Abstract
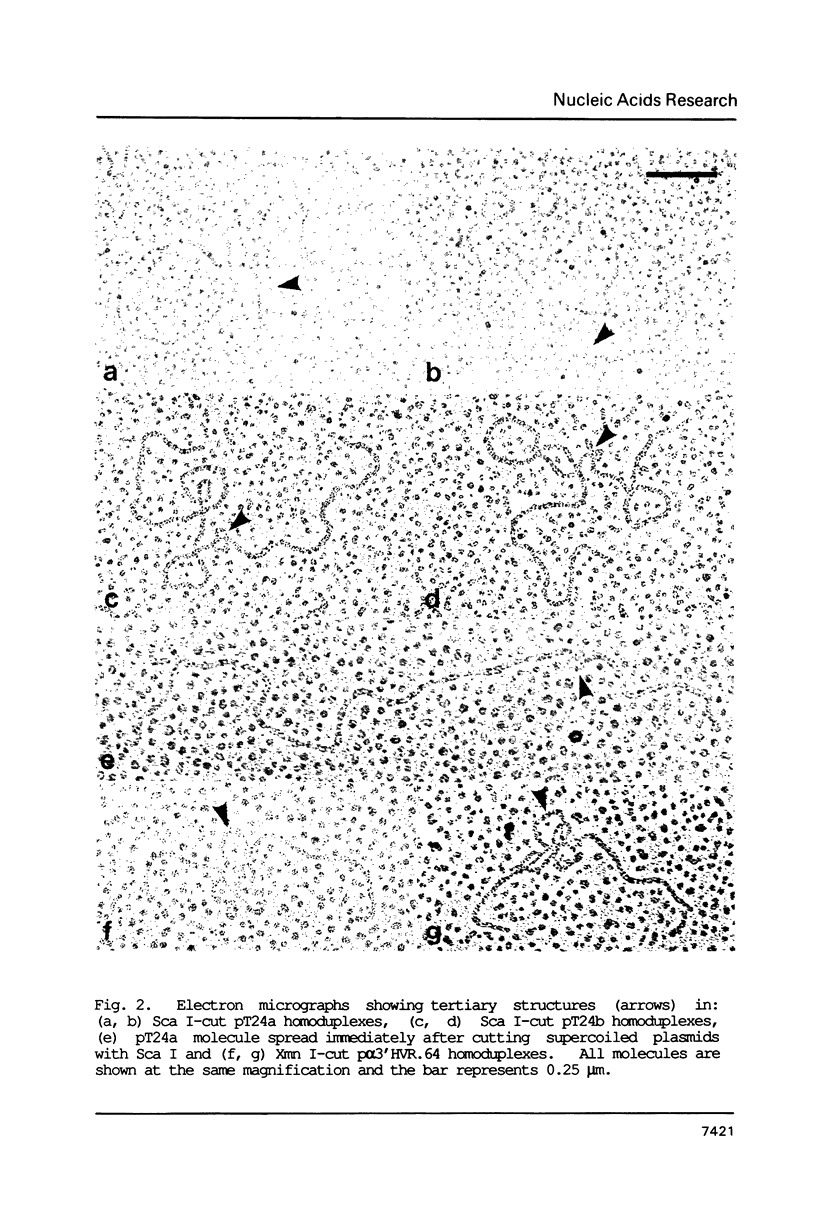
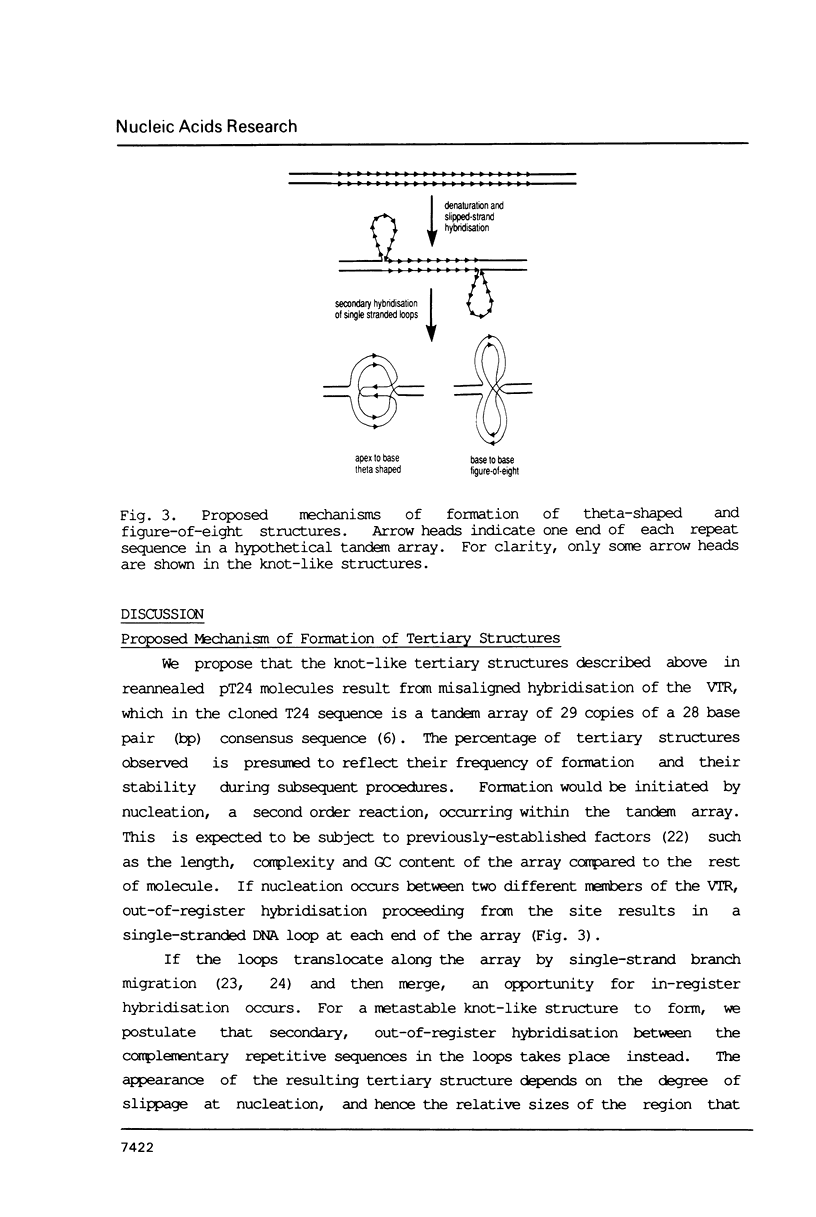
DNA tertiary structures are shown to be formed by denaturation and reannealing in vitro of molecularly-cloned DNA containing multiple tandem repeat sequences. Electron microscopy of homoduplex DNA molecules containing the human c-Harvey-ras gene revealed knot-like structures which mapped to the position of the 812 bp variable tandem repeat (VTR) sequence. We propose that the structures result from slipped-strand mispairing within the VTR and hybridisation of homologous repetitive sequences in the single-stranded loops so produced. Similar structures were also found in freshly-linearized supercoiled plasmids. More complex knot-like structures were found in homoduplexes of a 4 kb tandem array from the hypervariable region 3' to the human alpha-globin locus. Formation of such DNA tertiary structures in vitro also provides a practical method for identifying and mapping direct tandem repeat arrays that are at least 800 bp long.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Soll L., Chow L. T. Underwound loops in self-renatured DNA can be diagnostic of inverted duplications and translocated sequences. J Mol Biol. 1977 Jul 15;113(4):579–589. doi: 10.1016/0022-2836(77)90223-6. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Chou J., Roizman B. Isomerization of herpes simplex virus 1 genome: identification of the cis-acting and recombination sites within the domain of the a sequence. Cell. 1985 Jul;41(3):803–811. doi: 10.1016/s0092-8674(85)80061-1. [DOI] [PubMed] [Google Scholar]
- Deiss L. P., Chou J., Frenkel N. Functional domains within the a sequence involved in the cleavage-packaging of herpes simplex virus DNA. J Virol. 1986 Sep;59(3):605–618. doi: 10.1128/jvi.59.3.605-618.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejman P. V., Sitaram N., Hsieh W. T., Gelernter J., Gershon E. S. The effects of field inversion electrophoresis on small DNA fragment mobility and its relevance to DNA polymorphism research. Appl Theor Electrophor. 1988;1(1):29–34. [PubMed] [Google Scholar]
- Goodbourn S. E., Higgs D. R., Clegg J. B., Weatherall D. J. Molecular basis of length polymorphism in the human zeta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5022–5026. doi: 10.1073/pnas.80.16.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heighway J., Thatcher N., Cerny T., Hasleton P. S. Genetic predisposition to human lung cancer. Br J Cancer. 1986 Apr;53(4):453–457. doi: 10.1038/bjc.1986.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs D. R., Wainscoat J. S., Flint J., Hill A. V., Thein S. L., Nicholls R. D., Teal H., Ayyub H., Peto T. E., Falusi A. G. Analysis of the human alpha-globin gene cluster reveals a highly informative genetic locus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5165–5169. doi: 10.1073/pnas.83.14.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman A. P., Nicholls R. D., Weatherall D. J., Clegg J. B., Higgs D. R. Molecular characterisation of a hypervariable region downstream of the human alpha-globin gene cluster. EMBO J. 1986 Aug;5(8):1857–1863. doi: 10.1002/j.1460-2075.1986.tb04437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kim J., Sharp P. A., Davidson N. Electron microscope studies of heteroduplex DNA from a deletion mutant of bacteriophage phiX-174. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1948–1952. doi: 10.1073/pnas.69.7.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., DiMartino N. A., Colb M., Parkinson D. R. Unique allelic restriction fragments of the human Ha-ras locus in leukocyte and tumour DNAs of cancer patients. 1985 Jan 31-Feb 6Nature. 313(6001):369–374. doi: 10.1038/313369a0. [DOI] [PubMed] [Google Scholar]
- Levinson G., Gutman G. A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987 May;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- Mace H. A., Pelham H. R., Travers A. A. Association of an S1 nuclease-sensitive structure with short direct repeats 5' of Drosophila heat shock genes. Nature. 1983 Aug 11;304(5926):555–557. doi: 10.1038/304555a0. [DOI] [PubMed] [Google Scholar]
- Malamy M. H., Fiandt M., Szybalski W. Electron microscopy of polar insertions in the lac operon of Escherichia coli. Mol Gen Genet. 1972;119(3):207–222. doi: 10.1007/BF00333859. [DOI] [PubMed] [Google Scholar]
- McKeon C., Schmidt A., de Crombrugghe B. A sequence conserved in both the chicken and mouse alpha 2(I) collagen promoter contains sites sensitive to S1 nuclease. J Biol Chem. 1984 May 25;259(10):6636–6640. [PubMed] [Google Scholar]
- Mosharrafa E., Pilacinski W., Zissler J., Fiandt M., Szybalski W. Insertion sequence IS2 near the gene for prophage lambda excision. Mol Gen Genet. 1976 Aug 10;147(1):103–109. doi: 10.1007/BF00337943. [DOI] [PubMed] [Google Scholar]
- Murphy G. L., Connell T. D., Barritt D. S., Koomey M., Cannon J. G. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989 Feb 24;56(4):539–547. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- Radice P., Pierotti M. A., Borrello M. G., Illeni M. T., Rovini D., Della Porta G. HRAS1 proto-oncogene polymorphisms in human malignant melanoma: TaqI defined alleles significantly associated with the disease. Oncogene. 1987;2(1):91–95. [PubMed] [Google Scholar]
- Reeders S. T., Breuning M. H., Davies K. E., Nicholls R. D., Jarman A. P., Higgs D. R., Pearson P. L., Weatherall D. J. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 1985 Oct 10;317(6037):542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Colby W. W., Capon D. J., Goeddel D. V., Levinson A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984 Nov 1;312(5989):71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- Singer M. F. Highly repeated sequences in mammalian genomes. Int Rev Cytol. 1982;76:67–112. doi: 10.1016/s0074-7696(08)61789-1. [DOI] [PubMed] [Google Scholar]
- Thein S. L., Oscier D. G., Flint J., Wainscoat J. S. Ha-ras hypervariable alleles in myelodysplasia. Nature. 1986 May 1;321(6065):84–85. doi: 10.1038/321084a0. [DOI] [PubMed] [Google Scholar]
- Thompson B. J., Camien M. N., Warner R. C. Kinetics of branch migration in double-stranded DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2299–2303. doi: 10.1073/pnas.73.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmuza S. L., Smiley J. R. Signals for site-specific cleavage of HSV DNA: maturation involves two separate cleavage events at sites distal to the recognition sequences. Cell. 1985 Jul;41(3):793–802. doi: 10.1016/s0092-8674(85)80060-x. [DOI] [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wohlrab F., McLean M. J., Wells R. D. The segment inversion site of herpes simplex virus type 1 adopts a novel DNA structure. J Biol Chem. 1987 May 5;262(13):6407–6416. [PubMed] [Google Scholar]
- Yu Y. T., Manley J. L. Structure and function of the S1 nuclease-sensitive site in the adenovirus late promoter. Cell. 1986 Jun 6;45(5):743–751. doi: 10.1016/0092-8674(86)90788-9. [DOI] [PubMed] [Google Scholar]



