Abstract
While unilateral spatial neglect after left brain damage is undoubtly less common than spatial neglect after a right hemisphere lesion, it is also assumed to be less severe. Here we directly test this latter hypothesis using a continuous measure of neglect severity: the so-called Center of Cancellation (CoC). Rorden and Karnath (2010) recently validated this index for right brain damaged neglect patients. A first aim of the present study was to evaluate this new measure for spatial neglect after left brain damage. In a group of 48 left-sided stroke patients with and without neglect, a score greater than −0.086 on the Bells Test and greater than −0.024 on the Letter Cancellation Task turned out to indicate neglect behavior for left brain damaged patients. A second aim was to directly compare the severity of spatial neglect after left versus right brain injury by using the new CoC measure. While neglect is less frequent following left than right hemisphere injury, we found that when this symptom occurs it is of similar severity in acute left brain injury as in patients after acute right brain injury.
Keywords: Spatial neglect, Left brain damage, Center of cancellation (CoC), Attention, Exploration, Stroke, Human
1. Introduction
Spatial neglect is a well known phenomenon mostly occurring after right brain damage (RBD). Nevertheless, there are also some reports describing spatial neglect following left brain damage (LBD) (e.g., Becker & Karnath, 2007; Beis et al., 2004; Kleinman et al., 2007; Maeshima, Shigeno, Dohi, Kajiwara, & Komai, 1992; Ogden, 1985a; Ringman, Saver, Woolson, Clarke, & Adams, 2004). However, these reports vary tremendously regarding the incidence and severity of left hemisphere neglect; values ranged from 2.4% (Becker & Karnath, 2007) to 65% (Stone, Halligan, & Greenwool, 1993). It is likely that this variability reflects exclusion criteria (e.g., some left hemisphere patients have dense aphasia and therefore can not complete many neglect tests), tests used to define neglect, and the corresponding cut-offs used to identify neglect (for discussion Bowen, McKenna, & Tallis, 1999; Karnath & Rorden, 2011; Stone et al., 1991). Moreover, such tests were often used as simple binary classifiers to detect the presence or absence of neglect, despite the existing continuous spectrum of neglect severity. Thus, our aim was to explore neglect severity in acute LBD patients with a simple continuous measure known as the 'Center of Cancellation' (CoC) which has proved sensitive at detecting neglect severity in right hemisphere stroke patients (Rorden & Karnath, 2010) to see (i) if this measure is also sensitive to left hemipshere neglect and (ii) to determine whether acute neglect is as severe following LBD as RBD.
Cancellation tasks are popular clinical and scientific tools for the investigation of neglect patients. Recently, Rorden and Karnath (2010, www.mricro.com/cancel/) developed a new tool for measuring neglect severity in such tasks by means of a continuous variable: the CoC. This measure expresses the mean horizontal coordinate for the detected items of each test. Individuals who miss no items or show a symmetrically distributed pattern of errors receive a CoC score near zero. Individuals who only detect the rightmost/leftmost items, i.e. show very severe left/right-sided neglect, receive a score close to +1/−1. This measure avoids the various disadvantages from previously suggested measures or indices, such as the number of omissions/cancellations (on the whole sheet or on the left side, respectively), lateralization indices, or the use of power functions. For example, counting the number of errors or hits cannot distinguish between spatially biased performance versus inattentive performance. Some patients may miss items specifically on the contralesional side of the test sheet whereas others may miss the same number of targets but evenly distributed across the sheet. While the first observation is indicative of spatial neglect; the latter does not support this diagnosis. Lateralization indices, i.e. the number of targets detected on the left half of a test sheet divided by the total number of targets detected, also do not solve this problem, i.e. they may not represent a reliable measure of severe neglect either (see Rorden & Karnath 2010 for details).
To validate the new CoC measure, Rorden and Karnath (2010) evaluated a group of 110 individuals with right hemisphere injury. A CoC score greater than +0.081 on the Bells Test and +0.083 on the Letter Cancellation Task after an acute right hemisphere brain lesion turned out to indicate neglect behavior on other measures. A first aim of the present study is to elaborate the CoC measure for left brain damaged neglect patients in the Bells Test (Gauthier, Dehaut, & Joanette, 1989) and the Letter Cancellation Task (Weintraub & Mesulam, 1985) by applying the same procedure as Rorden and Karnath (2010) for right brain damaged neglect patients.
One probable reason for the variable incidence reported for LBD neglect may be the exclusion of aphasic and/or heavily impaired patients. An important aspect of the present study was to avoid an a priori bias due to the systematic exclusion of patients suffering from severe language deficits. Therefore we also included patients who – due to severely disturbed comprehension – could only perform the Albert´s Test (Albert, 1973). Fullerton (1986) pointed out that the Albert´s Test can be performed from almost every subject compared to other (more difficult) tests. Indeed, the Albert´s Test can easily be explained to patients with even severe aphasia, using non-verbal gestures and examples of the required cancellation behavior performed by the examiner.
Beyond incidence, the severity of spatial neglect after left brain damage has been heavily debated. Indeed, these factors are not independent: if neglect is less severe following LBD it may appear to have a lower incidence as milder patients do not achieve a cutoff-threshold. In line, most of the early studies found neglect after LBD to be less severe than after RBD (e.g. Albert, 1973; Chedru, 1976; Gainotti, Messerli, & Tissot, 1972; Ogden, 1987) while only two studies reported neglect after LBD to be equally severe compared to neglect after RBD (Arrigoni & De Renzi, 1964; Costa, Vaughn, Horwitz, & Ritter, 1969). During the last twenty years only two further investigations addressed this question. Both found spatial neglect after LBD less severe than after RBD (Ringman et al., 2004; Stone et al., 1991). However, all of these previous studies have in common that they either used binary classifiers to identify the presence or absence of spatial neglect or used gross ordinary scales (e.g. "severe/moderate/no neglect") to quantify neglect severity. A re-investigation of this question by using a continuous measure of neglect severity is therefore required. Therefore, the second aim of the present study thus is to directly compare the severity of spatial neglect after left versus right brain injury by using the new CoC measure of neglect severity.
A final confounding factor in assessing LBD neglect may be the type of task used to measure spatial neglect. In a recent review of literature regarding RBD neglect, Karnath and Rorden (2011) have noted that there seem to be a series of syndromes that anatomically and behaviorally dissociate from each other. For example, individuals with more posterior injury appear to have allocentric deficits (missing information on the contralesional side of objects, such as lines), whereas more anterior injury is correlated with egocentric deficits (missing items on the left side of space). With regards to LBD, Kleinman and colleages (2007) suggested that allocentric deficits are more common than egocentric deficits, whereas the reverse is true following RBD. Therefore, studies of neglect need to be careful not to pool across different underlying syndromes. Similar to most of the previous studies of both RBD and LBD neglect, here we focus on the pathological egocentric syndrome. Recently, we revealed that while spatial neglect is relatively infrequent (just ~4% of LBD patients), the core anatomy of egocentric LBD spatial neglect is homologous to the perisylvian regions implicated for RBD patients with egocentric neglect (Suchan & Karnath, 2011).
In sum, our aim was to investigate LBD egocentric neglect using the CoC measure. As this provides a continuous measure of impairment, we can determine when spatial neglect is present and whether it is less severe following LBD relative to RBD. By including the simple Albert’s task we can examine whether exclusion of heavily impaired patients (who can not complete more complicated cancellation tasks) might explain the discrepancy in the reports of LBD neglect severity. For this purpose we re-analyzed a large database including RBD and LBD patients recruited in previous studies (Rorden & Karnath, 2010; Suchan & Karnath, 2011). This gave us the opportunity to base our statistical analysis on two large samples of patients with LBD versus RBD.
2. Methods
We investigated data from 48 stroke patients with focal left-hemisphere brain lesions admitted to the Center of Neurology at Tübingen University, Germany (Table 1). Fourty-four subjects were part of a recent sample (Suchan & Karnath, (2011) five patients of this sample had to be excluded due to the requirements for independent CoC validation [see below]). Four newly admitted patients with spatial neglect were added. Thus, the present sample consisted of 21 patients with first ever circumscribed left-hemisphere stroke and spatial neglect and 27 patients with LBD but without spatial neglect. The latter group had been selected to match the following variables of the patients with spatial neglect: age, handedness, frequencies of aphasia, hemiparesis, and visual field defects (Table 1). For comparison of neglect severity after left- and right brain damage the data of the group with LBD were contrasted with those from a sample of RBD subjects (n = 53 with spatial neglect, n = 57 without spatial neglect) from a previous investigation (for details see Rorden & Karnath, 2010).
Table 1.
Demographic and clinical data of left brain damaged patients with and without spatial neglect.
| Neglect | No neglect | ||
|---|---|---|---|
| Number | 21 | 27 | |
| Sex | 16f, 5m | 17f, 10m | |
| Age (yr) | Mean (range) | 65 (21–83) | 61 (32–86) |
| Aetiology | 14 infarct, 7 haemorrhage |
20 infarct, 7 haemorrhage |
|
| Handedness | 20 right, 1 ambidextrous |
26 right, 1 ambidextrous |
|
| Time since lesion – scanning (d) | Mean (SD) | 4.10 (4.9) | 3.67 (5.7) |
| Time since lesion – clinical examination (d) | Mean (SD) | 5.57 (4.7) | 5.41 (4.0) |
| Paresis of contralesional side | percent present | 90 | 78 |
| Aphasia | percent present | 90 | 84 |
| Hemianopia | percent present | 10 | 13 |
| Letter cancellation (CoC) | Mean (SD) | −0.36 (0.3) | 0.00 (0.0) |
| left | Mean (SD) | 23.6 (9.1) | 28.8 (1.9) |
| right | Mean (SD) | 12.3 (8.8) | 28.5 (2.2) |
| Bells test (CoC) | Mean (SD) | −0.36 (0.3) | −0.03 (0.04) |
| left | Mean (SD) | 12.8 (3.6) | 14.1 (1.4) |
| right | Mean (SD) | 5.1 (4.7) | 13.9 (1.6) |
| Albert’s test (CoC) | Mean (SD) | −0,62 (0.3) | 0,00 (0.0) |
| left | Mean (SD) | 12.6 (4.9) | 18.0 (0.0) |
| right | Mean (SD) | 3.6 (7.1) | 18.0 (0.0) |
| Copying (% omitted) | Mean (SD) | 40% (30) | 2% (4) |
f: female; m: male; d: days, CoC: Center of Cancellation
The lesions were demonstrated by magnetic resonance imaging (MRI) or by spiral computed tomography (CT). Patients with diffuse or bilateral brain injury, patients with tumors, as well as patients in whom MRI or CT scans revealed no obvious lesion were excluded. The patients or their relatives gave their informed consent to participate in the study, which was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
We examined the patients' performance on four traditional paper-and-pencil tests: three cancellation tasks and a copying task, each presented on a horizontally oriented 21 × 29.7 cm sheet of paper. The Letter Cancellation Task (Weintraub & Mesulam, 1985) requires marking 60 target letters 'A' distributed amid distractors letters. The Bells Test (Gauthier et al., 1989) requires identifying 35 bell symbols distributed on a field of other symbols. The Albert´s Test (Albert, 1973) consists of seven columns of black lines that all have to be cancelled. Further, patients were asked to copy a complex multi-object scene consisting of four figures (a fence, a car, a house and a tree) (Copying task, Johannsen & Karnath, 2004).
In order to validate the CoC index, we used an independent clinical diagnosis of spatial neglect. This allowed us to determine if CoC scores reliably discriminate patients with neglect from those without neglect. Identical to our previous study of neglect severity following right hemisphere lesions (Rorden & Karnath, 2010), for the Bells Test we identified an individual as having spatial neglect if they showed biased performance on the Letter Cancellation Task or the Copying task. If both of these two tests could not be conducted, the Albert´s Test was used instead. An individual thus was diagnosed as having spatial neglect when the Letter Cancellation Task and/or the Copying task fulfilled these criteria. If one of these tests could not be performed, the Albert´s test was used to categorize a patient as showing or not showing the disorder. This classification of the patients was then used to validate their performance (CoC value) in the Bells Test. For validation of the Letter Cancellation Task a similar procedure was used. In that case, the neglect diagnosis was based on the performance in the Bells Test and/or the Copying task. If both of these two tests could not be conducted, the Albert´s Test was used, respectively. This classification of the patients was then used to validate their performance (CoC value) in the Letter Cancellation Task. In eight of the 21 LBD patients with spatial neglect, aphasia was so severe that only the Albert´s Test could be performed.
We used previously established thresholds for the diagnosis of spatial neglect in these tests. The disorder was diagnosed when patients omitted more than four contralaterally located targets in the Letter Cancellation Task, more than five contralaterally targets in the Bells Test, more than one contralateral located targets in the Albert´s Test, or when they showed a score higher than 1 (i.e. > 12.5% omissions) in the copying task. The basis for these criteria is as follows: Weintraub and Mesulam (1985) found that four targets on each side may go undetected in normal subjects over the age of 80 in their Letter Cancellation Task. A comparable criterion was reported by Gauthier et al. (1989) for the Bells Test. They found that more than five omitted bells indicated neglect. Albert (1973) found that more than one omitted lines is indicative for neglect. For the Copying task we followed the criterion reported by Johannsen and Karnath (2004).
To calculate the CoC indices for the Letter Cancellation Task, the Bells Test, and the Albert's Test, respectively, the software described by Rorden and Karnath (2010) was used (www.mrico.com/cancel). The software calculates the mean horizontal coordinate for the detected items of each test. The center of mass is calculated in terms of distribution of items on the page (scaled such that the left-most item had a position of −1 and the right-most item had a position of +1).
2.1 Evaluation of the CoC measure in LBD patients
Parallel to our previous study in RBD patients (Rorden & Karnath, 2010), the analysis focused on the Letter Cancellation Task and the Bells Test. We computed CoC measures for the two cancellation tasks separately, as numerous factors influence performance on cancellation tasks, for example the number and density of items (Sarri, Greenwood, Kalra, & Driver, 2009). As suggested in our previous study (Rorden & Karnath, 2010), the mean ± 2.326 standard deviations (this value corresponds to p < 0.01 for a one-tailed test) of the non-neglect group was used to create cut-off thresholds for the two tests, respectively. For this purpose the observed, negative or positive values of the CoC scores were used.
2.2 Determination of neglect severity of LBD versus RBD patients
Neglect severity was measured based on the CoC values from the Bells Test and the Letter Cancellation task. For the comparison between LBD and RBD patients the absolute values of the CoC scores were used. A first analysis included only those LBD patients who were able to perform the Bells Test as well as the Letter Cancellation Task. Therefore, in our initial analysis eight subjects with severe comprehension deficits were excluded. To avoid an a priori bias due to the systematic exclusion of patients suffering from severe aphasia (and potentially severe additional neglect), a second analysis was performed in which we also included those eight patients. For this purpose, we estimated the performance of these eight patients for the Bells Test and the Letter Cancellation Task by means of a linear regression analysis based on their CoC values of the Albert´s Test. Due to the low number of individuals with left brain damage who were able to perform all three cancellation tasks, we calculated the regression equation on the basis of all left as well as right brain damaged patients, who had performed all three cancellation tasks (n = 24). This procedure was justified because there is no clinical or scientific evidence that the within-group relation between the performance in the Albert's test and the performance in the Bells/Letter Cancellation Task is systematically different between left and right brain damaged patients. It should be noted that this procedure thus does not damp the validity to subsequently explore whether or not there are significant differences in neglect severity between RBD and LBD patients and that this exploration is not based on a circular arrangement.
Overall lesion volume size was evaluated using MRIcron software (Rorden, Karnath, & Bonilha, 2007, www.mrico.com). Statistics were performed using SPSS (SPSS Inc., Chicago, IL, U.S.A).
3. Results
3.1 Evaluation of the CoC measure in LBD patients
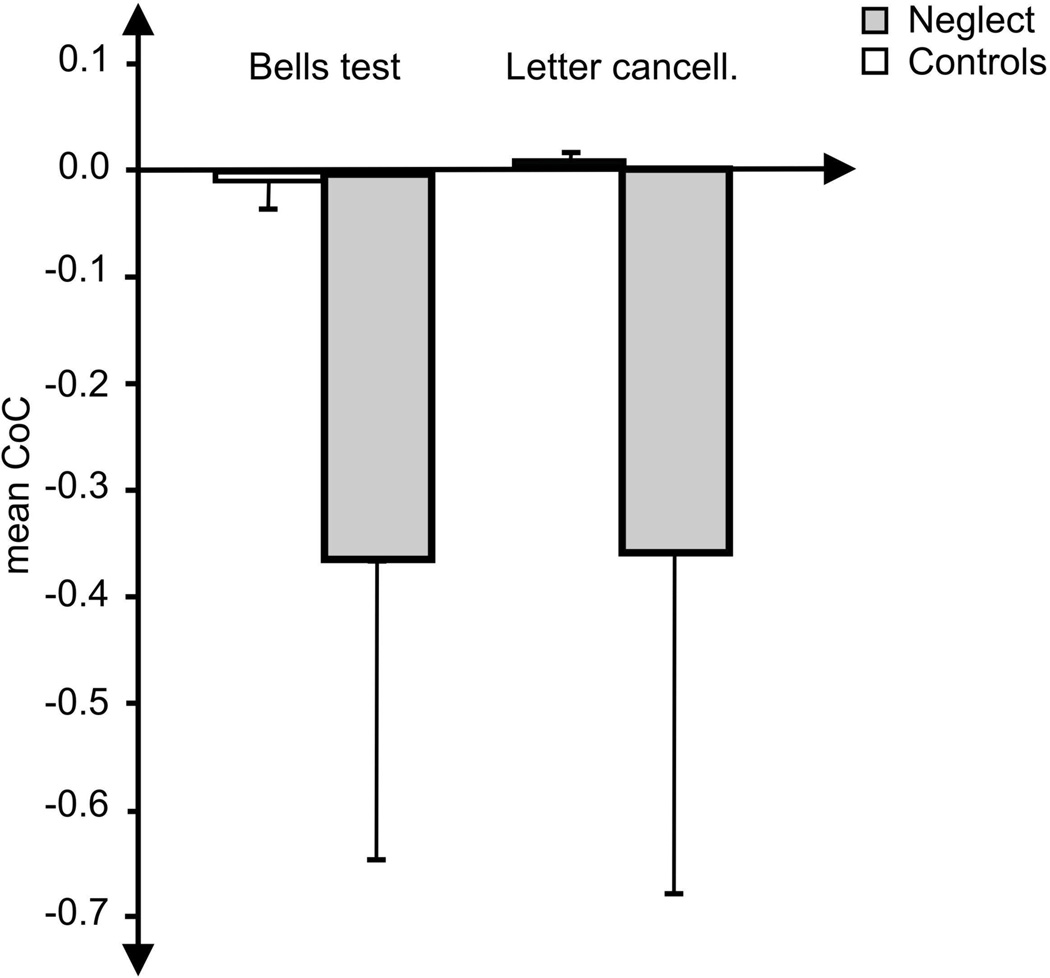
When we compared the CoC measure against the two independent traditional clinical binary classifiers for spatial neglect, the acute left-sided stroke patients without spatial neglect showed a mean CoC score of −0.0032 with a standard deviation of 0.0356 for the Bells Test, and a mean of +0.0006 with a standard deviation of 0.0104 for the Letter Cancellation Task (Fig. 1). Applying the criterion for calculating the cut-off threshold (mean ± 2.326 SD; see methods section above), this leads to a cut-off value of −0.086 for the Bells Test and −0.024 for the Letter Cancellation task.
Figure 1.
Mean CoC value (and standard deviation) of the left brain damaged patients with spatial neglect (grey bar) and without neglect (white bar) measured in the Bells test (left panel) and the Letter Cancellation Task (right panel).
To validate the sensitivity of these cut-off values, we applied this threshold to the remaining patients who were classified as having neglect based on independent measures. The CoC threshold was able to correctly detect all of the individuals with neglect based solely on their performance on either one of the two cancellation tasks. On the other hand, when this threshold was applied to the patients without neglect (the population used to define our threshold), a total of two individuals was classified as having neglect with CoC scores higher than the respective cut-off score (one based on the performance in the Bells test and one based on performance on the Letter Cancellation task). Therefore, of the present LBD sample, our CoC cut-off scores based on the performance from a single cancellation test (Letter Cancellation Task or Bells Test) agreed with the independent traditional scoring method applied to three tests in 95.8% of the cases.
3.2 Comparison of neglect severity following LBD versus RBD
For evaluating a possible difference in neglect severity between left- and right brain damaged patients with spatial neglect the 21 LBD patients with neglect were compared to the 57 RBD patients with spatial neglect from our previous study (Rorden & Karnath, 2010).
To address possible confounding variables determining neglect severity we analysed age, hemianopia, overall lesion volume, as well as time since lesion (TSL), that means the time between stroke-onset and neuropsychological testing. We found no differences regarding age between the LBD (mean (m) = 65.23y, SD = 18.5) and RBD (m = 62.7, SD = 13.1) neglect groups (unpaired T-test = 0.579, p = 0.564). Further there was no significant difference regarding hemianopia between LBD (present in 10.4%) and RBD (present in 25.4%) patients (Chi2 = 3.494, p = 0.062). In contrast, lesion volume was significantly smaller in LBD patients (m = 6.2% of overall left hemisphere volume, SD = 4.9, RBD: m = 16.2%, SD = 11.1, T = −5.008, p < 0.001) and TSL was significantly longer (T = −4.105, p < 0.001) in the RBD neglect group (m = 16.7, SD = 19.0) compared to the LBD neglect group (m = 4.9, SD = 4.9). These two variables thus were taken into account as covariates when we statistically tested the CoC score between LBD and RBD patients for the Bells test and the Letter cancellation Task by means of an analysis of covariance (ANCOVA), respectively. It involved the independent factor group (LBD vs. RBD) and the absolute values of the CoC score as the dependent variable.
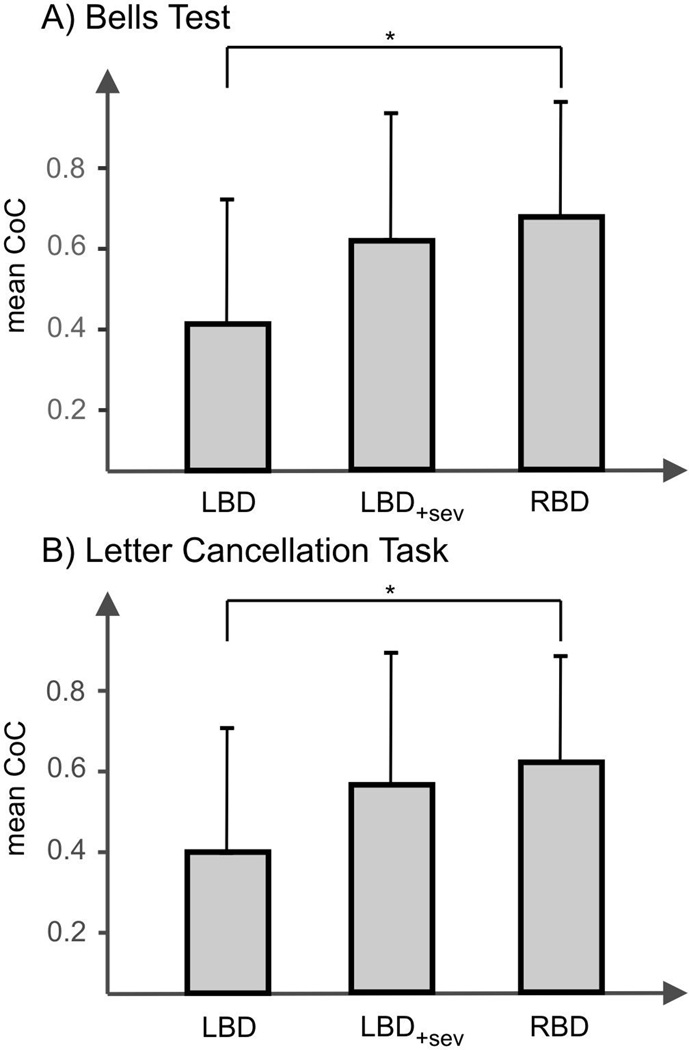
In a first analysis, we only included those patients who actually performed the Letter Cancellation Test and/or the Bells Test. This analysis thus excluded the eight patients who were only able to complete the Albert´s Test, due to severe comprehension deficits. Even when lesion volume and TSL were taken into account for both tests, the analysis revealed a significantly smaller absolute CoC score of the LBD patients (m = 0.365, SD = 0.29) compared to the RBD patients (m = 0.640, SD = 0.28) for the Bells test (F1.0 = 5.381, p = 0.024) (Fig. 2a). Likewise, we found the absolute CoC score for the Letter Cancellation task significantly smaller in LBD patients (m = 0.362, SD = 0.32) compared to RBD patients (m = 0.583, SD = 0.26) (F1.0 = 4.113, p = 0.047) (Fig. 2b).
Figure 2.
Mean of the absolute CoC values (and standard deviation) for (A) the Bells Test and (B) the Letter Cancellation Task. The bar termed 'LBD' indicates this measure for the left brain damaged neglect patients if the heavily impaired patients (n = 8) who were just able to perform the Albert´s test but not the other cancellation tasks were excluded. The bar termed 'LBD+sev' indicates this measure for the left brain damaged neglect patients if all (continously admitted) subjects were included, i.e. also those 8 subjects who were just able to perform the Albert´s test but not the other cancellation tasks. (The performance of these eight patients for the Bells Test and the Letter Cancellation Task were estimated by means of a linear regression analysis based on their CoC values of the Albert´s Test). The bar termed 'RBD' indicates this measure for the right brain damaged neglect patients. For the latter bar data from Rorden and Karnath (2010) were used.
*, significant difference p < .05
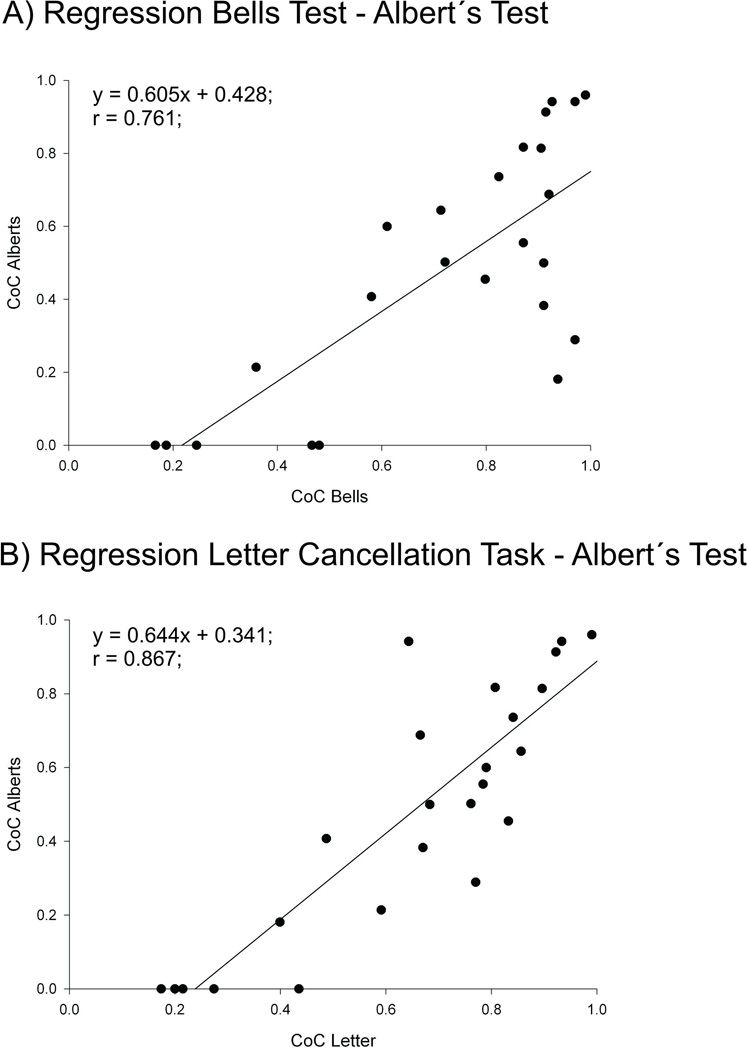
To include also those heavily impaired eight patients which were just able to perform the Albert´s Test, we estimated the performance of these eight patients for the Bells Test and the Letter Cancellation Task by means of a linear regression analysis based on their CoC values of the Albert´s Test. For this purpose, a linear regression between Bells Test and Albert´s Test as well as Letter Cancellation Task and Albert´s Test was conducted based on the CoC values from those left and right brain damaged patients who had performed all three tests (Fig. 3). We then repeated the analyses of covariance (see above; again using TSL and lesion volume as covariates) but now with the inclusion of estimated CoC values for the Bells Test and the Letter Cancellation task from those eight subjects with severe comprehension impairments. No statistical differences between the absolute CoC score of LBD and RBD neglect patients for the Bells Test (LBD: m = 0.610, SD = 0.34; RBD: m = 0.640, SD = 0.28; F1.0 = 0.011, p = 0.917) (Fig. 2a) and the Letter Cancellation Task (LBD: m = 0.570, SD = 0.33; RBD: m = 0.583, SD = 0.26; F1.0 = 0.003, p = 0. 954) were obtained (Fig. 2b).
Figure 3.
Results of the linear regression anlysis for (A) the Bells Test and the Albert´s Test and (B) the Letter Cancellation task and the Albert´s Test from all left and right brain damaged patients with spatial neglect, who performed all three tests (Bells Test, Letter Cancellation Task, Albert´s Test).
Due to the obvious difference regarding sample size of LBD versus RBD patient groups, differences in variance could have an impact on the robustness of statistical results from the ANCOVA. By applying the Levene-test we did not find significant differences for all of our calculations (Tests without the severely impaired patients: Bells test: LBD SD = 0.287, RBD: SD = 0.281, F(1) = 0.75, p = 0.78; Letter Cancellation Task: LBD: SD = 0.324, RBD: SD = 258, F(1) = 1.371, p = 0.246; tests including severely impaired patients: Bells Test: LBD SD = 0.336, RBD: SD = 0.281, F(1) = 0.945, p = 0.334) except for the Letter Cancellation Task after inclusion of the heavily impaired patients (LBD SD = 0.329, RBD: SD = 0.281, F(1) = 4.650, p = 0.034). To cope with the latter we performed the Bootstrapping method (Bortz, 1999) and found – like in the initial analysis – no significant difference between LBD and RBD patients (F(1) = 0.03, p = 0.954).
4. Discussion
By applying the same procedure as Rorden and Karnath (2010) in a sample of RBD patients, we examined the CoC for both the Bells Test and the Letter Cancellation Task in a group of 48 individuals who had acute left hemisphere stroke. We used independent binary classifiers from two clinical tests to generate normative data for the CoC scores. A score greater than −0.086 on the Bells Test and greater than −0.024 on the Letter Cancellation Task proved accurate at detecting the neglect patients in our sample of acute left-sided stroke patients. It suggests that the CoC is a robust continuous measure of neglect severity not only in RBD (Rorden & Karnath, 2010) but also in individuals with LBD. Thus, our data were able to confirm that the CoC index is at least as accurate as traditional evaluations to diagnose spatial neglect.
The second part of the study compared neglect severity from patients suffering from a stroke in the left and the right hemisphere. When we included only those subjects who were able to comprehend the instructions related to the Bells Test and the Letter Cancellation Task we revealed neglect to be significantly less severe in patients with LBD compared to RBD. This result is in line with previous reports on this issue (e.g. Chedru, 1976; Gainotti, Caltagirone, & Miceli, 1977; 1972; Ogden, 1985b). However, for this first analysis, we had to exclude eight subjects with LBD who were not able to accomplish the Bells Test and the Letter Cancellation Task. When we instead used the performance of these eight subjects on the Albert's Test to estimate their performance on the Bells Test and the Letter Cancellation Task by means of linear regression, we no longer revealed a significant difference between neglect severity of LBD and RBD neglect patients. Therefore, while egocentric neglect does appear more frequently following RBD, when this symptom is present it is of similar severity if all subjects from a continuous sample of stroke patients are considered (i.e. when one considers LBD individuals who also suffer from dense comprehension deficits). This finding is consistent with the notion that dense aphasia is predictive of more severe neglect (potentially as both are more likely following extensive lesions that completely disrupt the underlying functional perisylvian modules). Nevertheless, we would like to note that our data rely on (in part) predicted values rather than on ‘real data’. A comparison of neglect severity between LBD and RBD patients based on standardized tasks completed by both groups, of course, would be optimal. Although the Albert´s Test would fulfill this aspect it is not suitable as well. Since the Albert´s Test is too easy in less impaired patients, it causes ceiling effects. Future development of more suitable test procedures is thus required.
Reviewing the literature, many different tests or test batteries mainly including cancellation tasks, copying tasks and line bisection were used to identify the presence or absence of spatial neglect in LBD versus RBD patients (for review see Bowen et al., 1999). Since not all of these tests are equally easy to apply in acute stroke patients, the experimenters' choice for the respective neglect tests is an important factor for in- or exclusion of aphasic patients (Colombo, De Renzi, & Faglioni, 1976). We speculate that one reason for previous reports suggesting acute spatial neglect after LBD to be less severe than after RBD might be due to the fact that patients with severe aphasia (and probably more severe neglect) were not always included. This assumption is underpinned by the finding of Basso (1973) that LBD patients with aphasia were more impaired in neglect tests – depending on different subtests – compared to patients without aphasia. In addition, Colonna and Faglioni (1966) found aphasia as the main influencing factor for differences in neglect severity between LBD and RBD patients.
Unfortunately, many studies comparing neglect severity in LBD versus RBD patients give no information regarding the inclusion, exclusion or adjustments made for aphasic patients (e.g. Chedru, 1976; Gainotti et al., 1972; Maeshima et al., 1992; Ringman et al., 2004). Some studies reported how many subjects had to be excluded due to severe aphasia (Colonna & Faglioni, 1966; Egelko, Riley, Simon, Diller, & Ezrachi, 1988). Mosidze (1994) even excluded all aphasic patients from the analysis and found just one of the remaining LBD patients suffering from spatial neglect. None of these previous studies used the Albert´s Test as a diagnostic tool to measure spatial neglect. Therefore, it is very likely that neglect severity was not assessed in LBD patients with severe aphasia. To our knowledge only the investigations by Stone and colleagues (1991) as well as Ogden (1985a) have used the Albert's Test as a diagnostic tool; in both studies it was part of a larger test battery including 8 and 5 different tests, respectively. Since Stone et al. (1991) reported that they excluded LBD patients due to their aphasia [Ogden (1985a) did not give any information how she dealed with aphasic patients] it must be assumed that the subjects' performance on the Albert's Test alone had no particular consequence for the inclusion of patients for these experiments.
Former studies already praised the Albert´s Test for usefulness in testing individuals with severe aphasia (Colombo et al., 1976; Fullerton et al., 1986). This task is easy to understand and can be explained and demonstrated to the patients even without verbal commands. Moreover, the empirical comparison of the Albert's Test with other clinical cancellation tests (Bells Test, Letter Cancellation Task, Star Cancellation) has revealed that it is the easiest of these tests (Ferber & Karnath, 2001). The present study underlines this previous experience with the Albert's Test, as we were able to administer this task with heavily impaired and/or aphasic patients. Its application can avoid biases in testing spatial neglect especially after LBD. If the severity of neglect is determined without such aphasic bias in LBD patients, we observed that spatial neglect after a left hemisphere lesion is as severe as after right hemisphere damage.
It should be noted that the present work focuses on acute symptoms. While we found that spatial neglect, when present, is of similar severity in acute LBD and RBD patients, it is possible that these patients have a different prognosis. This is an important question for future research.
Nevertheless, spatial neglect after left-sided brain damage in humans is a rare phenomenon. A clear asymmetry exists in favour of right hemisphere injury causing full-blown spatial neglect. This is interesting since homologous cortical regions are associated with spatial orienting and attention in the left as well as the right hemisphere (Suchan & Karnath, 2011). The latter study thus speculated that a representation of spatial orienting in left hemisphere language areas might be a phylogenetic relict in humans, though this representation stays subdominant in the vast majority of individuals.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (KA 1258/10-1) and the National Institutes of Health (R01 NS054266).
References
- Albert ML. A simple test of visual neglect. Neurology. 1973;23:658–664. doi: 10.1212/wnl.23.6.658. [DOI] [PubMed] [Google Scholar]
- Arrigoni G, De Renzi E. Constructional apraxia and hemispheric locus of lesions. Cortex. 1964;1:170–197. [Google Scholar]
- Basso A, De Renzi E, Faglioni P, Scotti G, Spinnler H. Neuropsychological evidence for the existence of cerebral areas critical to the performance of intelligence tasks. Brain. 1973;96:715–728. doi: 10.1093/brain/96.4.715. [DOI] [PubMed] [Google Scholar]
- Becker E, Karnath H-O. Incidence of visual extinction after left versus right hemisphere stroke. Stroke. 2007;38:3172–3174. doi: 10.1161/STROKEAHA.107.489096. [DOI] [PubMed] [Google Scholar]
- Beis J-M, Keller C, Morin N, Bartolomeo P, Bernati T, Chokron S, et al. Right spatial neglect after left hemisphere stroke: Qualitative and quantitative study. Neurology. 2004;63:1600–1605. doi: 10.1212/01.wnl.0000142967.60579.32. [DOI] [PubMed] [Google Scholar]
- Bortz J. Statistik für Sozialwissenschaftler. 5 ed. Heidelberg: Springer Verlag; 1999. [Google Scholar]
- Bowen A, McKenna K, Tallis RC. Reasons for variability in the reported rate of occurence of unilateral spatial neglect after stroke. Stroke. 1999;30:1196–1202. doi: 10.1161/01.str.30.6.1196. [DOI] [PubMed] [Google Scholar]
- Chedru F. Space representation in unilateral spatial neglect. Journal of Neurology, Neurosurgery and Psychiatry. 1976;39:1057–1061. doi: 10.1136/jnnp.39.11.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo A, De Renzi E, Faglioni P. The occurrence of visual neglect in patients with unilateral cerebral disease. Cortex. 1976;12:221–231. doi: 10.1016/s0010-9452(76)80003-2. [DOI] [PubMed] [Google Scholar]
- Colonna A, Faglioni P. The performance of hemisphere-damaged patients on spatial intelligence test. Cortex. 1966;2:293–307. [Google Scholar]
- Costa A, Vaughn HG, Horwitz M, Ritter W. Patterns of behavioral deficit associated with visual spatial neglect. Cortex. 1969;5:242–263. doi: 10.1016/s0010-9452(69)80033-x. [DOI] [PubMed] [Google Scholar]
- Egelko S, Riley E, Simon D, Diller L, Ezrachi O. Unilateral spatial neglect: Biases in contralateral search and fine spatial attention. Archives of Clinical Neuropsychology. 1988;3:213–225. [PubMed] [Google Scholar]
- Ferber S, Karnath H-O. How to assess spatial neglect -line bisection or cancellation tasks? Journal of Clinical and Experimental Neuropsychology. 2001;23:599–607. doi: 10.1076/jcen.23.5.599.1243. [DOI] [PubMed] [Google Scholar]
- Fullerton KJ, McSherry D, Stout RW. Albert´s Test: A neglected test of perceptual neglect. Lancet. 1986;327:430–432. doi: 10.1016/s0140-6736(86)92381-0. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Caltagirone C, Miceli G. Poor performance of right brain-damaged patients on Raven's coloured matrices: Derangement of general intelligence or of specific abilities? Neuropsychologia. 1977;15:675–680. doi: 10.1016/0028-3932(77)90071-9. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Messerli P, Tissot R. Qualitative analysis of unilateral spatial neglect in relation to laterality of cerebral lesions. Journal of Neurology, Neurosurgery and Psychiatry. 1972;35:545–550. doi: 10.1136/jnnp.35.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L, Dehaut F, Joanette Y. The Bells Test: A quantitative and qualitative test for visual neglect. International Journal of Clinical Neuropsychology. 1989;11:49–54. [Google Scholar]
- Johannsen L, Karnath H-O. How efficient is a simple copying task to diagnose spatial neglect in its chronic phase? Journal of Clinical and Experimental Neuropsychology. 2004;26:251–256. doi: 10.1076/jcen.26.2.251.28085. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Rorden C. The anatomy of spatial neglect. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman JT, Newhart M, Davis C, Heidler-Gary J, Gottesman RF, Hillis AE. Right hemispatial neglect: Frequency and characterization following acute left hemisphere stroke. Brain and Cognition. 2007;64:50–59. doi: 10.1016/j.bandc.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima S, Shigeno K, Dohi N, Kajiwara T, Komai N. A study of right unilateral spatial neglect in left hemisphere lesions: The difference between right-handed and non-right-handed post-stroke patients. Acta Neurologica Scandinavia. 1992;85:418–424. doi: 10.1111/j.1600-0404.1992.tb06040.x. [DOI] [PubMed] [Google Scholar]
- Mosidze VM, Mkheidze RA, Makashvili MA. Disorders of visuo-spatial attention in patients with unilateral brain damage. Behavioural Brain Research. 1994;65:121–122. doi: 10.1016/0166-4328(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Ogden JA. Anterior-posterior interhemispheric differences in the loci of lesions producing visual hemineglect. Brain and Cognition. 1985a;4:59–75. doi: 10.1016/0278-2626(85)90054-5. [DOI] [PubMed] [Google Scholar]
- Ogden JA. Contralesional neglect of constructed visual images in right and left brain-damaged patients. Neuropsychologia. 1985b;23:273–277. doi: 10.1016/0028-3932(85)90112-5. [DOI] [PubMed] [Google Scholar]
- Ogden JA. The 'neglected' left hemisphere and its contribution to visuospatial neglect. In: Jeannerod M, editor. Neurophysiological and neuropsychological aspects of spatial neglect. 1 ed. North Holland: Elsevier Science Publishers; 1987. pp. 215–233. [Google Scholar]
- Ringman JM, Saver JL, Woolson RF, Clarke WR, Adams HP. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63:468–474. doi: 10.1212/01.wnl.0000133011.10689.ce. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O. A simple measure of neglect severity. Neuropsychologia. 2010;48:2758–2763. doi: 10.1016/j.neuropsychologia.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, Bonilha L. Improving lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Sarri M, Greenwood R, Kalra L, Driver J. Task-related modulation of visual neglect in cancellation tasks. Neuropsychologia. 2009;47:91–103. doi: 10.1016/j.neuropsychologia.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SP, Halligan PW, Greenwool RJ. The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age and Ageing. 1993;22:48–52. doi: 10.1093/ageing/22.1.46. [DOI] [PubMed] [Google Scholar]
- Stone SP, Wilson B, Wroot A, Halligan PW, Lange LS, Marshall JC, et al. The assessment of visuo-spatial neglect after acute stroke. Journal of Neurology, Neurosurgery and Psychiatry. 1991;54:345–350. doi: 10.1136/jnnp.54.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchan J, Karnath H-O. Spatial orienting by left hemisphere language areas: A relict from the past? Brain. 2011;134:3059–3070. doi: 10.1093/brain/awr120. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam M-M. Mental state assessment of young and elderly adults in behavioral neurology. In: Mesulam M-M, editor. Principles of behavioral neurology. Philadelphia: F.A. Davis Company; 1985. pp. 71–123. [Google Scholar]