Abstract
Objective
To determine the effects of topical 0.5% tropicamide on anterior segment morphology (ASM) and intraocular pressure (IOP) in normal and glaucomatous cats.
Animals used
Normal cats and cats with inherited primary congenital glaucoma (PCG).
Procedures
Control IOP curves were performed in untreated normal and PCG cats. In the first experiment, tropicamide was applied OD in eight normal and nine PCG cats. IOP and pupillary diameter (PD) were measured at 0, 30, and 60 min, then hourly until 8 h post-treatment. In a second experiment, six normal and seven PCG cats received tropicamide OD. High-resolution ultrasound images were obtained at 0, 1, 5, and 10 h post-treatment to measure ASM changes. IOP and PD were measured OD at 0, 1, 2, 3, 5, 7, and 9 h.
Results
In untreated normal cats IOP OU decreased throughout the day. In PCG cats IOP OU had wide fluctuations over time. In normal cats IOP response varied in the treated eye but did not change significantly in untreated eyes. IOP significantly increased from baseline in both eyes of all treated PCG cats. Increases in IOP were associated with some ASM changes. Cats with PCG had a significantly smaller angle recess areas, diminished ciliary clefts and decreased iris-lens contact. ASM changes were not strongly correlated with IOP in all cats.
Conclusions
The ASM of PCG cats is markedly different from normal cats, and clinically significant increases in IOP OU occur in cats with PCG after tropicamide treatment. The mechanism for this increase remains unclear.
Keywords: anterior segment morphology, cat, glaucoma, high-resolution ultrasound, intraocular pressure, tropicamide
INTRODUCTION
Tropicamide is a nonselective muscarinic antagonist which produces a short-lived mydriasis and cycloplegia.1–3 When applied topically it is rapidly absorbed within a few minutes from the eye into the systemic circulation.1 Despite being considered a very safe drug when used topically,3 it has been associated with an acute transient sialoadenomegaly,4 sialorrhea, conjunctival hyperemia, chemosis, blepharospasm, and nictitating membrane protrusion in cats.5 Published studies present conflicting data regarding the effects of topical application of mydriatics on pupillary diameter (PD) in normal cats, particularly with regard to contralateral effects.5 In comparable studies, intraocular pressure (IOP) was found to be either increased in both eyes,6 or in the treated eye exclusively. 7 In cats with primary congenital glaucoma (PCG), we have previously shown that IOP increases 30 min after topical application of 1%tropicamide,8 however, the duration of this effect was not studied, and the underlying cause of this observed IOP increase was not established. In addition, our previous studies were not designed to evaluate the response in the contralateral eye in PCG cats.
In this study, we used glaucomatous cats that were known to be homozygous for a completely penetrant, autosomal recessive form of inherited PCG. All affected cats exhibit bilaterally symmetric disease, with characteristic clinical features that include mild to moderate buphthalmos and enlargement and elongation of the ciliary processes, that is detectable within the first few weeks of life. In all affected eyes IOP elevation is characterized by pronounced fluctuation in circadian IOP that is sustained throughout life and results in optic disk cupping and progressive glaucomatous damage to the retina and optic nerve. No consistent biomicroscopic differences are observed in the anterior segment morphology (ASM) of adolescent vs. older affected cats.9–12
Use of high-resolution ultrasound (HRUS) and ultrasound biomicroscopy to evaluate ocular ASM has been described in humans and conscious veterinary patients.13,14 Ultrasonography may serve as a useful tool to characterize mechanisms of IOP alteration in response to topical medications in normal animals as well as patients affected by glaucoma.13
The objective of this study was to examine the effect of topical application of 0.5% tropicamide on IOP, to identify any contralateral effect, and to assess the relationship between ASM and the observed changes in IOP and PD in glaucomatous and normal cats.
MATERIALS AND METHODS
All procedures involving animals were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison.
All cats were selected from a research colony maintained at the University of Wisconsin-Madison and were housed under standard laboratory conditions, subject to a consistent 12-h light/dark cycle (6 am/6 pm). All cats had a complete ophthalmic examination including slit-lamp biomicroscopy (SL5; Kowa Optomed Inc., Torrance, CA, USA), indirect ophthalmoscopy (Omega 500; Heine Optotechnik, Herrsching, Germany) and rebound tonometry (TonoVet®; Icare Finland Oy, Helsinki, Finland). Cats were determined to be normal based on the absence of any ocular lesions on ophthalmic examination. Glaucomatous cats were known to be homozygous for PCG. Aside from consistently elevated IOP and the characteristic abnormalities described previously, glaucomatous cats were free of secondary or unrelated ocular abnormalities, such as lens luxation or corneal lesions. All cats were accustomed to being handled at least weekly for ocular examinations and IOP measurements.
In each phase of the study, IOP measurements for each eye were represented by the mean of three valid readings obtained at each time point by a single consistent observer (FE) using the same Tonovet® rebound tonometer. Readings were considered valid when either no ‘bar’ was visible on the display, or a low ‘bar’ was visible next to a flashing reading on the display. The same disposable probe was used between eyes and animals unless several invalid IOP readings were obtained in succession. Although the manufacturers recommend changing the probe between patients (not eyes), this recommendation is directed at the prevention of spread of infection between patients and does not impact IOP readings derived. However, all subjects in this study were derived from a single colony housed at University of Wisconsin-Madison and none showed evidence of ocular disease at the time of the study, with the exception of PCG. The first eye to have IOP measurements was randomly selected. No topical anesthetic was used prior to rebound tonometry.
Measurement of maximal horizontal PD was also performed by the same examiner, using a digital caliper held approximately 10 mm in front of the eye. All measurements were obtained under consistent illumination conditions (200–220 foot candles; Sekonic Handy Lumi model 246; Sekonic Co., Ltd, Tokyo, Japan) in the same room throughout each phase during this study.
The pattern and extent of the diurnal variation of IOP and PD in normal and glaucomatous cats was established. To account for the influence of diurnal variation on IOP and PD, IOP was measured at 8 am and then 30 min, 1 h and then hourly thereafter until 4 pm in untreated normal and PCG cats. No topical medications were used for this part of the study.
The study then consisted of two main phases. The first phase of the study was conducted to determine the effect of unilateral tropicamide administration on IOP and PD in both eyes of normal and glaucomatous cats. The second phase of the study examined the ASM of normal and glaucomatous cats using HRUS and evaluated changes in a number of ASM parameters in response to topical tropicamide application. The relationships between IOP and PD and ASM following tropicamide application were also examined in this phase.
Phase 1: unilateral tropicamide
Subjects consisted of eight normal domestic cats (five females and three males; mean age 2.2 years, range 0.5–5.9 years) and nine cats with PCG (seven females and two males; mean age 2.4 years, range 0.5–5.5 years). Within the normal group, three/eight cats had a Siamese phenotype (color point coat with blue irides) and five/nine cats in the glaucoma group had a Siamese phenotype. On the day of pharmacologic testing, one drop (approximately 30 µL) of 0.5% tropicamide ophthalmic solution (Bausch and Lomb, Inc., Tampa, FL, USA) was instilled OD. Rebound tonometry and PD measurements OU were performed as described above, immediately prior to treatment then at 30 min, 1 h and every hour thereafter until 8 h post-treatment.
Phase 2: HRUS and the relationship between ASM, IOP, and PD
Subjects consisted of six normal domestic cats (six females; mean age 2.5 years, range 1.1–5.7 years) and seven cats with PCG (six females and one male; mean age 1.8 years, range 0.5–5.4 years). Within the normal group, no cats had a Siamese phenotype and three/seven cats in the glaucoma group had a Siamese phenotype.
Tonometry and PD measurements were performed immediately prior to and at 1, 2, 3, 5, 7, and 9 h following the application of a single drop of 0.5% tropicamide OD. If, during the course of the experiment, IOP measurements were ≥70 mmHg for three consecutive readings, one drop of 2% dorzolamide (Trusopt®,Merck & Co. Inc., Whitehouse Station, NJ, USA) was applied in the affected eye and any subsequent data excluded from further statistical analysis. Immediately prior to and at 1, 5, and 10 h following the application of tropicamide, HRUS images were obtained with a 20-MHz probe (I3 B-scan; Innovative Imaging Inc, Sacramento, CA, USA) following application of 0.5% proparacaine hydrochloride. The probe marker was placed perpendicular to the limbus in the superior temporal quadrant, with every effort made to image the same clock hour at each time point. At least three images were collected at each time point for later analysis. Images chosen for analysis were those in which perpendicularity to anterior segment structures was established by a bright Descemet’smembrane, lens capsule and iris base, and/or the landmarks for measurement could be easily identified.
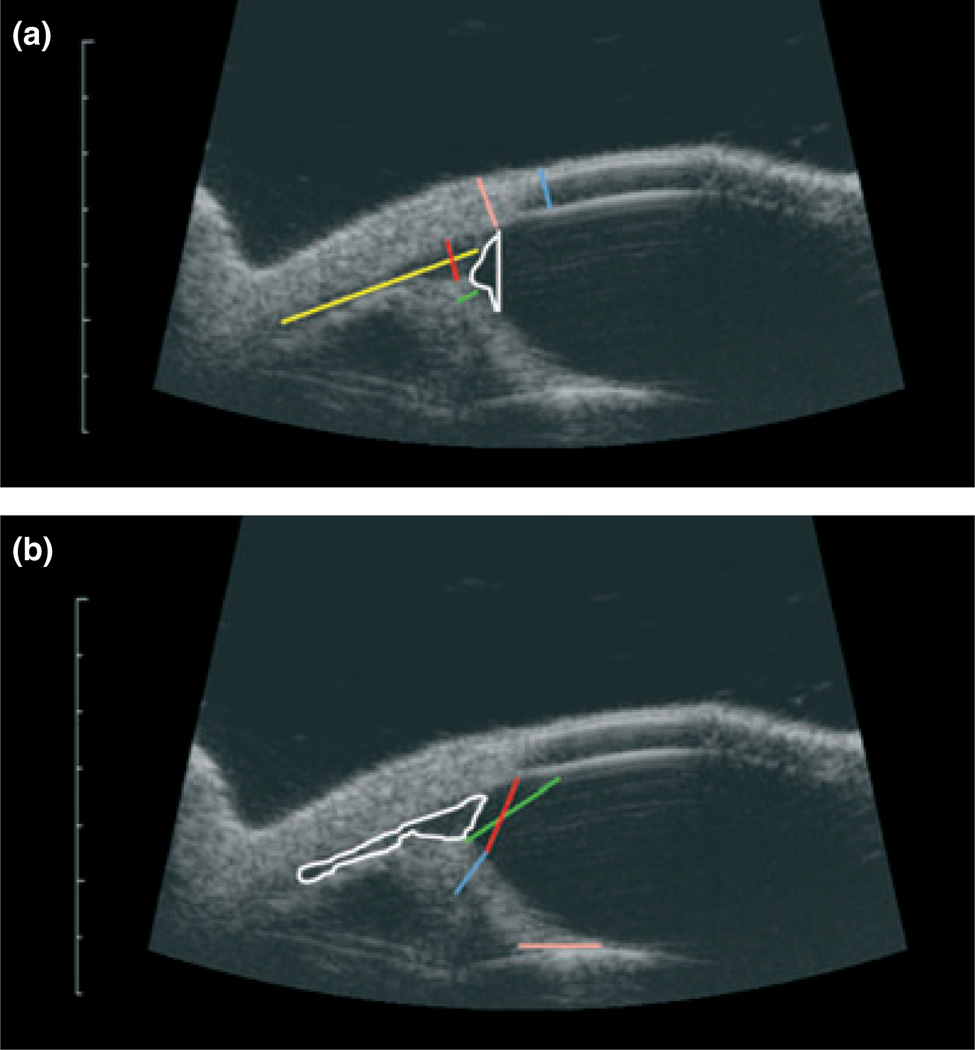
Images were evaluated using Image J software (Image J 1.42q; NIH, Bethesda, MD, USA). Eleven parameters adapted from prior studies in dogs and humans were evaluated for this study (Fig. 1).15,16 These measurements consisted of corneal thickness(CT), scleral thickness (ST), iris thickness (IT), maximal length of the ciliary cleft (MLCC), maximal width of the ciliary cleft (MWCC), area of the ciliary cleft (ACC), trabecular meshwork-iris distance (TMID), iridociliary process distance (ICPD), angle recess area (ARA), and iris-lens contact (ILC).15 The only parameter that was slightly different from previous descriptions15 was angle opening distance. Angle opening distance at 500 µm (AOD500 µm) was measured 500 µm anteriorly from the end of Descemet’s membrane to the anterior surface of the peripheral iris.
Figure 1. High-resolution ultrasound images of a normal feline eye with measurement parameters indicated.
Scale bar to left of each image indicates millimeters. (a) ARA = angle recess area, white; ST = scleral thickness, pink; CT = corneal thickness, blue; IT = iris thickness, green; MLCC = maximum length of ciliary cleft, yellow; MWCC = maximum width of ciliary cleft, red; and (b) ACC = area of ciliary cleft, white; TMID = trabecular meshwork-iris distance, red; ICPD = iridociliary process distance, red/blue; AOD500 µm = angle opening distance at 500 µm, green; ILC = iris lens contact, pink.
Data analysis
For phase one, experimental data (unilateral application of tropicamide), IOP and PD measurements were each compared at different time points by either paired Student’s t-test or repeated measurements anova, with Tukey–Kramer post-test where applicable, for each group. Comparison of IOP and PD values between groups was by unpaired Student’s t-test (Instat® version 3.10; GraphPad Software, Inc., San Diego, CA, USA). A multivariate anova (mixed effects model) was used to examine the effect of time on IOP and PD in both treated and untreated cats (SAS version 9.1.3; SAS Institute Inc., Cary, NC, USA). The degree of correlation between IOP and PD was determined by Spearman rank correlation. Statistical significance was set at P < 0.05.
For phase two, experimental data ASM, IOP, and PD measurements were each compared at different time points by either paired Student’s t-test or repeated measures anova for each group. An unpaired t-test was used to compare ASM parameters between normal and glaucomatous cats (Instat® version 3.10). When ASM was significantly different from baseline, a Spearman rank correlation was used at each time point to determine if there was a correlation between the significant ASM parameters and either IOP or PD. Statistical significance was set at P < 0.05.
RESULTS
Diurnal IOP
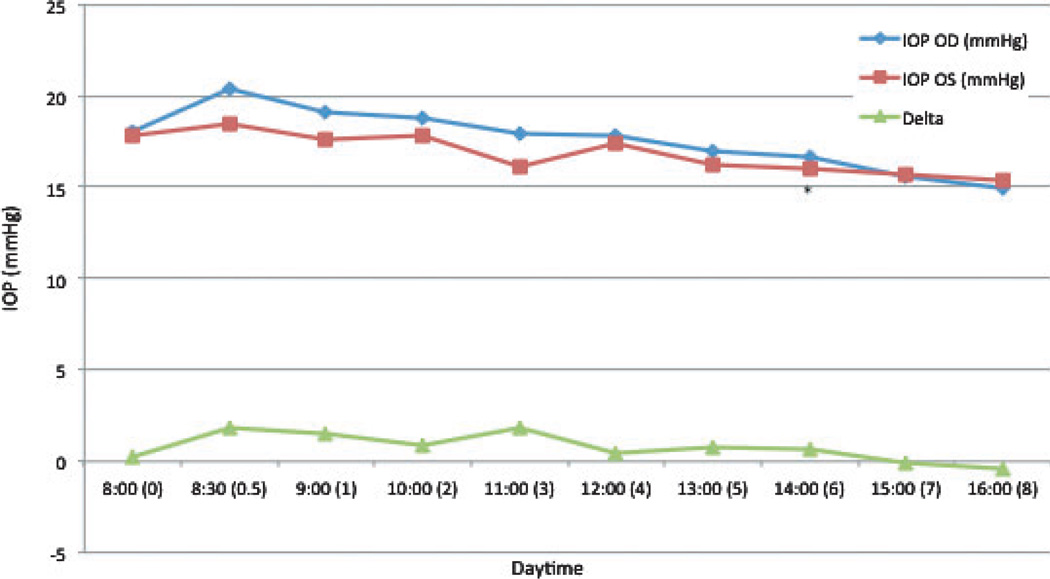
In untreated normal cats, IOP had a tendency to decrease over the course of the diurnal phase. In the left eye (OS), IOP was significantly lower than the 8 am measurement at 2 pm (6 h) (P = 0.023; 10% mean decrease) (Fig. 2).
Figure 2. Mean intraocular pressure (IOP) in untreated normal cats.
Time 0 = 8 am. Delta = difference in IOP between the right and left eyes. * = mean value significantly different from baseline (P < 0.05).
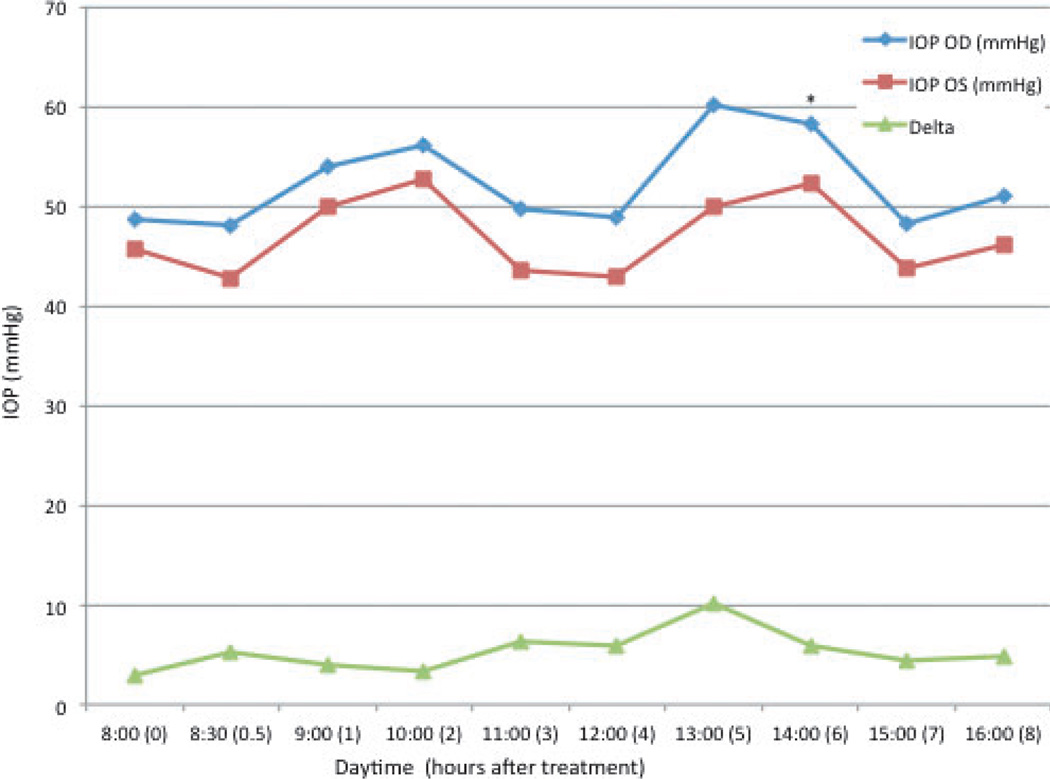
There was pronounced fluctuation in diurnal IOP in untreated glaucomatous cats (Fig. 3). In the group of untreated cats with PCG, mean IOP in the right eye (OD) ranged from 48.1 to 60.1 mmHg and mean IOP OS ranged from 42.8 to 52.7 mmHg. No consistent and significant differences in IOP relative to baseline were observed at any time point, with the exception of a significant but relatively modest increase in IOP OD, observed at 6 h (2 pm) (P = 0.043; 20% mean increase).
Figure 3. Mean intraocular pressure (IOP) in untreated PCG cats.
Time 0 = 8 am. Delta = difference in IOP between the right (OD) and left (OS) eyes. * = mean value significantly different from baseline (P < 0.05).
Phase 1: unilateral tropicamide
Intraocular pressure – normal cats
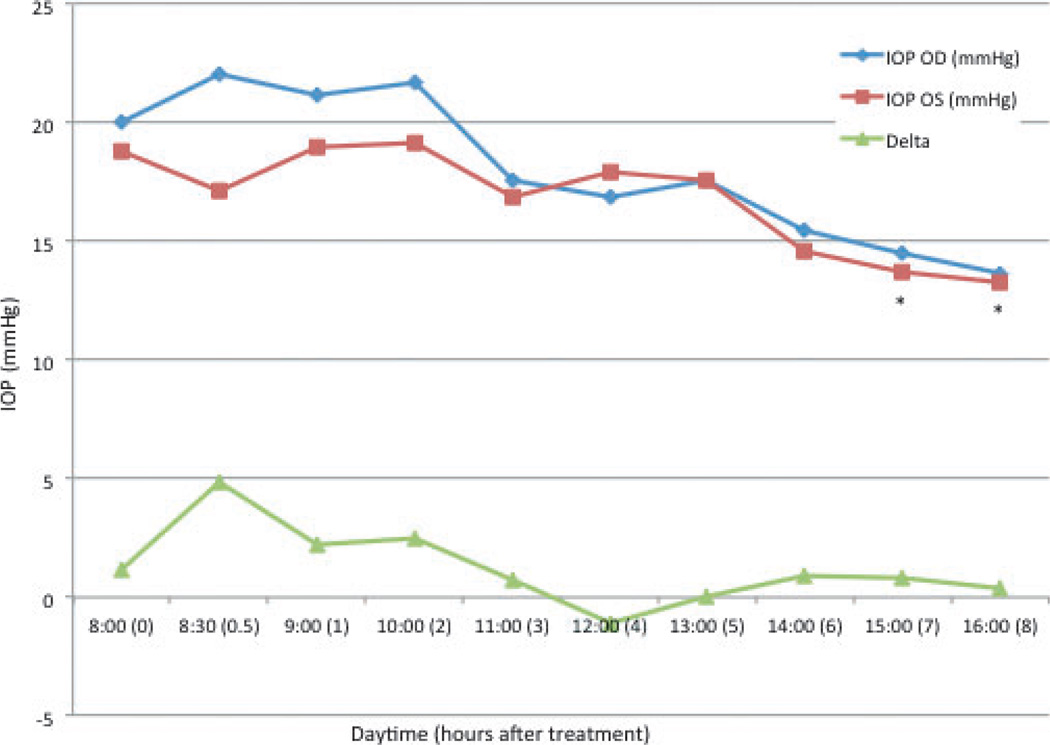
In normal cats, mean baseline IOP was 20 ± 7.4 mmHg (range 12.3–35 mmHg) OD and 18.8 ± 5.7 mmHg (range 11.3–30 mmHg) OS. In treated eyes (OD) there was no significant change in IOP relative to baseline at any time point following tropicamide application. In the untreated, contralateral eyes (OS), a significant decrease in IOP relative to baseline was noted at 7 h (13.7 ± 3.6 mmHg, P = 0.028) and 8 h (13.25 ± 1.9 mmHg, P = 0.021) (Fig. 4). Between eyes, IOP was significantly higher in the treated eye when compared to the untreated eye at 30 min (P = 0.019) and 1 h (P = 0.033) but was not significantly different at any other time point.
Figure 4. Mean intraocular pressure (IOP) in normal cats after topical application of tropicamide in the right eye.
Time 0 = 8 am, immediately prior to application of tropicamide. Delta = difference in IOP between the right (OD) and left (OS) eyes. * = mean value significantly different from baseline (P < 0.05).
In three of four normal cats younger than 13 months, IOP in the treated eye increased 45% relative to baseline by 30 min, but decreased 20% in the remaining young cat. In contrast, IOP increased 5% in two of four older cats (mean age 4.6 years) 30 min after tropicamide application, however, it decreased by 8%relative to baseline in the remaining two cats (2.8 years old).
Intraocular pressure – PCG cats
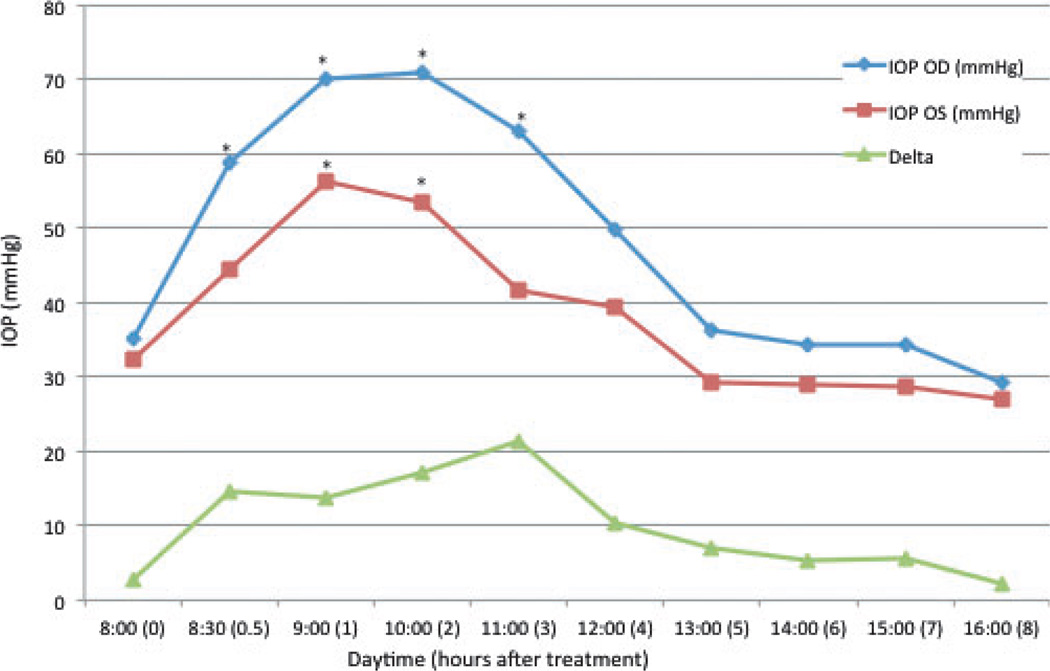
In PCG cats, mean baseline IOP was 35.2 ± 12.2 mmHg OD and 32.3 ± 13.1 mmHg OS. In the treated eyes (OD), a significant increase in IOP relative to baseline was observed for the first 3 h after application of tropicamide (Fig. 5). Maximal effect was observed 2 h after topical administration of tropicamide (70.9 ± 12.9 mmHg; representing a 101.5 ± 5.8% increase). When comparing treated with untreated eyes in cats with PCG, IOP was not significantly greater in the treated eyes than in the untreated eyes at baseline but was significantly greater in the treated compared to the untreated eyes at 30 min, 1, 2, 3, and 4 h after application of tropicamide. The maximal difference between eyes occurred at 2 h (P = 0.001) after unilateral tropicamide application. All PCG cats had a significant increase in IOP in the treated eye, however, the magnitude of this increase was greater in the four youngest cats (less than 13 months), in which maximal IOP occurred at 1 h (mean increase was 166%), while in older cats (n = 5), maximal IOP increase occurred at 2 h (mean increase was 71%).
Figure 5. Mean intraocular pressure (IOP) in PCG cats after topical application of tropicamide in the right eye.
Time 0 = 8 am, immediately prior to application of tropicamide. Delta = difference in IOP between the right (OD) and left (OS) eyes. * = mean value significantly different from baseline (P < 0.05).
In the untreated left eyes, IOP was significantly increased relative to baseline at 1 h (56.2 ± 16.7 mmHg, P = 0.009) and 2 h (53.6 ± 14.5 mmHg, P = 0.009) after application of tropicamide to the fellow eye.
Mixed effects modeling (multivariate anova) confirmed that IOP was significantly influenced by time from tropicamide application and by the presence of PCG.
Pupil diameter – normal cats
In normal cats, baseline PD was 5.1 ± 1.7 mm OD and 5.7 ± 1.5 mm OS. PD measurements in the treated eyes (OD) were significantly greater than baseline at all time points for 5 h after tropicamide application. Maximal pupil dilation (11.7 ± 1.5 mm) was observed at 1 h following application of tropicamide. In the untreated eyes, a significant increase in PD relative to baseline was only noticed at 30 min (7.1 ± 2.4 mm, P = 0.021) and a significant decrease relative to baseline was noted at 7 h (3.7 ± 2.3 mm, P = 0.010).
A modest, but still positive correlation between IOP and PD was found in normal cats (Spearman r = 0.6205, P < 0.0001 and Spearman r = 0.5917, P < 0.0001), for the treated and untreated eyes, respectively.
Pupil diameter – PCG cats
In PCG cats, baseline PD was 5.9 ± 2.1 mm OD and 6.8 ± 2.0 mm OS. PD measurements in the treated eyes (OD) were significantly greater than baseline at all time points for 5 h after tropicamide application. Maximal pupil dilation (13.6 ± 0.7 mm, P < 0.0001) was observed at 1 h following application of tropicamide. In the untreated eyes, a significant increase in PD relative to baseline was only noticed at 30 min and 1 h (8.9 ± 1.7 mm, P = 0.0006 and 9.6 ± 2.1 mm, P = 0.0007, respectively).
In PCG cats, there was a positive correlation between PD and IOP, although this was stronger in the treated eye (Spearman r = 0.8153, P < 0.0001 and Spearman r = 0.5828, P < 0.0001, for the treated and untreated eyes, respectively).
Phase 2: HRUS and the relationship between ASM, IOP, and PD
Anterior segment morphology
When comparing ASM of normal cats and PCG cats at baseline (Fig. 6a,b), the following parameters were all significantly smaller in glaucomatous cats: ARA (P = 0.030), IT (P < 0.0001), maximal length and width of the ciliary cleft (P = 0.0001 and 0.0011, respectively), ACC (P = 0.0009), ILC (P = 0.0012), ICPD (P = 0.041), and ST (P = 0.043). CT, AOD500 µm, and TMID were not significantly different between groups.
Figure 6.
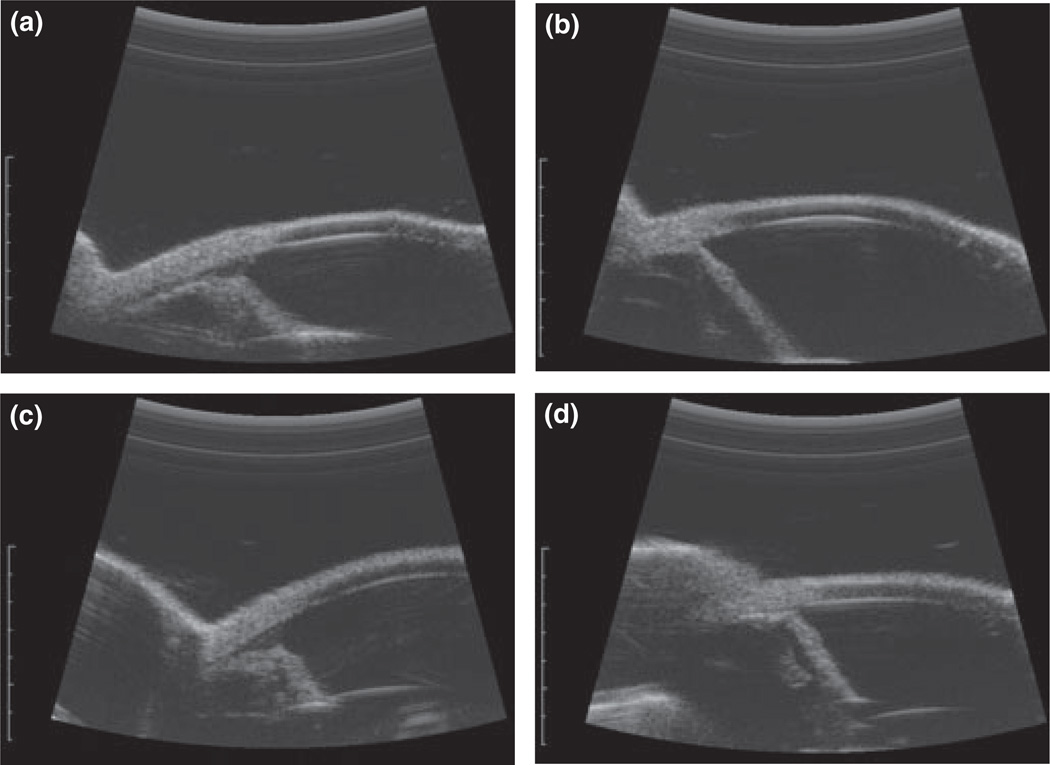
High-resolution ultrasound images of the right eyes of (a) a normal cat, prior to topical treatment with 0.5% tropicamide (IOP: 28 mmHg; PD: 7.3 mm); (b) a PCG cat prior to topical treatment with 0.5% tropicamide (IOP 39.7 mmHg; PD: 4.5 mm); (c) the same normal cat as depicted in (a), 1 h after topical treatment with 0.5% tropicamide(IOP: 24.3 mmHg; PD: 11.6 mm), and (d) the same PCG cat as depicted in (b), 1 h after topical treatment with 0.5% tropicamide (IOP: 85 mmHg; PD: 12.9 mm).
Response to tropicamide – normal cats
In the eyes of normal cats treated with 0.5% tropicamide, mean baseline IOP OD was 20.1 ± 5.3 mmHg (range 15–28 mmHg) and 18.1 ± 3.6 mmHg (range 14–23.3 mmHg) OS. In this second phase of the study, there was a significant increase in IOP OD relative to baseline that occurred 3 h after tropicamide administration (OD 28.2 ± 5.9 mmHg P = 0.019, range 20.3–36.7 mmHg, mean increase 40.4%). All cats had an increased IOP at 3 h after topical tropicamide treatment. In normal cats younger than 16 months (4/6 cats), mean IOP OD increase was 49.6% relative to baseline by 3 h. In contrast IOP OD increased 24.7% in older cats (mean age 5.2 years) 3 h after tropicamide application.
In the right eye of normal cats treated with 0.5% tropicamide, CT, ST, IT, ARA, maximal length and width of the ciliary cleft, AOD500 µm, and ACC did not significantly change over time following tropicamide application (Fig. 6c).
One hour after treatment, TMID (P = 0.030) and ICPD (P = 0.010) were significantly increased from baseline. ILC (P = 0.006) was significantly decreased 1 h after treatment (Table 1). In normal cats 1 h following treatment, there was no correlation between IOP or PD and either in the TMID (IOP: r = 0.2319, P = 0.658; PD: r = 0.1160, P = 0.803); ICPD (IOP: r = 0.1429, P = 0.803; PD: r = 0.2000, P = 0.714), or ILC (IOP: r = −0.6000, P = 0.242; PD: r = −0.4286, P = 0.419).
Table1.
Anterior segment morphology measurements obtained from high-resolution ultrasound images of normal and cats with primary congenital glaucoma prior to and following topical tropicamide administration. Measurements represent means from the right eye (±SD). Shaded values were significantly different from baseline (P < 0.05).
| Animal group |
Time point |
CT (mm) | ST (mm) | ARA (mm2) | IT (mm) | MLCC (mm) |
MWCC (mm) |
AOD500 µm (mm) |
TMID (mm) |
ICPD (mm) |
ILC (mm) |
ACC (mm2) |
IOP (mmHg) |
PD (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal (n = 6) |
pre-tx | 0.657(0.072) | 0.853(0.086) | 0.769(0.209) | 0.701(0.043) | 1.688(0.408) | 0.211(0.080) | 1.202(0.270) | 0.825(0.160) | 1.475(0.202) | 1.650(0.314) | 0.242(0.129) | 20.1(5.3) | 7(0.8) |
| 1 h | 0.714(0.042) | 0.923(0.142) | 0.959(0.206) | 0.757(0.106) | 1.147(0.313) | 0.242(0.114) | 1.346(0.158) | 0.964(0.175) | 1.624(0.256) | 1.156(0.258) | 0.154(0.145) | 25.5(4.1) | 11.1(0.7) | |
| 5 h | 0.671(0.057) | 0.876(0.126) | 0.854(0.167) | 0.748(0.118) | 1.286(0.467) | 0.220(0.056) | 1.366(0.394) | 0.842(0.204) | 1.581(0.268) | 1.438(0.348) | 0.148(0.106) | 22.7(2.0) | 11(1.3) | |
| PCG (n = 7) |
pre-tx | 0.590(0.064) | 0.747(0.082) | 0.248(0.122) | 0.454(0.077) | 0.085(0.224) | 0.006(0.015) | 1.185(0.286) | 0.686(0.317) | 1.165(0.269) | 0.971(0.199) | 0.003(0.009) | 41.7(15.6) | 6.6(1.7) |
| 1 h | 0.582(0.063) | 0.764(0.061) | 0.227(0.080) | 0.489(0.058) | 0.390(0.450) | 0.050(0.053) | 1.155(0.376) | 0.678(0.340) | 1.350(0.300) | 0.735(0.116) | 0.016(0.020) | 75.1(11.1) | 12.8(1.3) | |
| 5 h | 0.610(0.067) | 0.796(0.065) | 0.221(0.119) | 0.508(0.103) | 0.328(0.563) | 0.034(0.044) | 1.026(0.200) | 0.578(0.235) | 1.139(0.333) | 0.975(0.255) | 0.013(0.020) | 49.7(24) | 9.4(2.4) |
CT = corneal thickness; ST = sclera thickness; ARA = angle recess area; IT = iris thickness; MLCC = maximal length ciliary cleft; MWCC = maximal width ciliary cleft; AOD500 µm = angle opening distance at 500 µm; TMID = trabecular meshwork-iris distance; ICPD = iridociliary process distance; ILC = iris-lens contact; ACC = area of the ciliary cleft; IOP = intraocular pressure; PD = pupil diameter. No parameters were significantly different from baseline at 10 h post-tropicamide (data not shown in this Table)
Response to tropicamide – PCG cats
In PCG cats, mean baseline IOP OD was 41.7 ± 15.6 mmHg and 37.8 ± 13.7 mmHg OS. As in phase one of the study, there was a significant increase in IOP OU relative to baseline that occurred within the first 3 h after tropicamide administration, with maximal effect observed at 1 h after tropicamide administration (OD 75.1 ± 11.1 mmHg, P = 0.002; OS 71.3 ± 11.2 mmHg, P = 0.0003). Three PCG cats were rescued by application of 2% dorzolamide at 5 h after tropicamide application (one was treated OS and two were treated OU). In the right eye of PCG cats treated with 0.5% tropicamide, CT, IT, ARA, MLCC, MWCC, AOD500 µm, TMID, and ACC did not change significantly over time (Fig. 6d). ICPD (P = 0.046) was significantly increased at 1 h, ST was significantly increased at 5 h (P = 0.041) whereas, ILC was significantly decreased at 1 h (P = 0.024) (Table 1).
In PCG cats, there was no correlation between IOP or PD and either the ICPD (IOP: r = 0.07143, P = 0.906; PD: r = 0.6429, P = 0.139), or ILC (IOP: r = −0.03571, P = 0.964; PD: r = −0.1071, P = 0.840) 1 h after treatment. However, 5 h after treatment, ST was positively correlated with IOP (IOP: r = 0.8108, P = 0.034; PD: r = 0.3604, P = 0.444).
DISCUSSION
The results of this study indicate that unilateral tropicamide application in glaucomatous cats causes a dramatic increase in IOP, especially in the treated eye. This is in agreement with our previous findings.8 We also found that this effect was more pronounced in younger animals.
The range of IOP in normal cats prior to treatment in our study (11.3–35 mmHg) was comparable to a previous report using TonoVet (11–33 mmHg).17 While overall there was no clinically significant increase in IOP in the treated eyes of normal cats in this study, a subset of younger cats demonstrated a significant increase in IOP. This finding was in concordance with previous research that found that older cats had a less significant change in IOP after treatment with tropicamide when compared to younger cats.6 In addition, IOP in a subpopulation of the normal cats in our study decreased after treatment. This variability in response may partially explain the contradictory results of previous studies, in which the treated eye had an increased IOP7 or no change in IOP.21 Another possible explanation for inconsistencies between studies is the different methods for measuring IOP, as some have used an applanation tonometer with or without topical anesthesia,6,18 while we used a rebound tonometer with no topical anesthesia. A rebound tonometer was used in the present study as it has been shown to be accurate and precise in measuring IOP in normal and glaucomatous cats.17,19
In untreated glaucomatous cats there was a pronounced fluctuation in diurnal IOP and as reported previously, these fluctuations were relatively symmetric.10 Despite the fact that IOP differences between eyes of untreated animals were statistically significant at some time points, these differences were small and were considered clinically insignificant. IOP fluctuations were more dramatic in the animals receiving tropicamide.
In PCG cats, the IOP in the untreated eye increased after tropicamide application to the contralateral eye, while in normal cats the IOP generally showed no significant change or actually decreased. The decrease in IOP observed in the contralateral eye in normal cats was consistent with the normal feline diurnal pattern of IOP, as IOP has a tendency to decrease in the afternoon.20 Diurnal IOP variation in untreated glaucomatous cats followed a similar pattern but exhibited accentuated fluctuations. In previous studies in PCG cats, peak IOPs occurred early in the nocturnal phase.10 Therefore, this increase in IOP in the untreated eye of PCG cats following contralateral tropicamide application is likely to be a clinically significant finding.
Pupil dilation was observed in both eyes of normal and glaucomatous cats following unilateral tropicamide application in our study. However, the mydriatic effect was longer lasting in the treated eye. With regard to this contralateral response, conflicting results have been previously reported, where pupil dilation occurred in the treated eye only, or in both eyes.6,7 We found that changes in pupil size were longer lasting than IOP changes after topical application of tropicamide. In addition, although the IOP significantly increased in the contralateral eye of unilaterally treated PCG cats, neither the increase was as dramatic, nor as long lasting as the IOP increase in the treated eye. There are a number of possible explanations for the contralateral effects observed in both normal and glaucomatous cats. Tropicamide has been detected in the serumafter topical ophthalmic application in humans, although its serum levels decline very rapidly to an undetectable concentration in a few hours.1 In addition, when tropicamide was given orally to cats, an increase in IOP and PD was observed.18,21 Thus, the contralateral effect observed in this study and in previous studies could be explained in terms of systemic, specifically oral absorption, particularly as no effort was made to occlude the lacrimal puncta after topical application of the drug. Another possible explanation for the contralateral effects seen in this study could be a contamination of the rebound tonometer probe with tropicamide, as the probe tip was not changed between animals or eyes at every single measurement. However, we have shown in our laboratory that cross-contamination is unlikely with the Tonovet probe, based on the lack of weight changes in the probe before and after IOP measurement (G. J. McLellan and P. E. Miller, unpublished data). Also, an increase in IOP was observed at the first post-treatment time point (30 min) after topical treatment, which suggests that cross-contamination is unlikely to account for the contralateral effect.
There are a number of possible explanations for the differences observed in the magnitude and duration of both ipsilateral and contralateral effect between normal and glaucomatous cats. As the iris in cats with PCG is hypoplastic, it is possible that they have a smaller density of melanocytes, which could reduce binding of tropicamide by melanin, and in turn could reduce the time to onset of maximal effect and enhance drug activity.24 However, Siamese cats are known to have deficient pigmentation of the anterior uveal stroma22 and we did not observe a difference in drug response between Siamese and non-Siamese animals in either group in our study. Regardless of the underlying mechanism for IOP elevation, any alterations in aqueous humor dynamics that arise as a result of tropicamide are likely to have greater effects on animals with PCG, in which there is significant pre-existing compromise to the aqueous outflow pathways.
Anterior segment morphology in normal and glaucomatous cats was significantly different. In PCG cats, the ciliary cleft was typically too small to measure, in marked contrast to normal cats; the IT was reduced, and the ARA was much smaller in cats with PCG relative to normal cats. In addition, the ST was significantly less in glaucomatous cats at all time points. All glaucomatous cats exhibit a degree of buphthalmos, 9 and it is possible that the difference in ST between normal and glaucomatous cats may reflect either progressive scleral thinning or may represent a phenotypic feature of the underlying extracellular matrix abnormalities associated with this form of congenital glaucoma. The role that this apparent difference in ST may have played in the response of these cats to tropicamide remains unclear, however, in normal cats there was no obvious difference in ST when comparing animals that had an increase or a decrease in IOP. In depth study of the scleral morphology and biomechanics in cats with PCG was not within the scope of the present study but is currently under investigation. ASM differences between normal and PCG cats were somewhat expected given the considerable clinical differences observed on ophthalmic examination.
Quantitative evaluation of ASM in HRUS images from PCG cats proved challenging. In addition to the virtual lack of a ciliary cleft, it proved difficult to identify the termination of Descemet’s membrane in cats with PCG. These difficulties in identifying important landmarks undoubtedly had a negative impact on the reliability and reproducibility of a number of other ASM parameters such as CT, sclera thickness, ARA, AOD500 µm, and TMID.
Determination of a causal relationship between ASM changes and IOP increases proved elusive. Most changes in the ASM in both groups occurred at 1 h, whereas maximal IOP and PD occurred at 3 h post-treatment in normal cats and at 1 h in PCG cats. In a previous study in humans, ILC and ICPD 40 min after topical tropicamide had a tendency to increase in normal patients, and to decrease in primary open angle glaucoma (POAG) patients.14 This is in contrast with our findings in all cats, where the ILC was significantly decreased and the ICPD was significantly increased at 1 h after treatment. In humans, the AOD500 µm in normal and POAG had a tendency to increase in both groups, while in this study the AOD500 µm had a tendency to decrease in PCG cats and increase in normal cats. In addition, IT increased in all groups in both humans and cats.14
No strong correlation was found between ASM parameters and IOP in cats, with the exception of the ST in glaucomatous cats which increased at the last measured time point. An increase in the ST was also found in normal cats, but was of no statistical significance. The increase in the ST in PCG cats occurred after the time of the IOP peak, which implies that there is no direct causal relationship between the change in ST and IOP increase. It is conceivable that subtle differences in orientation of the HRUS probe at this time point may have resulted in an oblique rather than perpendicular image, with artifactual increase in ST. However, a similar finding was also observed in a previous study on POAG in humans, where the sclera had a tendency to become thicker after treatment with tropicamide in both normal patients and those with POAG.14
The relationships between IOP, PD, and ASM are undoubtedly complex and dynamic. For example, high IOP may contribute to further collapse of an already compromised aqueous outflow pathway. In addition, PD in cats with PCG is likely affected by varying degrees of iris hypoplasia, as well as both afferent and efferent neuropathic effects of sustained and fluctuating IOP elevations that characterize this disease. Together these effects would confound our ability to identify correlations between IOP, PD, and ASM.
The IOP increases observed after tropicamide could be at least partially explained by a mechanical obstruction of the aqueous humor outflow at a different location that was not evaluated (or visible) via HRUS (e.g., further ciliary cleft collapse or trabecular meshwork changes related to cycloplegic effects of tropicamide). The potential effects of topical tropicamide on intrascleral or episcleral blood vessels were not assessed. As these veins have smooth muscle and adrenergic innervation,23 it is possible that tropicamide interferes with the smooth muscle activity in the blood vessel walls which in turn could influence IOP, but would not be detected by HRUS. Other key factors that may be considered include blockage of the pumping action of the ciliary muscle25, change in the secretion rate of the aqueous humor, or a change in the scleral rigidity.26
Several limitations of this study may have affected our results. First, the number of subjects in both groups was relatively small, limiting our ability to identify small changes in the very variable ASM parameters. Second, our glaucomatous subjects studied represented a homogeneous population of animals with a single form of PCG that is not truly representative of the most common type of feline glaucoma encountered in practice.27 Our study population also had a low ratio of males to females, which could have influenced our results.28
In summary, application of tropicamide in one eye can change bilateral IOP in normal cats and those with PCG. Age may play a role in IOP variation. Based on our findings, IOP should be evaluated before dilating the pupils to avoid glaucoma misdiagnosis in normal cats, and to prevent a potentially detrimental IOP increase in glaucomatous cats. Elevation in IOP cannot be solely explained by the ASM changes observed.
ACKNOWLEDGMENTS
The authors thank Laura Ramsey and Jeremy P Kemmerling for their technical support in the conduct of this study, as well as Nicholas Keuler MS for his advice on statistical analyses.
This study was supported by NIH grants K08 EY018609 and P30 EY0016665, and by the Companion Animal Fund, UW-Madison School of Veterinary Medicine.
REFERENCES
- 1.Vuori ML, Kaila T, Lisalo E, et al. Systemic absorption and anti-cholinergic activity of topically applied tropicamide. Journal of Ocular Pharmacology. 1994;10:431–437. doi: 10.1089/jop.1994.10.431. [DOI] [PubMed] [Google Scholar]
- 2.Gettes BC. Tropicamide, a new cycloplegic mydriatic. Archives of Ophthalmology. 1961;65:632–635. doi: 10.1001/archopht.1961.01840020634005. [DOI] [PubMed] [Google Scholar]
- 3.Merrill DLGD, Zavell S. Bis-tropamide, a new parasympatholytic. Current Therapeutic Research. 1960;2:43–50. [Google Scholar]
- 4.Willis M, Martin CL, Stiles J, et al. Acute, transient sialoadenomegaly in two cats following topical administration of tropicamide. Veterinary & Comparative Ophthalmology. 1997;7:206–208. [Google Scholar]
- 5.Gelatt KN, Bogges T, Cure T. Evaluation of mydriatics in the cat. Journal of the American Animal Hospital Association. 1973;9:283–287. [Google Scholar]
- 6.Stadtbaumer K, Kostlin RG, Zahn KJ. Effects of topical 0.5% tropicamide on intraocular pressure in normal cats. Veterinary Ophthalmology. 2002;5:107–112. doi: 10.1046/j.1463-5224.2002.00226.x. [DOI] [PubMed] [Google Scholar]
- 7.Stadtbaumer K, Frommlet F, Nell B. Effects of mydriatics on intraocular pressure and pupil size in the normal feline eye. Veterinary Ophthalmology. 2006;9:233–237. doi: 10.1111/j.1463-5224.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 8.McLellan GJ, Kuehn M, Sigle K. Effect of topical 1% tropicamide on intraocular pressure in cats with primary congenital glaucoma; 36th Annual Meeting of the American College of Veterinary Ophthalmologists; 2005. p. 53. [Google Scholar]
- 9.McLellan GJ, Betts DM, Sigle K, et al. Congenital glaucoma in the Siamese cat – a new spontaneously occurring animal model for glaucoma research; 35th Annual Meeting of the American College of Veterinary Ophthalmologists; 2004. p. 36. [Google Scholar]
- 10.Sigle KJ, Camano-Garcia G, Carriquiry AL, et al. The effect of dorzolamide 2% on circadian intraocular pressure in cats with primary congenital glaucoma. Veterinary Ophthalmology. 2011 doi: 10.1111/j.1463-5224.2011.00913.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLellan GJ, Kuehn MH, Ellinwood NM, et al. A feline model of primary congenital glaucoma – histopathological and genetic characterization. Association for Research in Vision and Ophthalmology – Annual Meeting. 2006;47:175. [Google Scholar]
- 12.McLellan GJ, Seo K, Finch A, et al. SD-OCT imaging of the retina and optic nerve in normal and glaucomatous cats; 41st Annual Meeting of the American College of Veterinary Ophthalmologists; 2010. p. 123. [Google Scholar]
- 13.Bentley E, Miller PE, Diehl KA. Use of high-resolution ultrasound as a diagnostic tool in veterinary ophthalmology. Journal of the American Veterinary Medical Association. 2003;223:1617–1622. doi: 10.2460/javma.2003.223.1617. [DOI] [PubMed] [Google Scholar]
- 14.Marchini G, Babighian S, Tosi R, et al. Comparative study of the effects of 2% ibopamine 10% phenylephrine, and 1% tropicamide on the anterior segment. Investigative Ophthalmology and Visual Science. 2003;44:281–289. doi: 10.1167/iovs.02-0221. [DOI] [PubMed] [Google Scholar]
- 15.Bentley E, Miller PE, Diehl KA. Evaluation of intra- and interobserver reliability and image reproducibility to assess usefulness of high-resolution ultrasonography for measurement of anterior segment structures of canine eyes. American Journal of Veterinary Research. 2005;66:1775–1779. doi: 10.2460/ajvr.2005.66.1775. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa H, Liebmann JM, Ritch R. Quantitative assessment of the anterior segment using ultrasound biomicroscopy. Current Opinion in Ophthalmology. 2000;11:133–139. doi: 10.1097/00055735-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Rusanen E, Florin M, Hässig M, et al. Evaluation of a rebound tonometer (Tonovet®) in clinically normal cat eyes. Veterinary Ophthalmology. 2010;13:31–36. doi: 10.1111/j.1463-5224.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt KS, Hacker DV, Kass PH, et al. Effects of systemic administration of 0.5% tropicamide on intraocular pressure, pupillary diameter, blood pressure, and heart rate in normal cats. Veterinary Ophthalmology. 2006;9:137–139. doi: 10.1111/j.1463-5224.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 19.McLellan GJ, Kemmerling JP, Kiland JA. Evaluation of rebound and applanation tonometry in normal and glaucomatous cats; 40th Annual Meeting of the American College of Veterinary Ophthalmologists; 2009. p. 78. [Google Scholar]
- 20.Del Sole MJ, Sande PH, Bernades JM, et al. Circadian rhythm of intraocular pressure in cats. Veterinary Ophthalmology. 2007;10:155–161. doi: 10.1111/j.1463-5224.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt KS, Hacker DV, Kass P, et al. Effects of systemic and topical administration of 0.5% tropicamide on intraocular pressure, pupillary diameter, blood pressure, and heart rate in normal cats; 35th Annual Meeting of the American College of Veterinary Ophthalmologists; 2004. p. 81. [DOI] [PubMed] [Google Scholar]
- 22.Thibos LN, Levick WR, Morstyn R. Ocular Pigmentation in White and Siamese Cats. Investigative Ophthalmology and Visual Science. 1980;19:475–486. [PubMed] [Google Scholar]
- 23.Phelps CD, Armaly MF. Measurement of episcleral venous pressure. American Journal of Ophthalmology. 1978;85:35–42. doi: 10.1016/s0002-9394(14)76662-0. [DOI] [PubMed] [Google Scholar]
- 24.Larsson BS. Interaction between chemicals and melanin. Pigment Cell Research. 1993;6:127–133. doi: 10.1111/j.1600-0749.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 25.Velasco Cabrera J, Eiroa Mozos P, Garcia Sanchez J, et al. Changes in intraocular pressure due to cycloplegia. Contact Lens Association of Ophthalmologists. 1998;24:111–114. [PubMed] [Google Scholar]
- 26.Schimek RA, Lieberman WJ. The influence of Cyclogyl and Neosynephrine on tonographic studies of miotic control in open-angle glaucoma. American Journal of Ophthalmology. 1961;51:781–784. doi: 10.1016/0002-9394(61)91813-x. [DOI] [PubMed] [Google Scholar]
- 27.Wilcock BP, Peiffer RL, Jr, Davidson MG. The causes of glaucoma in cats. Veterinary Pathology. 1990;27:35–40. doi: 10.1177/030098589002700105. [DOI] [PubMed] [Google Scholar]
- 28.Ofri R, Shub N, Galin Z, et al. Effect of reproductive status on intraocular pressure in cats. American Journal of Veterinary Research. 2002;63:159–162. doi: 10.2460/ajvr.2002.63.159. [DOI] [PubMed] [Google Scholar]