Abstract
The evasion of apoptosis is a key characteristic of cancer, and thus strategies to selectively induce apoptosis in cancer cells hold considerable promise in personalized anticancer therapy. Structurally similar procaspase activating compounds PAC-1 and S-PAC-1 restore procaspase-3 activity through the chelation of inhibitory zinc ions in vitro, induce apoptotic death of cancer cells in culture, and reduce tumor burden in vivo. IP or IV administrations of high doses of PAC-1 are transiently neurotoxic in vivo, while S-PAC-1 is safe even at very high doses and has been evaluated in a Phase I clinical trial of pet dogs with spontaneously occurring lymphoma. Here we show that PAC-1 and S-PAC-1 have similar mechanisms of cell death induction at low concentrations (less than 50 µM), but at high concentrations PAC-1 displays unique cell death induction features. Cells treated with a high concentration of PAC-1 have a distinctive gene expression profile, unusual cellular and mitochondrial morphology, and an altered intracellular Ca2+ concentration, indicative of endoplasmic reticulum (ER) stress-induced apoptosis. These studies suggest strategies for anticancer clinical development, specifically bolus dosing for PAC-1 and continuous rate infusion for S-PAC-1.
Keywords: Apoptosis, small molecule, zinc chelation, ER stress, blood-brain barrier, calcium release, punctate mitochondria, constant rate infusion, bolus dose, transcript profiling
Introduction
The discovery of compounds that induce apoptotic cell death is a major aim of anticancer drug discovery. As cancer cells characteristically evade apoptosis through inactivating mutations and aberrant expression levels of key proteins, the challenge is to reactivate the apoptotic cascade, or to exploit those parts that remain functional.1, 2 Among the current arsenal of compounds that target the apoptotic pathway are small molecule disruptors of the p53-MDM2 interaction,3, 4 inhibitors of Bcl-2,5 and ligands for XIAP.6 Since major apoptotic pathways converge on the activation of executioner caspases-3/-7 from their less active procaspase zymogens, one appealing apoptosis-inducing strategy is the direct activation of executioner procaspases with a small molecule, bypassing defective upstream apoptotic circuitry. Procaspase-3 levels are elevated in many tumor types compared to normal tissue,7–13 therefore a procaspase-3 activating compound could have value as a selective personalized anticancer drug. To date, the first discovered procaspase-activating compounds are procaspase-3/-7 activators PAC-1,14–16 S-PAC-1,17 other PAC-1 derivatives,18, 19 and procaspase-3/-6 activator 1541.20, 21
PAC-1 and its sulfonamide derivative S-PAC-1 activate procaspase-3 in vitro through the chelation of inhibitory zinc ions,15, 17, 18 induce apoptotic death in cultured cancer cells selectively,14, 17 and are effective in murine tumor models and pet dogs with lymphoma.14, 16, 17 In cancer cells, a fluorescent derivative of PAC-1 co-localizes with sites of caspase-3/-7 activity, suggesting that PAC-1 acts to chelate labile inhibitory Zn2+ from procaspase-3/-7 in the cell to induce apoptotic death.18 When administered via IP or IV injection, high doses of PAC-1 elicit transient neuroexcitation (seizure and ataxia) in mice and dogs, thus S-PAC-1 was developed as a viable procaspase activator for in vivo therapeutic use.17 This compound is well tolerated at doses in excess of 350 mg/kg and 60 mg/kg in mice and dogs, respectively, and showed promising results in a small Phase I clinical trial of pet dogs with lymphoma.17 As PAC-1 and S-PAC-1 elicit drastically different neurologic responses in vivo, we sought to further characterize similarities and differences between PAC-1 and S-PAC-1 in an effort to understand how each compound can be best utilized in anticancer therapy. In this work, we report further mechanistic similarities between low concentrations (< 50 µM) of PAC-1 and S-PAC-1 but differences at high concentrations, suggesting different dosing strategies and anticancer applications for each compound.
Experimental Section
Cell lines and reagents
All cell lines were purchased from ATCC, grown in a 37 °C, 5% CO2, humidified environment, in media containing 1% penicillin/streptomycin. Cells were used within six months of purchase/resuscitation with no further authentication. Cell culture conditions are as follows: Human cell lines U-937 lymphoma and HeLa cervical cancer in RPMI 1640 or DMEM with 10% fetal bovine serum (FBS), HL-60 human leukemia in IMEM with 20% FBS, and Neuro-2a murine neuroblastoma cells in EMEM with 10% fetal calf serum (FCS). PAC-1 and S-PAC-1 were synthesized as previously reported.14, 18 The following compounds were purchased: thapsigargin (Tg, Santa Cruz Biotechnology), N,N,N',N'-Tetrakis(2-pyridylmethyl)ethylenediamine (TPEN, Sigma), and digitonin (Calbiochem). Compounds were dissolved in DMSO (Sigma) for cell culture studies, or 2-hydroxypropyl-β-cyclodextrin (HPβCD, CDT) for cell permeability and blood-brain barrier studies.
Cellular Zn2+ and Ca2+ measurements
The following genetically encoded FRET sensors were used in this study: ZapCY2 was used to study cytosolic Zn2+,22 D3cpv for cytosolic Ca2+,23 and D1ER for ER Ca2+ levels.24 HeLa cells were transfected with sensor constructs in 3.5 cm glass bottom imaging dishes using TransIt (Mirus), 1 µg DNA: 5 µl TransIt, and imaged 48h post-transfection with an Axiovert 200M inverted fluorescence microscope (Zeiss) with a 1.3NA 40X oil immersion objective, and a Cascade 512B CCD camera (Roper scientific), equipped with a Xenon arc lamp (XBO75). To study cytosolic Zn2+, cells were imaged in phosphate-free HEPES-buffered Hanks’ Balanced Salt Solution (HHBSS), pH = 7.4 to prevent precipitation of zinc. At the end of each experiment, cells were treated with 150 µM TPEN, followed by 10 µM digitonin and a Zn2+/EGTA buffered solution with free Zn2+ buffered at 9.5 nM. These perturbations allow determination of low and high FRET ratios, respectively. The 9.5 nM Zn2+ solutions were made from Zn2+ /EGTA - buffered solutions, as described.22 To study cytosolic Ca2+, cells were imaged in HEPES-buffered Hanks’ Balanced Salt Solution (HHBSS), pH = 7.4. At the end of each experiment, cells were treated with 5 µM ionomycin and 10 mM CaCl2 to identify the maximum FRET ratio of the sensor. Details regarding image acquisition and analysis are in the Supplemental Data. All experiments were conducted at least three times. Statistical analysis was performed using ANOVA with a post-hoc test or Student’s t-test using KaleidaGraph.
Gene expression profiling and Connectivity Map analysis
U-937 or HL-60 cells (3,000,000 cells/sample) were incubated in the presence of 25 µM PAC-1 or S-PAC-1, 100 µM PAC-1, or DMSO vehicle (0.2% final DMSO v/v) for 6 hours in a 12-well plate in culture media for the respective cell lines as described above. RNA isolation, whole genome transcript profiling was performed on the Illumina HumanHT-12 bead array for seven compounds and DMSO control from three independent experiments. Data processing and comparison to the Connectivity Map database were all performed exactly as previously reported,25 and is described in more detail in the Supplementary Data.
Transmission Electron Microscopy (TEM) and Confocal microscopy
U-937 cells were harvested and transferred to a 24-well plate at 500,000 cells/well in 500 µL RPMI 1640 media with 10% FBS. DMSO, PAC-1, or S-PAC-1, were dissolved from DMSO stocks in RPMI 1640 with 10% FBS as pre-mixed concentrated solutions. Compound solutions of 500 µL were added to the wells with cells, so that the final concentration of compound was 100 µM PAC-1, 100 µM S-PAC-1 (in 1% v/v DMSO), or DMSO for a compound exposure of 1, 2, and 3 hours. The cells were washed once with PBS, and pelleted. Karnovsky’s fixative (500 µL/sample) was filtered through a 0.22 micron filter and fixative was added directly to the cell pellet. The preparation and imaging of samples by TEM were carried out in part by the Center for Microanalysis of Materials of the Frederick Seitz Materials Research Laboratory Central Facilities, University of Illinois. Images of several cells in each sample were taken by film and scanned. Shown are representative cell images over three experiments.
HeLa cells were harvested and grown in Lab-Tek 4-well chambered 1.0 borosilicate coverglass (Nunc) dishes to ~50% confluency in RPMI 1640 media. The cells were washed with Hanks’ Balanced Salt Solution (HBSS). The cells were treated with 25 and 100 µM PAC-1, 100 µM S-PAC-1, and 10 µM thapsigargin pre-mixed in 500µM HBSS for exposure times up to 12h, and stained with Mitotracker Red CMXRos (Invitrogen) and SYBR Green I (Invitrogen) in HBSS. For details regarding order of treatment and staining, see the Supplemental Data. The cells, in 500 µL HBSS, were imaged on a LSM700 confocal microscope (Zeiss) using a 40X oil immersion objective. Shown cell images are representative of the cell population over at least three experiments. Cell viability was diagnostically assessed by flow cytometry via FITC-annexin V and propidium iodide as described in the Supplementary Data.
Cell cytotoxicity
U-937 cells were harvested and 500,000 cells/sample were exposed to DMSO for 1h, and 100 µM PAC-1 or S-PAC-1 (as described above for transmission electron microscopy experiments) for exposure times of 4, 8, 12, and 24 hours. Following compound exposure time, the cells were centrifuged in the 24-well plate, washed once in 500 µL RPMI 1640, centrifuged again, and resuspended in 500 µL RPMI 1640. At 24h, cell viability was assessed by Annexin-V/propidium iodide (AV/PI) double staining and flow cytometry. Data collected from at least 10,000 cells was analyzed using the BD FACSDiva Software, with percentage of whole cells with negative staining for FITC-annexin V and propidium iodide reported as viable cells. The percentage of viable cells was averaged over three replicate experiments, statistical analysis was performed as a Student’s t-test in Excel.
Neuronal cell permeability assay and Blood-brain barrier (BBB) penetration study
Neuro-2a cells were grown to ~80% confluence in 10 cm culture dishes and were detached with trypsin-EDTA, and suspended in EMEM with 10% FCS. Cells were centrifuged, washed twice with chilled PBS, then resuspended in serum free media containing 50 µM PAC-1 or S-PAC-1 dissolved in HPβCD to a final volume of 10 mL in conical tubes and gently agitated on a laboratory orbital shaker set at 40 rpm and 37 °C for 30 minutes. Cells were washed with chilled PBS, centrifuged and residual supernatant removed three times. Resultant cell pellets were lysed with 1mL of M-PER, centrifuged, and residual cell lysates were collected and analyzed by HPLC for PAC-1 or S-PAC-1 concentrations. Experiments were conducted in triplicate; statistical analysis was performed as a Student’s t-test in Excel.
BBB penetration study was performed as follows: 12 week old, female, C57/BL6 mice were administered PAC-1 or S-PAC-1 in HPβCD at 75mg/kg via lateral tail vein injection. Five minutes post lateral tail vein injection, mice were sacrificed, blood was collected by lacerating the right auricle with iris scissors. An 18 gauge angiocatheter was inserted through the left ventricle and all residual circulatory volume was removed by perfusing 3.6 mL of 0.9% saline solution over 6 minutes via an analog peristaltic pump. Brains were harvested from the cranial vault, weighed, and homogenized in 3 mL of ice cold methanol. Homogenized samples were centrifuged at 4000 rpm for 10 minutes and supernatant and tissue debris separated. The resultant supernatant was then centrifuged at 10,000 rpm for an additional 10 minutes and clarified supernatants (3 mL) were analyzed, along with serum, by HPLC to determine compound concentrations. Experiments were conducted in triplicate, statistical analysis was performed as a Student’s t-test in Excel.
Results
Effect of PAC-1 and S-PAC-1 on intracellular Zn2+ concentration
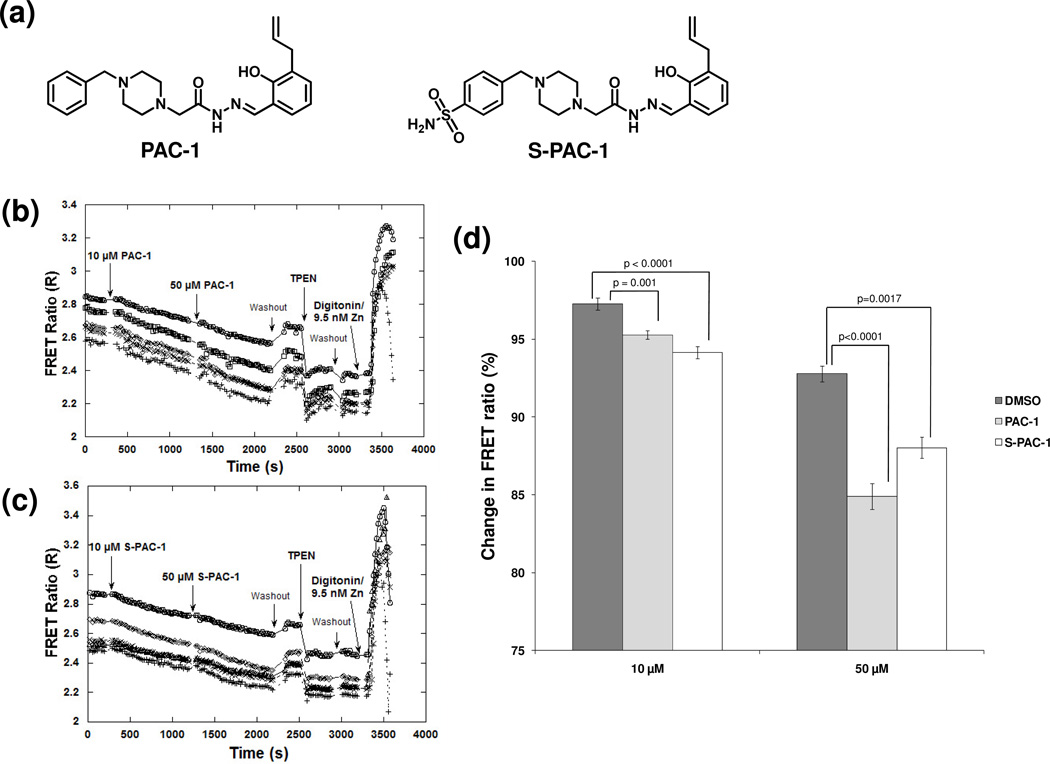
PAC-1 and S-PAC-1 (Figure 1a) have been shown to activate procaspase-3 in vitro through the chelation of inhibitory zinc ions,15, 17, 18 and both compounds chelate zinc with comparable Kd values (52 nM for PAC-1 and 46 nM for S-PAC-1);17, 18 however, the effect of these compounds on the cellular zinc pool has not been examined. Genetically-encoded Zn2+ sensors have been developed to study changes in intracellular Zn2+ concentrations in live cells.22, 26 A conformational change in the sensor upon Zn2+-binding leads to increased fluorescence resonance energy transfer (FRET), offering an opportunity to visualize the changes in the cellular labile zinc pool upon treatment with PAC-1 or S-PAC-1. Zn2+ levels are proportional to the FRET ratio (FRET/CFP signal) which can be measured by fluorescence microscopy. HeLa cells, transfected with the ZapCY2 sensor (apparent Kd’ for Zn2+: 811 pM)22, were exposed to PAC-1 or S-PAC-1 for 15 min at 10 µM, the approximate concentration at which both compounds induce half-maximal cytotoxicity at 72 h in a variety of cell lines.14, 17, 18 Upon treatment with either PAC-1 or S-PAC-1, the FRET ratio decreased, indicating a decrease in intracellular free Zn2+ (Figure 1b–d). The FRET ratio decreased further upon exposure to 50 µM PAC-1 or S-PAC-1 for 15 min (Figure 1b, 1c). To demonstrate that the decrease in FRET was due to chelation of labile zinc, cells were washed and exposed to 150 µM TPEN, a cell permeable Zn2+ chelator with a Kd of 2.6 × 10−16 M.27 The FRET ratio of the sensor decreased further upon treatment with this powerful Zn2+ chelator. Finally, when cells were permeabilized with digitonin and treated with a solution containing 9.5 nM Zn2+, the FRET ratio of the sensor increased. Together, these experiments suggest that PAC-1 and S-PAC-1 penetrate cells and chelate intracellular Zn2+.
Figure 1. Changes in intracellular zinc concentration upon PAC-1 and S-PAC-1 treatment.
a) Structures of PAC-1 and S-PAC-1. FRET ratio changes in HeLa cells using the genetically-encoded zinc sensor ZapCY2. Graphs of a representative individual experiment show a decrease in intracellular free zinc in five cells treated with b)10 µM PAC-1 followed by 50 µM PAC-1, or c) 10 µM S-PAC-1 followed by 50 µM S-PAC-1. d) The decrease in intracellular zinc for both PAC-1 and S-PAC-1 is significant over multiple experiments. Error bars are standard error of the mean, p-values are from DMSO (10 cells or n=2 experiments), PAC-1 (21 cells or n=4 experiments), S-PAC-1 (14 cells or n=3 experiments).
Effect of PAC-1 on global gene expression
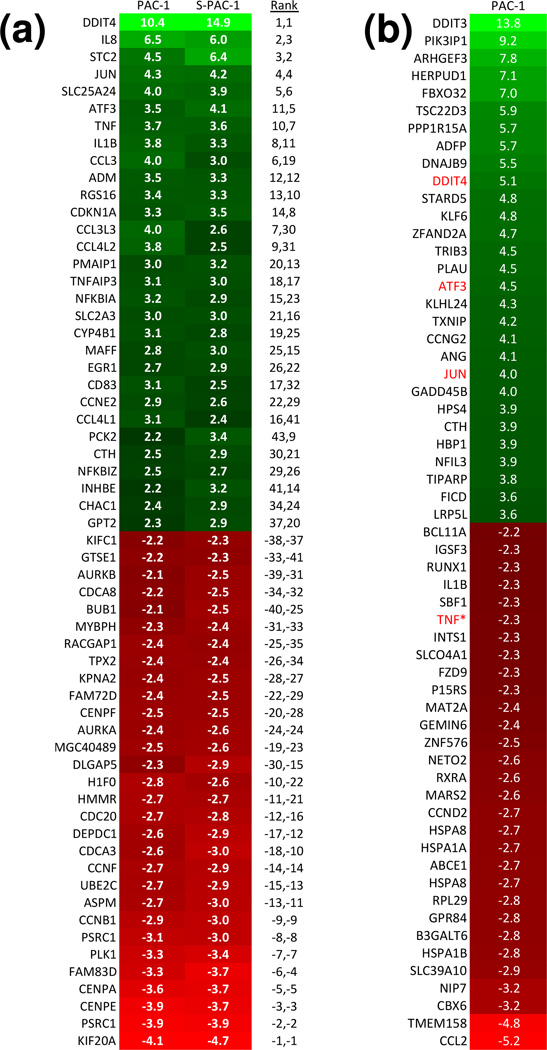
Transcript profiling can be a useful tool for studying the effect of compounds on global gene expression,28 and was thus used in the assessment of PAC-1 and S-PAC-1. First, mRNA was isolated from HL-60 cells that were treated with PAC-1 and S-PAC-1 (25 µM for each) for 6 hours, and a transcript profile was generated. At this low concentration, PAC-1 and S-PAC-1 have a very similar pattern of up- and downregulated transcripts. DDIT4, IL8, STC2, JUN, SLC25A24, ATF3, TNF were all among the most highly upregulated transcripts in both treatment groups compared to DMSO control (Figure 2a). The Spearman rank correlation value,29 a measure of similarity, is 0.928 for PAC-1 and S-PAC-1 profiles (using the top 200 up- and downregulated genes). These results indicate that at 25 µM, PAC-1 and S-PAC-1 act on cancer cells in a remarkably similar manner. The transcript profile for 25 µM PAC-1 was also carried out in U-937 cells, and the results largely matched those obtained in HL-60 cells; numerous significantly up- and downregulated genes with a p-value cut-off of 0.05 were shared between transcript profiles in the two cell lines (Supplemental Figure 1).
Figure 2. Expression profiles for PAC-1 and S-PAC-1.
a) HL-60 cells were treated with PAC-1 or S-PAC-1 at 25 µM for 6 hours. Shown are the most up- or down-regulated transcripts (p < 0.05) compared to DMSO control cells. White text: Fold-change values, black text: the ranks of transcripts in individual profiles. b) U-937 cells were treated with PAC-1 at 100 µM for 6 hours. Shown are the 30 most up- or down-regulated transcripts compared to DMSO control cells. White text: fold change values, red text: common genes with PAC-1 treatments at low (25 µM) concentrations. TNF (indicated with an *) is downregulated at high PAC-1 concentrations but shows upregulation at low PAC-1 concentrations.
In a second transcript profile experiment, the expression signature of cells treated with high (100 µM) and low (25 µM) concentrations of PAC-1 in U-937 cells were compared. The transcript profiles between 25 µM and 100 µM PAC-1 have a Spearman rank correlation value of 0.436, indicating that the expression signatures are quite different. When compared to vehicle-treated cells, only three genes, DDIT4, ATF3, and JUN, are shared in the top 30 most upregulated genes for both high and low PAC-1 treatments. TNF, which ranks among the top 30 most upregulated genes for 25 µM PAC-1-treated cells, is among the top 30 most downregulated transcripts in the 100 µM PAC-1 profile (Figure 2b). The transcript profiling data also suggest potential pathways affected and mechanisms of action at the high concentration of PAC-1. Three of the top 10 most highly upregulated transcripts, DDIT3 (CHOP transcription factor), PI3KIP1 (PI3K interacting protein 1), and HERPUD1(Herp), are associated with ER stress and unfolded protein response (UPR) pathways.30–32 As such, we hypothesized that at high concentrations, PAC-1 may induce cell death via the ER stress pathway.
Further supporting this hypothesis is the similarity of the expression signatures of PAC-1 and thapsigargin using the Connectivity Map. The Connectivity Map database contains 7000 gene expression profiles representing about 1300 individual compounds.33 In comparison with the expression signature of 100 µM PAC-1, thapsigargin was the top permuted compound from the database with the most similar gene expression signature (Supplemental Table S1). As a sarco/endoplasmic reticulum calcium ATPase (SERCA) inhibitor, thapsigargin induces ER stress as the ER calcium stores are depleted by an inhibition of the ability of the ER to pump Ca2+.34 Thus, this similar profile to thapsigargin supports ER stress as a possible mechanism for PAC-1 at high concentrations.
Effect of PAC-1 on cellular and mitochondrial morphology
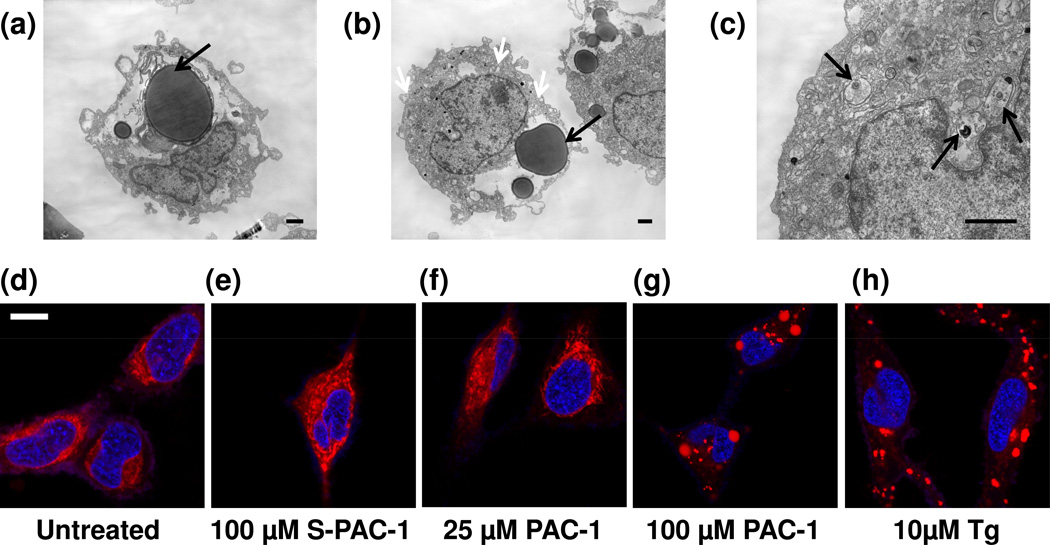
Transmission electron microscopy (TEM) was utilized to study the effect of a high concentration of PAC-1 on general cellular morphology. U-937 cells were exposed to 100 µM PAC-1 or S-PAC-1 for 1–3 hours. Following treatment, cells were immediately washed, fixed, and imaged by TEM. Compared to DMSO control cells, the remarkable changes to cellular morphology upon 100 µM PAC-1 treatment include large lysosome-like structures surrounded with myelin-like membranes, dilated ER, and myelin-like inclusions or packaging in the mitochondria (Figure 3a–c, Supplemental Figure 2b). A few cells undergoing apoptosis and an apoptotic body were observed in the 3h 100 µM PAC-1 treatment sample (Supplemental Figure 2c–d), however there were no phenotypes indicative of necrosis or autophagy observed in any of the examined cells. The cells treated with 100 µM S-PAC-1 for up to 3h resembled the 1h DMSO control cells (Supplemental Figure 2a, e–g).
Figure 3. Changes in cellular and mitochondrial morphology.
T.E.M. images of U-937 cells treated with a) 100 µM PAC-1 for 1h, 5kx, b) 100 µM PAC-1 for 1h, 4kx (black arrows: large lysosome-like structures, white arrows: dilated ER), and c) 100 µM PAC-1 for 1h, 15kx (arrows: dark myelin-like mitochondrial inclusions). Images are representative of several cells, n = 3 experiments, all scale bars indicate 1 micron. Confocal images at 40× of HeLa cells stained with Mitotracker Red (false-colored red) and SYBR Green (false-colored blue) show no changes in mitochondria morphology at 60 min in d) untreated control, e) 100 µM S-PAC-1, f) 25 µM PAC-1, but punctate mitochondrial staining with g) 100 µM PAC-1, and h) 10µM thapsigargin (Tg). All confocal images are 40× magnification. Images are a single replicate, n ≥ 3 experiments, scale bar represents 10 microns.
The morphological changes visualized by electron microscopy in PAC-1-treated cells, especially mitochondrial changes (Figure 3c, and Supplemental Figure 2b), motivated further investigation of the effect of PAC-1 on mitochondrial morphology. HeLa cells were treated with 25 µM and 100 µM PAC-1, 10 µM thapsigargin (ER stress inducing compound), and 100 µM S-PAC-1. The mitochondria were stained with Mitotracker Red and visualized by confocal microscopy. After a 60 min exposure of 100 µM PAC-1 and 10 µM thapsigargin, the mitochondrial staining was localized and punctate, compared to the diffuse mitochondrial staining observed in untreated cells or cells treated with 25 µM PAC-1 or 100 µM S-PAC-1 (Figure 3d–h). Punctate mitochondrial staining was noted with 100 µM PAC-1 treatment at exposure times as early as 30 min (Supplemental Figure 3a). Cell viability was diagnostically measured at the exposure times by cell flow cytometry of a sample population of 5,000 cells. Even at 12h, when ~50% of HeLa cells exposed to 25 µM PAC-1 and 100 µM S-PAC-1 remained viable, no changes in mitochondrial morphology were noted (Supplemental Figure 3b–h). These data suggest that at high concentrations, PAC-1 induces a unique general cellular morphology compared to control and S-PAC-1-treated cells. PAC-1, at a high concentration, also induces a mitochondrial phenotype similar to thapsigargin. The staining of mitochondria was diffuse and unaltered with a low concentration of PAC-1 or high concentration S-PAC-1, even at longer exposure times when the cell population was undergoing death, indicating that a high concentration of PAC-1 behaves very differently than it does at a lower concentration or compared to equimolar amounts of S-PAC-1.
Effect of a high concentration of PAC-1 on cytosolic and ER Ca2+ concentration
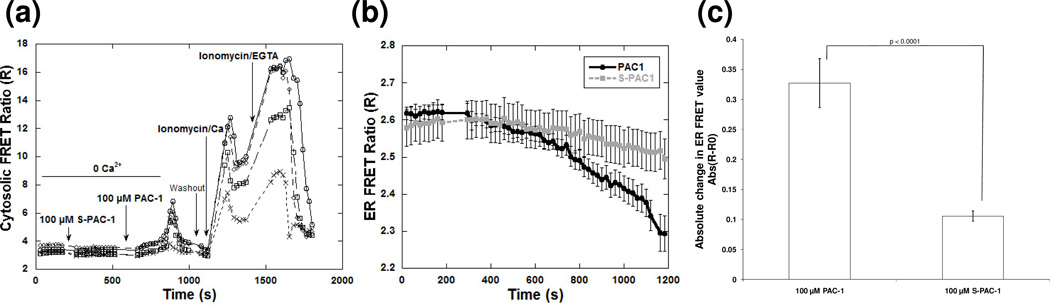
The similarity of thapsigargin and high concentrations of PAC-1 in both the expression profile and mitochondrial morphology experiments suggests that both compounds may be acting by a similar mechanism. Thapsigargin is known to cause an increase in cytosolic Ca2+ as the calcium stores in the ER are released.34 Thus, we used genetically-encoded calcium sensors to examine whether PAC-1 induced changes in cytosolic Ca2+ levels in live cells.23 Treatment of HeLa cells expressing the calcium sensor D3cpv with 100 µM PAC-1 in calcium-free media led to an increase in cytosolic Ca2+ (Figure 4a). A lower increase was observed upon treatment of the cells with 50 µM PAC-1 (Supplemental Figure 4a). However, under the same experimental conditions 100 µM S-PAC-1 showed no significant change in the FRET of the sensor (Figure 4a, Supplemental Figure 4a).
Figure 4. Changes in cytosolic and ER calcium levels upon PAC-1 and S-PAC-1 treatment.
Graphs of single representative experiments show a) an increase in the FRET ratio in four individual HeLa cells expressing genetically-encoded cytosolic calcium sensor D3cpV upon treatment with 100 µM PAC-1 but not S-PAC-1. Following treatment with PAC-1 or S-PAC-1, cells were washed and treated with ionomycin/Ca2+ to demonstrate the maximum FRET ratio and confirm sensor functionality in each cell. b) A greater decrease in the FRET ratio of genetically-encoded ER calcium sensor D1ER was observed upon treatment of 100 µM PAC-1 compared to S-PAC-1 (average of at least 9 cells or n=3 experiments) and c) the absolute change in FRET value is shown to demonstrate that 100 µM PAC-1 elicits a significantly different ER calcium response than S-PAC-1. Error bars represent standard deviation, p < 0.0001.
The increase in cytosolic Ca2+ induced by 100 µM PAC-1 was observed in absence of extracellular calcium, suggesting that PAC-1 induced release of Ca2+ from the ER. To test this hypothesis, a genetically-encoded calcium sensor that localizes to the ER, D1ER, was used to measure calcium levels within the ER.24 Treatment of HeLa cells with 100 µM PAC-1 for 15 minutes caused the FRET ratio of the ER-localized calcium sensor to decrease, indicating a decrease in ER Ca2+ (Figure 4b, c). A less significant decrease was noted for 100 µM S-PAC-1 treatments. Furthermore, compared to the decrease in ER Ca2+ observed for 100 µM PAC-1-treated cells, 50 µM PAC-1 elicited a smaller decrease, while 10 µM PAC-1 elicited no marked decrease in ER Ca2+, indicating that this effect is most noted at high PAC-1 concentrations (Supplemental Figure 4b). Together, the data from both cytosolic and ER Ca2+ sensors demonstrate that a high concentration of PAC-1, but not S-PAC-1, triggers a marked decrease in ER Ca2+, and a concomitant increase in cytosolic Ca2+. The release of Ca2+ from the ER is a distinct indicator and trigger of an acute ER stress response, and leads to ER stress-related apoptosis,35, 36 indicating that PAC-1 may behave as an ER stress inducing compound at high concentrations.
Cell permeability of PAC-1 and S-PAC-1
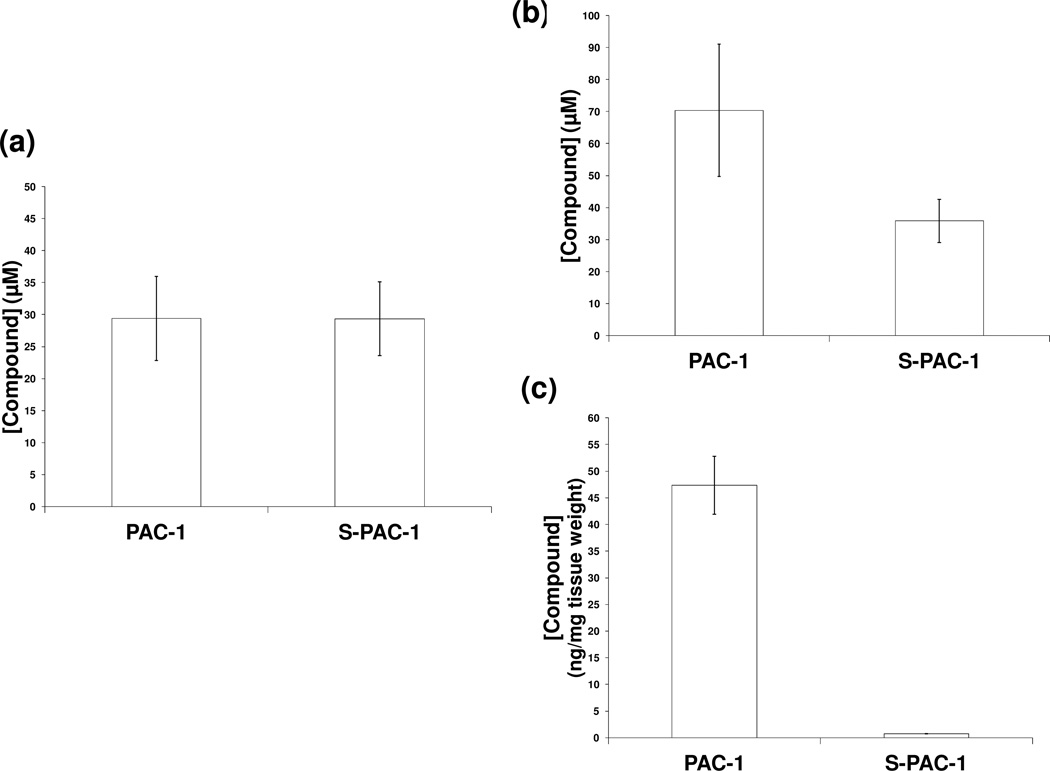
The divergent in vivo neuroexciation induced by PAC-1 and S-PAC-1 may be attributed to differences in the ability to penetrate neuronal cell membranes, or differences in BBB permeability.17 To explore the first hypothesis, Neuro-2a murine neuroblastoma cells were treated with either PAC-1 or S-PAC-1 (both in HPβCD) at 50 µM for 30 minutes. Following compound exposure and cell lysis, the lysates were analyzed by HPLC for intracellular compound concentration. Both PAC-1 and S-PAC-1 were detected in the cell lysates at equivalent concentrations (PAC-1: 29.4 ± 6.5 µM and S-PAC-1: 29.4 ± 5.7 µM) (Figure 5a). These results demonstrate that both PAC-1 and S-PAC-1 are equally capable of penetrating neuronal cell membranes, and together with evidence that both compounds enter cells and chelate intracellular zinc (Figure 2), suggest that cell permeability does not play an essential role in the different in vivo neuroexcitation induced by the two compounds.
Figure 5. Neuronal cellular membrane and BBB permeabilities of PAC-1 and S-PAC-1.
a) Mean concentrations of intracellular PAC-1 or S-PAC-1 within Neuro-2a cells following incubation with 50 µM PAC-1 or S-PAC-1 for 30 minutes. Experiment performed in triplicate, p > 0.5. To measure the BBB permeability of PAC-1 and S-PAC-1, C57BL/6 mice received PAC-1 or S-PAC-1 at 75mg/kg (dissolved in HPβCD) via lateral tail vein injection. Concentrations of PAC-1 and S-PAC-1 within b) serum (p < 0.005) and c) brain tissue (p < 0.005) 5 minutes post-injection. Cohort size per group (n=4). Error bars indicate standard deviation.
BBB penetrance of PAC-1 and S-PAC-1
We previously hypothesized that the differences in the BBB permeability of PAC-1 and S-PAC-1 might contribute to the observed neuroexcitation induced by PAC-1 in vivo.17 The calculated logBB is a predictive value based on the ClogP of a compound and its total polar surface area.37, 38 The PAC-1 calculated logBB value is −0.07 (ratio of PAC-1 concentrations in brain:blood of 46:54), while S-PAC-1 has a calculated logBB value of −1.26 (ratio of S-PAC-1 concentrations in brain:blood of 4:96).17 We performed an in vivo study of BBB penetrance in which two cohorts of four C57/BL6 mice were injected with 75 mg/kg PAC-1 or S-PAC-1 (both formulated in HPβCD) via the lateral tail vein and subsequently sacrificed 5 minutes post-injection. Immediately following sacrifice, both serum and perfused brain samples were submitted for HPLC analysis of PAC-1 and S-PAC-1 concentration. While PAC-1 and S-PAC-1 serum concentrations differed by approximately 2-fold (70.4 ± 20.7 µM versus 35.8 ± 6.8 µM, respectively), the concentration of PAC-1 in the brain was 62 times greater than S-PAC-1 in the brain (47.3 ± 5.4 ng/mg versus 0.76 ± 0.05 ng/mg tissue, respectively) (Figure 5b, c). These data support the hypothesis that PAC-1 and S-PAC-1 have significantly different permeability to the BBB, as well as support the predictive power of the logBB calculations for these compounds. These results suggest that BBB penetration is a prerequisite for the observed transient neuroexcitation induced by PAC-1 when high concentrations are administered in vivo.
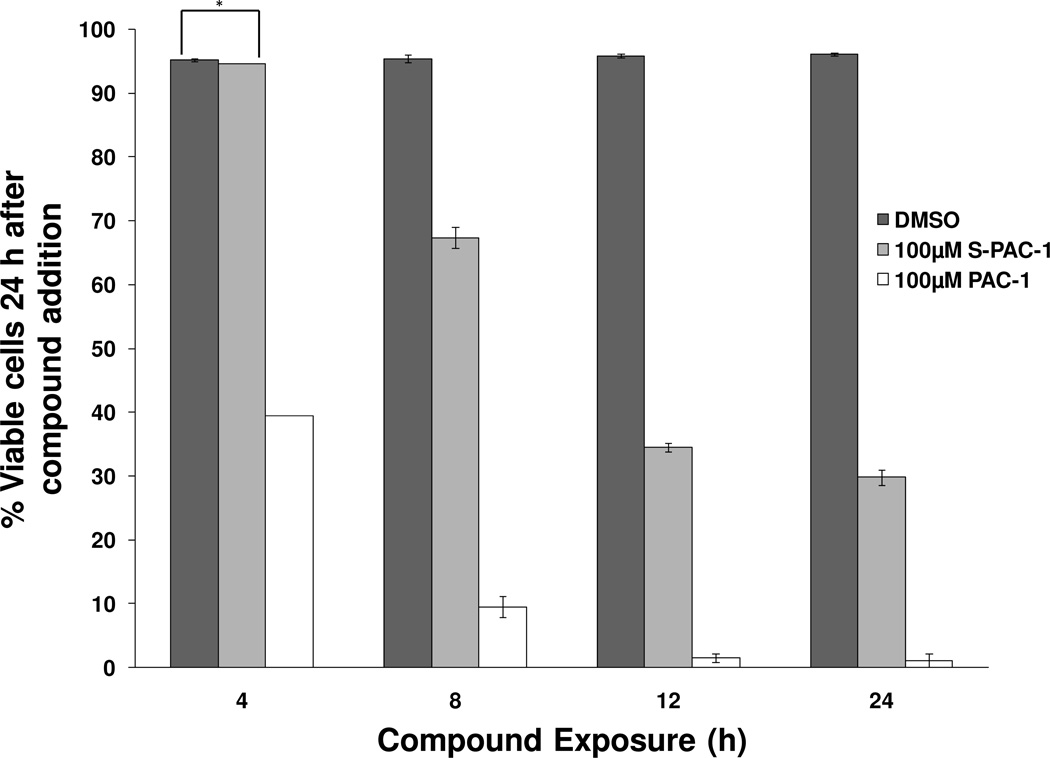
Effect of exposure time of PAC-1 on cell viability
Cell culture studies indicate that at high concentrations PAC-1 act to induce cell death through an ER stress-related mechanism, in addition to procaspase activation. Additionally, PAC-1 when administered at high doses via IP or IV injection, induces transient neurotexcitation in vivo, setting it apart from S-PAC-1.17 These studies prompted further investigation into the clinical implications of PAC-1 and S-PAC-1 as anticancer compounds. Previously, high concentrations of PAC-1 (50 and 100 µM) and S-PAC-1 (50 µM) were reported to induce cell death by apoptosis via the sequential staining of phosphatidylserine and nuclear DNA by AV/PI.14, 17 An examination of the effect of PAC-1 exposure on cell viability in cultured cells lends information on potential dosing strategies of PAC-1 in vivo, especially in light of the additional ER stress-related mechanism by which PAC-1 at high concentrations induces cell death. Thus, U-937 cells were treated with 100 µM PAC-1, 100 µM S-PAC-1, and DMSO for various exposure times. After treatment, the cells were washed and incubated in fresh media. At 24 hours, the cells were stained with AV/PI to assess cell viability by flow cytometry. While PAC-1 and S-PAC-1 induce death with a similar potency in a 24- or 72-hour continuous exposure,17 at exposure times as short as 4 hours, 100 µM PAC-1 induced large amounts of cell death as assessed at 24 hours. Furthermore, this effect was not observed with 100 µM S-PAC-1 (Figure 6, Supplemental Figure 5). Since PAC-1 and S-PAC-1 have comparable cell permeability, as assessed in the Neuro2a cell permeability assay, the unique ability of short exposures of a high concentration of PAC-1 to induce potent cell death may be related to the additional ER stress-related mechanism of PAC-1. Furthermore, the capability of short exposures of highly concentrated PAC-1 to induce cell death suggests that a transient exposure of the compound may be sufficient in inducing cancer cell death when a high serum concentration of PAC-1 is achieved in vivo.
Figure 6. Short exposure to PAC-1 induces cell death.
U-937 cells were treated with 100 µM PAC-1, 100 µM S-PAC-1, or DMSO for various exposure times, and cell viability was assessed by flow cytometry of the cells double stained AV/PI. Shown are the percent viable cells at 24h averaged over triplicate data. Error bars indicate standard error of the mean, * indicates p > 0.005, all other relationships between samples/exposure time, p < 0.005.
Discussion
In this study, we report two major findings: 1) PAC-1 and S-PAC-1 at low concentrations (less than 50 µM) induce death through a similar mechanism, and at high concentrations, short exposures of PAC-1 kill cells potently through an ER stress-related cell death mechanism. 2) PAC-1 and S-PAC-1 have similar cell membrane permeability, yet major differences in exposure times to induce cell death and BBB penetrance, leading to different clinical implications. At low concentrations (50 µM or lower), the evidence supports the hypothesis that PAC-1 and S-PAC-1 serve as zinc-chelating procaspase activating compounds in the cell. In addition to their ability to activate procaspase-3 and induce apoptotic death, PAC-1 and S-PAC-1 have comparable zinc-binding Kd values and cytotoxic IC50 values.17, 18 In this study, low concentrations of PAC-1 and S-PAC-1 elicit highly correlated transcript profiles in cells and cause a decrease in intracellular zinc concentrations. These observations contribute additional evidence that both compounds chelate labile zinc to activate procaspase-3 in the cell.
High concentration PAC-1 as an ER stress inducing compound
Evidence presented herein suggests that at high concentrations, PAC-1 induces cell death through a mechanism that is related to ER stress, in addition to its ability to activate procaspase-3/-7 via zinc chelation. A high concentration of PAC-1 produces a distinct gene expression signature that is highly similar to ER stress inducer thapsigargin. Cellular morphology is altered in cells treated with a high concentration of PAC-1, in particular the dilated ER39, 40 and enlarged lysosomes, indicative of an ER stress-related response.41 The release of Ca2+ through thapsigargin treatment has been shown to promote the hemolytic fusion of multiple lysosomes into large lysosomes in fibroblasts.42 Comparisons of mitochondrial morphology show that a high concentration of PAC-1 induces punctuate mitochondrial staining also observed in thapsigargin treatment, as shown in Figure 4 and in other studies.43, 44 The rapid mitochondrial changes may be due to mitochondrial Ca2+ uptake, leading to release of cytochrome c and apoptosis through apoptosome formation,45–47 and this has been observed in thapsigargin treatment.48 Furthermore, Seervi and co-workers recently observed cytochrome c release in cells treated with high concentrations of PAC-1 independent of bax, bak, bcl-2, and bcl-xL expression, supporting our hypothesis that mitochondrial release of cytochrome c may be induced through ER stress; importantly, Seervi observed no cytochrome c release in cells treated with low (< 30 µM) concentrations of PAC-1.49 Data presented herein show that PAC-1 at high concentrations causes an increase in cytosolic calcium concentration within minutes of treatment, a hallmark of many ER stress inducing compounds, including thapsigargin34 and tunicamycin.50
Since ER stress inducing compounds thapsigargin (SERCA inhibitor) and tunicamycin (N-linked protein glycosylation inhibitor) have different targets yet share similar ER stress-related phenotypes, it is possible that a high concentration of PAC-1 elicits a similar ER stress-related phenotype by acting at a completely different target than thapsigargin and tunicamycin. Furthermore, caspases have been found to be directly associated with the ER during the UPR pathways. For example, caspase-8 is thought to cleave ER membrane protein BAP31 to induce a release of Ca2+ from the ER,51, 52 procaspase-12 may accumulate and activate near the ER in the IRE1 pathway,53 or executioner procaspase-7 may activate procaspase-12 near the ER.46 ER stress may also induce caspase-2 cleavage of BID.54
The induction of ER stress holds strong potential as an anticancer strategy. It has been proposed that cancer cells are already in a general state of ER stress due to accumulation of mutated and misfolded proteins, hypoxic tumor environment, and general dysregulation of cellular homeostasis.55 Therefore ER stress inducing compounds could cause a more acute stress response in tumor cells than normal cells. Indeed, ER stress inducers, such as tunicamycin and bortezomib, have been investigated as novel anticancer drugs as single entity agents or combined with other chemotherapeutics.56–58 Therefore, PAC-1 at high concentrations may hold potential therapeutic promise in its role as an ER stress inducing compound.
Clinical implications for low and high concentrations of PAC-1
Experiments revealing that PAC-1 has a significant BBB penetrance support the hypothesis that the more positive calculated logBB value for PAC-1 is a predictor of BBB permeability, as well as the hypothesis that IP or IV injection of high doses of PAC-1 elicits a transient neurologic response in vivo by crossing the BBB. Although, the neurologic effect observed in vivo in mice and dogs is transient not life-threatening,17 the therapeutic use of low concentrations of PAC-1 could be a viable anticancer strategy that avoids this phenotype altogether.16 The studies reported herein suggest that at these low dosages PAC-1 acts primarily as a zinc chelating procaspase-3 activator.
The ability of 100 µM PAC-1 to induce potent cell death 24 hours after a short compound exposure is likely linked to ER stress, as short treatments of thapsigargin59 and tunicamycin60 also have been shown to elicit a similar strong cytotoxic response. The sufficiency of short exposures of compound to trigger death is unusual, and can be an attractive feature of anticancer drugs, allowing the compound to be administered via a bolus dosing regimen instead of constant rate infusion. Short compound exposure also may decrease dosing frequency for patients if the drug has effective cytotoxicity with one bolus dose. For example, the frequency of dasatinib dosing was reduced for treatment of chronic myeloid leukemia after the discovery that the compound is cytotoxic and effective after short exposures.61, 62 Although PAC-1 elicits a transient neurologic response in vivo, a high peak plasma concentration (>50 µM) of PAC-1 can be achieved in mice with a short bolus IV infusion of the compound with slight to moderate neurological response.17 However, at high doses, PAC-1 most likely will act in its ER stress-related mode as an anticancer therapy.
S-PAC-1 has been pursued as an anticancer therapy in a Phase I clinical trial for patient pet dogs with lymphoma.17 The initial bolus dose of S-PAC-1 achieved a peak plasma concentration no greater than ~70 µM in the patient dogs with a continuous infusion of a maintenance achieving a blood serum concentration less than 50 µM. The experiments reported herein confirm that at these concentrations, S-PAC-1 acts as a zinc-chelating procaspase activating compound. Furthermore, should S-PAC-1 be used at higher doses in future studies, it is unlikely that the ER stress induction observed with high concentrations of PAC-1 will be a confounding issue for the clinical use of S-PAC-1.
In summary, at low concentrations PAC-1 and S-PAC-1 act as zinc-chelating procaspase-3 activators, and at high concentrations PAC-1 also induces death through an ER stress-related mechanism. However, PAC-1 more readily penetrates the BBB and elicits a transient neurologic response in vivo when given via IP or IV injection. An intriguing clinical candidate would combine the safety of S-PAC-1 and the cytotoxicity features of PAC-1: a next-generation PAC-1 derivative with a highly negative logBB that induces cancer cell death with short exposures. A compound with these properties could be a potent and effective antitumor agent, and the search for such a derivative is underway.
Supplementary Material
Acknowledgments
We thank Justin Lamb (Broad Institute) for advice regarding use of the Connectivity Map database, the Keck facility (UIUC) for performing the microarray experiment and data processing, Lou Ann Miller and the Center for Microanalysis of Materials of the Frederick Seitz Materials Research Laboratory Central Facilities (UIUC) for transmission electron microscopy, Barbara Pilas and the Flow Cytometry Facility at the Roy J. Carver Biotechnology Center (UIUC), and Institute for Genomic Biology Core Facility (UIUC).
Grant Support
This work was supported by NIH grant R01-CA120439, University of Illinois, Ruth L. Kirschstein National Research Service Award F31-CA130138-01S1 (D.C.W), NIH Chemistry-Biology Interface Training Grant NRSA 1-T32-GM070421 and the Medicinal Chemistry Division of the American Chemical Society (Q.P.P), and NIH grant GM084027 (to A.E.P).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supporting Information
Supporting Figures, Supplementary Information, and additional experimental protocols. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 4.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A. 2006;103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Nikolovska-Coleska Z, Yang CY, Qian D, Lu J, Qiu S, Bai L, Peng Y, Cai Q, Wang S. Design of small-molecule peptidic and nonpeptidic Smac mimetics. Acc Chem Res. 2008;41:1264–1277. doi: 10.1021/ar8000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy S, Bayly CI, Gareau Y, Houtzager VM, Kargman S, Keen SL, Rowland K, Seiden IM, Thornberry NA, Nicholson DW. Maintenance of caspase-3 proenzyme dormancy by an intrinsic "safety catch" regulatory tripeptide. Proc Natl Acad Sci U S A. 2001;98:6132–6137. doi: 10.1073/pnas.111085198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Donovan N, Crown J, Stunell H, Hill AD, McDermott E, O'Higgins N, Duffy MJ. Caspase 3 in breast cancer. Clin Cancer Res. 2003;9:738–742. [PubMed] [Google Scholar]
- 9.Krepela E, Prochazka J, Liul X, Fiala P, Kinkor Z. Increased expression of Apaf-1 and procaspase-3 and the functionality of intrinsic apoptosis apparatus in non-small cell lung carcinoma. Biol Chem. 2004;385:153–168. doi: 10.1515/BC.2004.034. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Gong M, Cui Y, Ma K, Chang D, Wang TY. Upregulation of caspase-3 expression in esophageal cancer correlates with favorable prognosis: an immunohistochemical study from a high incidence area in northern China. Dis Esophagus. 2010;23:487–492. doi: 10.1111/j.1442-2050.2009.01043.x. [DOI] [PubMed] [Google Scholar]
- 11.Fink D, Schlagbauer-Wadl H, Selzer E, Lucas T, Wolff K, Pehamberger H, Eichler HG, Jansen B. Elevated procaspase levels in human melanoma. Melanoma Res. 2001;11:385–393. doi: 10.1097/00008390-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Izban KF, Wrone-Smith T, Hsi ED, Schnitzer B, Quevedo ME, Alkan S. Characterization of the interleukin-1beta-converting enzyme/ced-3-family protease, caspase-3/CPP32, in Hodgkin's disease: lack of caspase-3 expression in nodular lymphocyte predominance Hodgkin's disease. Am J Pathol. 1999;154:1439–1447. doi: 10.1016/s0002-9440(10)65398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrobel G, Maldyk J, Kazanowska B, Rapala M, Maciejka-Kapuscinska L, Chaber R. Immunohistochemical expression of procaspase-3 and its clinical significance in childhood non-Hodgkin lymphomas. Pediatr Dev Pathol. 2011;14:173–179. doi: 10.2350/10-01-0779-OA.1. [DOI] [PubMed] [Google Scholar]
- 14.Putt KS, Chen GW, Pearson JM, Sandhorst JS, Hoagland MS, Kwon JT, Hwang SK, Jin H, Churchwell MI, Cho MH, Doerge DR, Helferich WG, Hergenrother PJ. Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat Chem Biol. 2006;2:543–550. doi: 10.1038/nchembio814. [DOI] [PubMed] [Google Scholar]
- 15.Peterson QP, Goode DR, West DC, Ramsey KN, Lee J, Hergenrother PJ. PAC-1 Activates Procaspase-3 in Vitro through Relief of Zinc-Mediated Inhibition. J Mol Biol. 2009;388:144–158. doi: 10.1016/j.jmb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas PW, Schmit JM, Peterson QP, West DC, Hsu DC, Novotny CJ, Dirikolu L, Churchwell MI, Doerge DR, Garrett LD, Hergenrother PJ, Fan TM. Pharmacokinetics and derivation of an anticancer dosing regimen for PAC-1, a preferential small molecule activator of procaspase-3, in healthy dogs. Invest New Drugs. 2011;29:901–911. doi: 10.1007/s10637-010-9445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson QP, Hsu DC, Novotny CJ, West DC, Kim D, Schmit JM, Dirikolu L, Hergenrother PJ, Fan TM. Discovery and canine preclinical assessment of a nontoxic procaspase-3-activating compound. Cancer Res. 2010;70:7232–7241. doi: 10.1158/0008-5472.CAN-10-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson QP, Hsu DC, Goode DR, Novotny CJ, Totten RK, Hergenrother PJ. Procaspase-3 Activation as an Anti-Cancer Strategy: Structure-Activity Relationship of PAC-1, and its Cellular Co-Localization with Procaspase-3. J Med Chem. 2009;52:5721–5731. doi: 10.1021/jm900722z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu DC, Roth HS, West DC, Botham RC, Novotny CJ, Schmid SC, Hergenrother PJ. Parallel synthesis and biological evaluation of 837 analogues of procaspase-activating compound 1 (PAC-1) ACS Comb Sci. 2012;14:44–50. doi: 10.1021/co2001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolan DW, Zorn JA, Gray DC, Wells JA. Small-molecule activators of a proenzyme. Science. 2009;326:853–858. doi: 10.1126/science.1177585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zorn JA, Wille H, Wolan DW, Wells JA. Self-assembling small molecules form nanofibrils that bind procaspase-3 to promote activation. J Am Chem Soc. 2011;133:19630–19633. doi: 10.1021/ja208350u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci U S A. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci U S A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bair JS, Palchaudhuri R, Hergenrother PJ. Chemistry and biology of deoxynyboquinone, a potent inducer of cancer cell death. J Am Chem Soc. 2010;132:5469–5478. doi: 10.1021/ja100610m. [DOI] [PubMed] [Google Scholar]
- 26.Dittmer PJ, Miranda JG, Gorski JA, Palmer AE. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J Biol Chem. 2009;284:16289–16297. doi: 10.1074/jbc.M900501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+ J Biol Chem. 1985;260:2719–2727. [PubMed] [Google Scholar]
- 28.Palchaudhuri R, Hergenrother PJ. Transcript profiling and RNA interference as tools to identify small molecule mechanisms and therapeutic potential. ACS Chem Biol. 2011;6:21–33. doi: 10.1021/cb100310h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keum C, Woo JH, Oh WS, Park SN, No KT. Improving gene expression similarity measurement using pathway-based analytic dimension. BMC Genomics. 2009;10(Suppl 3):S15. doi: 10.1186/1471-2164-10-S3-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das SK, Mondal AK, Elbein SC. Distinct gene expression profiles characterize cellular responses to palmitate and oleate. J Lipid Res. 2010;51:2121–2131. doi: 10.1194/jlr.M004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 32.Kokame K, Agarwala KL, Kato H, Miyata T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J Biol Chem. 2000;275:32846–32853. doi: 10.1074/jbc.M002063200. [DOI] [PubMed] [Google Scholar]
- 33.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, Reich M, Hieronymus H, Wei G, Armstrong SA, Haggarty SJ, Clemons PA, Wei R, Carr SA, Lander ES, Golub TR. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 34.Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 35.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norinder U, Haeberlein M. Computational approaches to the prediction of the blood-brain distribution. Adv Drug Deliv Rev. 2002;54:291–313. doi: 10.1016/s0169-409x(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 38.Clark DE. In silico prediction of blood-brain barrier permeation. Drug Discov Today. 2003;8:927–933. doi: 10.1016/s1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- 39.Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett. 2004;357:127–130. doi: 10.1016/j.neulet.2003.12.080. [DOI] [PubMed] [Google Scholar]
- 40.Chiang PC, Chien CL, Pan SL, Chen WP, Teng CM, Shen YC, Guh JH. Induction of endoplasmic reticulum stress and apoptosis by a marine prostanoid in human hepatocellular carcinoma. J Hepatol. 2005;43:679–686. doi: 10.1016/j.jhep.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 41.Linder S, Shoshan MC. Lysosomes and endoplasmic reticulum: targets for improved, selective anticancer therapy. Drug Resist Updat. 2005;8:199–204. doi: 10.1016/j.drup.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Bakker AC, Webster P, Jacob WA, Andrews NW. Homotypic fusion between aggregated lysosomes triggered by elevated [Ca2+]i in fibroblasts. J Cell Sci. 1997;110(Pt 18):2227–2238. doi: 10.1242/jcs.110.18.2227. [DOI] [PubMed] [Google Scholar]
- 43.Hom JR, Gewandter JS, Michael L, Sheu SS, Yoon Y. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J Cell Physiol. 2007;212:498–508. doi: 10.1002/jcp.21051. [DOI] [PubMed] [Google Scholar]
- 44.Hom J, Yu T, Yoon Y, Porter G, Sheu SS. Regulation of mitochondrial fission by intracellular Ca2+ in rat ventricular myocytes. Biochim Biophys Acta. 2010;1797:913–921. doi: 10.1016/j.bbabio.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 46.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 47.Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23:143–149. doi: 10.1016/j.ceb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seervi M, Joseph J, Sobhan PK, Bhavya BC, Santhoshkumar TR. Essential requirement of cytochrome c release for caspase activation by procaspase-activating compound defined by cellular models. Cell Death Dis. 2011;2:e207. doi: 10.1038/cddis.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buckley BJ, Whorton AR. Tunicamycin increases intracellular calcium levels in bovine aortic endothelial cells. Am J Physiol. 1997;273:C1298–C1305. doi: 10.1152/ajpcell.1997.273.4.C1298. [DOI] [PubMed] [Google Scholar]
- 51.Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandra D, Choy G, Deng X, Bhatia B, Daniel P, Tang DG. Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol. 2004;24:6592–6607. doi: 10.1128/MCB.24.15.6592-6607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 54.Upton JP, Austgen K, Nishino M, Coakley KM, Hagen A, Han D, Papa FR, Oakes SA. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:3943–3951. doi: 10.1128/MCB.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moenner M, Pluquet O, Bouchecareilh M, Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007;67:10631–10634. doi: 10.1158/0008-5472.CAN-07-1705. [DOI] [PubMed] [Google Scholar]
- 56.Leleu X, Xu L, Jia X, Sacco A, Farag M, Hunter ZR, Moreau AS, Ngo HT, Hatjiharissi E, Ho AW, Santos DD, Adamia S, O'Connor K, Ciccarelli B, Soumerai J, Manning RJ, Patterson CJ, Roccaro AM, Ghobrial IM, Treon SP. Endoplasmic reticulum stress is a target for therapy in Waldenstrom macroglobulinemia. Blood. 2009;113:626–634. doi: 10.1182/blood-2007-10-116848. [DOI] [PubMed] [Google Scholar]
- 57.Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.07.016. in press, [DOI] [PubMed] [Google Scholar]
- 58.Schleicher SM, Moretti L, Varki V, Lu B. Progress in the unraveling of the endoplasmic reticulum stress/autophagy pathway and cancer: implications for future therapeutic approaches. Drug Resist Updat. 2010;13:79–86. doi: 10.1016/j.drup.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Peiro C, Vallejo S, Cercas E, Llergo JL, Lafuente N, Matesanz N, Rodriguez-Manas L, Sanchez-Ferrer CF. Thapsigargin induces apoptosis in cultured human aortic smooth muscle cells. J Cardiovasc Pharmacol. 2000;36:676–680. doi: 10.1097/00005344-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 60.Carlberg M, Dricu A, Blegen H, Kass GE, Orrenius S, Larsson O. Short exposures to tunicamycin induce apoptosis in SV40-transformed but not in normal human fibroblasts. Carcinogenesis. 1996;17:2589–2596. doi: 10.1093/carcin/17.12.2589. [DOI] [PubMed] [Google Scholar]
- 61.Shah NP, Kasap C, Weier C, Balbas M, Nicoll JM, Bleickardt E, Nicaise C, Sawyers CL. Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer Cell. 2008;14:485–493. doi: 10.1016/j.ccr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Saglio G, Hochhaus A, Goh YT, Masszi T, Pasquini R, Maloisel F, Erben P, Cortes J, Paquette R, Bradley-Garelik MB, Zhu C, Dombret H. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116:3852–3861. doi: 10.1002/cncr.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.