 Jan 1;198(1):135–138. [PubMed] [Google Scholar]
- 42.McCalla TL, Moore CP, Collier LL. Phacoclastic uveitis with secondary glaucoma in a cat. Companion Animal Practice. 1988;2(11):13–17. [Google Scholar]
- 43.Peiffer RL, Jr, Wilcock BP, Yin H. The pathogenesis and significance of pre-iridal fibrovascular membrane in domestic animals. Veterinary Pathology. 1990 Jan;27(1):41–45. doi: 10.1177/030098589002700106. [DOI] [PubMed] [Google Scholar]
- 44.Dubielzig RR, Everitt J, Shadduck JA, Albert DM. Clinical and morphologic features of post-traumatic ocular sarcomas in cats. Veterinary Pathology. 1990 Jan;27(1):62–65. doi: 10.1177/030098589002700111. [DOI] [PubMed] [Google Scholar]
- 45.Dubielzig RR, Hawkins KL, Toy KA, Rosebury WS, Mazur M, Jasper TG. Morphologic features of feline ocular sarcomas in 10 cats: light microscopy, ultrastructure, and immunohistochemistry. Veterinary and Comparative Ophthalmology. 1994;4(1):7–12. [Google Scholar]
- 46.Dubielzig RR, Steinberg H, Garvin H, Deehr AJ, Fischer B. Iridociliary epithelial tumors in 100 dogs and 17 cats: a morphological study. Veterinary Ophthalmology. 1998;1:223–231. doi: 10.1046/j.1463-5224.1998.00046.x. [DOI] [PubMed] [Google Scholar]
- 47.Dubielzig RR, Ketring KL, McLellan GJ, Albert DM. Veterinary Ocular Pathology A Comparative Review. Oxford: Saunders Elsevier; 2010. The Uvea; pp. 245–322. [Google Scholar]
- 48.Zeiss CJ, Johnson EM, Dubielzig RR. Feline intraocular tumors may arise from transformation of lens epithelium. Veterinary Pathology. 2003 Jul;40(4):355–362. doi: 10.1354/vp.40-4-355. [DOI] [PubMed] [Google Scholar]
- 49.Olivero DK, Riis RC, Dutton AG, Murphy CJ, Nasisse MP, Davidson MG. Feline lens displacement : a retrospective analysis of 345 cases. Progress in Veterinary and Comparative Ophthalmology. 1991;1(4):239–244. [Google Scholar]
- 50.La Croix N, van der Woerdt A, Silverman RH, Blocker T, Hoffman A. 34th Annual Meeting of the American College of Veterinary Ophthalmologists. Coeur d’Alene; Idaho: 2003. Feline malignant glaucoma/aqueous misdirection:16 cases. abstract. [Google Scholar]
- 51.Czederpiltz JM, La Croix NC, van der Woerdt A, Bentley E, Dubielzig RR, Murphy CJ, et al. Putative aqueous humor misdirection syndrome as a cause of glaucoma in cats: 32 cases (1997-2003) Journal of the American Veterinary Medical Association. 2005 Nov 1;227(9):1434–1441. doi: 10.2460/javma.2005.227.1434. [DOI] [PubMed] [Google Scholar]
- 52.Macri FJ, Dixon RL, Rall DP. Aqueous humor turnover rates in the cat. I. Effect of acetazolamide. Investigative Ophthalmology. 1965 Oct;4(5):927–934. [PubMed] [Google Scholar]
- 53.Chiou GCY, Liu HK, Trzeciakowski J. Studies of action mechanism of antiglaucoma drugs with a newly developed cat model. Life Sciences. 1980;27(25-26):2445–2451. doi: 10.1016/0024-3205(80)90520-2. [DOI] [PubMed] [Google Scholar]
- 54.Crumley WR, Rankin AJ. The effect of topical 2% dorzolamide solution on aqueous humor flow rate and intraocular pressure in normal cats. 41st Annual Conference of the American College of Veterinary Ophthalmologists; San Diego, CA. 2010. p. 58. abstract. [Google Scholar]
- 55.Rainbow ME, Dziezyc J. Effects of twice daily application of 2% dorzolamide on intraocular pressure in normal cats. Veterinary Ophthalmology. 2003;6(2):147–150. doi: 10.1046/j.1463-5224.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 56.Dietrich UM, Chandler MJ, Cooper T, Vidyashankar A, Chen G. Effects of topical 2% dorzolamide hydrochloride alone and in combination with 0.5% timolol maleate on intraocular pressure in normal feline eyes. Veterinary Ophthalmology. 2007 Nov-Dec;10(Suppl 1):95–100. doi: 10.1111/j.1463-5224.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 57.Gray HE, Willis AM, Morgan RV. Effects of topical administration of 1% brinzolamide on normal cat eyes. Veterinary Ophthalmology. 2003;6(4):285–90. doi: 10.1111/j.1463-5224.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 58.McLellan GJ, Lin T-L, Hildreth S, Petersen C, Leon A, Jens JK, et al. Diurnal Intraocular Pressure and Response to Topically Administered 1% Brinzolamide in a Spontaneous Feline Model of Primary Congenital Glaucoma. Annual Meeting of the Association for Research in Vision and Ophthalmology; Fort Lauderdale, FL. 2009. abstract. [Google Scholar]
- 59.Liu HK, Chiou GC, Garg LC. Ocular hypotensive effects of timolol in cat eyes. Archives of Ophthalmology. 1980 Aug;98(8):1467–1469. doi: 10.1001/archopht.1980.01020040319022. [DOI] [PubMed] [Google Scholar]
- 60.Wilkie DA, Latimer CA. Effects of topical administration of timolol maleate on intraocular pressure and pupil size in cats. American Journal of Veterinary Research. 1991 Mar;52(3):436–440. [PubMed] [Google Scholar]
- 61.Colasanti BK, Trotter RR. Effects of selective beta 1- and beta 2-adrenoreceptor agonists and antagonists on intraocular pressure in the cat. Investigative Ophthalmology & Visual Science. 1981 Jan 1;20(1):69–76. [PubMed] [Google Scholar]
- 62.Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture] Investigative Ophthalmology & Visual Science. 1991;32(13):3145–3166. [PubMed] [Google Scholar]
- 63.Liu JH, Bartels SP, Neufeld AH. Effects of timolol on intraocular pressure following ocular adrenergic denervation. Current Eye Research. 1984 Sep;3(9):1113–1117. doi: 10.3109/02713688409000810. [DOI] [PubMed] [Google Scholar]
- 64.Regnier A. Clinical Pharmacology and Therapeutics part 2 Antimicrobials, Anti-inflammatory Agents, and Antiglaucoma Drugs. In: Gelatt KN, editor. Veterinary Ophthalmology. 3. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 297–336. [Google Scholar]
- 65.Wilkie DA, Latimer CA. Effects of topical administration of 2.0% pilocarpine on intraocular pressure and pupil size in cats. American Journal of Veterinary Research. 1991 Mar;52(3):441–444. [PubMed] [Google Scholar]
- 66.Rankin AJ, Krohne SG, Glickman NW, Glickman LT, Stiles J. Laser flaremetric evaluation of experimentally induced blood-aqueous barrier disruption in cats. American Journal of Veterinary Research. 2002;63(5):750–756. doi: 10.2460/ajvr.2002.63.750. [DOI] [PubMed] [Google Scholar]
- 67.Wang YL, Toris CB, Zhan G, Yablonski ME. Effects of topical epinephrine on aqueous humor dynamics in the cat. Experimental Eye Research. 1999 Apr;68(4):439–445. doi: 10.1006/exer.1998.0623. [DOI] [PubMed] [Google Scholar]
- 68.Anderson JA, Richman JB, Mindel JS. Effects of echothiophate on enzymatic hydrolysis of dipivefrin. Archives of Ophthalmology. 1984 Jun;102(6):913–916. doi: 10.1001/archopht.1984.01040030733032. [DOI] [PubMed] [Google Scholar]
- 69.Toris CB, Tafoya ME, Camras CB, Yablonski ME. Effects of apraclonidine on aqueous humor dynamics in human eyes. Ophthalmology. 1995 Mar;102(3):456–461. doi: 10.1016/s0161-6420(95)31000-7. [DOI] [PubMed] [Google Scholar]
- 70.Miller PE, Rhaesa SL. Effects of topical administration of 0.5% apraclonidine on intraocular pressure, pupil size, and heart rate in clinically normal cats. American Journal of Veterinary Research. 1996 Jan;57(1):83–86. [PubMed] [Google Scholar]
- 71.Studer ME, Martin CL, Stiles J. Effects of 0.005% latanoprost solution on intraocular pressure in healthy dogs and cats. American Journal of Veterinary Research. 2000 Oct;61(10):1220–1224. doi: 10.2460/ajvr.2000.61.1220. [DOI] [PubMed] [Google Scholar]
- 72.Bartoe JT, Davidson HJ, Horton MT, Jung Y, Brightman AH. The effects of bimatoprost and unoprostone isopropyl on the intraocular pressure in normal cats. Veterinary Ophthalmology. 2005;8(4):247–252. doi: 10.1111/j.1463-5224.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 73.Regnier A, Lemagne C, Ponchet A, Cazalot G, Concordet D, Gelatt KN. Ocular effects of topical 0.03% bimatoprost solution in normotensive feline eyes. Veterinary Ophthalmology. 2006 Jan-Feb;91:39–43. doi: 10.1111/j.1463-5224.2005.00435.x. [DOI] [PubMed] [Google Scholar]
- 74.Bhattacherjee P, Paterson CA. Studies on prostanoid receptors in ocular tissues. Journal of Ocular Pharmacology. 1994;10(1):167–175. doi: 10.1089/jop.1994.10.167. [DOI] [PubMed] [Google Scholar]
- 75.Sharif NA, Kaddour-Djebbar I, Abdel-Latif AA. Cat iris sphincter smooth-muscle contraction: comparison of FP-class prostaglandin analog agonist activities. Journal of Ocular Pharmacology and Therapeutics. 2008 Apr;24(2):152–163. doi: 10.1089/jop.2007.0076. [DOI] [PubMed] [Google Scholar]
- 76.Chen J, Woodward DF. Prostanoid-induced relaxation of precontracted cat ciliary muscle is mediated by EP2 and DP receptors. Investigative Ophthalmology and Visual Science. 1992;33(11):3195–3201. [PubMed] [Google Scholar]
- 77.Bhattacherjee P, Williams BS, Paterson CA. Responses of intraocular pressure and the pupil of feline eyes to prostaglandin EP1 and FP receptor agonists. Investigative Ophthalmology and Visual Science. 1999 Nov;40(12):3047–3053. [PubMed] [Google Scholar]
- 78.Toris C, Yablonski M, Wang Y-L, Hayashi M. Prostaglandin A2 increases uveoscleral outflow and trabecular outflow facility in the cat. Experimental Eye Research. 1995;61:649–657. doi: 10.1016/s0014-4835(05)80015-6. [DOI] [PubMed] [Google Scholar]
- 79.Toris CB, Camras CB, Yablonski ME, Brubaker RF. Effects of exogenous prostaglandins on aqueous humor dynamics and blood-aqueous barrier function. Survey of Ophthalmology. 1997 Feb;41(Suppl 2):S69–75. doi: 10.1016/s0039-6257(97)80010-0. [DOI] [PubMed] [Google Scholar]
- 80.Ward D. Effects of latanoprost on aqueous humor flow rate in normal dogs. 36th Annual Conference American College of Veterinary Ophthalmologists; Nashville, TN. 2005. abstract. [Google Scholar]
- 81.Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Survey of Ophthalmology. 2008 Nov;53(Suppl1):S107–120. doi: 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhan GL, Miranda OC, Bito LZ. Steroid glaucoma: corticosteroid-induced ocular hypertension in cats. Experimental Eye Research. 1992 Feb;54(2):211–218. doi: 10.1016/s0014-4835(05)80210-6. [DOI] [PubMed] [Google Scholar]
- 83.Bhattacherjee P, Paterson CA, Spellman JM, Graff G, Yanni JM. Pharmacological validation of a feline model of steroid-induced ocular hypertension. Archives of Ophthalmology. 1999 Mar;117(3):361–364. doi: 10.1001/archopht.117.3.361. [DOI] [PubMed] [Google Scholar]
- 84.Godel V, Feiler-Ofry V, Stein R. Systemic steroids and ocular fluid dynamics. II. Systemic versus topical steroids. Acta Ophthalmologica (Copenhagen) 1972;50(5):664–676. doi: 10.1111/j.1755-3768.1972.tb06607.x. [DOI] [PubMed] [Google Scholar]
- 85.Leibowitz HM, Bartlett JD, Rich R, McQuirter H, Stewart R, Assil K. Intraocular pressure-raising potential of 1.0% rimexolone in patients responding to corticosteroids. Archives of Ophthalmology. 1996 Aug;114(8):933–937. doi: 10.1001/archopht.1996.01100140141005. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg LF, Burchfield JC, Krupin T, Bock CJ, Jr, Goldenfeld M, O’Grady RB. Cat model for intraocular pressure reduction after transscleral Nd:YAG cyclophotocoagulation. Current Eye Research. 1995 Apr;14(4):255–261. doi: 10.3109/02713689509033523. [DOI] [PubMed] [Google Scholar]
- 87.Bingaman DP, Lindley DM, Glickman NW, Krohne SG, Bryan GM. Intraocular gentamicin and glaucoma: a retrospective study of 60 dog and cat eyes (1985-1993) Veterinary and Comparative Ophthalmology. 1994;4(3):113–119. [Google Scholar]
- 88.Koch SA. Intraocular prosthesis in the dog and cat: the failures. Journal of the American Veterinary Medical Association. 1981;179(9):883–885. [PubMed] [Google Scholar]
- 89.McLaughlin SA, Ramsey DT, Lindley DM, Gilger BC, Gerding PA, Whitley RD. Intraocular silicone prosthesis implantation in eyes of dogs and a cat with intraocular neoplasia: nine cases (1983 - 1994) Journal of the American Veterinary Medical Association. 1995;207(11):1441–1443. [PubMed] [Google Scholar]
- 90.Naranjo C, Dubielzig RR. Histopathological Study of the Causes for Failure of Intrascleral Prosthesis in Dogs and Cats. 39th Annual Meeting of the American College of Veterinary Ophthalmologists; Boston, MA. 2008. abstract. [DOI] [PubMed] [Google Scholar]
- 91.Nasisse MP, van Ee RT, Munger RJ, Davidson MG. Use of methyl methacrylate orbital prostheses in dogs and cats: 78 cases (1980-1986) Journal of the American Veterinary Medical Association. 1988 Feb 15;192(4):539–542. [PubMed] [Google Scholar]
- 92.Hamor RE, Roberts SM, Severin GA. Use of orbital implants after enucleation in dogs, horses, and cats: 161 cases (1980-1990) Journal of the American Veterinary Medical Association. 1993 Sep 1;203(5):701–706. [PubMed] [Google Scholar]
- 93.Bill A. Acetazolamide and intrascleral venous pressure. Archives of Ophthalmology. 1963 Feb;69:236–240. doi: 10.1001/archopht.1963.00960040242016. [DOI] [PubMed] [Google Scholar]


