Abstract
Docosapentaenoic acid (DPAn-6, 22:5n-6) is an n-6 polyunsaturated fatty acid (PUFA) whose brain concentration can be increased in rodents by dietary n-3 PUFA deficiency, which may contribute to their behavioral dysfunction. We used our in vivo intravenous infusion method to see if brain DPAn-6 turnover and metabolism also were altered with deprivation. We studied male rats that had been fed for 15 weeks post-weaning an n-3 PUFA adequate diet containing 4.6% alpha-linolenic acid (α-LNA, 18:3n-3) or a deficient diet (0.2% α-LNA), each lacking docosahexaenoic acid (22:6n-3) and arachidonic acid (AA, 20:4n-6). [1-14C]DPAn-6 was infused intravenously for 5 min in unanesthetized rats, after which the brain underwent high-energy microwaving, and then was analyzed. The n-3 PUFA deficient compared with adequate diet increased DPAn-6 and decreased DHA concentrations in plasma and brain, while minimally changing brain AA concentration. Incorporation rates of unesterified DPAn-6 from plasma into individual brain phospholipids were increased 5.2–7.7 fold, while turnover rates were increased 2.1–4.7 fold. The observations suggest that increased metabolism and brain concentrations of DPAn-6 and its metabolites, together with a reduced brain DHA concentration, contribute to behavioral and functional abnormalities reported with dietary n-3 PUFA deprivation in rodents.
Keywords: docosapentaenoic, docosahexaenoic, deficient, turnover, kinetics, brain, diet, n-6, PUFA, arachidonic, rat, phospholipase A2
INTRODUCTION
Docosapentaenoic acid (DPAn-6, 22:5n-6) is an n-6 polyunsaturated fatty acid (PUFA) present in a trace amounts in brain under normal dietary conditions [1]. However, dietary n-3 PUFA deprivation can increase its concentration, in rough proportion to docosahexaenoic acid (DHA, 22:6n-3) loss, in brain, liver, plasma and other tissues [2–7]. DPAn-6 is synthesized from arachidonic acid (AA, 20:4n-6) and/or linoleic acid (LA, 18:2n-6), mainly in the liver [8–11]
The physiological and biochemical actions of DPAn-6 are not fully understood. Its structure is similar to that of DHA, and it is enriched in brain ethanolamine glycerophospholipids (EtnGpl) and phosphatidylserine (PtdSer), as is DHA [6, 12, 13]. However, DPAn-6 is a poor substitute for DHA for maintaining normal brain function, since its accumulation in the brain of diet-deprived n-3 PUFA rats is associated with abnormal behavior and brain functions [14–16]. Effects include increased scores depression and aggression tests, and deficits in olfactory discrimination and learning tasks. One report suggested that increased brain DPAn-6 arising from dietary DPAn-6 supplementation decreased DHA efficiency in reducing amyloid-β accumulation in the 3xTg mouse model for Alzheimer disease, whereas dietary DPAn-6 with DHA reduced early-stage brain phospho-tau protein levels in this model [17]. Dietary DPAn-6 may not compete with DHA incorporation into tissue if DHA is present in the diet [18].
AA and DHA play critical roles in normal brain function. These PUFAs are released by different phospholipase A2 (PLA2) enzymes, and can serve as secondary signaling molecules or be converted to bioactive eicosanoids or docosanoids, respectively, by cyclooxygenase (COX) and lipoxygenase (LOX) enzymes [19–21].
We reported that recycling (turnover associated with deacylation and reacylation) of AA in brain phospholipids can be changed by several drugs or by diet [22–25], and that the changes are associated with altered expression of Ca+-dependent cytosolic PLA2 (cPLA2) Type IVA. This enzyme is thought to be selective for AA hydrolysis based on in vitro studies [26]. However, an n-3 PUFA deficient that increased the rat brain DPAn-6 concentration but did not change AA turnover or concentration also increased cPLA2 IVA expression [27]. Thus, cPLA2 IVA also may regulate brain DPAn-6 turnover and metabolism in vivo. Support for this interpretation would be consistent with showing that DPAn-6 turnover is elevated in rats fed an n-3 PUFA deficient diet having a high DPAn-6 content. A high content and increased turnover would suggest, furthermore, that DPAn-6 contributes to signaling and downstream metabolism in such animals.
In the present study, we used our published in vivo fatty acid infusion model [15, 28–31] to test these predictions. Male rats were fed an n-3 PUFA adequate diet (containing 4.6% α-linolenic acid (α-LNA)) or deficient diet (containing 0.2% α-LNA) for 15 weeks after weaning; neither diet contained AA or DHA [15, 31]. While unanesthetized, a rat was infused intravenously with [1-14C]DPAn-6 for 5 min, then brain lipid concentrations, DPAn-6 incorporation rates, turnovers and other kinetic parameters were determined and compared between groups [28–30].
MATERIALS AND METHODS
Materials
[1-14C]DPAn-6 in ethanol was purchased from Moravek Biochemicals (Brea, CA, USA). The specific activity was 54 mCi/mmol and the purity was > 94%, determined by high performance liquid chromatography (HPLC) and scintillation counting. Diheptadecanoate phosphatidylcholine (di-17:0 PC), free heptadecanoic acid (17:0), and thin-layer chromatography (TLC) standards (cholesterol, triglycerides, cholesteryl esters and individual phospholipids) were obtained from Aventi Polar Lipids (Alabaster, Alabama). Standards for fatty acid methyl esters (FAMEs) for gas chromatography (GC) and HPLC were purchased from NuChek Prep (Elysian, MN, USA). Silica gel 60 plates were purchased from EM Separation Technologies (Gibbstown, NJ, USA). 6-p-Toluidine-2-naphthalene sulfonic acid was from Acros Organics (Geel, Belgium). Fatty acyl-CoAs were purchased from Sigma-Aldridge (St. Louis, MO, USA). Solvents were HPLC grade and were purchased from Fisher Scientific (Fair Lawn, NJ, USA) or EMD Chemicals (Gibbstown, NJ, USA). Other chemicals and reagents were purchased from Sigma-Aldrich or Fisher Scientific.
Animals
The protocol was approved by the Animal Care and Use Committee of the Eunice Kennedy Schriver National Institute of Child Health and Human Development and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23). Fischer-344 (CDF) male rat pups (19 days old) and their surrogate mothers were purchased from Charles River Laboratories (Portage, MI, USA). The pups were allowed to nurse until weaning at 21 days of age. Lactating rats had free access to water and rodent chow formulation NIH-3118-4, which contained 4% (wt/wt) crude fat (Zeigler Bros., Gardners, PA, USA) and whose fatty acid composition has been reported (17, 30). α-LNA, EPA and DHA contributed 5.1%, 2.0% and 2.3% of total fatty acids, respectively, whereas linoleic acid (LA, 18:2n-6) and AA contributed 47.9% and 0.02%, respectively. After weaning, the pups were divided randomly into n-3 PUFA adequate (n = 8) and deficient (n = 8) diet groups as described below, and fed the diet for 15 weeks. They had free access to food and water, their food being replaced every 2 or 3 days. They were housed in an animal facility with regulated temperature, humidity, and a 12 h light/12 h dark cycle.
n-3 PUFA adequate and deficient diets
The n-3 PUFA adequate and deficient diets were prepared by Dyets Inc. (Bethlehem, PA, USA), based on the AIN-93G formulation [15, 32, 33], and each contained 10% fat [31]. The n-3 PUFA adequate diet contained hydrogenated coconut oil (6 g/100 g diet), safflower oil (3.23 g/100 g) and flaxseed oil (0.77 g/100 g) (Table 1) [9, 11, 13, 15, 31, 34]. The n-3 deficient diet contained hydrogenated coconut oil (6.62 g/100 g) and safflower oil (3.38 g/100 g). Detailed dietary compositions are given in Supplementary Table 1.
Table 1.
Fatty acid concentration in plasma of the rat at 15 weeks
| Fatty acid | Unesterified fatty acids | Phospholipids | Triacylglycerol | Cholesterol esters | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Adequate | Deficient | Adequate | Deficient | Adequate | Deficient | Adequate | Deficient | |
| nmol/ml plasma | ||||||||
| n-6 PUFA | ||||||||
| 18:2 | 245 ± 31 | 231 ± 38 | 301 ± 41 | 244 ± 40* | 303 ± 129 | 217 ± 137 | 184 ± 37 | 139 ± 19** |
| 18:3 | 3.8 ± 0.5 | 5.4 ± 1.9* | 2.3 ± 0.5 | 2.9 ± 0.5* | 5.9 ± 2.5 | 7.3 ± 5.1 | 10 ± 3 | 10 ± 2 |
| 20:3 | 4.9 ± 1.4 | 5.5 ± 1.1 | 28 ± 7 | 19 ± 8* | 7.0 ± 3.3 | 7.4 ± 3.9 | 10 ± 2 | 5 ± 2*** |
| 20:4 | 32 ± 3 | 47 ± 9** | 743 ± 101 | 765 ± 88 | 66 ± 21 | 118 ± 52* | 993 ± 188 | 1008 ± 141 |
| 22:4 | 6.5 ± 1.3 | 16 ± 16** | 5.9 ± 0.9 | 22 ± 2*** | 11 ± 5 | 26 ± 14* | 38 ± 10 | 35 ± 23 |
| 22:5 | 2.9 ± 0.2 | 14 ± 3*** | 9.4 ± 1.8 | 143 ± 17 | 3.1 ± 1.1 | 20 ± 10*** | 0.7 ± 0.2 | 13 ± 2*** |
| Total n-6 PUFA | 299 ± 38 | 319 ± 46 | 1089 ± 142 | 1226 ± 135 | 396 ± 157 | 396 ± 211 | 1235 ± 228 | 1209 ± 162 |
| n-3 PUFA | ||||||||
| 18:3 | 28 ± 4 | 0.6 ± 0.2*** | 2.5 ± 0.8 | ND | 23 ± 12 | 0.6 ± 0.5** | 1.8 ± 0.4 | ND |
| 20:5 | 5.5 ± 1.3 | ND | 8.5 ± 3.5 | ND | 17 ± 7.2 | ND | 18 ± 8 | ND |
| 22:5 | 8.7 ± 1.4 | 0.5 ± 0.3*** | 20 ± 1 | 1.2 ± 0.3*** | 16 ± 7 | ND | 0.5 ± 0.2 | ND |
| 22:6 | 14 ± 3 | 0.7 ± 0.2*** | 140 ± 16 | 13 ± 3*** | 33 ± 13 | 1.9 ± 1.5*** | 26 ± 6 | 4.3 ± 0.6*** |
| Total n-3 PUFA | 56 ± 8 | 1.9 ± 0.3*** | 172 ± 19 | 14 ± 3*** | 89 ± 38 | 2.5 ± 1.8*** | 46 ± 13 | 4.3 ± 0.6*** |
| Saturated | ||||||||
| 16:0 | 499 ± 55 | 513 ± 40 | 526 ± 51 | 508 ± 43 | 473 ± 211 | 419 ± 285 | 88 ± 11 | 81 ± 10 |
| 18:0 | 84 ± 9 | 90 ± 13 | 886 ± 126 | 877 ± 110 | 70 ± 45 | 75 ± 65 | 12 ± 2 | 14 ± 3 |
| Total saturated | 583 ± 63 | 603 ± 48 | 1412 ± 170 | 1385 ± 147 | 542 ± 239 | 476 ± 301 | 100 ± 12 | 94 ± 13 |
| Monounsaturated | ||||||||
| 16:1n-7 | 97 ± 15 | 114± 4* | 22 ± 7 | 23 ± 5 | 79 ± 45 | 75 ± 65 | 41 ± 16 | 34 ± 9 |
| 18:1n-9 | 266 ± 31 | 280 ± 37 | 100 ± 21 | 96 ± 16 | 332 ± 146 | 277 ± 200 | 41 ± 9 | 35 ± 7 |
| 18:1n-7 | 66 ± 7 | 67 ± 6 | 69 ± 12 | 71 ± 10 | 81 ± 32 | 75 ± 38 | 15 ± 3 | 14 ± 2 |
| Total mono | 428 ± 49 | 461 ± 51 | 191 ± 39 | 191 ± 30 | 492 ± 221 | 427 ± 302 | 98 ± 28 | 83 ± 17 |
| Total | 1366 ± 141 | 1385 ± 133 | 2864 ± 360 | 2815 ± 301 | 1518 ± 651 | 1302 ± 810 | 1479 ± 275 | 1391 ± 190 |
| n-6/n-3 | 5.1 | 169 | 6.4 | 89 | 4.4 | 158 | 27 | 281 |
| 22:5n-6# | 12 ± 2 | 23 ± 5*** | ||||||
Values are means ± SD (n = 8 for both groups).
Values are measured in plasma after DPAn-6 infusion.
p < 0.05
p < 0.01
p < 0.001, differs significantly from mean in adequate group., ND = not detected (<0.1nmol/ml plasma)
We determined fatty acid concentrations (μmol/g food) and composition (% of total fatty acid) of each diet (Supplementary Table 2). Total lipids extracted from random ~0.6 g samples (n = 3) were methylated, and the resulting fatty acid methyl esters (FAMEs) were separated by GC as described below. As illustrated in Supplementary Table 2, the n-3 PUFA adequate diet contained α-LNA at 6.7 μmol /g diet (4.6% of total fatty acid), whereas the deficient diet contained α-LNA at 0.2 μmol /g (0.2% of total fatty acid), which is 10% of the minimum requirement for rodents (42.8 μmol/g). Both diets contained LA at 35.8–40.5 μmol /g (27.2–27.9 % of total fatty acids), close to the minimum requirement for n-3 PUFA diet adequacy in rodents [4, 14]. Other n-3 and n-6 PUFAs, including AA and DHA, were absent from both.
Surgery
Polyethylene catheters (PE 50, Intramedic™, Clay Adams™, Becton Dickinson, Sparks, MD, USA) filled with heparinized saline (100 IU/ml) were surgically implanted into the right femoral artery and vein of a rat anesthetized with 1–3% halothane (Shirley Aldred & Co, England). The skin was closed with staples and treated with 1% lidocaine (Hospira, Illinois, USA) for pain control. The rat was wrapped loosely in a fast-setting plaster cast taped to a wooden block, and allowed to recover from anesthesia for 3–4 h. Body temperature was maintained at 36–38°C using a feedback-heating element (Indicating Temperature Controller, Yellow Springs Instruments, Yellow Springs, OH, USA).
Radiotracer infusion
After recovering from anesthesia, the rat was infused via the femoral vein catheter with 150 μCi/kg [1-14C]DPAn-6 ([1-14C]DPA) [34–37]. [1-14C]DPA in ethanol was dried under nitrogen gas, and the residue was dissolved in HEPES buffer (pH 7.4) containing 50 mg/ml fatty acid-free bovine serum albumin. The mixture was sonicated at 40°C for 20 min and mixed by vortexing. The solution (1.3 ml/rat) was infused intravenously over 5 min at a rate of 0.223(1+e−0.32t) ml/min (t in min) using a computer-controlled variable speed pump (No. 22; Harvard Apparatus, South Natick, MA, USA), to rapidly establish steady-state plasma radioactivity [34, 35, 38]. Aliquots of arterial blood (180 μl) were collected in centrifuge tubes (polyethylene-heparin lithium fluoride-coated, Beckman) at 0, 0.25, 0.5, 0.75, 1.5, 3, 4, and 5 min after starting infusion. At 5 min, the rat was euthanized with an overdose of sodium pentobarbital (Ovation Pharmaceuticals, Deerfield, IL, USA) (100 mg/kg i.v.). The head was immediately subjected to high-energy focused beam microwave irradiation (5.5 kW, 4.8 sec) (Model S6F, Cober Electronics, Stamford, CT, USA). The brain was weighed and frozen with dry ice in methylbutane, then stored at −80°C until assay. Arterial blood samples were centrifuged at 10,000 g for 1 min, and plasma was removed and stored at −80°C.
Separation and analysis of stable lipids in plasma and brain
Approximately 0.8 g brain and 150 µl plasma were used for lipid extraction by the Folch procedure [39]. The aqueous extraction phases were washed once with an equal volume of chloroform to remove residual lipid. Aqueous and total lipid radioactivity from brain and plasma were counted (see below). Total lipid extracts from plasma and brain were separated into neutral lipid subclasses by TLC on silica gel 60 plates using heptane /diethyl ether /glacial acetic acid (60:40:3, v/v/v) [40]. Total lipid extract from brain was separated into phospholipid classes by TLC on silica gel 60 plates using chloroform/methanol/glacial acetic acid/water (60:40:1:4, v/v/v/v) to separate ethanolamine glycerophospholipid (EtnGpl), choline glycerophospholipid (ChoGpl), phosphatidylserine (PtdSer), and phosphatidylinositol (PtdIns) [41]. Authentic standards of triacylglycerol, phospholipids, cholesterol, cholesteryl ester and unesterified fatty acids for neutral lipid separation and of individual phospholipids for phospholipid separation were run on the plates to identify the lipids. The plates were dried and sprayed with 0.03% 6-p-Toluidine-2-naphthalene sulfonic acid in 50 mM Tris-HCl buffer (pH 7.4) (w/v). The lipid bands were visualized under ultraviolet light. The bands were scraped, and the silica gel was used to directly quantify radioactivity by scintillation counting, to prepare FAMEs (described below).
Quantification of radioactivity
Samples for measuring radioactivity were placed in scintillation vials and dissolved in liquid scintillation cocktail (Ready Safe™ plus 1% glacial acetic acid). Radioactivity was determined using a liquid scintillation analyzer (2200CA, TRI-CARB®, Packard Instruments, Meriden, CT, USA).
Fatty acid methyl ester preparation
Unesterified and esterified fatty acids (total phospholipids, individual phospholipids, triacylglycerol, and cholesteryl esters), which were separated by TLC, were methylated with 1% H2SO4-methanol for 3 h at 70°C [13, 42]. Before methylation, appropriate quantities of di-17:0 PC (for phospholipids, triacylglycerol and cholesteryl esters) or of 17:0 (for free fatty acids) were added as internal standards. Prepared FAMEs were analyzed by GC and HPLC, as described below.
GC analysis
Fatty acid concentrations (nmol/g brain wet wt) in brain and plasma lipids were determined using a GC (6890N, Agilent Technologies, Palo Alto, CA, USA) equipped with an SP™-2330 fused silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) (Supelco, Bellefonte, PA, USA) and a flame ionization detector [13]. Concentrations were calculated by proportional comparison of peak areas to the area of the 17:0 internal standard.
HPLC analysis
To quantify radioactivity of fatty acids in brain and plasma lipids, FAMEs were quantified by HPLC by the method of Aveldano et al. [43] with the following modifications. Total lipid extraction of plasma was used to prepare the FAMEs. Total lipids of brain were alkalinized with KOH solution, extracted twice with n-hexane to extract esterified lipids, and the hexane phase was dried and methylated as described above. The FAMEs were dissolved in acetonitrile, and the solution was fractionated by reversed phase column-HPLC using a pump (System GOLD 126, Beckman Coulter, Fullerton, CA, USA) outfitted with a UV detector (UV/VIS-151, Gilson, Middleton, Wisconsin, USA) and an on-line continuous scintillation counter β-RAM detector (β-RAM Model 2, IN/US Systems, FL, USA) mixing in liquid scintillation cocktail (INFLOW™ 2:1, IN/US Systems). The reversed-phase column, Luna 5 μ C18 (5 μm particle size, 4.6 × 250 mm), was obtained from Phenomenex (Torrance, CA, USA). For the brain FAME samples, HPLC eluate was collected every 30 sec and subjected to liquid scintillation counting to obtain a radioactivity profile. Chromatography was performed using a linear gradient system of water and acetonitrile. The acetonitrile was held at 85% for 30 min, increased to 100% over 10 min, and held at 100% for 20 min. The flow rate was 1.0 ml/min. The UV detector was set at 205 nm.
Eight samples from each group were equally pooled to analyze HPLC profiles of FAMEs. The analysis was carried out in duplicate. The percentages of radioactivity in [1-14C]DPA and of other [14C]fatty acids in brain and plasma total lipid fractions were determined from these HPLC profiles.
Analysis of long chain acyl-CoAs
Long chain acyl-CoAs were extracted from brain using an affinity chromatography method with slight modification [44]. To brain (~ 0.8 g) was added 2 ml of 25 mM KH2PO4 and an appropriate amount of heptadecanoyl-CoA (17:0-CoA) as an internal standard, and the sample was sonicated for 30 sec on ice with a probe sonicator (Model W-225, Misonix, Farmingdale, NY, USA). To the sample was added 2 ml of 2-propanol and the sample was sonicated for 30 sec. Then, 0.25 ml of saturated (NH4)2SO4 was added to the homogenate to precipitate protein, and 4 ml of acetonitrile was added before the sample was mixed vigorously for 5 min at 3,000 rpm. The supernatant was diluted with 1.25 vol of 25 mM KH2PO4. The solution was passed 3 times through an oligonucleotide purification cartridge (ABI Masterpiece™, OPC®, Applied Biosystems, Foster City, CA, USA), and the cartridge was washed with 10 ml of 25 mM KH2PO4. Acyl-CoA species were eluted with 0.4 ml of isopropanol /1 mM glacial acetic acid (75:25 v/v).
Extracted acyl-CoAs were separated on a reversed phase HPLC column (Symmetry, 5 μm particle size, 4.6 mm × 250 mm, Waters Corporation, Milford, MA, USA), using a pump coupled with a UV/VIS detector (System Gold, Model 168, Beckman). Chromatography was performed using a linear gradient system of 75 mM KH2PO4 and acetonitrile. At the start, acetonitrile was 44% and held for 1 min, then increased to 49% over 25 min, increased to 68% over 10 min, held at 68% for 4 min, returned to 44% over 6 min, and held for 6 min (52 min total run time). The flow rate was 1.0 ml/min. UV detection was set at 260 nm for integration of concentrations and at 280 nm for identifying acyl-CoAs (260/280 = 4:1) [44]. Peaks were identified from the retention times of acyl-CoA standards. The acyl-CoA standard for DPAn-6 was prepared from the free fatty acid and free CoA by an enzymatic method [45]. Endogenous acyl-CoA concentrations (nmol/g brain) were calculated by direct proportional comparison with the peak area of the 17:0-CoA internal standard. The DPA-CoA peak was collected in each sample, and its radioactivity was counted with a liquid scintillation counter.
Calculations
The general pulse-labeling equations for determining the in vivo kinetics of fatty acid incorporation and turnover brain, following intravenous infusion with a radiolabeled fatty acid, are described elsewhere [28, 30, 35, 38, 46]. The equations were used to examine brain DPAn-6 metabolism in the rats fed n-3 PUFA adequate or deficient diets.
Incorporation coefficients (ml/sec*g brain), representing transfer of unesterified [1-14C]DPA from plasma into stable brain lipid i, were calculated as follows:
| (Eq. 1) |
where (T) (nCi/g brain) is DPA radioactivity in i at time T (5 min) after starting tracer infusion, t is time after starting infusion, and (nCi/ml plasma) is plasma radioactivity of unesterified DPA [35].
The incorporation rate of unlabeled unesterified DPAn-6 from plasma into brain lipid i, Jin,i(DPA) was calculated as follows in units of nmol/s/g brain,
| (Eq. 2) |
where cplasma(DPA) is the concentration (nmol/ml) of unlabeled unesterified DPA in plasma.
A “dilution factor”λDPA-CoA represents the steady-state ratio of brain DPA-CoA specific activity to specific activity of unesterified DPAn-6 in plasma,
| (Eq. 3) |
The rate of incorporation JFA,i(DHA) of unlabeled DPAn-6 from the brain DPA-CoA pool (the true precursor pool for incorporation) into brain stable lipid i equals, in units of nmol/g/sec,
| (Eq. 4) |
The turnover FFA,i(DPA) of DPAn-6 within brain stable lipid i equals,
| (Eq. 5) |
where cbrain,i(DPA) is the concentration of DPAn-6 in i. The corresponding half-life of DPAn-6 in i equals,
| (Eq. 6) |
Statistical analysis
Data are expressed as mean ± SD (n = 8). An unpaired Student's t-test was used to compare means in 2 groups having possible equal variance with Levene's test, whereas the Welch test was used to compare the means of 2 groups having unequal variances. p ≤ 0.05 was used as a cut-off for statistical significance.
RESULTS
Growth and tissue weight
Body and brain weight were not affected significantly by the n-3 PUFA deficient diet. The average initial body weight of 21-day old rats was 32 ± 3 g and 32 ± 4 g in the diet adequate and deficient groups, respectively (p = 0.132). Final body weight (at 15 weeks after weaning) was 386 ± 27 g and 389 ± 31 g in the adequate and deficient groups, respectively (p = 0.63). Microwaved brain weight was 1.4 ± 0.3 g and 1.4 ± 0.3 g in the adequate and deficient groups, respectively.
Fatty acid concentration in plasma
Table 1 summarizes concentrations of unesterified fatty acids, phospholipids, triacylglycerol, and cholesteryl esters in rat plasma before radiolabeled DPAn-6 infusion. These values agree with reported values [27, 35, 47]. Unesterified DPAn-6 concentrations were 2.9 ± 0.2 nmol/ml and 14 ± 3 nmol/ml in plasma of the diet adequate and deficient groups, respectively.
Unesterified DPAn-6 concentrations in individual studies were used to calculate incorporation rates of plasma unesterified DPAn-6 (Jin,i(DPA)) into brain lipid i, using Eq. 2. The plasma unesterified fatty acid concentrations were not changed by infusion, except for that of DPAn-6. After 5 min of infusion, unesterified plasma DPAn-6 concentrations were 12 ± 2 nmol/ml and 23 ± 5 nmol/ml in the diet adequate and deficient groups, respectively. Unesterified DPAn-6 concentrations in individual infusion studies were used to calculate dilution factors λDHA–C oA by Eq. 3, in each experiment.
Brain fatty acid concentrations
Brain DHA concentration was decreased by 35–44% in EtnGpl, ChoGpl, PtdSer, and PtdIns of the rats fed the n-3 PUFA deficient compared with adequate diet (Table 2). α-LNA, EPAn-3, and DPAn-3 were not detected in individual brain phospholipids in rats fed the deficient diet.
Table 2.
Fatty acid concentration in brain phospholipids
| Fatty acid | EtnGpl | ChoGpl | PtdSer | PtdIns | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Ade | Def | Ade | Def | Ade | Def | Ade | Def | |
| nmol/g brain | ||||||||
| n-6 PUFA | ||||||||
| 18:2 | 289 ± 31 | 225 ± 32** | 325 ± 35 | 257 ± 10** | 37 ± 6 | 27 ± 6** | 71 ± 12 | 50 ± 7** |
| 20:3 | 364 ± 48 | 343 ± 82 | 214 ± 52 | 183 ± 29 | 194 ± 64 | 154 ± 24 | 69 ± 16 | 62 ± 8 |
| 20:4 | 6754 ± 395 | 7308 ± 550* | 2812 ± 270 | 3237 ± 182** | 840 ± 158 | 815 ± 81 | 2436 ± 308 | 2296 ± 247 |
| 22:4 | 3776 ± 536 | 4255 ± 446* | 342 ± 43 | 430± 31*** | 794 ± 107 | 1039 ± 99** | 99 ± 19 | 102 ± 23 |
| 22:5 | 268 ± 33 | 4255 ± 409*** | 70 ± 12 | 1105 ± 109 *** | 194 ± 30 | 2397 ± 310*** | 16 ± 6 | 101 ± 21*** |
| Total | 11449 ± 833 | 16387 ± 1366*** | 3764 ± 375 | 5211 ± 261*** | 2059 ± 331 | 4430 ± 484*** | 2692 ± 359 | 2611 ± 282 |
| n-3 PUFA | ||||||||
| 18:3 | ND | ND | ND | ND | ND | ND | ND | ND |
| 20:5 | ND | ND | ND | ND | ND | ND | ND | ND |
| 22:5 | 167 ± 32 | 51 ± 7*** | 53 ± 9 | ND | 54 ± 6 | ND | 6 ± 3 | ND |
| 22:6 | 10885 ± 644 | 7112 ± 440*** | 2420 ± 366 | 1365 ± 96*** | 4987 ± 494 | 3099 ± 368*** | 316 ± 62 | 178 ± 42** |
| Total | 11052 ± 659 | 7163 ± 447*** | 2473 ± 373 | 1365 ± 96*** | 5041 ± 498 | 3099 ± 368*** | 323 ± 65 | 178 ± 42** |
| Saturated | ||||||||
| 16:0 | 2956 ± 280 | 2994 ± 190 | 20490 ± 1988 | 20751 ± 1278 | 340 ± 74 | 346 ± 25 | 753 ± 162 | 674 ± 63 |
| 18:0 | 8886 ± 535 | 9075 ± 573 | 7640 ± 1008 | 7390 ± 401 | 8473 ± 1354 | 8043 ± 708 | 2342 ± 349 | 2141 ± 203 |
Brain DPAn-6 concentration was increased by 16-fold in EtnGpl (p < 0.001), by 16-fold in ChoGpl (p < 0.001), by 12-fold in PtdSer (p < 0.001), and by 6-fold in PtdIns (p < 0.001) in rats fed the n-3 PUFA deficient compared with adequate diet (Table 2). Dietary deprivation slightly increased brain AA concentration in EtnGpl and ChoGpl and brain DTAn-6 concentration in EtnGpl, ChoGpl and PtdSer. Individual DPAn-6 concentrations in phospholipids were used to calculate DPAn-6 turnovers (FFA,i(DPA)) by Eq. 5.
Plasma radioactivity
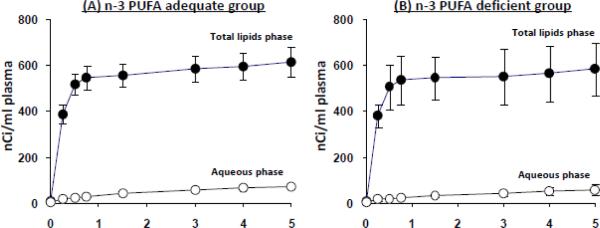
Figure 1 summarizes radioactivity in the total lipid and aqueous phases of plasma during the 5-min intravenous [1-14C]DPAn-6 infusion, in the dietary n-3 PUFA adequate and deficient rats. Steady-state lipid radioactivity was achieved within 1 min of infusion in both groups. At 5 min, > 90% of plasma radioactivity was within the total lipid phase in both dietary groups, while < 10% was in the aqueous phase. During the 5-min infusion, integrals of radioactivity in plasma total lipids equaled 164090 ± 12318 nCi.sec/ml and 159242 ± 31581 nCi.sec/ml in the n-3 PUFA adequate and deficient groups, respectively, not significantly different. Individual experimental values of integrated radioactivity were used to calculate k*i(DPA) by Eq. 1.
Figure 1.
Radioactivity in total lipid (●) and aqueous (◯) phases from plasma of n-6 PUFA adequate (A) and deficient (B) rats during i.v. infusion of [1-14C]DPA. Values are mean ± SD (n = 8 for both groups).
Radioactivity in neutral lipid classes was determined in plasma lipids at 5 min post infusion. Of total lipid radioactivity, > 92% was within unesterified fatty acids, and the remaining 8% was in phospholipids, triacylglycerol, cholesterol and cholesteryl esters. At 5 min after infusion, > 97% of plasma total lipid radioactivity was [1-14C]DPA in both groups (HPLC chromatograms not shown).
Brain radioactivity
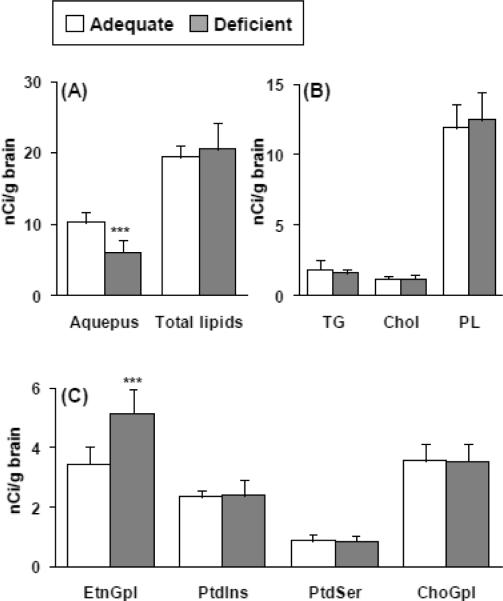
Radioactivity in different brain compartments after 5 min of [1-14C]DPA infusion is shown in Figure 2. In the diet adequate group, radioactivity equaled 19.3 ± 1.6 nCi/g brain in the total lipid phase (65% of total radioactivity) and 10.3 ± 1.4 nCi/g brain in the aqueous phase (35% of total radioactivity) (Figure 2A). In the deficient group, radioactivity equaled 20.5 ± 3.6 nCi/g brain in the total lipid phase (75% of total radioactivity) and 6.0 ± 1.7 nCi/g brain in the aqueous phase (25% of total radioactivity), significantly less than in the adequate group (p < 0.001). Of total lipid radioactivity, > 80% was in total phospholipid, 8% in triacylglycerol, and 12% in cholesterol in both groups (Figure 2B).
Figure 2.
Radioactivity of aqueous and total lipid phases (A), in neutral lipid classes (B), and phospholipid (C) of brain in n-3 PUFA adequate and deficient rats after 5-min i.v. infusion of [1-14C]DPA. Abbreviations: TG, triacylglycerol; PL, phospholipid; Chol, cholesterol; EtnGpl, ethanol glycerophospholipid; ChoGpl, choline glycerophospholipid; PtdSer, phosphatidylserine, PtdIns, phosphatidylinositol. Values are mean ± SD (n = 8 for both groups).
Radioactivities in individual phospholipids are shown in Figure 2C. Radioactivity in EtnGpl was significantly higher (5.16 ± 0.83 nCi/g brain vs. 3.45 ± 0.56 nCi/g brain, p < 0.001) in the diet-deficient than adequate group. There was no significant difference for radioactivity in ChoGpl (3.57 ± 0.59 nCi/g brain vs. 3.54 ± 0.61 nCi/ g brain), PtdSer (0.89 ± 0.21 nCi/g brain vs. 0.85 ± 0.19 nCi/g brain), or PtdIns (2.34 ± 0.23 nCi/g brain vs. 2.38 ± 0.52 nCi/g brain) between the adequate and deficient groups.
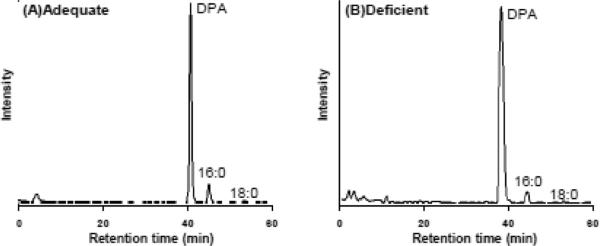
[14C]16:0 and [14C]18:0, expected β-oxidation products from [1-14C]DPA, were detected in total brain phospholipid (Fig. 3A and B), EtnGpl (chromatogram not shown), ChoGpl (chromatogram not shown), PtdSer (chromatogram not shown), and PtdIns (chromatogram not shown) of both groups. Percent radioactivity of fatty acids in individual phospholipids is shown in Table 3.
Figure 3.
HPLC chromatograms of fatty acid methyl esters in total phospholipids of the brain of rats fed n-3 PUFA adequate (A) or deficient diet (B). Eight samples for each group were equally pooled to analyze HPLC profiles of FAMEs.
Table 3.
Radioactivity composition of fatty acid in brain stable lipids of the rats
| Fatty acid | TPL | EtnGpl | ChoGpl | PtdSer | PtdIns | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Ade | Def | Ade | Def | Ade | Def | Ade | Def | Ade | Def | |
| % of total radioactivity | ||||||||||
| DPAn-6 | 89.8 | 94.9 | 94.7 | 98.5 | 71.8 | 86.9 | 86.8 | 94.9 | 78.3 | 92.1 |
| 16:0 | 9.5 | 4.2 | 4.8 | 1.3 | 26.3 | 12.3 | 10.9 | 4.0 | 18.3 | 6.1 |
| 18:0 | 0.7 | 0.8 | 0.5 | 0.2 | 1.8 | 0.8 | 2.3 | 1.1 | 3.5 | 1.8 |
Eight samples for each group were equally pooled to analyze HPLC profiles of FAMEs.
Radioactivity due to DPAn-6 in individual phospholipids was calculated with individual values of total radioactivity (Figure 2) and composition (Table 3) in brain phospholipids. Calculated radioactivity of DPAn-6 is shown in Table 4 (Column 2). It was increased significantly only in EtnGpl of the diet deficient compared with adequate rats. Individual values of radioactivity of DPAn-6 were used to calculate incorporation coefficients k*i(DPA) by Eq. 1.
Table 4.
Incorporation coefficient (k*i(DPA)) and incorporation rates (Jin,i(DPA)) of plasma DPAn-6 into brain of the rats
| Radioactivity of DPA (nCi/g brain) | Incorporation coefficients, ki(DPA)* (ml/sec*g ×10−4) | Incorporation rates, Jin,I (DPA) (nmol/sec/g × 10−4) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Adequate | Deficient | Adequate | Deficient | Adequate | Deficient | |
| Total phospholipids | 10.6 ± 1.6 | 11.7 ± 1.9 | 0.639 ± 0.082 | 0.743 ± 0.070* | 1.87 ± 0.22 | 10.1 ± 1.5*** |
| EtnGpl | 3.27 ± 0.53 | 5.08 ± 0.82** | 0.198 ± 0.034 | 0.324 ± 0 039*** | 0.579 ± 0.099 | 4.43 ± 0.85*** |
| PtdIns | 1.83 ± 0.18 | 2.19 ± 0.48 | 0.111 ± 0.011 | 0.138 ± 0.020** | 0.324 ± 0.040 | 1.91 ± 0.42*** |
| PtdSer | 0.772 ± 0.184 | 0.805 ± 0.181 | 0.0462 ± 0.0078 | 0.0516 ± 0.0177 | 0.135 ± 0.024 | 0.700 ± 0.157*** |
| ChoGpl | 2.56 ± 0.42 | 3.08 ± 0.53 | 0.156 ± 0.029 | 0.195 ± 0.015** | 0.456 ± 0.088 | 2.67 ± 0.47*** |
Values are mean ± SD (n = 8 for both groups).
p < 0.05,
p < 0.01,
p<0.001 differs significantly from mean in adequate group.
Unlabeled brain acyl-CoA concentrations and associated radioactivity
Unlabeled acyl-CoA concentrations and radioactivity of DPAn-6-CoA in rat brain were analyzed with the HPLC system (Table 5). The n-3 PUFA deprivation increased the DPA-CoA concentration by 12.2 fold (p < 0.001), while decreasing DHA-CoA concentration by 72% (p < 0.001). There was no significant difference in other acyl-CoA concentrations between the two dietary groups. Moreover, radioactivity of DPA-CoA was unaffected by the deficient diet (Table 5). Individual values of DPA-CoA concentration and of DPA-CoA radioactivity were used to calculate the dilution factor λDPA–CoA by Eq. 3.
Table 5.
Fatty acyl-CoA concentrations and radioactivity in brain from n-3 PUFA adequate and deficient rats following 5 min of [1-14C]DPA infusion
| Acyl-CoA | Concentration (nmol/g brain) | Radioactivity (nCi/g brain) | ||
|---|---|---|---|---|
|
|
||||
| Adequate | Deficient | Adequate | Deficient | |
| 14:0-CoA, 18:3n-3-CoA, EPA-CoA | 0.80 ± 0.17 | 0.70 ± 0.17 | ||
| 16:0-CoA | 9.6 ± 1.8 | 9.0 ± 2.7 | ||
| 18:0-CoA | 3.6 ± 0.9 | 3.6 ± 2.7 | ||
| 18:1-CoA | 10.7 ± 2.7 | 10.2 ± 3.1 | ||
| 18:2n-6-CoA | 0.52 ± 0.09 | 0.42 ± 0.13 | ||
| 20:4n-6-CoA | 0.81 ± 0.19 | 1.1 ± 0.2 | ||
| DPA(22:5n-6)-CoA | 0.060 ± 0.012 | 0.73 ± 0.26*** | 0.92 ± 0.23 | 1.00 ± 0.45 |
| DHA(22:6n-3)-CoA | 0.88 ± 0.14 | 0.25 ± 0.08*** | ||
Values are mean ± SD (n = 8 for both groups).
p < 0.01,
p < 0.001 differs significantly from mean in adequate group.
DPA incorporation and turnover rates in the rat brain
Incorporation coefficients k*i(DPA) (Table 4) of plasma-derived unesterified DPAn-6 into brain phospholipids were calculated using Eq. 1 with individual experimental values of integrated plasma radioactivity (Figure 1) and of radioactivity in stable brain lipids (Table 4). k*i(DPA) was increased significantly by 1.2–1.6 fold in total phospholipid, EtnGpl, PtdIns and ChoGpl, in rats fed the n-3 PUFA deficient compared with adequate diet.
Individual unesterified plasma DPAn-6 concentrations (Table 2) prior to [1-14C]DPA infusion, and calculated values of k*i(DPA) (Table 4), were used to calculate incorporation rates Jin,i(DPA) of plasma unesterified DPAn-6 into brain lipids i by Eq. 2 (Table 4). Jin,i(DPA) was increased 5.2–7.7 fold into total brain phospholipid, EtnGpl, PtdIns, PtdSer, and ChoGpl in rats fed the n-3 PUFA deficient compared with adequate diet; higher changes in incorporation rates than coefficient reflected the higher plasma unesterified DPAn-6 concentration in the deprived rats (Table 3). Thus, a general effect of deprivation was to increase rates of plasma DPAn-6 incorporation into brain phospholipids.
The dilution factor, λDPA–C oA, the ratio of steady-state brain DPA-CoA specific activity to plasma unesterified DPAn-6 specific activity, was calculated using Eq. 3. λDPA–C oA equaled 0.30 ± 0.12 and 0.055 ± 0.019 in the n-6 PUFA adequate and deprived rats, respectively, at the end of the 5-min tracer infusion (p = 0.001) (Table 6).
Table 6.
The dilution factor (λDPA–CoA), incorporation rates of DPA-CoA (JFA,i(DPA)), turnover rates (FFA,i(DPA)) and half-lives of DPA in the rat brains
| λ DPA–CoA | JFA (nmol/sec/g × 10−4) | FFA (%/hr) | Half-life (hr) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Adequat | Deficien | Adequat | Deficien | Adequat | Deficien | Adequat | Deficien | |
| e | t | e | t | e | t | e | t | |
| Total phospholipid s | 0.30 ± 0.12 | 0.055 ± 0.019** | 6.93 ± 2.47 | 219± 131*** | 0.446 ± 0.177 | 1.08 ± 0.63** | 177 ± 65 | 73 ± 25** |
| EtnGpl | 2.15 ± 0.77 | 99.1 ± 70.0*** | 0.298 ± 0.125 | 0.838 ± 0.599** | 274 ± 116 | 105 ± 40** | ||
| PtdIns | 1.22 ± 0.49 | 40.8 ± 24.6 *** | 0.322 ± 0.180 | 15.0 ± 99.3*** | 29 ± 18 | 5.8 ± 2.4** | ||
| PtdSer | 0.511 ± 0.210 | 15.4 ± 9.8** | 0.0955 ± 0.0389 | 0.242 ± 0.174* | 845 ± 367 | 398 ± 220* | ||
| ChoGpl | 1.73 ± 0.75 | 59.5 ± 40.3*** | 0.934 ± 0.486 | 1.91 ± 1.19*** | 94 ± 47 | 45 ± 18 | ||
Values are mean ± SD (n = 8 for both groups).
p < 0.05,
p < 0.01, differs significantly from mean in adequate group.
Individual values of incorporation rates from plasma Jin,i(DPA) and of dilution coefficients λDPA–C oA were used to calculate incorporation rates JFA,i(DPA) of DPAn-6 from the brain DPA-CoA precursor pool into stable lipids. As illustrated in Table 6, deprivation significantly increased JFA,i(DPA) by 29–46 fold into individual phospholipids.
Turnovers FFA,i(DHA) in brain lipids i were calculated using Eq. 5 with individual values of JFA,i(DPA) and of DPAn-6 concentration (Table 3). As illustrated in Table 6, n-3 PUFA deprivation significantly increased DPAn-6 turnover by 2.1 – 4.7 fold in the different phospholipids. Corresponding half lives, calculated using Eq. 6 from individual turnover rates, FFA,i(DHA), were shortened by 51–79% (Table 6).
DISCUSSION
In this study, we used our in vivo fatty acid infusion method and model to quantify incorporation rates and turnovers of DPAn-6 in brain lipids of unanesthetized male rat,, which had been fed an n-3 PUFA adequate or deficient diet for 15 weeks after weaning [14, 15, 28, 29]. Compared to rats fed the adequate diet, those fed the deficient diet had an increased plasma concentration of unesterified DPAn-6, increased brain concentration of esterified DPAn-6, and increased incorporation rates of unesterified DPAn-6 from plasma and from the brain DPAn-6 CoA pool into brain phospholipids. Turnovers of DPAn-6 in phospholipids were increased. Plasma and brain DHA concentrations were reduced by deprivation.
The results suggest that the behavioral, neurophysiological and neurochemical changes reported to accompany dietary n-3 PUFA deprivation in developing rodents arise not only from reduced brain DHA content and metabolism [15, 48, 49], but also from increased brain DPAn-6 content and metabolism [14–16, 48, 50]. The immaturity of the rat brain after birth makes it particularly vulnerable to disturbed PUFA dietary composition, whereas the adult brain may be less vulnerable because of its ability to conserve PUFAs [14, 51]. The rat brain does not reach maturity until about 3 months of age; incorporation of plasma unesterified palmitic acid (16:0) peaks at 20 days then declines 4-fold to adulthood, while brain glucose consumption is low at birth and then rises over time until adulthood [52–54]. There also is clinical evidence that adequate PUFA nutrition is important for postnatal human brain development, likely because the human brain also is immature at birth [52, 55, 56].
Brain AA and DHA are derived largely from unesterified plasma AA and DHA, since these long-chain PUFAs cannot be synthesized de novo in vertebrates, nor elongated in brain to a significant extent (< 1%) from their respective shorter-chain PUFA precursors, LA and α-LNA [10, 35, 57–59]. DPAn-6 in the rat is largely synthesized in the liver from shorter-chain n-6 PUFAs including AA [9, 10], secreted into blood within lipoproteins, then delivered to brain in its unesterified form [11, 49].
The n-3 PUFA deficient diet in the current study increased the unesterified plasma DPAn-6 concentration 4.8-fold (from 2.9 nmol/ml to 14 nmol/ml) and the esterified DPAn-6 concentration in total brain phospholipids 14-fold (from 548 nmol/g brain to 78.6 nmol/g brain) (Tables 1 and 3), likely by upregulating its elongation from AA in the liver [9, 49, 60–62]. Increased DPAn-6 concentrations also were reported in liver and erythrocyte membranes of obese patients with nonalcoholic steatohepatitis [63], and in plasma of adults fed PUFAs through a gastric tube [64].
Incorporation coefficients of DPAn-6 from plasma into brain phospholipids were increased significantly in the diet-deprived rats (Table 4). Incorporation rates Jin,i(DPA) were elevated to a greater extent, by 5.2–7.7 fold, reflecting the elevated plasma unesterified DPAn-6 concentration, while half-lives were shortened. The dilution coefficient λDPA-CoA was reduced in the n-3 PUFA deficient rats, reflecting increased DPAn-6 turnover and release from brain phospholipid [30]. In similarly unanesthetized n-3 PUFA deprived rats, brain DHA half-life was prolonged and DHA loss from brain was reduced [13]. AA concentrations were increased in EtnGpl and ChoGpl in this study (Table 2), but the AA concentration was changed minimally if at all in other deprivation studies [13, 15, 27].
Despite evidence that AA turnover in brain phospholipids is not increased even with more severe multigenerational dietary n-3 PUFA deprivation [27], expression of cPLA2 IVA, thought from in vitro studies to be selective for AA hydrolysis [26, 65–67], is increased nevertheless [24, 49]. Since an elevated enzyme activity is associated with increased DPAn-6 turnover, representing largely deacylation-reacylation of the PUFA [30, 68, 69], cPLA2 IVA likely can hydrolyze DPAn-6 as well as AA from phospholipid, but this remains to be tested in vitro.
In this regard, cPLA2 IVA can mediate in vivo neurotransmission by releasing AA as a second messenger from synaptic membrane phospholipid, following drug or neurotransmitter-induced activation of a neuroreceptor to which the enzyme is coupled [70–74]. The enzyme also can be activated via cytokine receptors during neuroinflammation and excitotoxicity [75–77]. Activation of brain cPLA2 IVA accompanied by an increased DPAn-6 concentration during deprivation thus may promote DPAn-6 release and metabolism in during neurotransmission and with neuroinflammation.
Effects of increased brain DPAn-6 concentration and metabolism remain to be elucidated. The loss of a single double bond from DHA to DPA replacement in the membrane causes a more even distribution of chain densities along the bilayer, which could alter function of integral membrane proteins [78]. In the brain of the n-3 PUFA adequate animals, radioactive β-oxidation products (16:0 and 18:0) represented 10% of total phospholipid radioactivity, whereas their contribution was 5% in the deprived animals, suggesting reduced β-oxidation following transfer of DPAn-6 into brain mitochondria [79].
Increased dietary DPAn-6 has been reported to potentiate the efficiency of DHA to reduce β-amyloid accumulation in 3×Tg mice, a model for Alzheimer disease, and dietary DPAn-6 plus DHA supplementation reduced brain phospho-tau and β-amyloid levels in these mice [17]. DPAn-6 can be a substrate for cyclooxygenases (COX) and lipoxygenases (LOX); bioactive 12-hydroxy-5,8,10,14-eicosatetraenoic acid is a major metabolite [80]. The reaction between DPAn-6 and 15-LOX generated 17(S)-hydroxy-DPAn-6 and 10, 17(S)-hydroxy-DPAn-6 [81], which have anti-inflammatory effects. 17(S)-hydroxy-DPAn-6, but not 10, 17(S)-hydroxy-DPAn-6, was detected in blood, trachea and heart of rats fed DPAn-6. The effects of DPAn-6 metabolites likely differ from those of DHA.
In summary, dietary n-3 PUFA deprivation, for 15 weeks post-weaning in rats, decreased plasma and brain DHA concentrations, while increasing plasma and brain DPAn-6 concentrations, incorporation of unesterified plasma DPA into brain phospholipids, and DPAn-6 turnover in the phospholipids. Increased brain DPAn-6 concentrations and metabolism in the face reduced brain DHA concentrations and metabolism may contribute to altered behaviors and metabolism reported in rats and other species following n-3 PUFA deprivation.
Supplementary Material
Highlights.
Dietary n-3 PUFA increased DPAn-6 in rat brain. The diet increased DPA incorporation and turnover rates in rat brain. The change may be associated with increased cytosolic cPLA2 IVA. These could contribute to behavioral and functional abnormalities by the diet.
ACKNOWLEDGEMENTS
This research was supported entirely by the Intramural Research Program of the National Institute on Aging. The authors thank the NIH Fellows' Editorial Board for editorial assistance.
Abbreviations
- AA
arachidonic acid
- DPA
docosapentaenoic acid (22:5)
- DHA
docosahexaenoic acid (22:6n-3)
- EPA
eicosapentaenoic acid (20:5n-3)
- FAME
fatty acid methyl ester
- GC
gas chromatography
- LA
linoleic acid (18:2n-6)
- α-LNA
α-linolenic acid (18:3n-3)
- PUFA
polyunsaturated fatty acid
- PL
phospholipids
- TG
triacylglycerol
- EtnGpl
ethanolamine glycerophospholipid
- ChoGpl
choline glycerophospholipid
- PtdSer
phosphatidylserine
- PtdIns
phosphatidylinositol
- cPLA2
cytosolic phospholipase A2
- sPLA2
secretory PLA2
- iPLA2
calcium independent PLA2
- COX
cyclooxygenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Igarashi M, Gao F, Kim HW, Ma K, Bell JM, Rapoport SI. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim Biophys Acta. 2009;1791:132–139. doi: 10.1016/j.bbalip.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Galli C, White HB, Jr., Paoletti R. Lipid alterations and their reversion in the central nervous system of growing rats deficient in essential fatty acids. Lipids. 1971;6:378–387. doi: 10.1007/BF02531374. [DOI] [PubMed] [Google Scholar]
- [3].Anderson RE, Benolken RM, Dudley PA, Landis DJ, Wheeler TG. Proceedings: Polyunsaturated fatty acids of photoreceptor membranes. Exp Eye Res. 1974;18:205–213. doi: 10.1016/0014-4835(74)90149-3. [DOI] [PubMed] [Google Scholar]
- [4].Bourre JM, Durand G, Pascal G, Youyou A. Brain cell and tissue recovery in rats made deficient in n-3 fatty acids by alteration of dietary fat. J Nutr. 1989;119:15–22. doi: 10.1093/jn/119.1.15. [DOI] [PubMed] [Google Scholar]
- [5].Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N., Jr. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J Lipid Res. 2001;42:419–427. [PubMed] [Google Scholar]
- [6].Greiner RS, Catalan JN, Moriguchi T, Salem N., Jr. Docosapentaenoic acid does not completely replace DHA in n-3 FA-deficient rats during early development. Lipids. 2003;38:431–435. doi: 10.1007/s11745-003-1080-2. [DOI] [PubMed] [Google Scholar]
- [7].Lim SY, Hoshiba J, Moriguchi T, Salem N., Jr. N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr Res. 2005;58:741–748. doi: 10.1203/01.PDR.0000180547.46725.CC. [DOI] [PubMed] [Google Scholar]
- [8].Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- [9].Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- [10].Igarashi M, DeMar JC, Jr., Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- [11].Igarashi M, DeMar JC, Jr., Ma K, Chang L, Bell JM, Rapoport SI. Upregulated liver conversion of α-linolenic acid to docosahexaenoic acid in rats on a 15 week n-3 PUFA-deficient diet. J Lipid Res. 2007;48:152–164. doi: 10.1194/jlr.M600396-JLR200. [DOI] [PubMed] [Google Scholar]
- [12].Murthy M, Hamilton J, Greiner RS, Moriguchi T, Salem N, Jr., Kim HY. Differential effects of n-3 fatty acid deficiency on phospholipid molecular species composition in the rat hippocampus. J Lipid Res. 2002;43:611–617. [PubMed] [Google Scholar]
- [13].DeMar JC, Jr., Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- [14].Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- [15].DeMar JC, Jr., Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- [16].Greiner RS, Moriguchi T, Slotnick BM, Hutton A, Salem N. Olfactory discrimination deficits in n-3 fatty acid-deficient rats. Physiol Behav. 2001;72:379–385. doi: 10.1016/s0031-9384(00)00437-6. [DOI] [PubMed] [Google Scholar]
- [17].Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27:4385–4395. doi: 10.1523/JNEUROSCI.0055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stark KD, Lim SY, Salem N., Jr. Artificial rearing with docosahexaenoic acid and n-6 docosapentaenoic acid alters rat tissue fatty acid composition. J Lipid Res. 2007;48:2471–2477. doi: 10.1194/jlr.M700317-JLR200. [DOI] [PubMed] [Google Scholar]
- [19].Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev. 2006;64:S24–33. doi: 10.1301/nr.2006.may.s24-s33. discussion S72–91. [DOI] [PubMed] [Google Scholar]
- [20].Farooqui AA, Horrocks LA, Farooqui T. Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem Phys Lipids. 2000;106:1–29. doi: 10.1016/s0009-3084(00)00128-6. [DOI] [PubMed] [Google Scholar]
- [21].Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int J Dev Neurosci. 2000;18:383–399. doi: 10.1016/s0736-5748(00)00013-7. [DOI] [PubMed] [Google Scholar]
- [22].Rapoport SI, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry. 2002;59:592–596. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- [23].Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol Psychiatry. 2006;59:401–407. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- [24].Rao JS, Ertley RN, DeMar JC, Jr., Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- [25].Kim HW, Rao JS, Rapoport SI, Igarashi M. Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain. Biochimica et biophysica acta. 2011;1811:111–117. doi: 10.1016/j.bbalip.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- [27].Contreras MA, Chang MC, Rosenberger TA, Greiner RS, Myers CS, Salem N, Jr., Rapoport SI. Chronic nutritional deprivation of n-3 alpha-linolenic acid does not affect n-6 arachidonic acid recycling within brain phospholipids of awake rats. J Neurochem. 2001;79:1090–1099. doi: 10.1046/j.1471-4159.2001.00658.x. [DOI] [PubMed] [Google Scholar]
- [28].Rapoport SI, Chang MC, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- [29].Rapoport SI. In vivo fatty acid incorporation into brain phospholipids in relation to plasma availability, signal transduction and membrane remodeling. J. Mol. Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. [DOI] [PubMed] [Google Scholar]
- [30].Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: Review and critical analysis. Brain Res Brain Res. Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- [31].Igarashi M. Unpublished results in, 2008. [Google Scholar]
- [32].Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- [33].Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr. 1993;123:1923–1931. doi: 10.1093/jn/123.11.1923. [DOI] [PubMed] [Google Scholar]
- [34].Igarashi M, DeMar JC, Jr., Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from α-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- [35].DeMar JC, Jr., Ma K, Chang L, Bell JM, Rapoport SI. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem. 2005;94:1063–1076. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- [36].Igarashi M, Ma K, Chang L, Bell JM, DeMar JC, Jr., Rapoport SI. α-linolenic acid is minimally converted to docosahexaenoic acid in brain and liver of adult rats fed a DHA-containing diet. Soc. Neurosci. Abstr. 2005;35:188.114. [Google Scholar]
- [37].Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI, Demar JC., Jr. Low liver conversion rate of α-linolenic to docosahexaenoic acid in awake rats on a high-docosahexaenoate-containing diet. J Lipid Res. 2006;47:1812–1822. doi: 10.1194/jlr.M600030-JLR200. [DOI] [PubMed] [Google Scholar]
- [38].Washizaki K, Smith QR, Rapoport SI, Purdon AD. Brain arachidonic acid incorporation and precursor pool specific activity during intravenous infusion of unesterified [3H]arachidonate in the anesthetized rat. J Neurochem. 1994;63:727–736. doi: 10.1046/j.1471-4159.1994.63020727.x. [DOI] [PubMed] [Google Scholar]
- [39].Folch J, Lees M, Stanley G.H. Sloane. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- [40].Skipski VP, Good JJ, Barclay M, Reggio RB. Quantitative analysis of simple lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1968;152:10–19. doi: 10.1016/0005-2760(68)90003-9. [DOI] [PubMed] [Google Scholar]
- [41].Skipski VP, Barclay M, Reichman ES, Good JJ. Separation of acidic phospholipids by one-dimensional thin-layer chromatography. Biochim Biophys Acta. 1967;137:80–89. doi: 10.1016/0005-2760(67)90010-0. [DOI] [PubMed] [Google Scholar]
- [42].Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr. 1994;60:189–194. doi: 10.1093/ajcn/60.2.189. [DOI] [PubMed] [Google Scholar]
- [43].Aveldano MI, VanRollins M, Horrocks LA. Separation and quantitation of free fatty acids and fatty acid methyl esters by reverse phase high pressure liquid chromatography. J Lipid Res. 1983;24:83–93. [PubMed] [Google Scholar]
- [44].Deutsch J, Grange E, Rapoport SI, Purdon AD. Isolation and quantitation of long-chain acyl-coenzyme A esters in brain tissue by solid-phase extraction. Anal Biochem. 1994;220:321–323. doi: 10.1006/abio.1994.1344. [DOI] [PubMed] [Google Scholar]
- [45].Taylor DC, Weber N, Hogge LR, Underhill EW. A simple enzymatic method for the preparation of radiolabeled erucoyl-CoA and other long-chain fatty acyl-CoAs and their characterization by mass spectrometry. Anal Biochem. 1990;184:311–316. doi: 10.1016/0003-2697(90)90686-4. [DOI] [PubMed] [Google Scholar]
- [46].Rapoport SI. In vivo approaches and rationale for quantifying kinetics and imaging brain lipid metabolic pathways. Prostaglandins Other Lipid Mediat. 2005;77:185–196. doi: 10.1016/j.prostaglandins.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [47].Contreras MA, Chang MC, Kirkby D, Bell JM, Rapoport SI. Reduced palmitate turnover in brain phospholipids of pentobarbital-anesthetized rats. Neurochem Res. 1999;24:833–841. doi: 10.1023/a:1020997728511. [DOI] [PubMed] [Google Scholar]
- [48].Contreras MA, Greiner RS, Chang MC, Myers CS, Salem N, Jr., Rapoport SI. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J Neurochem. 2000;75:2392–2400. doi: 10.1046/j.1471-4159.2000.0752392.x. [DOI] [PubMed] [Google Scholar]
- [49].Kim HW, Rao JS, Rapoport SI, Igarashi M. Regulation of rat brain polyunsaturated fatty acid (PUFA) metabolism during graded dietary n-3 PUFA deprivation. Prostaglandins, leukotrienes, and essential fatty acids. 2011 doi: 10.1016/j.plefa.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Salem N, Jr., Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- [51].Bourre JM, Dumont O, Pascal G, Durand G. Dietary alpha-linolenic acid at 1.3 g/kg maintains maximal docosahexaenoic acid concentration in brain, heart and liver of adult rats. J Nutr. 1993;123:1313–1319. doi: 10.1093/jn/123.7.1313. [DOI] [PubMed] [Google Scholar]
- [52].Nehlig A. Cerebral energy metabolism, glucose transport and blood flow: changes with maturation and adaptation to hypoglycaemia. Diabetes & metabolism. 1997;23:18–29. [PubMed] [Google Scholar]
- [53].Gottlieb A, Keydar I, Epstein HT. Rodent brain growth stages: an analytical review. Biology of the neonate. 1977;32:166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- [54].Tabata H, Bell JM, Miller JC, Rapoport SI. Incorporation of plasma palmitate into the brain of the rat during development. Brain Res. 1986;394:1–8. doi: 10.1016/0165-3806(86)90076-3. [DOI] [PubMed] [Google Scholar]
- [55].Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci. 2000;22:474–480. doi: 10.1159/000017478. [DOI] [PubMed] [Google Scholar]
- [56].Chugani HT, Hovda DA, Villablanca JR, Phelps ME, Xu WF. Metabolic maturation of the brain: a study of local cerebral glucose utilization in the developing cat. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1991;11:35–47. doi: 10.1038/jcbfm.1991.4. [DOI] [PubMed] [Google Scholar]
- [57].Purdon D, Arai T, Rapoport S. No evidence for direct incorporation of esterified palmitic acid from plasma into brain lipids of awake adult rat. J Lipid Res. 1997;38:526–530. [PubMed] [Google Scholar]
- [58].DeMar JC, Jr., Lee HJ, Ma K, Chang L, Bell JM, Rapoport SI, Bazinet RP. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- [59].Holman RT. Control of polyunsaturated acids in tissue lipids. J Am Coll Nutr. 1986;5:183–211. doi: 10.1080/07315724.1986.10720125. [DOI] [PubMed] [Google Scholar]
- [60].Gao F, Kiesewetter D, Chang L, Rapoport SI, Igarashi M. Quantifying conversion of linoleic to arachidonic and other n-6 polyunsaturated fatty acids in unanesthetized rats. J Lipid Res. 2010 doi: 10.1194/jlr.M005595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [61].Moriguchi T, Lim SY, Greiner R, Lefkowitz W, Loewke J, Hoshiba J, Salem N., Jr. Effects of an n-3-deficient diet on brain, retina, and liver fatty acyl composition in artificially reared rats. J Lipid Res. 2004;45:1437–1445. doi: 10.1194/jlr.M400087-JLR200. [DOI] [PubMed] [Google Scholar]
- [62].Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Elizondo A, Araya J, Rodrigo R, Poniachik J, Csendes A, Maluenda F, Diaz JC, Signorini C, Sgherri C, Comporti M, Videla LA. Polyunsaturated fatty acid pattern in liver and erythrocyte phospholipids from obese patients. Obesity (Silver Spring) 2007;15:24–31. doi: 10.1038/oby.2007.518. [DOI] [PubMed] [Google Scholar]
- [64].Glick NR, Fischer MH. Essential fatty acid-deprived tube-fed adults synthesize arachidonic and docosahexaenoic acids: a pilot analysis of the fatty acid status of people with profound developmental disabilities. Clin Biochem. 2008;41:1019–1021. doi: 10.1016/j.clinbiochem.2008.04.018. [DOI] [PubMed] [Google Scholar]
- [65].Yang HC, Mosior M, Ni B, Dennis EA. Regional distribution, ontogeny, purification, and characterization of the Ca2+-independent phospholipase A2 from rat brain. J Neurochem. 1999;73:1278–1287. doi: 10.1046/j.1471-4159.1999.0731278.x. [DOI] [PubMed] [Google Scholar]
- [66].Strokin M, Sergeeva M, Reiser G. Role of Ca2+-independent phospholipase A2 and n-3 polyunsaturated fatty acid docosahexaenoic acid in prostanoid production in brain: perspectives for protection in neuroinflammation. Int J Dev Neurosci. 2004;22:551–557. doi: 10.1016/j.ijdevneu.2004.07.002. [DOI] [PubMed] [Google Scholar]
- [67].Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768:43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- [68].Lands WEM, Crawford CG. Enzymes of membrane phospholipid metabolism. In: Martonosi A, editor. The Enzymes of Biological Membranes. vol. 2. Plenum; New York: 1976. pp. 3–85. [Google Scholar]
- [69].Sun GY, MacQuarrie RA. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann N Y Acad Sci. 1989;559:37–55. doi: 10.1111/j.1749-6632.1989.tb22597.x. [DOI] [PubMed] [Google Scholar]
- [70].Ong WY, Sandhya TL, Horrocks LA, Farooqui AA. Distribution of cytoplasmic phospholipase A2 in the normal rat brain. J. Hirnforsch. 1999;39:391–400. [PubMed] [Google Scholar]
- [71].Rapoport SI. Arachidonic acid and the brain. The Journal of nutrition. 2008;138:2515–2520. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jones CR, Arai T, Bell JM, Rapoport SI. Preferential in vivo incorporation of [3H]arachidonic acid from blood in rat brain synaptosomal fractions before and after cholinergic stimulation. J Neurochem. 1996;67:822–829. doi: 10.1046/j.1471-4159.1996.67020822.x. [DOI] [PubMed] [Google Scholar]
- [73].Bayon Y, Hernandez M, Alonso A, Nunez L, Garcia-Sancho J, Leslie C, Crespo M. Sanchez, Nieto ML. Cytosolic phospholipase A2 is coupled to muscarinic receptors in the human astrocytoma cell line 1321N1: characterization of the transducing mechanism. Biochem J. 1997;323(Pt 1):281–287. doi: 10.1042/bj3230281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vial D, Piomelli D. Dopamine D2 receptors potentiate arachidonate release via activation of cytosolic, arachidonic-specific phospholipase A2. J. Neurochem. 1995;64:2765–2772. doi: 10.1046/j.1471-4159.1995.64062765.x. [DOI] [PubMed] [Google Scholar]
- [75].Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- [76].Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13. [PubMed] [Google Scholar]
- [77].Dumuis A, Sebben M, Haynes L, Pin JP, Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988;336:68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- [78].Eldho NV, Feller SE, Tristram-Nagle S, Polozov IV, Gawrisch K. Polyunsaturated docosahexaenoic vs docosapentaenoic acid-differences in lipid matrix properties from the loss of one double bond. Journal of the American Chemical Society. 2003;125:6409–6421. doi: 10.1021/ja029029o. [DOI] [PubMed] [Google Scholar]
- [79].Gavino GR, Gavino VC. Rat liver outer mitochondrial carnitine palmitoyltransferase activity towards long-chain polyunsaturated fatty acids and their CoA esters. Lipids. 1991;26:266–270. doi: 10.1007/BF02537135. [DOI] [PubMed] [Google Scholar]
- [80].Milks MM, Sprecher H. Metabolism of 4,7,10,13,16-docosapentaenoic acid by human platelet cyclooxygenase and lipoxygenase. Biochim Biophys Acta. 1985;835:29–35. doi: 10.1016/0005-2760(85)90026-8. [DOI] [PubMed] [Google Scholar]
- [81].Dangi B, Obeng M, Nauroth JM, Teymourlouei M, Needham M, Raman K, Arterburn LM. Biogenic synthesis, purification, and chemical characterization of anti-inflammatory resolvins derived from docosapentaenoic acid (DPAn-6) J Biol Chem. 2009;284:14744–14759. doi: 10.1074/jbc.M809014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.