Abstract
The drugs in clinical use against African sleeping sickness are toxic, costly, or inefficient. We show that Trypanosoma brucei, which causes this disease, has very low levels of CTP, which are due to a limited capacity for de novo synthesis and the lack of salvage pathways. The CTP synthetase inhibitors 6-diazo-5-oxo-l-norleucine (DON) and α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (acivicin) reduced the parasite CTP levels even further and inhibited trypanosome proliferation in vitro and in T. brucei-infected mice. In mammalian cells, DON mainly inhibits de novo purine biosynthesis, a pathway lacking in trypanosomes. We could rescue DON-treated human and mouse fibroblasts by the addition of the purine base hypoxanthine to the growth medium. For treatment of sleeping sickness, we propose the use of CTP synthetase inhibitors alone or in combination with appropriate nucleosides or bases.
Up to 500,000 people are estimated to suffer from African sleeping sickness (1, 2), a fatal disease caused by the protozoan parasites Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense. These pathogens are transmitted by tsetse flies to their mammalian hosts, where they first establish an infection in the blood and lymph. In the second stage of the disease, the parasites invade the central nervous system. Most drugs used to treat sleeping sickness do not cross the blood–brain barrier; as soon as the trypanosomes have entered the central nervous system, only eflornithine and arsenicals such as melarsoprol are effective. However, the arsenicals are highly toxic, whereas eflornithine is ineffective against T. b. rhodesiense (3) and is very expensive.
Purine metabolism has been extensively studied in trypanosomes (4), and it is well established that they lack the ability to form purines de novo. They therefore absolutely depend on preformed purine bases or nucleosides for survival. Any purine source is fine, because they have all of the enzymes needed to interconvert IMP, AMP, and GMP. Much less attention has been paid to the synthesis of pyrimidines in trypanosomes (4); so far, no study of the pathways for CTP synthesis has been published.
We have previously reported that cultured bloodstream T. brucei have a very small CTP pool in comparison with other NTP pools and the CTP pools of other cell types (5). In this paper, we demonstrate that the low CTP pools are a consequence of slow synthesis by the parasite CTP synthetase. We suggest that this enzyme is an excellent target for chemotherapy, because we found that the trypanosomes, unlike mammalian cells, cannot compensate for the inhibition of CTP synthetase by the salvage of cytidine. The rest of our work is focused on the use of two irreversible glutamine analog CTP synthetase inhibitors, 6-diazo-5-oxo-l-norleucine (DON) and α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (acivicin). These drugs have been used extensively in clinical trials against various cancers, and at least acivicin is known to penetrate the blood–brain barrier (6). We found that both drugs inhibited the proliferation of cultured trypanosomes at much lower concentrations than the levels measured in the blood of cancer patients. Furthermore, treatment of T. brucei-infected mice with DON or acivicin reduced the number of trypanosomes in the blood to undetectable levels. We found that DON mainly affected de novo purine biosynthesis in mammalian cells. Because this pathway is not the target in trypanosomes, DON therapy can be combined with a purine base to minimize side effects, without losing efficacy against T. brucei.¶
Materials and Methods
Handling of Trypanosomes.
Bloodstream forms of the pleomorphic T. brucei brucei variant clone AnTat 1.1 (7) were grown in NMRI mice. Long, slender trypanosomes were purified on day 3 after infection, and short, stumpy trypanosomes were purified on day 5 after infection from blood by DEAE-cellulose chromatography (8). The trypanosomes were confirmed to be long, slender forms (33–35% of the cells had two kinetoplasts and one or two nuclei) and short, stumpy forms (only 6–7% with two kinetoplasts and one or two nuclei) by inspection of 4′,6-diamino-2-phenylindole-stained cells with a microscope.
Procyclic insect forms of T. brucei brucei AnTat 1.1 (7) were cultured at 27°C in SDM-79 medium (9) supplemented with 10% heat-inactivated FBS. Culture-adapted bloodstream forms of the T. brucei brucei cell line TC221 (10) were propagated at 37°C in Hirumi's modified Iscove's medium-9 (11) supplemented with 5% FBS in a humidified atmosphere containing 7% CO2.
NTP Pools.
Trypanosomes (1–3 × 108) were collected, and their NTPs were determined as described (5). Briefly, the nucleotides were extracted by a trichloroacetic acid precipitation procedure and quantified by HPLC. A connection of the HPLC to a flow scintillation analyzer (Radiomatic 150 TR; Packard) made it possible to monitor radioactivity in the chromatographic peaks.
Ribonucleoside and Nucleobase Analysis.
Two hundred microliters of culture medium was centrifuged through a Nanosep 3K filter (Pall Filtron, New York). Various amounts of the filtrate were separated on a 4.6 × 150 mm Discovery HPLC column (Sigma-Aldrich) that was run isocratically in 10 mM ammonium phosphate (pH 3.4) at 1 ml/min. The peaks were identified by their A260/A280 and by comparing their retention times with standard nucleosides and bases mixed with culture medium. Finally, we quantified the ribonucleosides and bases by comparing peak heights with a standard curve.
Conversion of [3H]Uracil into UTP, CTP, Cytidine, Uridine, RNA, and DNA.
Logarithmically growing trypanosomes (2 × 108) were collected (5-min centrifugation at 3,000 rpm) and resuspended in 20 ml of fresh culture medium. After 30 min of preincubation under standard growth conditions, they were incubated at a final concentration of 0.13 μM [5,6-3H]uracil (39 Ci/mmol) in their growth medium. Radioactivity in the UTP, CTP, cytidine, and uridine pools was determined by HPLC coupled to a flow scintillation analyzer (see above). Similar experiments were performed with 0.34 μM [6-3H]cytosine (14.7 Ci/mmol) or 0.22 μM [5-3H(N)]cytidine (22.9 Ci/mmol). All tritiated compounds were purchased from Moravek Biochemicals (Brea, CA). The amount of radioactivity in the RNA and DNA was determined from trichloroacetic acid-precipitated cell material, as described (12).
Glutamine Analogs.
DON and acivicin (Sigma-Aldrich) were stored as aqueous 5 mM solutions at −20°C. All experiments performed with these drugs were comparable because we used a physiological concentration of l-glutamine (0.584 g/liter) in all growth media.
DON Treatment of T. brucei-Infected Mice.
T. brucei-infected BALB/c mice (8 weeks old) were injected i.p. with 0.5 mg DON (25 mg DON/kg) dissolved in 200 μl 0.9% sodium chloride solution every third day. Control mice received only the vehicle.
Cultivation of Mammalian Cells.
Mammalian cells were cultivated as monolayers in DMEM (Sigma) supplemented with l-glutamine (0.584 g/liter) and 10% heat-inactivated horse serum at 37°C in a humidified atmosphere containing 7% CO2.
Results
Determination of Intracellular NTP Pools during the Various Stages of the T. brucei Life Cycle.
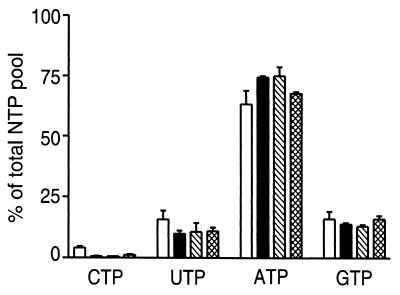
First, we wanted to check whether the low CTP level we had reported for cultured bloodstream forms (5) also appears in vivo. The NTP pools in long, slender (proliferating stage) and short, stumpy trypanosomes (nondividing stage), which were purified from T. brucei-infected mice (Fig. 1), were compared with the pools of in vitro cultured procyclic trypanosomes (corresponding to the midgut forms in the tsetse fly) and in vitro-cultured bloodstream forms. Again we observed that the CTP pools were much lower than the pools of UTP, ATP, and GTP (Fig. 1), especially in the three bloodstream forms of T. brucei.
Figure 1.
NTP pools in T. brucei from different life cycle stages. The samples are from procyclics (open bars), long slender (solid bars), short stumpy (hatched bars), and in vitro-cultured bloodstream forms (cross-hatched bars). All values are averages of two experiments, with standard deviations indicated by error bars, except the value for the cultured bloodstream forms, which is an average of 19 experiments. The values are represented relative (in %) to the total NTP pool (CTP + UTP + ATP + GTP).
T. brucei Lacks Salvage Pathways for CTP Synthesis.
Generally, CTP can be synthesized de novo from UTP or from salvage of cytidine. A few organisms, such as Giardia lamblia, also can salvage cytosine through a phosphoribosyltransferase (13). When we added up to 15 mM cytidine or 1 mM cytosine in the culture medium and harvested the trypanosomes 1 h later, there was no change in the size of the CTP pool. Similarly, if we gave the trypanosomes tritiated cytidine or cytosine, the CTP pool was not labeled. We confirmed by HPLC chromatography that the added cytidine was not degraded to uridine during the course of the experiment. We can conclude that there is no salvage of cytidine or cytosine in T. brucei.
The Synthesis of CTP Is Slow in T. brucei.
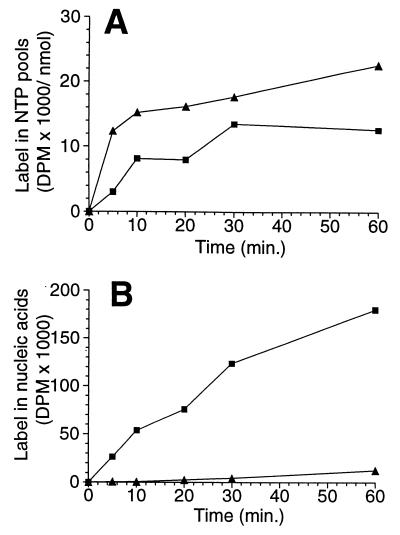
We wanted to investigate why the CTP pool is so low in the bloodstream form of T. brucei. This low level of CTP could be the consequence of deficient synthesis or of excessive degradation of CTP. First, we determined the rate of de novo CTP synthesis. Trypanosomes that were offered tritiated uracil in the culture medium incorporated label into both the UTP and the CTP pools (Fig. 2A). Thus we can conclude that trypanosomes have a de novo pathway for CTP synthesis. The accumulation of label in the UTP pool was rapid and was almost saturated after 5 min, whereas the CTP pool was labeled at a much slower rate. This observation suggested that slow de novo synthesis could be responsible for the low CTP level.
Figure 2.
Incorporation of tritium label into CTP, UTP, and nucleic acids in the T. brucei brucei cell line TC221. [3H]Uracil was added to cultivated bloodstream forms, and aliquots were collected and analyzed at various times (see Materials and Methods). (A) Incorporation of tritium into UTP (▴) and CTP (■). The values are plotted as specific activities (dpm/nmol of each nucleotide). (B) Incorporation of label into RNA + DNA (■) and DNA only (▴), shown as absolute values (dpm).
We also wanted to know whether the size of the CTP pool was controlled by a high degradation rate. We therefore checked the label in the first two degradation products of CTP catabolism, cytidine and uridine, by HPLC chromatography. No radioactivity was detectable in these compounds. On the contrary, label was readily incorporated into nucleic acids (Fig. 2B). Considering the detection limit of our experiments, we can conclude that at least 95% of the CTP and UTP in trypanosomes was used for nucleic acid synthesis, whereas less than 5% was degraded. A rapid degradation is therefore not responsible for the low CTP pool in T. brucei.
Testing CTP Synthetase Inhibitors on Cultured Bloodstream Forms of T. brucei.
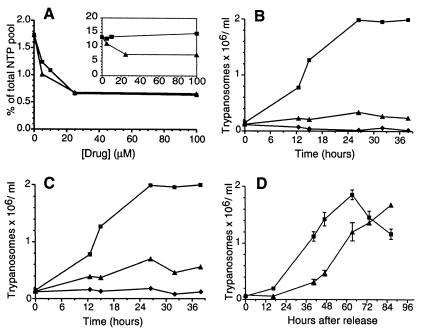
The absolute dependence of T. brucei on de novo synthesis for CTP production might be exploited for chemotherapy purposes, because the host can be rescued by cytidine. We tested two glutamine analog CTP synthetase inhibitors, DON and acivicin, and we measured how the NTP pools of cultured bloodstream forms of T. brucei changed during the 1-h incubation period. DON specifically lowered the CTP pool, and acivicin also lowered the GTP pool (Fig. 3A). None of the drugs affected the ATP or the UTP pools (data not shown). The inclusion of 160 μM guanine in the growth medium abolished the effect by acivicin on the GTP pool. Because the CTP level was still low, we conclude that the inhibition of CTP synthesis by acivicin was direct and was not due to an allosteric effect by GTP. The result with acivicin is in agreement with the situation in mammalian cells, where it is established that the main targets are CTP synthetase and GMP synthase (6). Trypanosomes lack the enzymes in the de novo purine biosynthesis pathway up to IMP but contain later enzymes such as GMP synthase (4). It is therefore not surprising that GTP synthesis was affected by acivicin. The inclusion of 5 mM cytidine or 1 mM cytosine in the growth medium did not affect the inhibition of CTP synthesis by DON or acivicin (data not shown).
Figure 3.
Effect of CTP synthetase inhibitors on cultured bloodstream T. brucei brucei (TC221) CTP pools and proliferation. (A) Analysis of CTP (main graph) and GTP (Inset) pools from trypanosomes treated with DON (■) or acivicin (▴) for 1 h. (B) The effect of DON on trypanosome proliferation. The parasites were treated with no drug (■), 1 μM DON (▴), or 5 μM DON (⧫), and the number of trypanosomes was recorded over time. (C) Effect of acivicin on trypanosome proliferation. The trypanosomes were treated with no drug (■), 1 μM acivicin (▴), or 5 μM acivicin (⧫). (D) The trypanosomes were treated with DON for 1 h, washed, and resuspended in drug-free medium. The number of trypanosomes was counted at various time points after the release (▴). A control experiment was performed, in which the trypanosomes were not exposed to DON but were otherwise treated in exactly the same way as the drug-treated trypanosomes (■). Each point is an average of two experiments, with the standard deviation indicated by error bars.
Next, we wanted to know how the drugs affected the growth of cultured bloodstream trypanosomes. DON and acivicin were both very active (Fig. 3 B and C, respectively); DON completely blocked proliferation, even at a concentration of only 1 μM. DON also inhibited the proliferation of T. b. rhodesiense (IC50 = 0.36 μM), which is a human pathogenic subspecies of T. brucei resistant to eflornithine. Cytidine (up to 5 mM) or cytosine (1 mM) could not rescue the trypanosomes from the proliferation block imposed by 1 μM DON (data not shown). Similarly, trypanosomes treated with 1 μM acivicin were not rescued by the combination of 160 μM guanine with 5 mM cytidine or 1 mM cytosine (data not shown). We confirmed by HPLC that the added cytidine and cytosine were not degraded to a significant extent in these experiments.
DON is not stable in the mammalian bloodstream and has a half-life of only 1.5–3 h in humans (14, 15). We therefore tested the effect of a short pulse of DON (5 μM DON for 1 h) on trypanosome proliferation (Fig. 3D). Control trypanosomes received the same treatment, except that the 1-h incubation was completed without DON. Inhibitor-treated trypanosomes required ≈20 h to recover from 1 h of DON exposure.
Treatment of Trypanosome-Infected Mice with DON.
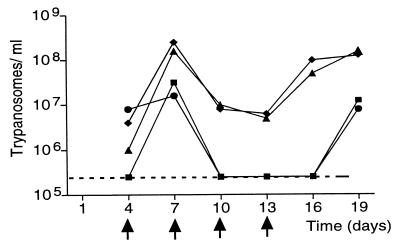
We tested DON on mice infected with the pleomorphic T. brucei strain Antat 1.1 (Fig. 4). This strain (7) gives a chronic infection in mice similar to that in humans, with the number of parasites increasing and decreasing in a cyclic manner. Four mice were given 2 × 104 trypanosomes i.p. on day 0. Four days later, two of the mice started to receive 25 mg/kg DON i.p. every third day. Whereas the parasitemia of the control mice was increasing and decreasing in a periodic pattern, the number of trypanosomes in the DON-treated mice was low at the first inspection (day 7) and below the detection limit by day 10. The trypanosomes remained undetectable as long as therapy continued, and they did not appear again until 6 days after the last dose of DON. Thereafter, the DON-treated mice developed parasitemias similar to those of the controls. The strong trypanocidal effect of DON is rather surprising, considering that the half-life of DON is only 5–8 min in the mouse bloodstream (17).
Figure 4.
Effects of DON on trypanosome-infected mice. Mice were infected with the pleomorphic T. brucei variant clone AnTat 1.1 on day 0, and the number of trypanosomes in the blood was counted (16) every third day, starting at day 4. Two of the mice (■ and ●) were exposed to DON on days 4, 7, 10, and 13. These days are marked with arrows. Two other mice treated with vehicle alone served as controls (⧫ and ▴). The dashed line at the bottom of the graph represents the detection limit.
Preliminary results suggest that not only DON but also acivicin is active in vivo. T. brucei-infected mice received a daily dose of 5 mg/kg acivicin for 12 days. By day 12, we could detect no trypanosomes in the blood of these mice. At this point the therapy was discontinued and the trypanosomes reappeared 3 days later. We conclude that acivicin is active in vivo, but more studies are needed to see whether acivicin at a higher dose or given for a longer exposure time eventually would eradicate the parasites completely.
Effects of Combinations of DON and Nucleosides/Bases on Mammalian Cells.
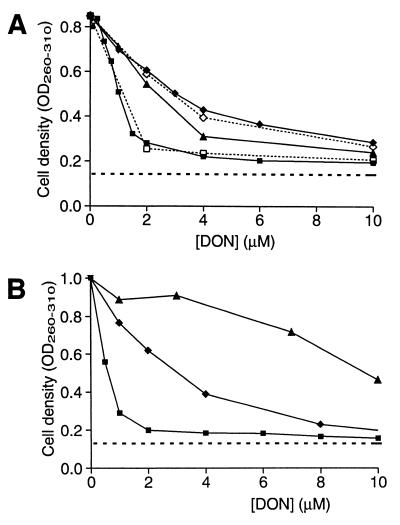
DON also inhibits the proliferation of mammalian cells. It would be advantageous to reduce the antiproliferative effect on mammalian cells and still retain the trypanocidal activity. The main target of DON in mammalian cells has not been completely determined, but one candidate is de novo purine synthesis (6). We could minimize the inhibition of de novo purine biosynthesis by adding hypoxanthine (a purine base) to the growth medium. In the human cells (Fig. 5A) there was a strong and saturating effect already at 2.5 μM hypoxanthine; increasing the concentration to 10 or 160 μM gave similar results (the 160 μM curve is not shown). In contrast, very high levels of hypoxanthine were needed to achieve a saturating effect on the mouse cells (Fig. 5B). We therefore analyzed the growth medium of mouse fibroblasts cultivated in the presence of 10 μM hypoxanthine by HPLC and found that the purine base was completely degraded during the course of the experiment. Xanthine oxidase, which is expressed in most tissues and cell types in mice, but is limited to the intestine and the liver in humans (19), is probably the cause of this degradation. In addition to hypoxanthine, other purine bases such as guanine rescued the human cells from DON cytotoxicity (data not shown); the addition of cytidine, however, had no effect whether it was used alone or in combination with hypoxanthine (Fig. 5A). We conclude that CTP synthetase, which was the main target of DON in T. brucei, is not an important target in mammalian cells. Instead, de novo purine biosynthesis seems to be the main pathway affected.
Figure 5.
Proliferation of human WS1 fibroblasts (ATCC no. CRL-1502) or mouse BALB/3T3 fibroblasts (ATCC no. CCL-163) in the presence of DON and hypoxanthine. The cells were seeded at a low density (105 cells per 5-cm dish), incubated with the drug to be studied, and collected 3 days later (before confluency). The cell density (OD260–310) was then determined by a procedure based on NaOH lysis (18). (A) Human WS1 fibroblasts were grown at various concentrations of DON in the absence (■) or presence of 2.5 μM (▴) or 10 μM (⧫) hypoxanthine. The open symbols represents experiments where 1 mM cytidine was present alone (□) or together with 10 μM hypoxanthine (◊). (B) Mouse BALB/3T3 cells were grown at various concentrations of DON in the absence (■) or presence of 10 μM (⧫) or 160 μM hypoxanthine (▴). The dashed line at the bottom of the graphs represents the initial cell density (day 0).
Discussion
Intrigued by the low levels of CTP in the bloodstream forms of T. brucei, we took a closer look at the metabolism of CTP in the trypanosomes. During these studies, we made two discoveries suggesting that CTP synthetase could be an Achille's heel in trypanosome metabolism. First, the CTP levels are controlled by synthesis rather than by degradation. Thus inhibition of CTP synthesis should have a direct effect on CTP levels. Second, there is no salvage of cytidine or cytosine in trypanosomes. This finding implies that T. brucei cannot use the salvage mechanism to compensate for the inhibition of CTP synthetase. In this paper, we have focused on two CTP synthetase inhibitors, the glutamine analogs acivicin and DON, that both inhibited trypanosome proliferation and CTP synthesis.
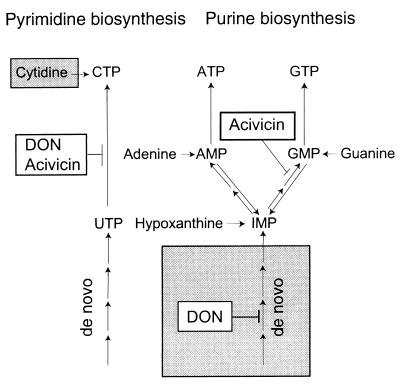
It is well established that acivicin passes through the blood–brain barrier in humans (6); such passage is an absolute requirement for treatment in the second stage of sleeping sickness. The extent to which DON can enter the central nervous system remains to be established; DON has at least been shown to have an affinity similar to that of acivicin for the protein that transports neutral amino acids through the blood–brain barrier (20). The pharmacological properties of acivicin and DON have been extensively studied as cancer drugs, and the steady-state levels in cancer patients receiving tolerable doses were 58 μM for DON applied as a 4-h continuous infusion (14) and 16 μM for acivicin applied as a 24-h continuous infusion (21). Although these levels are much higher than the dose required to block the proliferation of mammalian cells, efficacy against various tumors was limited. In the case of acivicin, the low response level has been explained as follows: tumors (22), as well as tissue-cultured mammalian cells (23, 24), compensate for the inhibition of CTP synthetase and GMP synthase by increasing the salvage of cytidine and guanine/guanosine that are present in extracellular space. Similarly, we could show that mouse and human cells can be rescued from DON inhibition by purine bases. In contrast, trypanosomes cannot compensate for the inhibition of CTP synthetase by salvage, and they are severely inhibited by both glutamine analogs, even at a concentration of only 1 μM. We suggest that DON therapy against T. brucei can be combined with a purine base to reduce the reported side effects (14, 15, 25) (Fig. 6). Similarly, cytidine and guanine/guanosine may reduce acivicin-associated side effects (Fig. 6). Unfortunately, these suggestions cannot easily be studied in trypanosome-infected mice, because mice degrade purines at a much faster rate than humans.
Figure 6.
The targets of DON and acivicin in T. brucei and mammalian cells. All pathways shown for the synthesis of UTP, CTP, ATP, and GTP exist in mammalian cells, whereas the trypanosomes lack salvage pathways for CTP synthesis (left shaded box) and de novo purine biosynthesis (right shaded box). DON targets the CTP synthetase in the trypanosomes, whereas it mainly targets de novo purine biosynthesis in mammalian cells. A combination therapy of DON and any of the purine bases (adenine, guanine, or hypoxanthine) would bypass the block on de novo purine biosynthesis, and it therefore would spare the human cells, whereas the trypanosomes would still be targeted. Acivicin is targets the CTP synthetase and GMP synthase in trypanosomes as well as in mammalian cells. However, the trypanosomes cannot compensate for the block on CTP synthetase by salvaging cytidine. Therefore, a combination therapy of acivicin with cytidine and guanine should spare the mammalian cells and kill the trypanosomes. For simplicity, we have not included purine nucleosides in the scheme; in principle, adenosine, guanosine, and inosine can replace adenine, guanine, and hypoxanthine. The salvage pathways for UTP synthesis are also omitted, because they are not relevant to our work.
The relapse of trypanosomes after drug removal observed in our short-term DON/acivicin-treated mice indicate that, like eflornithine, the glutamine analogs only block the proliferation of the parasites. A functional immune response is then required to eradicate the growth-arrested trypanosomes (26). More experiments would be needed to investigate whether the relapse can be prevented in mice. However, because the pharmacokinetics of the glutamine analogs are very different in humans and mice, we believe that this information would be of only limited value for the treatment of sleeping sickness.
We also have tested DON against the malaria parasite, Plasmodium falciparum, which was grown in cultured human erythrocytes (27); DON efficiently inhibited parasite proliferation with IC50 values ranging from 0.36 μM (strain NF54) to 0.52 μM (strain K1). Furthermore, preliminary results on P. berghei-infected mice confirm that DON is also active in vivo against rodent malaria. Like T. brucei, the malaria parasite lacks de novo purine biosynthesis and salvage pathways for CTP synthesis (4). Thus the glutamine analog CTP synthetase inhibitors, alone or in combination with relieving substances such as hypoxanthine or cytidine, might prove useful against both sleeping sickness and malaria.
Acknowledgments
We thank Anne Simmonds (Purine Research Laboratory, United Medical and Dental Schools, Guy's Hospital, London) for valuable information about purine metabolism in humans and mice. This work was supported by the Swedish International Development Cooperation Agency, the Swedish Natural Science Research Council; the Kempe Foundation, the Swedish Society for Medical Research, and the Strategic Nucleic Acid Research Program at the Swedish Foundation for Strategic Research. The patent was financed by the Swedish Foundation for Strategic Research and the Uminova Center, Sweden.
Abbreviations
- DON
6-diazo-5-oxo-l-norleucine
- acivicin
α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid
Footnotes
The combination of glutamine analog CTP synthetase inhibitors together with nucleobases and nucleosides against parasitic protozoa is covered by a Swedish patent application filed under the number SE 0001531-3.
References
- 1.Brun R. Karger-Gazette. 1999;63:5–7. [Google Scholar]
- 2.Seed J R. ASM News. 2000;7:395–402. [Google Scholar]
- 3.Iten M, Matovu E, Brun R, Kaminsky R. Trop Med Parasitol. 1995;46:190–194. [PubMed] [Google Scholar]
- 4.Hassan H F, Coombs G H. FEMS Microbiol Rev. 1988;54:47–84. doi: 10.1111/j.1574-6968.1988.tb02708.x-i1. [DOI] [PubMed] [Google Scholar]
- 5.Hofer A, Ekanem J T, Thelander L. J Biol Chem. 1998;273:34098–34104. doi: 10.1074/jbc.273.51.34098. [DOI] [PubMed] [Google Scholar]
- 6.Ahluwalia G S, Grem J L, Hao Z, Cooney D A. Pharmacol Ther. 1990;46:243–271. doi: 10.1016/0163-7258(90)90094-i. [DOI] [PubMed] [Google Scholar]
- 7.Van Meirvenne N, Janssens P G, Magnus E. Ann Soc Belge Med Trop. 1975;55:1–23. [PubMed] [Google Scholar]
- 8.Lanham S M, Godfrey D G. Exp Parasitol. 1970;28:521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- 9.Brun R, Schoenenberger M. Acta Trop. 1979;36:289–292. [PubMed] [Google Scholar]
- 10.Hirumi H, Hirumi K, Doyle J J, Cross G A M. Parasitology. 1980;80:371–382. doi: 10.1017/s0031182000000822. [DOI] [PubMed] [Google Scholar]
- 11.Hirumi H, Hirumi K. J Parasitol. 1989;75:985–989. [PubMed] [Google Scholar]
- 12.Nicander B, Reichard P. J Biol Chem. 1985;260:5376–5381. [PubMed] [Google Scholar]
- 13.Aldritt S M, Tien P, Wang S S. J Exp Med. 1985;161:437–445. doi: 10.1084/jem.161.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovach J S, Eagan R T, Powis G, Rubin J, Creagan E T, Moertel C G. Cancer Treat Rep. 1981;65:1031–1036. [PubMed] [Google Scholar]
- 15.Sullivan M P, Nelson J A, Feldman S, Nguyen B V. Cancer Chemother Pharmacol. 1988;21:78–84. doi: 10.1007/BF00262746. [DOI] [PubMed] [Google Scholar]
- 16.Herbert W J, Lumsden W H R. Exp Parasitol. 1976;40:427–431. doi: 10.1016/0014-4894(76)90110-7. [DOI] [PubMed] [Google Scholar]
- 17.Cooney D A, Jayaram H N, Milman H A, Homan E R, Pittillo R, Geran R I, Ryan J, Rosenbluth R J. Biochem Pharmacol. 1976;25:1859–1870. doi: 10.1016/0006-2952(76)90190-8. [DOI] [PubMed] [Google Scholar]
- 18.Suttle D P, Stark G R. J Biol Chem. 1979;254:4602–4607. [PubMed] [Google Scholar]
- 19.Moriwaki Y, Yamamoto T, Higashino K. Histol Histopathol. 1999;14:1321–1340. doi: 10.14670/HH-14.1321. [DOI] [PubMed] [Google Scholar]
- 20.Takada Y, Greig N H, Vistica D T, Rapoport S I, Smith Q R. Cancer Chemother Pharmacol. 1991;29:89–94. doi: 10.1007/BF00687316. [DOI] [PubMed] [Google Scholar]
- 21.Weiss G R, McGovren J P, Schade D, Kufe D W. Cancer Res. 1982;42:3892–3895. [PubMed] [Google Scholar]
- 22.Fischer P H, Pamukcu R, Bittner G, Willson J K. Cancer Res. 1984;44:3355–3359. [PubMed] [Google Scholar]
- 23.Neil G L, Berger A E, McPartland R P, Grindey G B, Bloch A. Cancer Res. 1979;39:852–856. [PubMed] [Google Scholar]
- 24.Lui M S, Kizaki H, Weber G. Biochem Pharmacol. 1982;31:3469–3473. doi: 10.1016/0006-2952(82)90628-1. [DOI] [PubMed] [Google Scholar]
- 25.Sklaroff R B, Casper E S, Magill G B, Young C W. Cancer Treat Rep. 1980;64:1247–1251. [PubMed] [Google Scholar]
- 26.Denise H, Barrett M P. Biochem Pharmacol. 2001;61:1–5. doi: 10.1016/s0006-2952(00)00477-9. [DOI] [PubMed] [Google Scholar]
- 27.Divo A A, Geary T G, Davis N L, Jensen J B. J Protozool. 1985;32:59–64. doi: 10.1111/j.1550-7408.1985.tb03013.x. [DOI] [PubMed] [Google Scholar]