Abstract
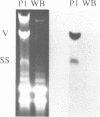
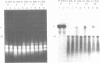
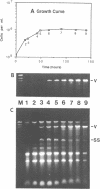
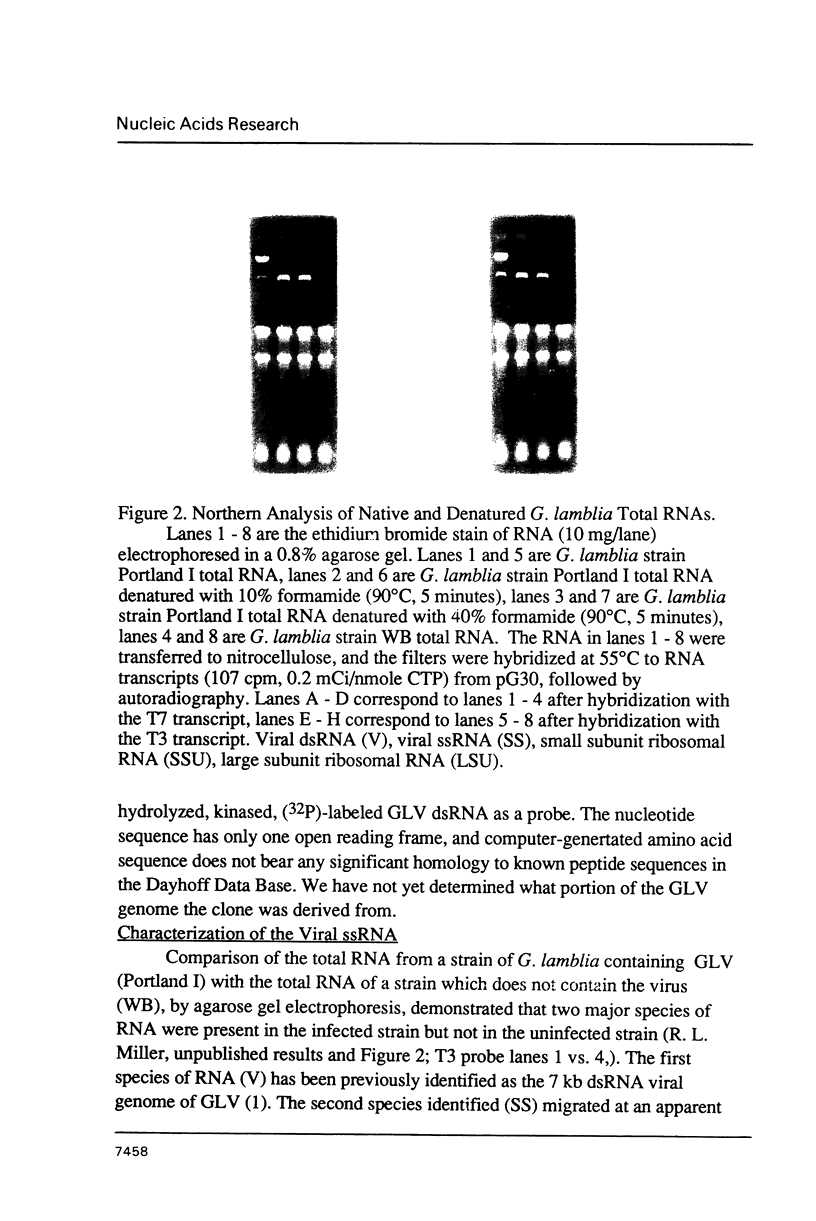
An isolate of Giardia lamblia infected with the double-stranded RNA virus (GLV) has two major species of RNA that are not present in an uninfected isolate. One of these species is the previously characterized double-stranded RNA genome of GLV (1). The second species of RNA appears to be a full length copy of one strand of the double-stranded RNA genome. This full length single-stranded RNA is not present in viral particles isolated from the growth medium. The cellular concentration of the single-stranded RNA changes during exponential and stationary phases of cell growth in a fashion consistent with a viral replicative intermediate or mRNA. The single-stranded species does not appear to be polyadenylated.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruenn J., Bobek L., Brennan V., Held W. Yeast viral RNA polymerase is a transcriptase. Nucleic Acids Res. 1980 Jul 11;8(13):2985–2997. doi: 10.1093/nar/8.13.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck K. W., Ackermann H. W., Bozarth R. F., Bruenn J. A., Koltin Y., Rawlinson C. J., Ushiyama R., Wood H. A. Six groups of double-stranded RNA mycoviruses. Intervirology. 1984;22(1):17–23. doi: 10.1159/000149529. [DOI] [PubMed] [Google Scholar]
- De Lange T., Liu A. Y., Van der Ploeg L. H., Borst P., Tromp M. C., Van Boom J. H. Tandem repetition of the 5' mini-exon of variant surface glycoprotein genes: a multiple promoter for VSG gene transcription? Cell. 1983 Oct;34(3):891–900. doi: 10.1016/0092-8674(83)90546-9. [DOI] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Esteban R., Fujimura T., Wickner R. B. Site-specific binding of viral plus single-stranded RNA to replicase-containing open virus-like particles of yeast. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4411–4415. doi: 10.1073/pnas.85.12.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Miller R. L., Wang A. L., Wang C. C. Identification of Giardia lamblia isolates susceptible and resistant to infection by the double-stranded RNA virus. Exp Parasitol. 1988 Jun;66(1):118–123. doi: 10.1016/0014-4894(88)90056-2. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Wang A. L., Wang C. C. Purification and characterization of the Giardia lamblia double-stranded RNA virus. Mol Biochem Parasitol. 1988 Apr;28(3):189–195. doi: 10.1016/0166-6851(88)90003-5. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R. Structure-independent nucleotide sequence analysis. Proc Natl Acad Sci U S A. 1979 May;76(5):2232–2235. doi: 10.1073/pnas.76.5.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Nemhauser I., Gottlieb P., Romantschuk M., Carton J., Frucht S., Strassman J., Bamford D. H., Kalkkinen N. Nucleotide sequence of the large double-stranded RNA segment of bacteriophage phi 6: genes specifying the viral replicase and transcriptase. J Virol. 1988 Apr;62(4):1180–1185. doi: 10.1128/jvi.62.4.1180-1185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering L. K., Engelkirk P. G. Giardia lamblia. Pediatr Clin North Am. 1988 Jun;35(3):565–577. doi: 10.1016/s0031-3955(16)36472-0. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. A linear double-stranded RNA in Trichomonas vaginalis. J Biol Chem. 1985 Mar 25;260(6):3697–3702. [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol. 1986 Dec;21(3):269–276. doi: 10.1016/0166-6851(86)90132-5. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded RNA replication in yeast: the killer system. Annu Rev Biochem. 1986;55:373–395. doi: 10.1146/annurev.bi.55.070186.002105. [DOI] [PubMed] [Google Scholar]