Abstract
The purpose of this study was to analyze the effect of strontium-substituted hydroxyapatite (Sr-HA) on bone osseointegration of the implants using fluorescence microscopy. We allocated 20 implants to two groups: Sr-HA group and HA group. Electrochemically deposited HA and Sr-HA coatings were applied onto the implants separately. All the implants were inserted into femur bone of rabbits. Oxytetracycline hydrochloride, alizarin-complexon, and calcein green were respectively administered 7, 28, and 46 d after the implantation. After eight weeks, femurs were retrieved and prepared for the fluorescence microscopy observation. We analyzed the bone mineral apposition rates (MARs), bone area ratios (BARs), and bone to implant contact (BIC) of the two groups. Fluorescence microscopic observation showed that all groups exhibited extensive early peri-implant bone formation. The MAR of the Sr-HA group was greater than that for pure HA from 7 to 28 d after implantation, but no significant difference was found at later stage. And the BIC showed difference at 7 and 28 d compared with pure HA. We concluded that Sr-HA coating can improve the bone osseointegration of the implant in the early stage compared with the HA coating.
Keywords: Strontium-substituted hydroxyapatite, Hydroxyapatite, Osseointegration
1. Introduction
Improving the clinical survival rates of titanium implant in poor-quality bone and to shorten the healing period required for osseointegration after implantation has been a crucial issue in the field of implantology. Numerous studies have attempted to use various bioactive coatings onto the surface of titanium implants in order to improve implant osseointegration (Sul, 2003; Jinno et al., 2004; Sul et al., 2005). Calcium phosphate coating has the excellent capacity to absorb proteins into the biomaterial surfaces, which is favorable for early healing events at the tissue/material interface, leading to increased bone formation (Suh et al., 2007). Among the calcium phosphates, hydroxyapatite (HA) coating has been a promising method to improve osseointegration (Cooper et al., 2006; Yang and He, 2009). The chemical resemblance of HA to the bone minerals ensures its ability to bond directly to bone tissue without an intervening fibrous layer. It has been found that inorganic material incorporation such as strontium (Sr), zinc (Zn), magnesium (Mg), sodium (Na), silver (Ag), silicon (Si), and yttrium (Y) in HA plays an important role in bone formation and also affects bone mineral characteristics, such as crystallinity, degradation behavior, and mechanical properties (Sato et al., 2006; Xue et al., 2008; Boanini et al., 2010; Yang L. et al., 2010; Yang F. et al., 2012).
Various in vitro and in vivo studies reported the beneficial effects of strontium ions (Sr2+) as a potential bioactive element to enhance bone healing. Strontium has a positive effect on osteoblast-related gene expression such as Cbfa1 and osteonectin, and the alkaline phosphatase (ALP) activity of mesenchymal stem cells (MSCs). Also it can inhibit the differentiation of osteoclasts (Sila-Asna et al., 2007; Park et al., 2008; 2010; Gentleman et al., 2010). Thus it enhances matrix deposition, inhibits bone absorbing activity of osteoclasts, and, ultimately, encourages rapid new bone formation. Consequently, strontium is thought to be effective in enhancing the bioactivity and biocompatibility of some biomaterials.
Based on their chemical resemblance, calcium ions (Ca2+) in HA can be replaced partially by Sr2+. Strontium-substituted calcium phosphates have been developed. Several methods have been introduced to fabricate strontium-substituted calcium phosphate ceramics and bone cements (Roy et al., 2011). The structural changes, growth and dissolution behavior, and mechanical and biological properties of these materials were also studied (Zhang et al., 2011). Strontium-substituted HA exhibited superior mechanical properties than pure HA. Recently, Gentleman et al. (2010) investigated the effects of bioactive glass series, in which 0–100% of the calcium was substituted by strontium. It showed that the metabolic activity of osteoblastic cells can be enhanced with a dose-dependent manner.
Although a lot of studies have been conducted in vitro, only few in vivo studies have been carried out to investigate the influence of the Sr-HA coating on the bone osseointegration of implants. So, in our study, Sr-HA coating and HA coating were applied onto the rough surface of screw-shaped titanium implants using electrochemical deposition. The implants were inserted into the femur of rabbits to investigate the characteristics of the bone-implant interface. We hypothesized that Sr-HA can improve the bone formation on the bone-implant interface.
2. Materials and methods
2.1. Preparation of roughened implants coated with HA or Sr-HA
A total of 20 screw-shaped titanium implants (Zhejiang Guangci Medical Appliance Co., Ltd., Ningbo, China) whose external diameter and length are 4.1 mm and 8 mm, respectively, were roughened as previously described (Yang G.L. et al., 2008). In brief, implants were polished, sandblasted, and washed with acetone, 75% alcohol, and distilled water in an ultrasonic cleaner. Subsequently, a solution containing HF and HNO3 was used to treat the implants at room temperature and then a solution containing HCl and H2SO4 was used at 80 °C. Implants were dried in an oven at 50 °C for 24 h.
Electrochemically deposited hydroxyapatite (HA) and strontium-substituted hydroxyapatite (Sr-HA) coatings were applied as previously described (He et al., 2009; Jiao and Wang, 2009). Implants were used as the working electrode (cathode) for the deposition of HA or Sr-HA, with a platinum (Pt) plate as the counter electrode. For Sr-HA, the electrolytes were prepared by dissolving analytical grade Ca(NO3)2·4H2O, NH4H2PO4 (3.6×10−4 mol/L), and SrCl2·6H2O into distilled water, with (Ca+Sr)/P molar ratio being 1.67. The concentration of (Ca2++Sr2+) was 6×10−4 mol/L in the electrolytes with Sr2+/(Ca2++Sr2+) molar ratio of 10%. The electrolyte without Sr2+ was served to deposit HA, and 0.1 mol/L NaNO3 was added to improve the conductivity. The deposition process was performed with a DC power source operated at 3.0 V at 85 °C for 1 h.
2.2. Experimental design and implantation procedure
The animals used in this experiment were treated with approval of the Institutional Animal Care and Use Committee of Zhejiang University, Hangzhou, China. Ten adult New Zealand white rabbits weighing 2.5 to 3.0 kg were used in the surgery, which was performed under sterile conditions in a veterinary operating theater. The surgical placement of implants has been reported in this model previously (He et al., 2009). Sumianxin II (0.1 to 0.2 ml/kg, intramuscularly (IM), the Military Veterinary Institute, Quartermaster University of PLA, Changchun, China) was injected to induce general anesthesia. Before the operation, lidocaine was used locally into the surgical site. Surgical sites were exposed at the medial surface of distal femur. The implants were placed randomly in the rabbits’ femurs. By intermittent drilling with a low rotary speed and profuse saline irrigation, each site was prepared and enlarged to a diameter of 4.1 mm. The implants were inserted without tapping until the implant abutments were level with the bone surface. All implants penetrated the first bone cortex only. After surgery, surgical sites were closed in layers and antibiotics (penicillin, 4×105 U/d) were given for the next 3 d.
2.3. Fluorescent bone labeling
Fluorescent labels were administered to the rabbits (eight weeks of observation) to monitor new bone formation (Nkenke et al., 2002). Oxytetracycline hydrochloride (FLUKA, 200 mg/ml, 20 mg/kg IM) was administered on Day 7 after implantation. Alizarin-complexon (Shanghai Sangon Biological Engineering Company, 30 mg/ml, 30 mg/kg IM) was administered on Day 28 post-implantation. Calcein green (Shanghai Sangon Biological Engineering Company, 25 mg/ml, 5 mg/kg IM) was administered on Days 46 and 53 following implantation.
2.4. Preparation of specimens for fluorescence microscopy
After eight weeks the animals were euthanized by injecting an overdose of sumianxin (2.0 ml/kg IM). The implants and surrounding tissues were dissected to specimens and stored in 10% neutral buffered formalin for 5 to 7 d. A macrocutting and grinding system (Exakt 310 CP series; Exakt Apparatebau, Norderstedt, Germany) was used to produce undecalcified cut and ground sections. One section with a thickness of 200 µm was taken from the central part of each specimen. A final thickness of approximately 50 µm of each section was produced for fluorescence microscopy.
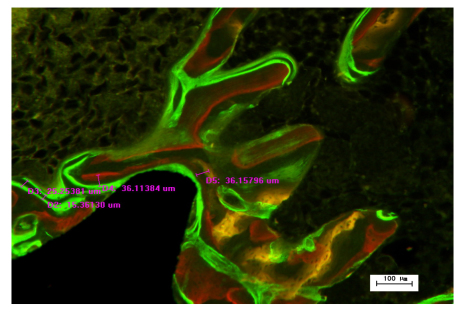
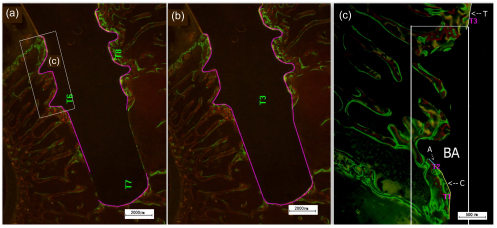
The fluorochrome labels were used to assess the bone mineral apposition rates (MARs) of HA and Sr-HA sections. Fluorochrome labels bind to sites of active bone deposition, shortly after administration. This enables identification of bone deposition at different time points. We calculated MAR by measuring the mean distance between the three different fluorescent labels, and dividing these distances by the difference in the time points at which the labels were administrated (Fig. 1) (Patel et al., 2002). The bone to implant contact (BIC) was measured as the percentage of the length of bone in direct contact with the implant surface (Fig. 2a). BIC in the total length of the implants including the apex was evaluated (Fig. 2b). BIC was divided into three groups according to the different fluorescence labels administered at different time points. Bone area ratio (BAR) was measured as the percentage of the area within the threads located inside the cancellous bone that contained newly formed bone (BA in Fig. 2c).
Fig. 1.
Measurement of bone MAR
Fig. 2.
Measurement of BIC and BAR of specimens
Purple line in (a) represents the bone in direct contact with the implant surface. Purple line in (b) represents the total length of the implants including the apex. In (c), “T” (oxytetracycline hydrochloride) represents the bone in direct contact with the implant surface on Day 7, “A” (alizarin-complexon) represents the new bone in direct contact with the implant surface on Day 28, “C” (calcein green) represents the new bone in direct contact with the implant surface on Day 53, and “BA” represents the area we employed to measure new BAR
Dynamic labeling of new bone formation was evaluated with Nikon Eclipse 80i fluorescence microscope (Nikon, Melville, NY, USA) with DXM1200F charge-coupled device (CCD). Fluorescence microscopic images were assessed at magnifications up to 100×. Image pro plus software was used for image analysis (Media Cybernetics, USA). Active bone formation was evaluated relative to the presence or absence, intensity, and width of the fluorochrome markers.
2.5. Statistical analysis
Each group contained 10 specimens. The data were tested for normal distribution. The mean value was used as the final result, and reported as mean±standard derivation (SD). One-way analysis of variance (ANOVA) test was performed to assess the presence of any significant differences between the two different surface treatments. P values of <0.05 were considered significant. The software of IBM SPSS statistics 19.0 was used for statistical analysis.
3. Results
3.1. Clinical observation
All rabbits survived the surgical procedure and recovered quickly from the surgery and appeared to be in good health throughout the test periods. At euthanasia, neither clinical signs of inflammation nor adverse tissue reactions were observed around the implants. All implants were still in situ.
3.2. Histological observation
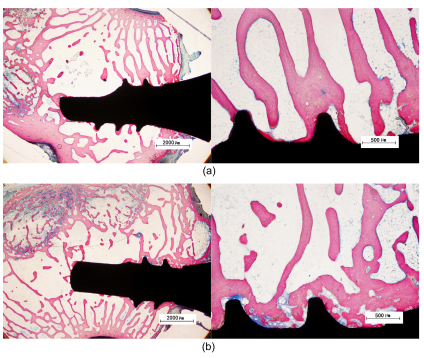
After eight weeks, newly formed bone was observed along the surface of all implants. In Fig. 3, we can find that the range of new bone formed in the Sr-HA group is much wider than that of the HA group. And the BIC is much more extensive compared to the HA group.
Fig. 3.
Histological sections of HA and Sr-HA coated implants after eight weeks
These figures show that the range of new bone formed in the Sr-HA group is much wider than that of the HA group. And the BIC is much more extensive in the Sr-HA group compared to the HA group. (a) HA group; (b) Sr-HA group
3.3. Fluorescence microscopy observation
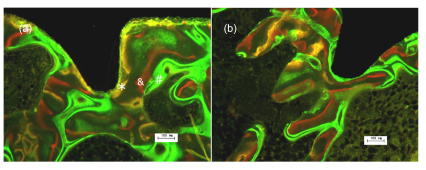
The obtained images showed areas labeled in green, yellow and red that represent regions of calcium precipitation labeled by fluorochromes at different moments of tissue mineralization. In Fig. 4, the oxytetracycline hydrochloride labeling (yellow) represents the regions where calcium precipitated on Day 7. The alizarin labeling (red) represents the regions where calcium precipitated on Day 28. The calcein labeling (green) represents the regions where calcium precipitated from Day 46 to Day 53. All groups exhibited extensive early peri-implant bone formation (wide diffuse yellow and red bands, narrow green band). Seven days after implantation, new bone tissue was observed attaching to all types of implants (Fig. 4). The deposits of the dyes were seen around the implants in the sequence of their administration. In the HA (Fig. 4a), most of the substances applied on Day 7 were closest to the implant surface in all implants, and the substances administered later were found further away from the implant surface. In the Sr-HA group (Fig. 4b), most green labels were found furthest away from the implant surface. That may be due to the formation of new bone around the old bone particles; so old bone particles were surrounded by newly formed bone.
Fig. 4.
Fluorescence microscopic images with labeling of oxytetracycline (* application date Day 7), alizarin-complexon (& application date Day 28) and calcein green (# application date Days 46 and 53)
The region where calcium precipitated from Day 46 to Day 53 is in close vicinity to the implant surface. (a) HA group; (b) Sr-HA group
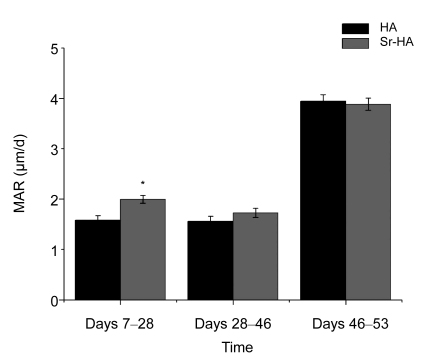
3.4. Bone mineral apposition rate
The MAR for all implants was calculated during Days 7 and 53 after implantation (Fig. 5). For the Sr-HA group, the MAR was significantly greater than that for pure HA from Day 7 to Day 28 (P<0.05). There was no statistically significant difference between the HA and Sr-HA groups in later periods.
Fig. 5.
MAR of implants after 7, 28, and 53 d of insertion
For the Sr-HA group, the MAR was significantly greater than that for pure HA from Day 7 to Day 28 (* P<0.05). There were no statistically significant differences between the HA and Sr-HA groups in the later periods
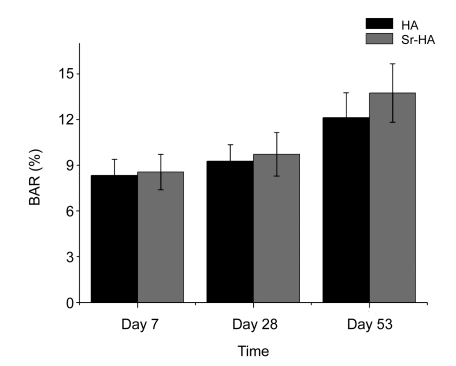
3.5. Bone area ratio
The BAR calculation results at Day 7 show that the new BAR was (8.34±1.06)% for the HA and (8.56±1.17)% for Sr-HA. There were no significant differences between HA and Sr-HA. On Day 28, the new BAR was (9.27±1.08)% for the HA and (9.73±1.43)% for Sr-HA. On Day 53, the new BARs of these two time points added together were (12.13±1.63)% for HA, and (13.74±1.92)% for Sr-HA. No statistically significant differences were found between the HA and Sr-HA groups at any time point (Fig. 6).
Fig. 6.
BAR of implants after 7, 28, and 53 d of insertion
No significant differences were found between the HA and Sr-HA groups at any time point
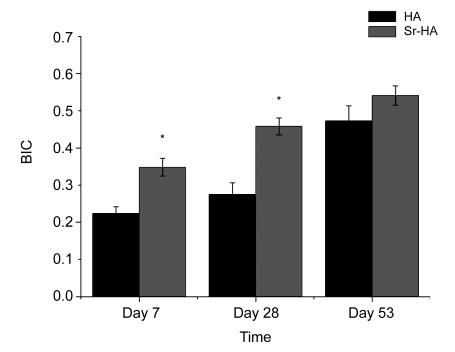
3.6. Bone to implant contact
Fig. 7 showed the mean BIC over the total implant length. For the Sr-HA group, the BIC showed significant differences on Days 7 and 28 compared with pure HA (P<0.05), but on Day 53 there were no differences.
Fig. 7.
BIC of implants after 7, 28, and 53 d of insertion
It showed the mean BIC over the total implant length. For the Sr-HA group, the BIC showed significant differences on Days 7 and 28 compared with pure HA (* P<0.05), but on Day 53 there were no differences
4. Discussion
Stability at placement and during function is a very important criterion for the success of dental implant. Primary stability may be a useful predictor for osseointegration (Friberg et al., 1999) and a high primary stability makes immediate loading more predictable (Szmukler-Moncler et al., 2000). The high incidence of bone implant failures is mainly blamed on incomplete osseointegration and stress shielding due to the differences in mechanical properties between the implant and surrounding bone. So, good osseointegration after implantation is essential not only for the long term but also the short term. In our study, electrochemically deposited Sr-HA and HA coatings were applied to the implants to investigate their effects on the bone osseointegration after the implantation. It was found that the MAR of the Sr-HA group was greater than that for pure HA from Day 7 to Day 28 after implantation, but no significant difference was found at later stage. And the BIC showed difference on Days 7 and 28 compared with pure HA. So, we concluded that compared to the HA coating, the Sr-HA coating has good bone integration properties in the early stage after implantation.
Our results were consistent with those of other studies reporting the stimulatory effect of Sr-HA on bone osseointegration (Gentleman et al., 2010; Ni et al., 2010). When implanted in bone fractures, Sr-HA was found to stimulate new bone formation. Xue et al. (2007) reported that plasma-sprayed Sr-HA coating exhibited the ability to induce apatite formation on its surface only after 1 d in simulated body fluid (SBF). The fact of apatite formation on Sr-HA coating indicates that it has good bioactivity. Sr-HA bioactive bone cement was evaluated in a sheep hip replacement model to investigate its effect on femoral bone remodeling, and the results showed that it has positive effect in alleviating femoral bone remodeling (Ni et al., 2010).
Generally, the coatings of the implants are only necessary as a matrix for early immobilization of osteoblast like cell and the development of vascularized bone tissue on the implant. At a later stage of implantation, these coatings are absorbed or degraded and replaced by new bones (Ye and Wang, 2007). Peri-implant bone formation is stimulated by the high level of free Ca2+ provided by the dissolution of the calcium phosphate coating (Hanawa et al., 1997). It is presumed that the difference in their solubility is partially responsible for the different observed kinetics for bone formation associated with these two coatings. Moreover, calcium phosphate dissolution ability appears to be essential for an optimal bone formation and fixation onto the implants at the early stage of implantation. Christoffersen et al. (1997) demonstrated that the replacement of partial Ca2+ in HA by Sr2+ could increase its solubility. Xue et al. (2007)’s X-ray diffraction (XRD) results revealed that Sr-HA coating has low crystallinity, which means that it has higher solubility. The dissolution of Sr-HA results in the release of Ca2+, which increases the ionic activity product of the apatite in surrounding fluid, and thus readily induces apatite precipitation. However, the rate of dissolution is likely to have a great influence on implant fixation, and fast absorption would lead to loss of mechanical fixation and disintegration of the coating. That could be the reason of our results that not only the MAR but also the BIC of the Sr-HA group showed significant improvement in the early stage after the implantation. However, in later stage, there was no significant difference between the Sr-HA and HA groups. In the future, more studies should be designed to find an optimum Sr-HA coating which has the best dissolution rate to insure a high quality of bone osseointegration.
In addition to the increasing solubility of HA after strontium incorporation, which could lead to apatite formation, another reason may be the stimulatory effect of released Sr2+ on osteoblastic cell mineralization. Strontium incorporation in HA significantly increased the ALP activity and the expression of Cbfa1 of osteoblastic cells (Ni et al., 2011). Furthermore, strontium incorporation in HA ceramic was found to affect osteoclastic proliferation, which reduces on increasing strontium content (Gentleman et al., 2010). In our study, most green labels which presented the early formed bone were found furthest away from the implant surface. That may be due to osteoblastic cells firstly assembled around the old bone particles, resulting in old bone particles surrounded by newly formed bone.
Zhang et al. (2011) compared the effect of strontium-substitution (10%, 40%, 100%) in HA on bone formation, and found that 10% Sr-HA group was the best in stimulating bone formation. In another study, the highest strontium concentration was found in 10% Sr-HA group; however, no significant differences in ALP activity, Cbfa1 gene expression, or the formation of mineralized nodules by the osteoblastic cells were found between 5% and 10% Sr-HA groups, suggesting that the stimulatory effect of strontium incorporation on cellular differentiation and mineralization does not always improve with the increase of strontium concentration. Although strontium incorporation in various materials favors osteoblastic cell behaviors, the optimal concentration of strontium remains to be investigated (Ni et al., 2011). The mechanism of action by which ions released from Sr-HA act on cells remains unclear, and further studies are needed to solve these problems.
5. Conclusions
This study has shown that the electrochemical Sr-HA coating evidently increases the bone MAR and the BIC from Day 7 to Day 28 after implantation compared with the HA group. It is concluded that Sr-HA coating improves bone osseointegration.
Footnotes
Project supported by the Excellent Youth Science Foundation of Zhejiang Provincial Natural Science Foundation of China (No. R2110374) and the Provincial Department Co-building Foundation of Health Bureau of Zhejiang Province, China (No. WKJ2011-2-009)
References
- 1.Boanini E, Gazzano M, Bigi A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010;6(6):1882–1894. doi: 10.1016/j.actbio.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Christoffersen MR, Christoffersen N, Kolthoff O. Effect of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection. Bone. 1997;20(1):47–54. doi: 10.1016/S8756-3282(96)00316-X. [DOI] [PubMed] [Google Scholar]
- 3.Cooper LF, Zhou Y, Takebe J, Guo J, Abron A, Holmen A, Ellingsen JE. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials. 2006;27(6):926–936. doi: 10.1016/j.biomaterials.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Friberg B, Sennerby L, Linden B, Lekholm U. Stability measurements of one-stage Brånemark implants during healing in mandibles. A clinical resonance frequency analysis study. Int J Oral Maxillofac Surg. 1999;28(4):266–272. doi: 10.1016/S0901-5027(99)80156-8. [DOI] [PubMed] [Google Scholar]
- 5.Gentleman E, Fredholm YC, Jell G, Lotfibakhshaiesh N, O′Donnell MD, Hill RG, Stevens MM. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials. 2010;31(14):3949–3956. doi: 10.1016/j.biomaterials.2010.01.121. [DOI] [PubMed] [Google Scholar]
- 6.Hanawa T, Kamira Y, Yamamoto S, Kohgo T, Amemyia A, Ukai H, Murakami K, Asaoka K. Early bone formation around calcium-ion-implanted titanium inserted into rat tibia. J Biomed Mater Res. 1997;36(1):131–136. doi: 10.1002/(SICI)1097-4636(199707)36:1<131::AID-JBM16>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.He F, Yang G, Wang X, Zhao S. Effect of electrochemically deposited nanohydroxyapatite on bone bonding of sandblasted/dual acid-etched titanium implant. Int J Oral Maxillofac Implants. 2009;24(5):790–799. [PubMed] [Google Scholar]
- 8.Jiao MJ, Wang XX. Electrolytic deposition of magnesium-substituted hydroxyapatite crystals on titanium substrate. Mater Lett. 2009;63(27):2286–2289. doi: 10.1016/j.matlet.2009.07.048. [DOI] [Google Scholar]
- 9.Jinno T, Kirk SK, Morita S, Goldberg VM. Effects of calcium ion implantation on osseointegration of surface blasted titanium alloy femoral implants in a canine total hip arthroplasty model. J Arthroplasty. 2004;19(1):102–109. doi: 10.1016/j.arth.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Ni GX, Lin JH, Chiu PKY, Li ZY, Lu WW. Effect of strontium-containing hydroxyapatite bone cement on bone remodeling following hip replacement. J Mater Sci Mater Med. 2010;21(1):377–384. doi: 10.1007/s10856-009-3866-2. [DOI] [PubMed] [Google Scholar]
- 11.Ni GX, Yao ZP, Huang GT, Liu WG, William W. The effect of strontium incorporation in hydroxyapatite on osteoblasts in vitro. J Mater Sci Mater Med. 2011;22(4):961–967. doi: 10.1007/s10856-011-4264-0. [DOI] [PubMed] [Google Scholar]
- 12.Nkenke E, Kloss F, Wiltfang J, Schultze-Mosgau S, Radespiel-Tröger M, Loos L, Neukam FW. Histomorphometric and fluorescence microscopic analysis of bone remodelling after installation of implants using an osteotome technique. Clin Oral Implants Res. 2002;13(6):595–602. doi: 10.1034/j.1600-0501.2002.130604.x. [DOI] [PubMed] [Google Scholar]
- 13.Park JW, Suh JY, Chung HJ. Effects of calcium ion incorporation on osteoblast gene expression in MC3T3-E1 cells cultured on microstructured titanium surfaces. J Biomed Mater Res. 2008;86A(1):117–126. doi: 10.1002/jbm.a.31618. [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Kim YJ, Jang JH. Enhanced osteoblast response to hydrophilic strontium and/or phosphate ions-incorporated titanium oxide surfaces. Clin Oral Implants Res. 2010;21(4):398–408. doi: 10.1111/j.1600-0501.2009.01863.x. [DOI] [PubMed] [Google Scholar]
- 15.Patel N, Best SM, Bonfield W, Gibson IR, Hing KA, Damien E, Revell PA. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J Mater Sci Mater Med. 2002;13(12):1199–1206. doi: 10.1023/A:1021114710076. [DOI] [PubMed] [Google Scholar]
- 16.Roy M, Bandyopadhyay A, Bose S. Induction plasma sprayed Sr and Mg doped nano hydroxyapatite coatings on Ti for bone implant. J Biomed Mater Res B Appl Biomater. 2011;99B(2):258–265. doi: 10.1002/jbm.b.31893. [DOI] [PubMed] [Google Scholar]
- 17.Sato M, Sambito MA, Aslani A, Kalkhoran NM, Slamovich EB, Webster TJ. Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials. 2006;27(11):2358–2369. doi: 10.1016/j.biomaterials.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Sila-Asna M, Bunyaratvej A, Maeda S, Kitaguchi H, Bunyaratavej N. Osteoblast differentiation and bone formation gene expression in stroutium-inducing bone marrow mesenchymal stem cell. Kobe J Med Sci. 2007;53(1-2):25–35. [PubMed] [Google Scholar]
- 19.Suh JY, Jeung OC, Choi BJ, Park JW. Effects of a novel calcium titanate coating on the osseointegration of blasted endosseous implants in rabbit tibiae. Clin Oral Implants Res. 2007;18(3):362–369. doi: 10.1111/j.1600-0501.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 20.Sul YT. The significance of the surface properties of oxidized titanium to the bone response: special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials. 2003;24(22):3893–3907. doi: 10.1016/S0142-9612(03)00261-8. [DOI] [PubMed] [Google Scholar]
- 21.Sul YT, Johansson C, Byon E, Albrektsson T. The bone response of oxidized bioactive and non-bioactive titanium implants. Biomaterials. 2005;26(33):6720–6730. doi: 10.1016/j.biomaterials.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 22.Szmukler-Moncler S, Piattelli A, Favero GA, Dubruille JH. Considerations preliminary to the application of early and immediate loading protocols in dental implantology. Clin Oral Implants Res. 2000;11(1):12–25. doi: 10.1034/j.1600-0501.2000.011001012.x. [DOI] [PubMed] [Google Scholar]
- 23.Xue WC, Hosick HL, Bandyopadhyay A, Bose S, Ding C, Luk KDK. Preparation and cell-materials interactions of plasma sprayed strontium-containing hydroxyapatite coating. Surf Coat Technol. 2007;201(8):4685–4693. doi: 10.1016/j.surfcoat.2006.10.012. [DOI] [Google Scholar]
- 24.Xue WC, Dahlquist K, Banerjee A, Bandyopadhyay A, Bose S. Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. J Mater Sci Mater Med. 2008;19:2669–2677. doi: 10.1007/s10856-008-3395-4. [DOI] [PubMed] [Google Scholar]
- 25.Yang F, Dong WJ, He FM, Wang XX, Zhao SF, Yang GL. Osteoblast response to porous titanium surface coated with zinc-substituted hydroxyapatite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2012;113(3):313–318. doi: 10.1016/j.tripleo.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 26.Yang GL, He FM. Effects of biomimetically and electrochemically deposited nano-hydroxyapatite coatings on osseointegration of porous titanium implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(6):782–789. doi: 10.1016/j.tripleo.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Yang GL, He FM, Zhao SS, Wang XX, Zhao SF. Effect of H2O2/HCl heat treatment of implants on in vivo peri-implant bone formation. Int J Oral Maxillofac Implants. 2008;23(6):1020–1028. [PubMed] [Google Scholar]
- 28.Yang L, Perez-Amodio S, Florence YF, Groot BD, Everts V, Blitterswijk CAV, Habibovic P. The effects of inorganic additives to calcium phosphate on in vitro behavior of osteoblasts and osteoclasts. Biomaterials. 2010;31(11):2976–2989. doi: 10.1016/j.biomaterials.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Ye W, Wang XX. Effect of supersaturation on the morphologies of hydroxyapatite crystal deposited on titanium surface with electrochemical technique. Key Eng Mater. 2007;330-332:601–604. doi: 10.4028/www.scientific.net/KEM.330-332.601. [DOI] [Google Scholar]
- 30.Zhang WB, Shen YH, Pan HB, Lin KL, Liu XG, Darvell BW, Lu WW, Chang J, Deng LF, Wang DP, et al. Effects of strontium in modified biomaterials. Acta Biomater. 2011;7(2):800–808. doi: 10.1016/j.actbio.2010.08.031. [DOI] [PubMed] [Google Scholar]