Abstract
A 70-year-old man was referred to our hospital due to abnormal liver function. A tumor of 92 mm × 61 mm was detected on ultrasound screening of the left liver lobe. Although the tumor was suspected to be intrahepatic bile duct carcinoma, he had chronic heart disease and was unable to undergo surgery. Therefore, he was followed without further testing. No increase in tumor serum markers or tumor size was observed for the subsequent 7 years. We continued to suspect intrahepatic bile duct carcinoma, and we decided to perform a tumor biopsy. Tumor biopsy findings indicated intrahepatic bile duct adenoma (BDA), which is a rare benign epithelial liver tumor typically ranging from 1 mm to 20 mm. We herein report a case of very large BDA followed for 7 years.
Keywords: Intrahepatic bile duct adenoma, Large tumor, Differential diagnosis, Benign liver tumor
INTRODUCTION
Intrahepatic bile duct adenoma (BDA) is a rare, benign epithelial liver tumor that typically ranges in size from 1 mm to 20 mm[1]. BDA is mainly found incidentally at laparotomy or autopsy[1-5]. Here, we report a case of very large BDA (about 90 mm in diameter) that was followed for 7 years.
CASE REPORT
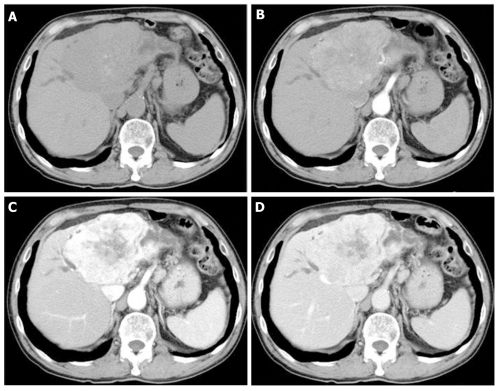
A 70-year-old man was referred to our hospital due to liver dysfunction (Table 1). Although no liver failure was shown, mild increased serum ALP and γ-GTP were observed. A tumor of 92 mm × 61 mm was observed on ultrasound screening of the left liver lobe. Unenhanced abdominal computed tomography (CT) showed a tumor of the same size with dilation of the peripheral bile duct, while dynamic contrast-enhanced CT revealed heterogeneous enhancement in the venous phase (Figure 1). Although the tumor was suspected to be intrahepatic bile duct carcinoma, he had chronic heart disease and was unable to undergo surgery. Therefore, he was followed without further testing. No increase in serum tumor markers, such as CEA and CA19-9, or in tumor size was observed during the subsequent 7 years.
Table 1.
Laboratory data at initial hospitalization
| WBC | 7000/uL | BS | 100 mg/dL |
| Neutro | 60.9% | CRP | 0.72 mg/dL |
| Eosin | 3.6% | Na | 139 mEq/L |
| Baso | 0.5% | K | 4.5 mEq/L |
| Mono | 9.1% | Cl | 103 mEq/L |
| Lympho | 25.9% | CEA | 2.5 ng/mL |
| RBC | 413 × 104/uL | CA19-9 | 46 U/mL |
| Hb | 12.8 g/dL | Alb | 4.2 g/dL |
| Ht | 41.2% | BUN | 20.8 mg/dL |
| Plt | 19.8 × 104/uL | Cr | 1.07 mg/dL |
| TP | 8.4 g/dL | D-Bil | 0.1 mg/dL |
| T-Bil | 0.9 mg/dL | ALT | 12 IU/L |
| AST | 22 IU/L | ALP | 360 U/L |
| PT | 56% | γ-GTP | 168 IU/L |
| ChE | 274 U/L | ||
WBC: White blood cell; BS: Blood suger; CRP: C-reactive protain; CEA: Carcinoembryonic antigen; BUN: Blood urea nitrogen ; ALT: Aspartate aminotransferase; ALP: Alkaline phosphatase; AST: Alanine aminotransferase; PT: Protronbin time; TP: Total protain; RBC: Red blood cell.
Figure 1.
Unenhanced and enhanced computed tomography images. A: Plain; B: Arterial phase; C: Equilibrium phase; D: Venous phase. Enhanced abdominal computed tomography scan showed a tumor of 92 mm × 61 mm in the left liver lobe, with dilatation of the peripheral bile duct. In addition, tumor showed more heterogeneous enhancement in the venous phase.
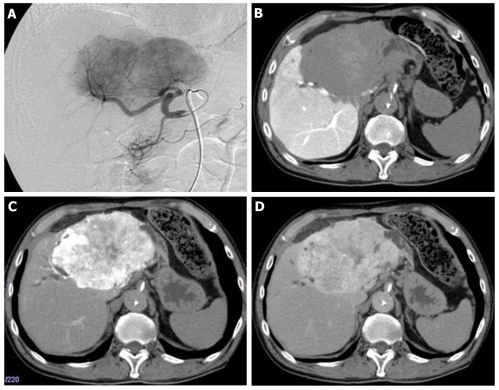
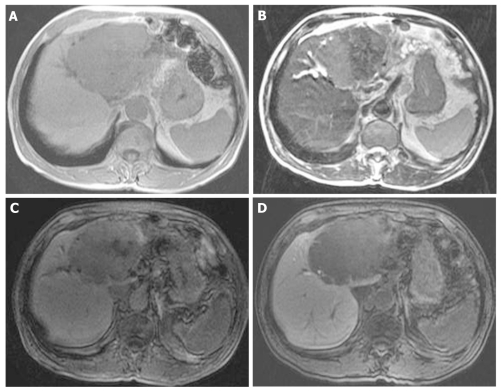
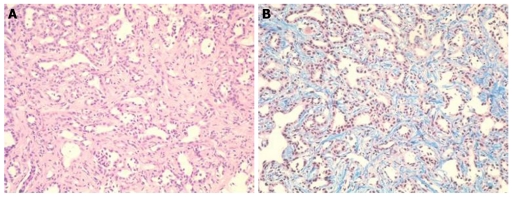
At that point, hepatic arterial angiography, abdominal CT angiography, gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced abdominal magnatic resonance image (MRI) and tumor biopsy were performed in order to confirm the diagnosis of intrahepatic bile duct carcinoma. Hepatic arterial angiography and CT angiography showed a hypervascular tumor of the left liver lobe with an enhancement effect persisting into the late phase, suggesting that this tumor had rich fibrous tissues (Figure 2). MRI confirmed that the tumor showed heterogeneous high intensity on T2-weighted images (Figure 3B) and low intensity on T1-weighted images (Figure 3A). Contrast-enhanced EOB MRI revealed that this tumor had relative hypointensity in comparison with the normal liver parenchyma on equilibrium and hepatobiliary phase (Figure 3C and D). Biopsy findings showed that the tumor consisted of small heterogeneous tubular ducts with fibrous tissues, without cell atypia or mitotic activity. Thus, diagnosis was confirmed as BDA (Figure 4).
Figure 2.
Follow-up hepatic arterial angiography and computed tomography images after 7 years. A: Hepatic arterial angiography showed hypervascular tumor of left liver lobe; B: Computed tomography (CT) angiography (portal phase) showed portal vascularity defect in the tumor; C-D: CT angiography (C: Arterial early phase; D: Arterial late phase) showed hypervascularity and a persistent enhancement effect.
Figure 3.
Follow-up magnatic resonance image images after 7 years. A: Large tumor of left lobe showed low intensity on T1-weighted images; B: Large tumor of left lobe showed heterogeneous high intensity on T2-weighted images; C-D: Large tumor of left lobe showed relative hypointensity in comparison with normal liver parenchyma on equilibrium and hepatobiliary phase of contrast-enhanced EOB magnatic resonance image.
Figure 4.
Tumor biopsy findings. Tumor consisted of small heterogeneous tubular ducts with fibrous tissues, without cell atypia or mitotic activity. A: Hematoxylin and eosin stain (× 400); B: Masson trichrome stain (× 400).
DISCUSSION
Although the actual incidence of BDA is unknown, it is a rare, benign and asymptomatic tumor of the liver, and is typically found incidentally[1-5]. Most BDAs are subcapsular, ranging from 1 mm to 20 mm in diameter[1]. However, in the present case, the tumor was very large, at 90 mm in diameter. Therefore, we initially suspected tumor intrahepatic bile duct carcinoma, and believed the patient to be end-stage. Thus, we followed him without treatment over a 7-year follow-up period.
Although various abdominal imaging approaches have been reported, most BDAs show hypervascular characteristics consisting of prolonged enhancement[6-9]. Our case showed the same findings as in these reports, and it was suggested that the delayed or prolonged enhancement on dynamic CT and MRI was due to the fibrous stroma within the tumor[8]. However, it has also been reported that the differential diagnosis between BDA and malignant tumor is very difficult using radiological methods[10]. In addition, although we obtained liver biopsy samples from some areas of the tumor, there may have been sampling errors due to the large tumor size and heterogeneous enhanced pattern. Therefore, we believe that this patient requires further follow-up.
To our knowledge, this is the first report of a very large BDA, and we confirmed no changes in size over a 7-year follow-up period. Although rare, BDA should be considered in the differential diagnosis of hepatic hypervascular tumors.
Footnotes
Peer reviewer: Naoya Sakamoto, MD, PhD, Associate Professor, Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8519, Japan
S- Editor Yang XC L- Editor A E- Editor Yang XC
References
- 1.Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1988;12:708–715. doi: 10.1097/00000478-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Edmondson HA. Tumors of the liver and intrahepatic bile duct. In: Atlas of tumor pathology, fascicle 25., editors. Washington DC: Armed Forces Institute of Pathology; 1958. pp. 19–29. [Google Scholar]
- 3.Craig JR, Peters RL, Edmondson HA. Tumors of the liver and intrahepatic bile ducts. In: Atlas of tumor pathology; 2nd series, fascicle 26., editors. Washington DC: Armed Forces Institute of Pathology; 1989. pp. 56–62. [Google Scholar]
- 4.Tajima T, Honda H, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Taguchi K, Shimada M, Masuda K. Radiologic features of intrahepatic bile duct adenoma: a look at the surface of the liver. J Comput Assist Tomogr. 1999;23:690–695. doi: 10.1097/00004728-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Cho C, Rullis I, Rogers LS. Bile duct adenomas as liver nodules. Arch Surg. 1978;113:272–274. doi: 10.1001/archsurg.1978.01370150044007. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Matsui O, Takashima T. A case of bile duct adenoma (Japanese). In Abdominal Radiology Study Group of Japan, eds. Atlas of diagnostic abdominal imaging, vol. 1. Osaka, Japan: Nihon Schering; 1993. pp. 26–27. [Google Scholar]
- 7.Maeda E, Uozumi K, Kato N, Akahane M, Inoh S, Inoue Y, Beck Y, Goto A, Makuuchi M, Ohtomo K. Magnetic resonance findings of bile duct adenoma with calcification. Radiat Med. 2006;24:459–462. doi: 10.1007/s11604-006-0044-z. [DOI] [PubMed] [Google Scholar]
- 8.Honda H, Onitsuka H, Yasumori K, Hayashi T, Ochiai K, Gibo M, Adachi E, Matsumata T, Masuda K. Intrahepatic peripheral cholangiocarcinoma: two-phased dynamic incremental CT and pathologic correlation. J Comput Assist Tomogr. 1993;17:397–402. [PubMed] [Google Scholar]
- 9.Kim YS, Rha SE, Oh SN, Jung SE, Shin YR, Choi BG, Byun JY, Jung ES, Kim DG. Imaging findings of intrahepatic bile duct adenoma (peribiliary gland hamartoma): a case report and literature review. Korean J Radiol. 2010;11:560–565. doi: 10.3348/kjr.2010.11.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakatsu M, Vilgrain V, Zins M, Vullierme M, Belghiti J, Menu Y. Radiologic features of papillary adenoma and papillomatosis of the biliary tract. Abdom Imaging. 1997;22:87–90. doi: 10.1007/s002619900147. [DOI] [PubMed] [Google Scholar]