Abstract
Sialic acid-binding immunoglobulin-like lectin (Siglec)-F is a mouse functional paralog of human Siglec-8 that induces apoptosis in human eosinophils, and therefore may be useful as the basis of treatments for a variety of disorders associated with eosinophil hyperactivity, such as asthma. The expression pattern and functions of this protein in various cell types remain to be elucidated. The aim of this study was to determine the expression of Siglec-F on mouse macrophages by immunocytochemical staining, and also to investigate the effects of Siglec-F engagement by a Siglec-F antibody on phagocytic activity of macrophages. The results showed that Siglec-F expression was detected on mouse alveolar macrophages, but not on peritoneal macrophages. Furthermore, Siglec-F engagement did not affect the phagocytic activity of alveolar macrophages in the resting state or in the activated state following stimulation by the proinflammatory mediator tumor necrosis factor alpha (TNF-α) or lipopolysaccharide (LPS). Siglec-F expression on alveolar macrophages may be a result of adaptation. Macrophages actively regulate immune responses via production of cytokines. Therefore, further investigation of the effects of Siglec-F engagement on immune mediators or cytokines released by alveolar macrophages is required.
Keywords: Siglec-F, Expression, Macrophages, Phagocytosis, Eosinophil
1. Introduction
Sialic acid-binding immunoglobulin-like lectins (Siglecs) are members of the immunoglobulin superfamily. Siglecs are single-pass transmembrane surface proteins that are characterized by their specificity for sialic acids attached to the terminal regions of cell-surface glycoconjugates. The molecular structure of Siglecs differs from that of other members of the immunoglobulin superfamily (Freeman et al., 1995; Angata and Varki, 2000). The specific binding of the extracellular domain of Siglecs to sialic acid residues on the glycosyl side-chains of glycolipids and glycoproteins mediates interactions with proteins and carbohydrates for a variety of purposes. The predominantly inhibitory function of the majority of Siglecs is indicated by the presence of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in the cytoplasmic domain (Angata et al., 2006; 2007).
To date, 15 Siglecs have been identified in humans and 9 in mice (Crocker et al., 2007). Through a nomenclature agreement, Siglecs unique to mice are lettered rather than numbered (Crocker et al., 1998). Despite the discovery of numerous Siglecs, the biological functions of these proteins are largely unknown, although it has been reported that Siglec-8 specifically induces eosinophil apoptosis (O′Reilly and Paulson, 2009), while Siglec-9, which is expressed on the leukocyte surface, specifically induces neutrophil death (von Gunten et al., 2005). Investigations of Siglec biology have focused on the mouse as a model organism due to the difficulties and limitations associated with this type of research in humans. Recent studies identified murine Siglec-F as the functional paralog of human Siglec-8 based on its expression on mouse eosinophils and its ability to adhere to the 6′-sulfo-sLex structure (Tateno et al., 2005; Bochner, 2009). Studies have demonstrated that cross-linking of Siglec-F with a specific antibody (Ab) induced mouse eosinophil apoptosis in vivo and in vitro (Zimmermann et al., 2008). Furthermore, administration of anti-Siglec-F Ab significantly reduced the level of allergen-induced airway inflammation and airway remodeling (Song et al., 2009; Cho et al., 2010). These findings indicate the potential value of Siglecs as a therapeutic target in humans. Further investigation of these proteins is required to provide proof-of-concept for targeting Siglecs in many diseases, such as asthma, allergies, and related disorders.
With the exception of myelin-associated glycoprotein (MAG)/Siglec-4, which is expressed only in the nervous system on the myelin sheath (Sun et al., 2004), the majority of Siglecs are expressed on the surface of immunocytes, where they act as adhesion and signaling molecules. These include sialoadhesin/Siglec-1, which is expressed on subsets of human macrophage, Siglec-5, which is expressed on monocytes and mature neutrophils, and Siglec-7, which is expressed on natural killer (NK) cells and monocytes (Crocker and Redelinghuys, 2008; Schauer, 2009). Siglecs play a particularly important role in macrophages. Apoptotic cells and secondary necrotic cells release their intracellular contents into the surrounding tissues. These contents are potential autoantigens and macrophages engulf these dying cells rapidly to prevent harmful inflammatory responses and autoimmunity. A variety of molecules on the surface of macrophages are involved in this process, such as c-mer proto-oncogene tyrosine kinase (MerTK), milk fat globule epidermal growth factor (EGF) factor 8 (MFG-E8), and T-cell immunoglobulin and mucin domain-containing molecule (Tim-4) (Nakayama et al., 2009). Multiple receptors may simultaneously recognize these “eat-me” signals on apoptotic cells. These observations demonstrated the importance of investigations of the expression and function of Siglecs in murine macrophages as a model to provide valuable information regarding Siglecs biology in humans. The aim of this study was to determine expression of Siglec-F on mouse macrophages by immunocytochemical staining, and also to investigate the effects of Siglec-F engagement by a Siglec-F Ab on macrophage phagocytic activity.
2. Materials and methods
2.1. Reagents
Thioglycollate broth, 2-mercaptoethanol (2-ME), and lipopolysaccharide (LPS) were purchased from Sigma (St. Louis, MO, USA); high-glucose Dulbecco’s modified Eagle’s medium (DMEM), Hank’s balanced salt solution (HBSS), penicillin, streptomycin, 1× nonessential amino acids, 1 mmol/L sodium pyruvate, and phosphate buffered saline (PBS) were purchased from Invitrogen (Grand Island, NY, USA); RPMI 1640 medium and endotoxin-free fetal bovine serum (FBS) were purchased from HyClone Laboratories (Logan, UT, USA). Purified rat anti-mouse Siglec-F Ab, rat anti-mouse IgG2α, and cell strainers (40 μm) were purchased from BD Biosciences (San Jose, CA, USA). Polink-1 horseradish peroxidase (HRP) detection system for rat primary antibodies, diaminobenzidine substrate kit, and hematoxylin and eosin (H&E) staining solutions were obtained from ZsGb Biotechnology (Beijing, China). Goat anti-rat IgG was purchased from Biosynthesis Biotechnology (Beijing, China). Tumor necrosis factor-α (TNF-α) was purchased from PrimeGene Biotechnology (Shanghai, China). Stem cell factor (SCF) and Flt3 ligand were purchased from PeproTech (Rocky Hill, NJ, USA). Interleukin-5 (IL-5) and fluorescein isothiocyanate (FITC)-conjugated anti-annexin V were purchased from R&D Systems (Minneapolis, MN, USA).
2.2. Animals
Female BALB/c mice [(20±2) g, aged 6‒8 weeks] were purchased from the Animal Center, Medical Institute of Sichuan University, China. Thymocytes were harvested from mice (aged 3‒4 weeks). These mice were housed under normal laboratory conditions [(21±2) °C] with free access to standard rodent chow and water for at least one week following delivery to allow recovery from transit stress. This study was performed in accordance with the Guidelines for the Care and Use of Animals of Sichuan University and was approved by the Sichuan University Animal Ethics Committee.
2.3. Isolation of macrophages
Murine peritoneal and alveolar macrophages were isolated following a previously described method by Zhang et al. (2008) with certain modifications. The importance of conducting all procedures under cold conditions should be emphasized.
2.3.1. Resident peritoneal macrophages
Briefly, untreated mice were euthanized by rapid cervical dislocation. The abdomen was soaked in 75% alcohol and a small incision was made along the midline with sterile scissors. The abdominal skin was manually retracted to expose the intact peritoneal wall. A needle was inserted through the peritoneal wall along the left side of the mouse. Cold harvest medium (HBSS, 5.0 ml) was injected into the peritoneal cavity and the fluid was aspirated from the peritoneum. These steps were repeated twice and the exudates were pooled. The exudate was then centrifuged for 10 min at 400×g (4 °C). The supernatant was discarded and the cell pellet was resuspended in DMEM by gently tapping the bottom of the centrifuge tube and pipetting up and down. The cell concentration was adjusted to 1×106 nucleated cells/ml in DMEM.
2.3.2. Thioglycollate-elicited peritoneal macrophages
A total of 3 ml of aged, sterile 3% thioglycollate broth (0.03 g/ml) was injected into the peritoneum of BALB/c mice. Mice were euthanized 3 d after injection. Macrophages were isolated using the procedure described for resident peritoneal macrophages (Section 2.3.1). Experiments using thioglycollate-elicited macrophages were performed the day after isolation, following removal of non-adherent cells by washing cells repeatedly with PBS.
2.3.3. Alveolar macrophages
A tracheotomy was performed on the mouse by making a midline incision in the anterior neck. Harvest medium (HBSS, 0.8 ml) was infused into the lung. The fluid was then gently drawn into the syringe and re-infused back into the lung three times in succession. This infusion/re-infusion process was repeated five times and the lung lavage fluid was pooled to give approximately 4 ml. Alveolar macrophages were isolated using the method described for resident peritoneal macrophages (Section 2.3.1).
2.4. Culture of mouse eosinophils derived from bone marrow cells
Eosinophils were generated by ex vivo culture of mouse bone marrow cells using the method previously described by Dyer et al. (2008). Briefly, bone marrow cells were suspended at 1×106 ml−1 in RPMI 1640 medium containing 20% FBS, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 1× nonessential amino acids, 1 mmol/L sodium pyruvate, and 50 µmol/L 2-ME and supplemented with 100 ng/ml SCF and 100 ng/ml Flt3 ligand from Days 0 to 4. On Day 4, cells were switched into new flasks and cultured at 1×106 ml−1 in basic medium containing 10 ng/ml recombinant mouse IL-5 (rmIL-5). On subsequent alternate days, one-half of the medium was replaced with fresh medium containing rmIL-5 and the concentration of the cells was adjusted to 1×106 ml−1 on each occasion. Cells were enumerated using a hemocytometer and viability was determined by trypan blue exclusion. The percentage of eosinophils was determined using a modified Giemsa preparation (Diff Quik).
2.5. Evaluation of effects of Siglec-F Ab cross-linking on eosinophil apoptosis
Eosinophils were incubated with anti-mouse Siglec-F Ab and eosinophil apoptosis was detected by flow cytometric analysis. Eosinophils were incubated without Ab and with anti-Fas Ab as controls. Annexin V labeling was performed to detect apoptosis in eosinophils as described previously by Saltan et al. (2011). Cultured cells were washed once in PBS and incubated with FITC-conjugated anti-annexin V (1:200, v/v) for 15 min in the dark at room temperature. Cells were resuspended in 200 µl ice-cold annexin V binding buffer and apoptosis was analyzed by flow cytometry (Beckman, USA) within 1 h.
2.6. Isolation of thymocytes and apoptosis induction
A model of apoptosis cells was established based on exposure of mouse thymocytes to ultraviolet radiation in vitro to explore the role of Siglec-F in macrophage phagocytosis (Vandivier et al., 2002; Morimoto et al., 2006; Bianchi et al., 2008). Thymuses obtained from mice (aged 3‒4 weeks) were gently pushed through a cell strainer (40 μm) to prepare a thymocyte suspension. Thymocytes were exposed to ultraviolet irradiation (254 nm) for 10 min to induce apoptosis followed by culture in DMEM containing 10% FBS at 37 °C for 3 h. Thymocytes prepared in this way have been shown to be approximately 90% annexin V-positive by flow cytometric analysis (Borges et al., 2009).
2.7. Macrophage culture and immunocytochemical staining
Peritoneal or bronchoalveolar lavage cells (1×106 ml−1) were seeded onto coverslips and cultured for 2 h in DMEM containing 100 U/ml penicillin, 100 g/ml streptomycin, and 10% heat-inactivated FBS at 37 °C in a humidified incubator containing 5% CO2. Non-adherent cells were removed by gentle washing three times with warm PBS. Adherent cells (macrophages) were cultured in the same medium for 24 h. In some experiments, peritoneal macrophages were co-cultured with or without LPS (1 μg/ml) and/or TNF-α (20 ng/ml) for 24 h.
Macrophages were fixed on the coverslips with 4% paraformaldehyde (0.04 g/ml) prior to processing for immunocytochemical staining with the Polink-1 HRP detection system according to the manufacturer’s instructions. After fixation, macrophages were immersed in 3% H2O2 for 8 min to block endogenous peroxidase activity. After washing three times with PBS, macrophages were co-incubated with Siglec-F Ab [diluted 1:150 (v/v) with PBS] for 24 h at 4 °C. As controls, macrophages were incubated with PBS or isotype control Ab. Cells were washed three times with PBS and incubated in the Polink-1 reagent mixture for 30 min at 37 °C. Cells were then washed three times with PBS and incubated with the reagent mixture from the diaminobenzidine substrate kit for 4 min. Finally, cells were washed extensively in water and counterstained with Mayer’s hematoxylin for 10 s. Positive staining of macrophages with Siglec-F was visualized as cells exhibiting brown coloration of the cell membrane.
2.8. Evaluation of effects of Siglec-F Ab cross-linking on phagocytic activity of alveolar macrophages in vitro
Alveolar macrophages were cultured on coverslips in 12-well plates (2×105 well−1) as described in Section 2.7. After incubation for 2 h, cells were washed with warm PBS to remove non-adherent cells. Adherent cells (alveolar macrophages) were co-cultured with apoptotic thymocytes for 90 min at an approximate ratio of 10:1 (apoptotic thymocytes:macrophages).
In order to investigate the effect of Siglec-F engagement on phagocytic function, alveolar macrophages were co-cultured with Siglec-F Ab [10 μg/ml, a concentration previously found to be saturating in eosinophil experiments (Zimmermann et al., 2008)] for 30 min in the presence or absence of polyclonal goat anti-rat IgG Ab, which was used to cross-link the Siglec-F Ab (Nutku et al., 2003; Zimmermann et al., 2008). For controls, cells were incubated with medium alone or in the presence of the secondary cross-linking Ab alone. In phagocytosis experiments on activated macrophages, before incubation with the Siglec-F Ab, alveolar macrophages were cultured in medium containing LPS (1 μg/ml) and/or TNF-α (20 ng/ml) for 24 h.
Alveolar macrophages were co-incubated with apoptotic thymocytes for 90 min at 37 °C before being washed with PBS, fixed with methyl alcohol, and stained with H&E. Phagocytosis was evaluated by counting 200 macrophages per well. Results were expressed as percent phagocytosis and phagocytosis index (Wang et al., 2009). Percent phagocytosis was calculated from the percentage of the number of macrophages containing at least one ingested thymocyte to the number of macrophages. Phagocytosis index was defined as the ratio of the number of thymocytes engulfed by macrophages to the number of macrophages. Each condition was tested in duplicate and repeated at least three times.
2.9. Statistical analysis
Data were expressed as mean±standard deviation (SD) and evaluated using Student’s t-test for two-sample comparisons or analysis of variance (ANOVA) for multi-sample comparisons. Use of these parametric statistics was deemed appropriate because phagocytosis of apoptotic thymocytes by macrophages has been shown to follow a Gaussian distribution (Licht et al., 1999). P<0.05 was considered statistically significant.
3. Results
3.1. Expression of Siglec-F on mouse alveolar macrophages and peritoneal macrophages
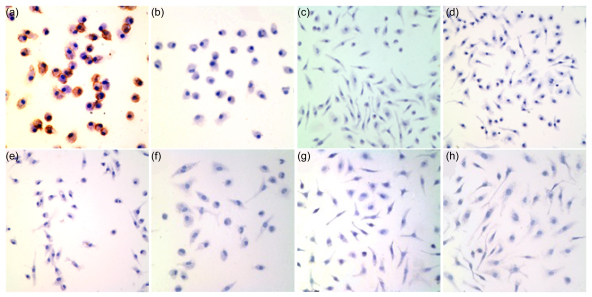
Peritoneal macrophages and alveolar macrophages, the two main subtypes of macrophages, differ considerably in morphology, biochemistry, secretory products, surface phenotype, and function. Immunocytochemical staining showed that Siglec-F was expressed on alveolar macrophages (Fig. 1a), but not on mouse peritoneal macrophages in the resting state (Fig. 1c). Immunocytochemical staining with the isotype control of anti-Siglec-F Ab was negative on both alveolar macrophages and peritoneal macrophages (Figs. 1b and 1d). Macrophage physiology changed dramatically in response to certain stimuli. Therefore, induction of Siglec-F expression on the peritoneal macrophages was investigated in response to thioglycollate. Peritoneal macrophages were activated by a single injection (3 ml) of aged, sterile 3% thioglycollate broth into the mouse peritoneal cavity or by co-incubation of peritoneal macrophages with the proinflammatory mediators LPS or/and TNF-α. Siglec-F was not expressed on activated peritoneal macrophages under either set of conditions (Figs. 1e‒1h).
Fig. 1.
Immunocytochemical staining of Siglec-F on macrophages
Siglec-F is expressed on mouse alveolar macrophages, but not on peritoneal macrophages in the resting or activating state. Siglec-F expression is exhibited as brown coloration of the cell membrane. Representative photomicrographs of Siglec-F and isotype control staining of macrophages are shown (original magnification, ×400). (a) Siglec-F on resting alveolar macrophages; (b) Isotype control for Siglec-F on resting alveolar macrophages; (c) Siglec-F on resident peritoneal macrophages in the resting state; (d) Isotype control on resident peritoneal macrophages in the resting state; (e) Siglec-F on peritoneal macrophages activated by thioglycollate; (f‒h) Siglec-F on cultural peritoneal macrophages activated in vitro for 24 h by LPS, LPS+TNF-α, and TNF-α, respectively
3.2. Effect of Siglec-F engagement on phagocytic activity of alveolar macrophages in vitro
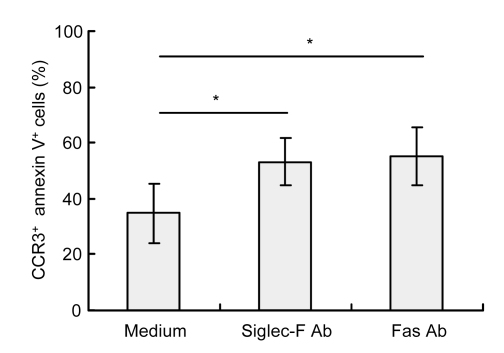
Previous studies have shown that Siglec-F stimulation by cross-linking is a key factor involved in mouse eosinophil apoptosis induction (Song et al., 2009). To confirm the efficacy of Siglec-F Ab as a cross-linking agent, murine bone marrow cells were cultured ex vivo to obtain eosinophils, which were then incubated with anti-mouse Siglec-F Ab. As a control, eosinophils were incubated without Ab or with anti-Fas Ab. Flow cytometric analysis revealed a significant increase in apoptotic eosinophils after 18 h (Fig. 2). The percentage of CC chemokine receptor 3 (CCR3)-positive and annexin V-positive cells increased from (34.94±10.67)% in the medium control to (53.21±8.42)% in Siglec-F Ab-incubated cells (n=5, P=0.013) and (55.28±10.45)% in Fas Ab-incubated cells (n=5, P=0.007). These results confirmed the efficacy of Siglec-F Ab as a cross-linking agent and demonstrated that eosinophils generated by ex vivo culture of bone marrow cells were similar to eosinophils generated in vivo, in that apoptosis is stimulated in both populations in response to Siglec-F engagement.
Fig. 2.
Apoptosis of eosinophils assessed by flow cytometry (CCR3+ annexin V+ cells)
Eosinophils were incubated without Ab or with rat anti-Siglec-F Ab or anti-Fas Ab for 18 h. Siglec-F Ab induced apoptosis of murine eosinophils generated by ex vivo culture of bone marrow cells. Data are expressed as mean±SD of five experiments. * P<0.05
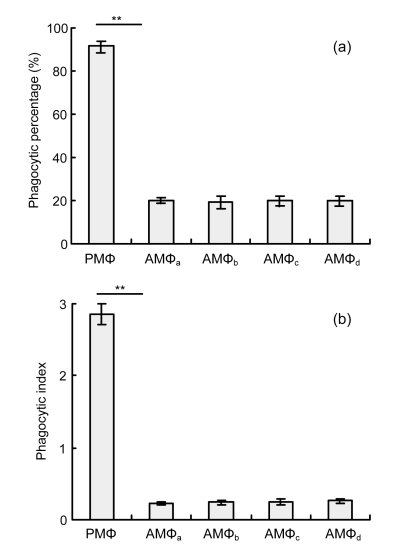
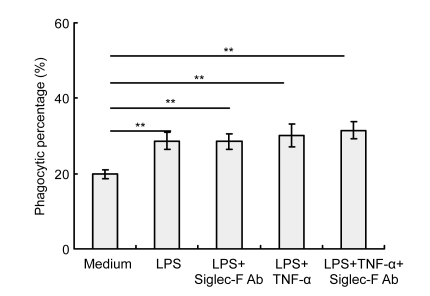
Alveolar macrophages were co-cultured with saturating concentrations of Siglec-F Ab for 30 min before addition of apoptotic thymocytes to determine the involvement of Siglec-F cross-linking in phagocytosis in these cells. Analyses of the percentage of phagocytic cells and phagocytic index revealed that phagocytic activity in resting alveolar macrophages was not altered by Siglec-F engagement (Figs. 3a and 3b). Furthermore, significantly lower phagocytic activity was observed in alveolar macrophages [(6.33±2.02)%] compared with peritoneal macrophages [(91.5±2.29)%] (P=0, Fig. 3a). This difference in activity was even more remarkable in comparisons of the number of macrophages that had ingested at least one apoptotic thymocyte and the phagocytic index, which was 0.23±0.02 for alveolar macrophages compared with 2.85±0.15 for peritoneal macrophages (P=0, Fig. 3b). Under activating conditions (LPS and/or TNF-α), the phagocytic activity of alveolar macrophages did not change significantly in response to Siglec-F engagement in vitro (Fig. 4). The phagocytic activity of alveolar macrophages [(28.67±2.25)%] was significantly enhanced in response to LPS, compared with that observed in untreated alveolar macrophages [(19.83±1.26)%] (P=0.001, Fig. 4).
Fig. 3.
Phagocytic activity of mouse resting alveolar macrophages in vitro
Adherent alveolar macrophages (AMΦ) and peritoneal macrophages (PMΦ) were obtained from normal BALB/c mice and were cultured in DMEM. AMΦ were further divided into four groups which were separately co-cultured for 30 min with: medium alone (AMΦa), anti-SiglecF Ab and polyclonal goat anti-rat IgG (AMΦb), anti-SiglecF Ab (AMΦc), or polyclonal goat anti-rat IgG (AMΦd). Cells were co-incubated with apoptotic thymocytes and processed as described in Section 2.8. Phagocytic activity was evaluated by counting 200 macrophages per condition. Siglec-F engagement did not influence phagocytic activity of mouse resting alveolar macrophages in vitro. (a) Phagocytic percentage; (b) Phagocytic index. Data represent mean±SD of three independent experiments. ** P<0.001, compared with AMΦa group
Fig. 4.
Phagocytic activity of activated mouse alveolar macrophages in vitro
Adherent alveolar macrophages were obtained from normal BALB/c mice (2×105 well−1) and were incubated on coverslips at 37 °C in 12-well plates. Alveolar macrophages were activated by LPS (1 μg/ml) and/or TNF-α (20 ng/ml) for 24 h in vitro prior to co-incubation for 30 min with or without anti-Siglec-F Ab and polyclonal goat anti-rat IgG followed by co-culture with apoptotic thymocytes (2×106 well−1) for 90 min. Phagocytic activity was evaluated by counting 200 macrophages per condition. Siglec-F engagement did not influence phagocytic activity of activated mouse alveolar macrophages in vitro. Data represent mean±SD of three independent experiments. ** P<0.001, compared with medium group
4. Discussion
Previous studies have shown that antibody-mediated cross-linking of Siglec-F expressed on mouse eosinophils stimulates apoptosis both in vivo and in vitro (Zimmermann et al., 2008). However, it is still unclear whether other inflammatory cells express Siglec-F. Mouse mast cells and basophils are difficult to isolate by routine methods. Therefore, in this study, expression and potential functions of Siglec-F were investigated in alveolar macrophages and peritoneal macrophages. Siglec-8 expression on human eosinophils was discovered prior to detection of Siglec-F expression on mouse eosinophils. However, due to the practical and ethical restrictions associated with research of the functional mechanisms of human Siglec-8 in vivo, investigations have focused on Siglec-8 homologues in animal models. Furthermore, despite identification of an increasing number of novel Siglecs, the exact biological functions of most of these molecules in mouse and human systems are still poorly understood. Therefore, this investigation focused primarily on the expression and function of Siglec-F on mouse macrophages.
Macrophages play an important role in the regulation of inflammation. Inflammatory responses are initiated through secretion of proinflammatory cytokines and resolved through phagocytosis of apoptotic and necrotic cells. Many signaling molecules expressed on macrophages are involved in macrophage phagocytosis. This study confirmed Siglec-F expression on mouse alveolar macrophages, but not on peritoneal macrophages. Alveolar macrophages are highly differentiated cells that are specifically adapted to the particular environment of the lungs. Therefore, it is intriguing to postulate that the expression of Siglec-F on alveolar macrophages is an adaptive response to this environment, which may indicate the biological function of Siglec-F. Furthermore, recent studies have shown that Siglec-F ligands are expressed endogenously on normal mouse airway epithelium and are enhanced in allergic inflamed lungs (Guo et al., 2007). It can be speculated that alveolar macrophages entering the airway encounter Siglec-F ligands, thus stimulating functional responses, although further investigations are required to validate this hypothesis.
Macrophages from different tissues differ considerably in morphology, biochemistry, secretory products, surface phenotype, and function. This study demonstrated that alveolar macrophages exhibit much lower phagocytic activity in response to apoptotic thymocytes compared with peritoneal macrophages. This conclusion is consistent with previous reports (Hu et al., 2000; 2004). Although the mechanism responsible for this marked difference in phagocytic activity remains to be elucidated, it is hypothesized that differences in the expression of surface receptors are a critical influencing factor.
The majority of Siglec family members contain ITIMs within cytoplasmic domains (Angata et al., 2006; 2007; Cao et al., 2008). Typically, receptors with ITIMs function as inhibitory receptors and suppress activation signals that emanate from receptors associated with immunoreceptor tyrosine-based activation motifs (ITAMs) through recruitment of tyrosine and inositol phosphatases. This suggests that Siglecs predominantly mediate inhibitory effects in these cells. It has been demonstrated that Siglec-8 is expressed mainly on the surface of human eosinophils and that antibody-mediated Siglec-8 cross-linking specifically induces apoptosis (Nutku et al., 2003). Siglec-8 is also expressed at low levels on human mast cells and basophils. Yokoi et al. (2008) observed that specific antibody-mediated cross-linking of Siglec-8 inhibited FcεRI-dependent histamine and prostaglandin D2 release, as well as Ca2+ flux, and that anti-IgE evoked contractions of human bronchial rings. The most prominent function of macrophages in inflammatory responses is removal of infiltrating apoptotic cells. In this study, it was confirmed that mouse alveolar macrophages express high levels of Siglec-F. Therefore, the effect of Siglec-F engagement on alveolar macrophage phagocytosis of apoptotic cells was investigated. It was demonstrated that antibody-mediated cross-linking of Siglec-F did not alter alveolar macrophage phagocytic activity in vitro, either in the resting state or following activation by inflammatory mediators such as LPS and/or TNF-α. However, the phagocytic activity of alveolar macrophage was enhanced by LPS and TNF-α pretreatment. Previous studies have shown that macrophage phagocytic activity is enhanced by LPS stimulation through toll-like receptor signaling (Corradin and Mauël, 1991; Shen et al., 2010). Alveolar macrophages are highly specialized mononuclear phagocytic cells located in the alveolar space. They are the first immune competent cells to encounter inhaled antigens and therefore play a critical role in regulating immune responses of the lungs. Recent studies identified Siglec expression predominantly on immune cells, indicating an important regulating role in the immune system (Crocker and Redelinghuys, 2008). The detection of high levels of Siglec-F expression on alveolar macrophages indicates the possibility that Siglec-F ligands are involved in regulating the functions of alveolar macrophages. However, the effects of Siglec-F engagement on the alveolar macrophage vitality and mediator release require further investigation.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30600265, 30871119, and 81070024) and the Science and Technology Department of Sichuan Province, China (No. 10FZ0043)
References
- 1.Angata T, Varki A. Cloning, characterization, and phylogenetic analysis of Siglec-9, a new member of the CD33-related group of Siglecs. Evidence for co-evolution with sialic acid synthesis pathways. J Biol Chem. 2000;275(29):22127–22135. doi: 10.1074/jbc.M002775200. [DOI] [PubMed] [Google Scholar]
- 2.Angata T, Hayakawa T, Yamanaka M, Varki A, Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 2006;20(12):1964–1973. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 3.Angata T, Tabuchi Y, Nakamura K, Nakamura M. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17(8):838–846. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi SM, Prince LR, McPhillips K, Allen L, Marriott HM, Taylor GW, Hellewell PG, Sabroe I, Dockrell DH, Henson PW, et al. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa . Am J Respir Crit Care Med. 2008;177(1):35–43. doi: 10.1164/rccm.200612-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39(3):317–324. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges VM, Vandivier RW, McPhillips KA, Kench JA, Morimoto K, Groshong SD, Richens TR, Graham RR, Muldrow AM, van Heule L, et al. TNF-α inhibits apoptotic cell clearance in the lung, exacerbating acute inflammation. Am J Physiol Lung Cell Mol Physiol. 2009;297(4):L586–L595. doi: 10.1152/ajplung.90569.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao H, Lakner U, de Bono B, Traherne JA, Trowsdale J. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol. 2008;38(8):2303–2315. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- 8.Cho JY, Song DJ, Lee SY, Miller M, Dayan S, Doherty TA, Varki A, Broide DH. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11(1):154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corradin SB, Mauël J. Phagocytosis of Leishmania enhances macrophage activation by IFN-γ and lipopolysaccharide. J Immunol. 1991;146(1):279–285. [PubMed] [Google Scholar]
- 10.Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochem Soc Trans. 2008;36(6):1467–1471. doi: 10.1042/BST0361467. [DOI] [PubMed] [Google Scholar]
- 11.Crocker PR, Clark EA, Filbin M, Grodon S, Jones Y. Siglecs: a family of sialic-acid binding lectins. Glycobiology. 1998;8(2):v–vi. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- 12.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 13.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman SD, Kelm S, Barber EK, Crocker PR. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85(8):2005–2012. [PubMed] [Google Scholar]
- 15.Guo JP, Myers A, Choi O, Lee HS, Zhu Z, Hudson SA, Brummet M, Bovin NV, Crocker PV, Bochner BS. Ligands for Siglec-8 and Siglec-F: binding characteristics and tissue distribution. J Allergy Clin Immunol. 2007;119(1):S299. doi: 10.1016/j.jaci.2006.12.542. [DOI] [Google Scholar]
- 16.Hu B, Sonstein J, Christensen PJ, Punturieri A, Curtis JL. Deficient in vitro and in vivo phagocytosis of apoptotic T cells by resident murine alveolar macrophages. J Immunol. 2000;165(4):2124–2133. doi: 10.4049/jimmunol.165.4.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu B, Jennings JH, Sonstein J, Floros J, Todt JC, Curtis JL. Resident murine alveolar and peritoneal macrophages differ in adhesion of apoptotic thymocytes. Am J Respir Cell Mol Biol. 2004;30(5):687–693. doi: 10.1165/rcmb.2003-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Licht R, Jacobs CW, Tax WJ, Berden JH. An assay for the quantitative measurement of in vitro phagocytosis of early apoptotic thymocytes by murine resident peritoneal macrophages. J Immunol Methods. 1999;223(2):237–248. doi: 10.1016/S0022-1759(98)00212-9. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto K, Janssen WJ, Fessler MB, McPhillips KA, Borges VM, Bowler RP, Xiao YQ, Kench JA, Henson PM, Vandivier RW. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol. 2006;176(12):7657–7665. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiquchi M, Azuma M, Yagita H, Okumura K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113(16):3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 21.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101(12):5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 22.O′Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30(5):240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saltan N, Kutlu HM, Hür D, İşcan A, Say R. Interaction of cancer cells with magnetic nanoparticles modified by methacrylamido-folic acid. Int J Nanomed. 2011;6:477–484. doi: 10.2147/IJN.S16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19(5):507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y, Kawamura I, Nomura T, Tsuchiya K, Hara H, Dewamitta SR, Sakai S, Qu H, Daim S, Yamamoto T, et al. Toll-like receptor 2- and MyD88-dependent phosphatidylinositol 3-kinase and Rac1 activation facilitates the phagocytosis of Listeria monocytogenes by murine macrophages. Infect Immun. 2010;78(6):2857–2867. doi: 10.1128/IAI.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183(8):5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Shaper NL, Itonori S, Heffer LM, Sheikh KA, Schnaar RL. Myelin-associated glycoprotein (Siglec-4) expression is progressively and selectively decreased in the brains of mice lacking complex gangliosides. Glycobiology. 2004;14(9):851–857. doi: 10.1093/glycob/cwh107. [DOI] [PubMed] [Google Scholar]
- 28.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15(11):1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 29.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PH, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169(7):3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 30.von Gunten S, Yousefi S, Seitz M, Jakob SM, Schaffner T, Seqer R, Takala J, Villiger PM, Simon HU. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 2005;106(4):1423–1431. doi: 10.1182/blood-2004-10-4112. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Cui X, Tai G, Ge J, Li N, Chen F, Yu F, Liu Z. A critical role of activin A in maturation of mouse peritoneal macrophages in vitro and in vivo. Cell Mol Immunol. 2009;6(5):387–392. doi: 10.1038/cmi.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, Ryu SD, Bickel CA, Hudson SA, Bobcher BS. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 2008;121(2):499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;83:14.1.1–14.1.14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63(9):1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Recommended reading
- 35.Boskabady MH, Keyhanmanesh R, Khameneh S, Doostdar Y, Khakzad MR. Potential immunomodulation effect of the extract of Nigella sativa on ovalbumin sensitized guinea pigs. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(3):201–209. doi: 10.1631/jzus.B1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang SC, Jiang DL, Chen M, Li D, Zhang JX. Clodronate-containing liposomes attenuate lung injury in rats with severe acute pancreatitis. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(11):828–835. doi: 10.1631/jzus.B1000044. [DOI] [PMC free article] [PubMed] [Google Scholar]