Abstract
Translational clinical research has emerged as an important priority for the national research enterprise, with a clearly stated mandate to deliver prevention strategies, treatments and cures based on scientific innovations faster to the public. Within this national effort, a lack of consensus persists concerning the need for clinical nurses with expertise and specialized training in study implementation and the delivery of care to research participants. This paper reviews efforts to define and document the role of practicing nurses in implementing studies and coordinating clinical research in a variety of clinical settings and differentiates this clinical role from the role of nurses as scientists and principal investigators. We propose an agenda for building evidence that having nurses provide and coordinate study treatments and procedures can potentially improve research efficiency, participant safety, and the quality of research data. We also provide recommendations for the development of the emerging specialty of clinical research nursing.
Translational Clinical Research: A New Arena for Nursing Practice
Clinical and translational research has emerged as an important, crosscutting priority for the national research enterprise. Calls for the acceleration of translational research initially focused on the “valley of death” between laboratory discoveries and clinical innovation and the testing and application of promising findings in clinical trials 1. The concept of translation was extended to include the concern that too often promising interventions were successfully tested in clinical trials, but that this transfer from bench to bedside only resulted in a transfer from “bedside to the academic bookshelf,” 2, page 229, with no perceptible change in clinical practice or patient outcomes. Translational research is now construed as a continuum with translation steps occurring from theories and discoveries in the basic sciences, through human testing, and then through multiple translation steps to application in a community setting and evaluation of long term outcomes and comparative effectiveness3–6.
Nurses have important roles across the knowledge creation and translation process, from concept development and basic scientific discovery through evaluation and dissemination research. However when nurses’ contributions to research are discussed, the conversation within the discipline is primarily about two basic roles: the role of the nurse scientist who leads the discovery side of the process, and the role of clinical nurses and their professional leadership in assessing, testing, applying and adopting practices based on research evidence (“evidence-based practice”). There has been very little explicit attention over the past 30 years given in mainstream nursing to the roles, required qualifications and impact of nurses who practice in the clinical research setting providing and coordinating the care of research participants. The purpose of this paper is to articulate the important contribution of clinical nursing to the care of research participants, coordination of clinical studies, and overall functioning of the clinical research enterprise.
What is the Clinical Specialty of Clinical Research Nursing?
Clinical research nursing is nursing practice with a specialty focus on the care of research participants in which nursing care delivery parallels the process of clinical research study implementation (Figure 1). In addition to providing and coordinating clinical care, clinical research nurses have a central role in assuring participant safety, maintenance of informed consent, integrity of protocol implementation, accuracy of data collection and recording, and appropriate follow up. Nursing care provided to research participants is driven by study requirements and the collection of research data as well as clinical indications. Patient assessment and clinical data collection may include clinical observations, clinical measurements, specimen collection and preparation, and documentation of research participant reported outcomes. Interventions and study procedures may include administration of investigational drugs, performance of an experimental or investigational surgical or radiological procedure, detailed clinical evaluation or phenotyping to characterize the natural history and etiology of a disease, or delivery of a psychosocial intervention. The participant’s response to the study intervention may make additional nursing care necessary. These care requirements range from educating the participant about self-monitoring to comprehensive physiological monitoring and life support in an intensive care unit. Because clinical research is an interdisciplinary process, the potential contribution of the clinical research nurse includes coordination of research and care as well as management of the interface between study-based care and community services.
Figure 1.
Care and Research in Clinical Research
Because of the intertwined link between care and research in the clinical research process, clinical research nurses must continually balance the requirements of the study with the clinical needs of individual participants7–11. This balance between clinical and research priorities is essential to the ethical implementation of clinical studies as well as the consistency, accuracy and efficiency of data collection12. Ultimately, achieving and maintaining this balance may be a significant factor in the participant’s perception of the integrity of the entire clinical research enterprise.
Nurses who practice in clinical research are often the first to experience and work with research participants on the actual delivery and management of a new therapy, drug or device. They may be the first to have a hint of implications that later become important to the general use of the new intervention – either an adverse event or a needed modification in delivery or implementation that is important to patient tolerance and adherence. Observations made by nurses who are the agents of implementation in a clinical trial may therefore have direct bearing on the speed with which translation to the next level of testing and use can occur13,14.
In order to achieve the best possible outcomes for research participants and the research process, clinical research nurses must not only have expert clinical skills and well developed critical thinking skills, but must also be well versed in the complex regulatory, ethical, and scientific aspects of clinical research. By managing study activities, monitoring participants for anticipated and unanticipated events, supporting and educating participants, interpreting and rephrasing descriptions of study-related care for participant and family, the clinical research nurse ensures that the study objectives are attained while upholding all the principles of participant rights, patient safety and continuity of care. Nurses improve recruitment of participants 15, and support individuals to make informed decisions about clinical trials participation 16.
The highest priority of clinical research is to protect human subjects from undue harm17 and undue burden from the research. The clinical research enterprise has special obligations to those willing to volunteer to participate in clinical studies and potentially place themselves in harm’s way for the benefit of others. The ability to conduct clinical studies depends upon individuals being willing to participate, so public trust is crucial. The public’s perception of both the safety of the studies and the integrity of the research enterprise are critical contributors to the public trust.
Clinical research nurses may serve as the first line of communication to participants and family members about study progress, evolving concerns and next steps. Because clinical research nurses play a vital role in detecting and documenting adverse events, their vigilance, clinical astuteness, and knowledge of the clinical context of the study are important factors in decreasing risks to participants. Identifying adverse events at the earliest time point requires disciplined training and an in-depth understanding of the scientific basis of the research and, where applicable, the investigational agent or device.
As the scope of clinical research is broadened to include more community-based sites, there will be greater reliance on local clinicians to deliver care in support of the research intervention as well as to monitor participant compliance and response to treatment. This expansion has implications for the roles of nurses coordinating studies in these sites as well as for nurses providing the care to research participants who may or may not have formal training in research implementation and human subject protection. It has been suggested 18 that as clinical care moves from the hospital setting to community-based settings, threats to patient safety shift from being treatment related to being diagnosis related. Greater reliance must be placed on adherence to therapy as opposed to direct delivery and oversight of care. This parallels the shift in treatment implementation responsibility from the care provider team to the patient in ambulatory settings, and has implications for the clinical research nurse’s role in promotion of adherence to an experimental protocol as well as the prevention of adverse events.
Role Development in Clinical Research Nursing
Although nurses are integral and assumed to be essential to the clinical research team, there is no generally accepted, standardized definition of the role of the clinical research nurse 8,12,19–22. The most concentrated effort to define the role of nurses in clinical research care has been the longstanding effort within the specialty of oncology nursing, beginning in the early 1980’s 23–28. This work resulted in an organizational focus within the Oncology Nursing Society: the Clinical Trials Special Interest Group 29, and the development of a survey instrument which is one of the few role profile instruments available for clinical research nursing 8.The effort culminated in a manual for clinical trials nursing and the formal development of clinical trials nursing competencies 30. During this same time period, there has been work in the United Kingdom defining and describing the roles and competencies of clinical research nurses. The National Health Service (NHS) initiated Agenda for Change in 2004 with associated job descriptions and profiles in an attempt to standardize terms and conditions for all non-medical jobs in the NHS across the United Kingdom (UK) 31. In association with the new UK Clinical Research Facility Network, a working group including the Royal College of Nursing and the National Cancer Research Network later developed the Competency Framework for Clinical Research Nurses 32. For the first time research nurses working in the UK had clearly described and relevant competencies linked to their roles with performance criteria and contextual factors to guide their professional development. More recent proposals have been made by members of the UKCRF Network as part of the UK Clinical Research Nurse Consortium, comprised of the Chief Executive and Registrar of the Nursing & Midwifery Council (the UK wide professional registration body), GCP Training Manager from the NIHR Clinical Research Network, Research & Development Advisor of the Royal College of Nursing, Directors and senior managers of UK Research Networks and facilities. Their position paper outlines the need for a more coordinated and strategic approach that will maximise the nursing, midwifery and health visiting contribution to improving the health and wealth of the UK through research. The paper acknowledges the financial constraints inherent in the research process and need to have clarity of roles within the multi-disciplinary team 33. This work builds on the premise that “well trained, competent, supervised, permanently employed research nurses are the best means of mitigating against poor clinical and research practice” 34. Development of clinical research nursing in other specialties, such as HIV care 35,36, gastroenterology 37, and urology 38 has added some conceptual clarity to current understanding of the practice, as has work in other countries with a high concentration of clinical trials such as Canada and New Zealand 39,40.
In spite of considerable effort to standardize terminology and communicate the roles of nurses within the clinical research infrastructure, confusion of titles and roles has persisted over the years with respect to nurses who provide direct clinical care in a setting in which clinical research is conducted or who coordinate a study in a clinical setting. It is therefore not surprising that an internal assessment conducted in 2007 at the NIH Clinical Center found five formal and 39 informal titles associated with nurses practicing on the clinical research patient care units or coordinating studies on behalf of individual NIH Institute principal investigators 41,42. Additionally, many titles applied to nurses in clinical research, such as Clinical Research Associate (CRA) or Clinical Research Coordinator (CRC), are also used for non-nurses who coordinate or support clinical research. Indeed certification for these titles does not require registered nurse licensure 43,44. It has been acknowledged that although roles such as a CRA may be filled by a nurse or a non-nurse, a staffing decision to use a nurse may be the most practical decision for a small research program, since a nurse can do both the clinical support activities as well as the study coordination and compliance management activities 45. Because clinical requirements vary significantly across studies, clarifying the scope and title for these roles is necessary to assure that the most qualified clinical research staff members are selected for a given study or research program.
Defining the Conceptual Domain of Practice for Clinical Research Nursing
It was recognized early in the exploration of conceptual definitions for clinical research nursing that the scope of practice for clinical research nurses extended beyond the dimension of direct clinical intervention to include study management, human subject protection, care coordination within the context of research participation, and contributions to clinical science as an active research team member 46,47. These elements became the basis for a five dimensional taxonomy of activities (see Table 1) proposed to describe the domain of practice of clinical research nurses, which was developed at the NIH Clinical Center and validated in a national sample of nurses involved with providing clinical research nursing services 7. This taxonomy is intended to serve as a blueprint for competency and core curriculum development for the specialty, and later potentially for certification standards.
Table 1. The Domain of Clinical Research Nursing Practice.
The overall domain of practice includes care provided to research participants, as well as coordination of care and services and activities to support protocol implementation, data collection, human subject protection and participation in team science
| Dimension | Definition |
|---|---|
| Clinical Practice | Provision of nursing care, education, and support, using the nursing process, to participants in clinical research and their families and significant others. Care requirements are determined by the scope of study participation, the clinical condition of the patient, and the requirements and clinical effects of research procedures and data collection |
| Study Management | Management of clinical and research support activities in order to assure patient safety, address clinical needs and assure protocol integrity and accurate data collection |
| Care Coordination and Continuity | Coordination of research and clinical activities to meet clinical needs, complete study requirements and manage linkage with referring and primary care providers |
| Human Subjects Protection | Facilitation of informed participation by diverse participants in clinical research |
| Contributing to the Science | Contributions as a research team member to the development of new ideas for study, explorations of innovations arising for clinical research and application of clinical research findings to practice |
The clinical research nursing practice domain was further conceptualized to cover in its scope two primary roles assumed by nurses practicing in clinical research settings. These roles represent the ends of a continuum with one role primarily concerned with patient care (Clinical Research Nurse or CRN) and the other role primarily concerned with study coordination (Research Nurse Coordinator or RNC). Actual roles vary along this continuum. The practice domain taxonomy was the basis for a role delineation survey instrument that was used in a survey of nurses in RNC and CRN roles at the NIH Clinical Center 48. The large sample size (n=412), and the ability of the role delineation instrument to differentiate roles focused on clinical implementation from roles focused on study coordination, supports broader application to characterize the roles of nurses practicing in clinical research across the spectrum of research settings.
Clinical research nurses are clinical staff nurses with a central focus on care of research participants. They support study implementation within the context of the care delivery setting and are primarily located in dedicated clinical research settings, such as the NIH Clinical Center, research units within the Clinical and Translational Science Award (CTSA) sites, clinical research programs within academic medical centers (such as designated NCI Cancer Centers), community based research programs and networks, or clinical research units run by industry or under contract to a sponsor. Staff nurses practicing in such clinical research settings are part of the permanent infrastructure of the research unit, and are available to any investigator or sponsor accessing the facility for support of research implementation and research participant clinical care.
Research nurse coordinators on the other hand are often hired by and report to a principal investigator and may be funded only for the support of a specific study or group of studies. Research nurse coordinators are primarily responsible for study coordination and data management, with a central focus on managing subject recruitment and enrollment, consistency of study implementation, data management and integrity, and compliance with regulatory requirements and reporting. They may rely on clinical staff to deliver “hands on” care including administration of investigational drugs or interventions.
The roles of clinical research nurse and research nurse coordinator are considered part of a career path in clinical research that in some cases could extend, with advanced education and experience, to the role of nurse scientist or principle investigator (Figure 2). There are several examples of this progression at the NIH Clinical Center which probably has the largest consolidated clinical research workforce in the U.S. However not all clinical research nurses are interested in becoming scientists and clinical scientists certainly do not have to start in research by providing care to research participants. There are many entry and exit points into and out of the clinical research career progression shown in Figure 2. It is important to note that the conceptual definition of clinical research nursing as a specialty is a clinical practice definition with a focus on research participant care and care coordination, and is not intended to refer to the role of nurse scientist as a clinical researcher.
Figure 2.
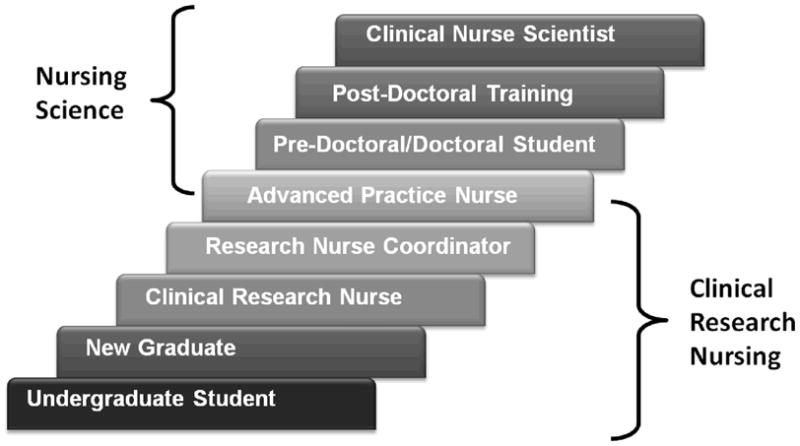
Nursing Career Path in Research at the NIH Clinical Center
Clinical Research Nursing in the Federally Funded US Clinical Research Enterprise
Clinical nursing resources have been a major part of two significant investments made by the NIH in the “critical mass” concept for clinical research. One of these investments is the NIH Clinical Center, the 240-bed clinical research hospital in Bethesda, Maryland, opened in 1952 49,50, and updated with a new state-of-the-art facility in 2005 51,52. The second was the national network of General Clinical Research Centers (GCRCs) located primarily in academic medical centers 53–55, and recently transformed into broader networks of linked research resources through the CTSAs. For over 50 years, the NIH Clinical Center and GCRC / CTSA programs provided a variety of clinical facilities and support services for investigators to conduct clinical research and represent a substantial investment in the clinical infrastructure to support ongoing implementation of clinical studies56. This investment included facilities, equipment and interdisciplinary staff, the majority of whom are specialty practice clinical research nurses.
Historically, the NIH Clinical Center and GCRCs / CTSA network provided a stable base upon which new researchers are able to build programs of research18,55,57. They have also provided research training for new investigators from every discipline. However, because of their concentrated and fixed cost, both structures have come under repeated scrutiny, which has intensified in the recent fiscal climate. As the NIH budget shrinks in terms of inflation-adjusted dollars, the NIH Clinical Center and newly funded CTSAs are facing financial pressures resulting in fiscal tension between the core clinical infrastructure and specific investigator-driven research. This situation puts particular pressure on the use of high cost resources such as registered nurses in the coordination and execution of clinical studies. Thus, there is an urgent need to clearly delineate the benefits of having highly skilled clinical research nurses as an integral part of the clinical research process.
The reengineering of federally funded clinical research driven by the NIH Roadmap initiative 58 and the launch of the CTSA program in 2006 59, fundamentally changed the clinical research infrastructure as it existed in academic medical centers and their communities of practice. The initial focus within nursing was to assure that nursing had a voice in the developing CTSA scientific and operational structures and facilitate inclusion of nurse scientists in emerging translational science interdisciplinary teams. Nursing leaders were urged to become actively involved in setting the research agenda within individual CTSAs and influence the scientific focus within the evolving CTSA network 60. As the transformational change catalyzed by the CTSA program gained momentum, the contribution of nurses to the interdisciplinary science agenda and the translation of research findings from early phase experiments to community practice settings became more visible61–63.
During the emergence of the CTSAs, the clinical practice environment of nurses working within the research infrastructure was not an area of focus and has not been formally articulated. The CTSA mechanism emphasizes interdisciplinary research and creation of networks of collaborating sites and resources that can reduce the time from discovery to application and extend findings rapidly to the community64,65. There was silence in calls for proposals, and in many of the CTSA grant responses, on the topic of how clinical resources would be supplied to research teams, or whether funded research support would include clinical nursing. This contrasts with the GCRC mechanism in which a structured presence of nursing services was specifically required 66,67. The allocation and use of clinical resources, including nurses, was left up to individual CTSA networks to decide, and has varied substantially as these entities have evolved. Lack of consensus has persisted within the clinical research community about the need for and use of clinical nurses with expertise and specialized training in clinical research practice to provide care and care coordination for research study participants.
Efforts within the NIH Clinical Center and the national network of GCRCs to define the roles and contributions of clinical research nurses took on a new urgency with the rapidly escalating pace of change in the clinical research environment. A Position Paper from the GCRC Nurse Managers Association posted on their website in February, 2006, and now available as an unpublished manuscript from the International Association of Clinical Research Nurses (IACRN) 68, called for a synthesis of the knowledge and experience from 45 years of GCRC clinical research nursing practice into the emerging innovative structures for clinical research, specifically calling for the retention of dedicated funded sites for clinical research that could sustain the kind of embedded expertise available in the GCRCs. The Position Paper called particular attention to the ongoing tension between clinical needs for interventions and research data collection requirements confronting clinicians attempting to combine care and research in one setting. This is a factor in dedicated research centers but becomes even more of an issue when clinical research is dispersed into an acute care or primary care environment69–78.
In 2006, nursing at the NIH Clinical Center launched a four-year strategic initiative, called CRN2010, to define clinical research nursing as a practice specialty. This included development and dissemination of tools and resources needed to support specialty practice and build the knowledge base needed for specialty practice certification. In 2007, these two efforts came together and the “National Clinical Research Nursing Consortium” was created, consisting of nurse managers from several GCRCs, about half of which had been funded as CTSAs and were in the process of transition, and nursing leadership from the NIH Clinical Center. This group met three times at Rockefeller University in New York, with the goal of outlining the issues related to the definition and use of clinical nursing resources which were urgently facing the clinical research enterprise. One outcome of these discussions was the creation of the International Association of Clinical Research Nurses in 2009 79. In the years since the first convening of the Consortium, significant progress has been made in defining the domain of clinical research nursing practice and laying the groundwork for core competency assessment, curriculum development and ultimately professional certification in the specialty.
Regulatory influences on Clinical Research Nursing
Standards of clinical research known as Good Clinical Practice (GCP) were developed to harmonize global efforts to protect human subjects as they participate in research and assure basic principles of quality control so that results from clinical research are reliable and valid80,81. Compliance with GCP provides the public and regulatory authorities with assurances that the rights, safety, and well being of research subjects are being protected. Furthermore, it helps to ensure that quality data can be submitted to regulatory authorities. The principles of GCP have been standard in industry-sponsored research for many years, and indeed set an international standard for the conduct of research involving human subjects. These standards do not specifically address the roles of clinical research nurses in supporting clinical research. Additional guidance and application of GCP principles have also been proposed by clinical societies, such as the American Society for Clinical Oncology, for use by their members in setting standards and institutional policy 82. As adherence to these standards becomes an expectation across all settings, it is important to document the contribution of clinical research nurses and their role in meeting these standards.
In addition to the overall guidance for conducting clinical research found in GCP guidelines, the practice of nurses is regulated by state licensing authorities and further delineated by institutional policy, including those working with human subjects in the context of clinical research. As licensed professionals, it is expected that nurses are informed about the scope of practice in the state in which they work, including delegation authorities. Activities such as medication administration vary by state in terms of who is allowed to administer, supervise administration and delegate administration of medications. This issue can cause significant confusion when research teams discuss, for example, assignment of the role of dosing and monitoring healthy subjects in a phase 1 trial or performing follow-up telephone evaluations to discover and document possible adverse reactions.
The Global Practice Environment for Clinical Research Nurses
Nurses have historically been integral to the conduct of industry sponsored trials on new drugs and devices within the U.S. and abroad. However the clinical research nursing workforce supporting pharmaceutical and device development is essentially invisible. Although nurses are evident in the leadership of international clinical research organizations such as the Association for Clinical Research Professionals (ACRP), Society for Clinical Research Associates (SoCRA), Association of Clinical Pharmacology Units (ACPU) and others, nurses are not specifically differentiated or counted in the membership of these organizations, which have a multidisciplinary, sponsor and product focused orientation, and which are currently the only providers of specialty certification in clinical research management. There is no reliable way in the nursing professional demographics data bases to find nurses who identify themselves as practicing in research (as opposed to conducting research), since that information is not specifically collected in surveys such as the National Sample Survey of Registered Nurses 83 or data collected by state boards of nursing. Anecdotal information from nurses practicing in commercial sites suggests that the FDA and its international counterparts, including the World Health Organization, are the primary drivers of standards and that scope of practice is primarily influenced by institutional policy and state regulations of nursing practice, which vary widely.
There is no denying the fact that clinical research has become a global enterprise. This has been driven in part by the escalating cost of bringing new drugs and treatments to market combined with investments in technology and clinical research infrastructure in rapidly developing countries such as China and India84,85. At the end of the 20th century, there was a growing understanding that the development and distribution of new pharmaceuticals and devices was a rapidly growing industry, generating its own financial, ethical and political growing pains 86. Globalization of the drug and device development industry has increased dramatically since then, with the U.S., Canada, the UK and Europe still holding the lead in terms of absolute volume of trials and participants, but with the greatest growth being seen in the developing countries of Asia 87, and more recently, South America 88. In addition to the opportunity presented by large numbers of potentially treatment-naïve participants that could be accessed in international sites, the opportunity to significantly increase accrual and conduct large-scale multisite studies of interventions is attractive to investigators 89. As the global clinical research enterprise grows, questions about the role of staff to support research, including the roles of clinical research nurses, become important. The Association of Clinical Research Professionals has a broad international membership, and has featured articles in its professional publications about the developing clinical research infrastructure in India 90, Austria and Hungary 91, and South Africa 92. Within this developing global enterprise, concerns about Good Clinical Practice standards being upheld, ethical treatment of human subjects, the clinical context of research done in international communities, and the quality of research data have all been raised 93–96. These issues create a dynamic environment for the emergence of an identity for clinical research nursing internationally.
Challenges Today
It is acknowledged that advances in the basic sciences in the U.S have exceeded the capacity of the clinical research infrastructure to respond with translational studies and clinical trials 4. A major limiting factor is the cost and complexity of implementing clinical studies, particularly studies that cross institutions, address questions relevant to vulnerable populations, or extend into community care settings. Such studies require careful screening of participants for eligibility and clinical appropriateness as well as intensive monitoring for compliance with study requirements, accurate data collection and documentation of adverse effects. Therapeutic trials using novel agents in specialties such as oncology, critical care, and obstetrics pose special challenges since they require nurses with advanced specialty skills, and often such nurses do not also have training in clinical research20,97,98.
As resources become increasingly constrained, and the focus for clinical translational research expands to include community and practice-based settings, we believe the infrastructure supporting clinical research must be redefined, including the potential roles and contributions of practicing clinical nurses in research. Even more important, the potential benefits must be outlined and demonstrated if these costly resources are used. Issues related to scope of practice and appropriate licensure and competency to assume principle investigator-delegated clinical tasks must also be addressed. In the most recent dramatic shift in Federal funding that is accompanying severe national fiscal constraints, there is acute awareness within the clinical research enterprise that resources are likely to be constrained or reduced for some time to come. In this environment, it is essential for nursing to be able to answer the following questions:
What unique contributions are made by a clinical nurse providing and coordinating clinical services for research participants? How are these services different from care driven by the clinical needs of the patient and service delivery mandate of a health providing organization? How is nursing care and coordination of care for research participants different from the work of a non-licensed clinical research coordinator?
What regulatory requirements affect the nature of work that can be delegated by a principal investigator to various licensed and unlicensed members of a clinical research team?
What is the benefit to having clinical nurses as part of a study team in terms of study efficiency, participant recruitment, satisfaction and retention, participant safety or other measurable outcomes?
What career paths and educational services need to be developed and offered to prepare a workforce that can meet the need? How early in the trajectory of nursing educational programs should information be provided to students about the clinical research enterprise and the potential roles of clinical nurses in providing care to participants and coordinating clinical studies?
A credible effort to answer these questions must be based on an understanding of the scope and contributions of nurses currently working within the clinical research enterprise as well as shared conceptual tools to describe their practice. In addition to agreement on specialty practice definitions, a basic understanding of the points in the clinical research process where nurses can make a difference, and on the nurse sensitive outcomes at those key contribution points is needed. These core steps are the foundation for defining the specialized practice of nurses in clinical research and assessing the sensitivity of clinical and operational outcomes of the research process to nursing involvement. This provides the basis for building evidence of the impact of professional nursing on the effectiveness of research teams, the safety and subjective experience of participants, and the quality and integrity of resulting data.
The Impact of Nurses Practicing in Clinical Research: Agenda for Building the Evidence
There is essentially no formal evaluation research that demonstrates the impact of nurses as study coordinators and care givers in a research setting on specific quality, safety or efficiency outcomes. Across the 30 year history of writing on this topic, there are themes that have emerged anecdotally as outcomes affected by clinical research nurses, including early assessment of adverse events, subject identification and recruitment, subject education, study management to improve efficiency, improved subject retention, better adherence of study subjects to treatment regimen, extension of PI effectiveness and availability (serving as PI-extender), maintenance of adequate informed consent, alerting research team to protocol violations, adapting clinical technology to support study implementation, training clinical staff, and facilitating the management and analysis of data.
Table 2 displays a possible beginning list of points of contribution and potential performance outcomes that may be influenced by clinical research nursing. These outcomes could become the basis for further work in describing and measuring the impact of clinical research nurses on the effectiveness of the clinical research enterprise. They could also provide the basis for a cost benefit analysis of staffing decisions.
Table 2. Potential Outcomes of Clinical Research Nursing.
| Contribution Area | Potential Outcome(s) |
|---|---|
| Intervention design and implementation planning within the clinical setting | Efficiency; intervention fidelity |
| Participant recruitment and consenting | Study accrual; adherence to human subjects protection standards |
| Participant education and support regarding self managed study procedures and evaluation | Participant safety; treatment fidelity; efficiency; subject retention; data quality |
| Direct administration of study treatments and evaluations | Participant safety; treatment fidelity; efficiency; data quality |
| Participant monitoring for response and adverse events | Participant safety; adherence to human subjects protection standards; data quality |
| Data preparation and management | Data quality; human subjects protection; efficiency; speed of dissemination |
Reengineering the clinical and translational research environment is imperative to achieving national science priorities. Clinical research nurses have a unique skill set and knowledge base that positions them to make significant and essential contributions to the clinical research enterprise. As institutions strive to provide quality clinical and translational research programs within the current fiscal climate the important principles of quality patient care and research integrity must remain a critical element. Maintaining equilibrium between the clinical and research needs in the care of the clinical research participant is vital. Nurses with clinical research expertise are a unique resource largely limited to academic medical centers. As the agenda for clinical and translational research broadens to include a variety of healthcare and community settings, ongoing work to clearly articulate this specialty practice will ensure the expertise of this unique group of nurses is readily accessible to those who will benefit from it most.
Acknowledgments
Grant Information:
This work was supported in part by the Intramural Research Program of the NIH, Clinical Center (Hastings and Fisher), Grant Number MO1-RR02172, from the National Center for Research Resources, a component of the National Institutes of Health, and the Children’s Hospital Boston, General Clinical Research Center (McCabe).
The following members of The National Clinical Research Nursing Consortium were also authors:
Wellcome Trust Clinical Research Facility, Southampton, England, UK - J. Allison;
The Rockefeller University, New York, N.Y. - D. Brassil and (formerly) M. Offenhartz
Georgetown University Medical Center, Washington, D.C. - S. Browning
New York University Langone Medical Center, New York, N.Y. - E. DeCandia and R. Medina;
Irving Institute for Clinical and Translational Research Columbia University, New York, N.Y. - J. Duer-Hefele;
Consultant in Clinical Research Nursing, Switzerland - K. McClary;
University of California, Davis Clinical and Translational Science Center, Sacramento, CA, - N. Mullen;
University of Texas Health Science Center, Houston, TX- M. Ottosen;
Yale- New Haven Hospital, New Haven, CT – S. Britt and T. Sanchez;
Partners HealthCare, Boston, MA - V. Turbini
Contributor Information
Clare E. Hastings, NIH Clinical Center.
Cheryl A. Fisher, NIH Clinical Center.
Margaret A. McCabe, Boston Children’s Hospital.
References
- 1.Butler D. Translational research: crossing the valley of death. Nature. 2008;453:840–2. doi: 10.1038/453840a. [DOI] [PubMed] [Google Scholar]
- 2.Peterson KA. National Institutes of Health Eliminates Funding for National Architecture Linking Primary Care Research. The Journal of the American Board of Family Medicine. 2007;20:229–31. doi: 10.3122/jabfm.2007.02.070012. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell SA, Fisher CA, Hastings CE, Silverman LB, Wallen GR. A thematic analysis of theoretical models for translational science in nursing: mapping the field. Nurs Outlook. 2010;58:287–300. doi: 10.1016/j.outlook.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung NS, Crowley WF, Jr, Genel M, et al. Central challenges facing the national clinical research enterprise. Jama. 2003;289:1278–87. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 5.VanLare JM, Conway PH, Sox HC. Five next steps for a new national program for comparative-effectiveness research. N Engl J Med. 2010;362:970–3. doi: 10.1056/NEJMp1000096. [DOI] [PubMed] [Google Scholar]
- 6.Woolf SH. The meaning of translational research and why it matters. Jama. 2008;299:211–3. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 7.Castro K, Bevans M, Miller-Davis C, et al. Validating the clinical research nursing domain of practice. Oncol Nurs Forum. 2011;38:E72–80. doi: 10.1188/11.ONF.E72-E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrenberger HE, Lillington L. Development of a measure to delineate the clinical trials nursing role. Oncol Nurs Forum. 2004;31:E64–8. doi: 10.1188/04.ONF.E64-E68. [DOI] [PubMed] [Google Scholar]
- 9.McCabe M, Cahill Lawrence CA. The clinical research nurse. Am J Nurs. 2007;107:13. doi: 10.1097/01.NAJ.0000287481.78601.38. [DOI] [PubMed] [Google Scholar]
- 10.Offenhartz M, McClary K, Hastings C. Nursing and realities of clinical research. Nurs Manage. 2008;39:34–9. doi: 10.1097/01.NUMA.0000340817.28894.46. [DOI] [PubMed] [Google Scholar]
- 11.Spilsbury K, Petherick E, Cullum N, Nelson A, Nixon J, Mason S. The role and potential contribution of clinical research nurses to clinical trials. J Clin Nurs. 2008;17:549–57. doi: 10.1111/j.1365-2702.2006.01872.x. [DOI] [PubMed] [Google Scholar]
- 12.Grady C, Edgerly M. Science, technology, and innovation: nursing responsibilities in clinical research. Nurs Clin North Am. 2009;44:471–81. doi: 10.1016/j.cnur.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muehlbauer PM, Klapec K, Locklin J, et al. Part II: Nursing implications of administering chemotherapy in interventional radiology or the operating room. Clin J Oncol Nurs. 2006;10:345–56. doi: 10.1188/06.CJON.345-356. [DOI] [PubMed] [Google Scholar]
- 14.Seidel GD, Locklin J, Muehlbauer PM. Part I: regional chematherapy clinical studies in nontraditional clinical settings. Clin J Oncol Nurs. 2006;10:338–44. doi: 10.1188/06.CJON.338-344. [DOI] [PubMed] [Google Scholar]
- 15.Best I. Central role of the research nurse in improving accrual of older persons to cancer treatment trials. J Clin Oncol. 2005;23:7752. doi: 10.1200/JCO.2005.02.6260. author reply -3. [DOI] [PubMed] [Google Scholar]
- 16.Biedrzycki BA. Decision making for cancer clinical trial participation: a systematic review. Oncol Nurs Forum. 2010;37:E387–99. doi: 10.1188/10.ONF.E387-E399. [DOI] [PubMed] [Google Scholar]
- 17.Murff HJ, Dittus RS. Near misses and research subjects. Qual Saf Health Care. 2006;15:228–9. doi: 10.1136/qshc.2006.018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi TK, Lee TH. Patient safety beyond the hospital. N Engl J Med. 2010;363:1001–3. doi: 10.1056/NEJMp1003294. [DOI] [PubMed] [Google Scholar]
- 19.Carter SC, Jester PM, Jones CT. Issues in clinical research manager education and training. Research Practitioner. 2007;8:48. [Google Scholar]
- 20.Davidson RM, McNeer JF, Logan L, et al. A cooperative network of trained sites for the conduct of a complex clinical trial: a new concept in multicenter clinical research. Am Heart J. 2006;151:451–6. doi: 10.1016/j.ahj.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Mori C, Mullen N, Hill EE. Describing the role of the clinical research nurse. Research Practitioner. 2007;8:220–8. [Google Scholar]
- 22.Mueller MR, Mamo L. The nurse clinical trial coordinator: benefits and drawbacks of the role. Res Theory Nurs Pract. 2002;16:33–42. doi: 10.1891/rtnp.16.1.33.52992. [DOI] [PubMed] [Google Scholar]
- 23.Arrigo C, Gall H, Delogne A, Molin C. The involvement of nurses in clinical trials. Results of the EORTC Oncology Nurses Study Group survey. Cancer Nurs. 1994;17:429–33. [PubMed] [Google Scholar]
- 24.Braccia DP. Implementation and management of clinical trials. Clin J Oncol Nurs. 2003;7:482–4. doi: 10.1188/03.CJON.482-484. [DOI] [PubMed] [Google Scholar]
- 25.Cassidy J, Macfarlane DK. The role of the nurse in clinical cancer research. Cancer Nurs. 1991;14:124–31. [PubMed] [Google Scholar]
- 26.Hubbard SM. Cancer treatment research: the role of the nurse in clinical trials of cancer therapy. Nurs Clin North Am. 1982;17:763–83. [PubMed] [Google Scholar]
- 27.McEvoy MD, Cannon L, MacDermott ML. The professional role for nurses in clinical trials. Semin Oncol Nurs. 1991;7:268–74. doi: 10.1016/0749-2081(91)90065-w. [DOI] [PubMed] [Google Scholar]
- 28.Ocker BM, Plank DM. The research nurse role in a clinic-based oncology research setting. Cancer Nurs. 2000;23:286–92. doi: 10.1097/00002820-200008000-00005. quiz 93-4. [DOI] [PubMed] [Google Scholar]
- 29. [Accessed July 14, 2011, 2011];Special Interest Groups Virtual Communities: Clinical Trial Nurses SIG. 2008 at http://clinicaltrial.vc.ons.org/
- 30.ONS ONS. Oncology Clinical Trials Nurse Competencies. 2010. 2010. [Google Scholar]
- 31. [Accessed July 21, 2011];How agenda for change works. 2011 at http://www.nhsemployers.org/PayAndContracts/AgendaForChange/Pages/Afc-AtAGlanceRP.aspx.
- 32.Competency Framework for Clinical Research Nurses. 2008 Accessed at http://www.rcn.org.uk/development/researchanddevelopment/rs/publications_and_position_statements/competencies.
- 33. [Accessed July 21, 2011];Maximising the nursing contribution to the UK clinical research agenda. 2011 at http://www.researchacademy.co.uk/the_uk_clinical_research_agenda.
- 34.Ledger T, Pulfrey A, Luke J. Developing clinical research nurses. Nurs Manag (Harrow) 2008;15:28–33. doi: 10.7748/nm2008.05.15.2.28.c8217. [DOI] [PubMed] [Google Scholar]
- 35.Cohen HL, Davis L, Hunter J, Carp D, Geromanos K, Sunkle S. Coordinating a large multicentered HIV research project. J Assoc Nurses AIDS Care. 1997;8:41–50. doi: 10.1016/S1055-3290(97)80036-2. [DOI] [PubMed] [Google Scholar]
- 36.Oberlink M. Patient care and research at the NIH Clinical Center. AIDS Patient Care. 1988;2:28–30. [Google Scholar]
- 37.Fay M. The role of nursing in clinical research trials. Gastroenterol Nurs. 1992;14:195–7. doi: 10.1097/00001610-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Poston RD, Buescher CR. The essential role of the clinical research nurse (CRN) Urol Nurs. 2010;30:55–63. 77. doi: 10.7257/1053-816x.2010.30.1.55. [DOI] [PubMed] [Google Scholar]
- 39.Bell J. Towards clarification of the role of research nurses in New Zealand: a literature review. Nurs Prax N Z. 2009;25:4–16. [PubMed] [Google Scholar]
- 40.Cairns JA, Yusuf S, Cook RJ, et al. Canadian Network and Centre for Trials Internationally (CANNeCTIN): a national network for Canadian-led trials in cardiovascular diseases and diabetes mellitus. Can J Cardiol. 2010;26:353–8. doi: 10.1016/s0828-282x(10)70408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro K, Bevans M, Cusack G, et al. 2007 Role Clarity Team Final Report. Vol. 2007. National Institutes of Health Clinical Center; Dec, 2007. Building the Foundation for the Clinical Research Nursing Domain of Practice. [Google Scholar]
- 42.Castro K, Bevans M, Cusack G, et al. Building the Foundation for the Clinical Research Nursing Domain of Practice: Poster Presentation. American Academy of Nursing 35th Annual Meeting; Scottsdale, Arizona. 2008. [Google Scholar]
- 43.CRA Certification. [Accessed July 25, 2011];Association of Clinical Research Professionals. at http://www.acrpnet.org/MainMenuCategory/Certification/CRACertification.aspx.
- 44.CRC Certification. [Accessed July 25, 2011]; at http://www.acrpnet.org/MainMenuCategory/Certification/CRCCertification.aspx.
- 45.Baer A, Bechar N, Cohen G, Devine S. Basic steps to building a research program. J Oncol Pract. 2010;6:45–7. doi: 10.1200/JOP.091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hastings C. Poster presentation: Putting Clinical Research Nursing on the NIH Roadmap. American Academy of Nursing Annual Conference; Washington, DC: DHHS/NIH; 2007. [Google Scholar]
- 47.Clinical Research Nursing 2010: Background and Overview. NIH Clinical Center Nursing and Patient Care Services; 2009. [Accessed June 20, 2010, 2011]. at http://www.cc.nih.gov/nursing/crn/crn_2010.html. [Google Scholar]
- 48.Bevans M. Defining CRN Practice: Results of a Role Delineation Study. Second Annual Conference: International Association of Clinical Research Nurses Preconference Session - Nursing Practice at America's Research Hospital; Bethesda, Maryland. 2010. [Google Scholar]
- 49.Topping NH. The United States Public Health Service's Clinical Center for Medical Research. J Am Med Assoc. 1952;150:541–5. doi: 10.1001/jama.1952.03680060013005. [DOI] [PubMed] [Google Scholar]
- 50.Masur J, Thompson N. The national clinical center for chronic disease research. Hospitals. 1949;23:45–56. [PubMed] [Google Scholar]
- 51.Gallin JI, Smits HL. Managing the interface between medical schools, hospitals, and clinical research. Jama. 1997;277:651–4. [PubMed] [Google Scholar]
- 52.Gallin JI, Varmus H. Revitalization of the Warren G. Magnuson Clinical Center at the National Institutes of Health. Acad Med. 1998;73:460–6. doi: 10.1097/00001888-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Nathan DG, Wilson JD. Clinical research and the NIH--a report card. N Engl J Med. 2003;349:1860–5. doi: 10.1056/NEJMsb035066. [DOI] [PubMed] [Google Scholar]
- 54.Phillipson E, Silverman M. Clinical research in a CIHR world. Canadian Institutes of Health Research. Clin Invest Med. 2002;25:26–46. [PubMed] [Google Scholar]
- 55.Robertson D, Tung CS. Linking molecular and bedside research: designing a clinical research infrastructure. J Mol Med. 2001;79:686–94. doi: 10.1007/s00109-001-0298-y. [DOI] [PubMed] [Google Scholar]
- 56.Gordon SM, Heft MW, Dionne RA, et al. Capacity for training in clinical research: status and opportunities. J Dent Educ. 2003;67:622–9. [PubMed] [Google Scholar]
- 57.Luft FC. General Clinical Research Centers in the United States make a healthy recovery. J Mol Med. 2001;79:679–80. doi: 10.1007/s00109-001-0297-z. [DOI] [PubMed] [Google Scholar]
- 58.Zerhouni EA. Clinical research at a crossroads: the NIH roadmap. J Investig Med. 2006;54:171–3. doi: 10.2310/6650.2006.X0016. [DOI] [PubMed] [Google Scholar]
- 59.Kaiser J. Biomedical research. A cure for medicine's ailments? Science (New York, NY) 2006;311:1852–4. doi: 10.1126/science.311.5769.1852. [DOI] [PubMed] [Google Scholar]
- 60.Knafl K, Grey M. Clinical Translational Science Awards: Opportunities and challenges for nurse scientists. Nurs Outlook. 2008;56:132–7. e4. doi: 10.1016/j.outlook.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Chesla CA. Translational research: essential contributions from interpretive nursing science. Res Nurs Health. 2008;31:381–90. doi: 10.1002/nur.20267. [DOI] [PubMed] [Google Scholar]
- 62.Sampselle CM, Pienta KJ, Markel DS. The CTSA mandate: are we there yet? Res Theory Nurs Pract. 2010;24:64–73. doi: 10.1891/1541-6577.24.1.64. [DOI] [PubMed] [Google Scholar]
- 63.Woods NF, Magyary DL. Translational research: why nursing's interdisciplinary collaboration is essential. Res Theory Nurs Pract. 2010;24:9–24. doi: 10.1891/1541-6577.24.1.9. [DOI] [PubMed] [Google Scholar]
- 64.Berglund L, Tarantal A. Strategies for innovation and interdisciplinary translational research: removal of barriers through the CTSA mechanism. J Investig Med. 2009;57:474–6. doi: 10.2310/JIM.0b013e3181982794. [DOI] [PubMed] [Google Scholar]
- 65.Reis SE, Berglund L, Bernard GR, Califf RM, Fitzgerald GA, Johnson PC. Reengineering the national clinical and translational research enterprise: the strategic plan of the National Clinical and Translational Science Awards Consortium. Acad Med. 2010;85:463–9. doi: 10.1097/ACM.0b013e3181ccc877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guidelines for the General Clinical Research Centers Program (M01) 2005. [Accessed September 25, 2010, 2010]. at. [Google Scholar]
- 67.Luft FC. The role of the general clinical research center in promoting patient-oriented research into the mechanisms of disease. J Mol Med. 1997;75:545–50. [PubMed] [Google Scholar]
- 68. [Accessed July 28, 2011];Position Statement: General Clinical Research Center Nurse Managers Position on Clinical Research Nursing. 2006 at http://iacrn.memberlodge.org/Resources/Documents/GCRC%20NM%20POSITION%20STATEMENT_06.pdf.
- 69.Alt-White AC, Pranulis MF. Addressing nurses' ethical concerns about research in critical care settings. Nurs Adm Q. 2006;30:67–75. doi: 10.1097/00006216-200601000-00010. [DOI] [PubMed] [Google Scholar]
- 70.Angus DC, Mira JP, Vincent JL. Improving clinical trials in the critically ill. Crit Care Med. 2010;38:527–32. doi: 10.1097/CCM.0b013e3181c0259d. [DOI] [PubMed] [Google Scholar]
- 71.Connelly LM. Staff nurses' responsibilities when caring for patients in a research study. Medsurg Nurs. 2009;18:385–6. 8. [PubMed] [Google Scholar]
- 72.Cossette S, D'Aoust LX, Morin M, Heppell S, Frasure-Smith N. The systematic development of a nursing intervention aimed at increasing enrollment in cardiac rehabilitation for acute coronary syndrome patients. Prog Cardiovasc Nurs. 2009;24:71–9. doi: 10.1111/j.1751-7117.2009.00038.x. [DOI] [PubMed] [Google Scholar]
- 73.Easter MM, Henderson GE, Davis AM, Churchill LR, King NM. The many meanings of care in clinical research. Sociology of health & illness. 2006;28:695–712. doi: 10.1111/j.1467-9566.2006.00537.x. [DOI] [PubMed] [Google Scholar]
- 74.Garfield SA, Malozowski S, Chin MH, et al. Considerations for diabetes translational research in real-world settings. Diabetes Care. 2003;26:2670–4. doi: 10.2337/diacare.26.9.2670. [DOI] [PubMed] [Google Scholar]
- 75.Lanter J. Clinical research and the development of new devices: considerations for nurses. Dimens Crit Care Nurs. 2007;26:117–20. doi: 10.1097/01.DCC.0000267805.49487.33. [DOI] [PubMed] [Google Scholar]
- 76.Moore S. A need to try everything: patient participation in phase I trials. J Adv Nurs. 2001;33:738–47. doi: 10.1046/j.1365-2648.2001.01715.x. [DOI] [PubMed] [Google Scholar]
- 77.Rickard CM, Roberts BL, Foote J, McGrail MR. Intensive care research coordinators: who are they and what do they do? Results of a binational survey. Dimens Crit Care Nurs. 2006;25:234–42. doi: 10.1097/00003465-200609000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Rosal MC, White MJ, Borg A, et al. Translational research at community health centers: challenges and successes in recruiting and retaining low-income Latino patients with type 2 diabetes into a randomized clinical trial. Diabetes Educ. 2010;36:733–49. doi: 10.1177/0145721710380146. [DOI] [PubMed] [Google Scholar]
- 79.International Association of Clinical Research Nurses. [Accessed July 25, 2011]; at http://iacrn.memberlodge.org/
- 80.ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice, E6. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 1996; [Accessed August 10, 2009]. at www.ich.org. [Google Scholar]
- 81.WHO. World Health Organization Handbook for Good Clinical Research practice (GCP) 2005. pp. 1–125. [Google Scholar]
- 82.ASCO. American Society of Clinical Oncology policy statement: oversight of clinical research. J Clin Oncol. 2003;21:2377–86. doi: 10.1200/JCO.2003.04.026. [DOI] [PubMed] [Google Scholar]
- 83.U.S. Department of Health and Human Services HRaSA. The Registered Nurse Population: Initial Findings from the 2008 national Sample Survey of Registered Nurses. 2010. [Google Scholar]
- 84.Federoff HJ, Rubin ER. A new research and development policy framework for the biomedical research enterprise. Jama. 2010;304:1003–4. doi: 10.1001/jama.2010.1270. [DOI] [PubMed] [Google Scholar]
- 85.Fitzgerald GA. Opinion: anticipating change in drug development: the emerging era of translational medicine and therapeutics. Nat Rev Drug Discov. 2005;4:815–8. doi: 10.1038/nrd1849. [DOI] [PubMed] [Google Scholar]
- 86.Rettig RA. The industrialization of clinical research. Health Aff (Millwood) 2000;19:129–46. doi: 10.1377/hlthaff.19.2.129. [DOI] [PubMed] [Google Scholar]
- 87.Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Discov. 2008;7:13–4. [Google Scholar]
- 88.Paschoale HS, Barbosa FR, Nita ME, Carrilho FJ, Ono-Nita SK. Clinical trials profile: professionals and sites. Contemp Clin Trials. 2010;31:438–42. doi: 10.1016/j.cct.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Vincent JL. Logistics of large international trials: the good, the bad, and the ugly. Crit Care Med. 2009;37:S75–9. doi: 10.1097/CCM.0b013e3181922c2d. [DOI] [PubMed] [Google Scholar]
- 90.Kumar A. The Clinical Trials Road in India: Looking for the Land Mines. The Monitor. 2007;21:17–21. [Google Scholar]
- 91.Mazalin KF, Klaus Pharmaceutical Clinical Research in Austria and Hungary. the Monitor. 2007;21:27–30. [Google Scholar]
- 92.Adbdul-Karrim S. Conducting Clinical Trials in South Africa. the Monitor. 2007;21:67–70. [Google Scholar]
- 93.Brosteanu O, Houben P, Ihrig K, et al. Risk analysis and risk adapted on-site monitoring in noncommercial clinical trials. Clin Trials. 2009;6:585–96. doi: 10.1177/1740774509347398. [DOI] [PubMed] [Google Scholar]
- 94.McGregor J. Does the use of human subjects in research in developing nations violate their human rights? If so, are reparations an appropriate response? Journal of Social Philosophy. 2006;37:441–63. [Google Scholar]
- 95.Shah S. Globalization of clinical research by the pharmaceutical industry. Int J Health Serv. 2003;33:29–36. doi: 10.2190/5FGJ-03AQ-BKW2-GLAA. [DOI] [PubMed] [Google Scholar]
- 96.Sweatman J. Good clinical practice: a nuisance, a help or a necessity for clinical pharmacology? Br J Clin Pharmacol. 2003;55:1–5. doi: 10.1046/j.1365-2125.2003.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carlson C, Reilly M, Hitchens A. An innovative approach to the care of patients on phase I and phase II clinical trials: the role of the experimental therapeutics nurse. J Pediatr Oncol Nurs. 2005;22:353–64. doi: 10.1177/1043454205281763. [DOI] [PubMed] [Google Scholar]
- 98.Green LA, White LL, Barry HC, Nease DE, Jr, Hudson BL. Infrastructure requirements for practice-based research networks. Ann Fam Med. 2005;3 (Suppl 1):S5–11. doi: 10.1370/afm.299. [DOI] [PMC free article] [PubMed] [Google Scholar]



