Abstract
While chemotherapy remains the most effective treatment for disseminated tumors, acquired or intrinsic drug resistance accounts for approximately 90% of treatment failure. Multidrug resistance (MDR), the simultaneous resistance to drugs that differ both structurally and mechanistically, often results from drug efflux pumps in the cell membrane that reduce intracellular drug levels to less than therapeutic concentrations. Expression of the MDR transporter P-glycoprotein (P-gp, MDR1, ABCB1) has been shown to correlate with overall poor chemotherapy response and prognosis. This review will focus on collateral sensitivity (CS), the ability of compounds to kill MDR cells selectively over the parental cells from which they were derived. Insights into CS may offer an alternative strategy for the clinical resolution of MDR, as highly selective and potent CS agents may lead to drugs that are effective at MDR cell killing and tumor resensitization. Four main mechanistic hypotheses for CS will be reviewed, followed by a discussion on quantitative and experimental evaluation of CS.
Keywords: Multidrug resistance, collateral sensitivity, P-glycoprotein, MDR1-selective, verapamil, MRP1
1. Introduction
While chemotherapy is currently the standard treatment for metastatic and hematological malignancies, drug resistance to chemotherapy, be it intrinsic or acquired, is often inevitable. Multidrug resistance (MDR) is characterized by the simultaneous resistance to drugs that differ both structurally and mechanistically (Gottesman et al., 2002). ATP binding cassette (ABC) transporters expressed in the plasma membrane are notorious mediators of MDR, actively effluxing a wide range of amphiphilic drugs irrespective of concentration gradient, thereby lowering intracellular concentrations to below therapeutic levels. ABC transporters play both excretory and protective physiological roles, being endogenously expressed in the liver, kidneys, and intestines, and preventing entry of xenobiotics at the blood-brain-barrier, testis, and placenta (Gottesman et al., 2002; Szakacs et al., 2006; Zhou, 2008). While crucial for physiological homeostasis, these transporters have been shown to affect drug absorption, distribution, and excretion, often unpredictably or undesirably, from tissues that express these transporters.
Three prominent ABC transporters that have been shown to modulate anticancer drug uptake in vitro are P-glycoprotein (P-gp, ABCB1, MDR1); multidrug resistance protein 1 (MRP1, ABCC1), and breast cancer resistance protein (BCRP, ABCG2). Of these, P-gp has been most extensively examined, and numerous anti-cancer drugs used in the clinic have been identified as substrates of P-gp, including paclitaxel, vinblastine, vincristine, daunorubicin, doxorubicin, and etoposide (Fox and Bates, 2007; Gottesman et al., 2002). Overexpression of P-gp has been shown to correlate with overall poor chemotherapy response and prognosis (Leonard et al., 2003). Studies have shown that 50% of human cancers express P-gp at easily detectable levels (Gottesman et al., 2002). While MRP1 and BCRP have not been correlated as closely with a MDR phenotype, there is limited evidence that intrinsic MRP1 expression in NSCLC and BCRP expression in leukemia leads to decreased response to chemotherapy and overall poor clinical outcome (Berger et al., 2005; Robey et al., 2010; Robey et al., 2007).
Numerous strategies to overcome P-gp-mediated MDR have been explored, including the design of novel drugs that evade recognition and efflux, inhibitors to block efflux and restore drug accumulation, and, more recently, the exploration of small molecules that are selectively lethal to P-gp-expressing cells (Hall et al., 2009a; Kelly et al., 2010; Nobili et al., 2011). Drug development strategies to resolve MDR have focused on medicinal chemistry approaches to identify analogs that evade P-gp, including epothilones, topoisomerase inhibitors, and second- and third-generation taxanes, which have shown initial success in clinical trials when administered to patients previously treated with cytotoxic P-gp substrates (Nobili et al., 2011). P-gp inhibitors have been used with limited clinical success, as the co-administration of a cytotoxic drug with an inhibitor often produces unpredictable or undesirable pharmacokinetics (Gottesman et al., 2002). In addition, expression of P-gp is by no means the only mechanism of MDR in clinical cancers, and simply overcoming or circumventing its activity would not be expected to cure all MDR cancers.
An alternative strategy to overcome and exploit clinical MDR is to identify compounds that selectively kill MDR cells but not the non-resistant parental cells from which they are derived, a phenomenon termed collateral sensitivity (CS) (Hall et al., 2009a). The term CS was first described qualitatively by Szybalski and Bryson in 1952 after observations that drug-resistant Escherichia coli displayed hypersensitivity to unrelated agents, thus acquiring a potentially exploitable weakness as a result of the drug selection process (Szybalski and Bryson, 1952). CS is a type of synthetic lethality1, wherein the genetic alterations accrued while developing resistance towards one agent is accompanied by the development of hypersensitivity towards a second agent. CS thus creates an “Achilles' heel” which can be exploited for the targeting and selective killing of MDR cells, and its efficacy is independent of the existence of other MDR mechanisms in cancer cells. Until recently there has been limited success at identifying MDR-selective compounds, with most agents that induce CS being unintentionally identified by after-the-fact observations that such agents show increased rather than decreased cytotoxicity towards MDR cell lines. The identification of highly selective and potent CS agents may lead to drugs that are highly effective at 1) preventing MDR through adjuvant administration during standard chemotherapeutic regimens or 2) resensitizing MDR tumors to commonly employed therapeutics through the selective killing of MDR cells in a heterogeneous tumor population (Fig. 1).
Fig. 1.
Scheme demonstrating how chemotherapeutics selectively kill the sensitive (black) sub-population of tumor cells from among a heterogenous malignant population. During the recovery phase multidrug resistant (striped) tumor cells re-populate, and repeated chemotherapeutic cycles result in an intractable multidrug resistant tumor. Treatment with CS agents can kill P-gp-expressing cells and/or reduce P-gp expression, potentially priming tumor cells for treatment with chemotherapeutics.
2. Putative Mechanisms of Collateral Sensitivity
The complex mechanisms by which CS agents exert selective killing of MDR cells have not been elucidated. At least four main hypotheses have been proposed to account for CS, each supported by limited experimental evidence. The hypotheses discussed herein attempt to explain CS by the ability of CS agents to 1) produce reactive oxygen species (ROS) via futile hydrolysis of ATP, 2) exploit energetic sensitivities, 3) extrude endogenous substrates which are essential for cell survival, or 4) perturb the plasma membrane.
2.1 Modulation of CS by Reactive Oxygen Species
The most recent hypothesis for CS is based on observations that numerous CS agents are substrates of P-gp (vide infra), stimulating ATPase activity and substrate extrusion from the plasma membrane into the extracellular environment (Laberge et al., 2009). Once back in the extracellular environment, substrates are thought to repeat this cycle as P-gp acts as a “hydrophobic vacuum cleaner”, a process known as futile cycling, leading to increased amounts of cellular ATP hydrolysis (Gottesman et al., 2009). As cells struggle to replenish ATP, they suffer from oxidative stress either as a direct result of increased ROS production from increased oxidative phosphorylation (such as O2−, H2O2, •OH production), or as an indirect result of a decrease in antioxidant defenses such as glutathione (Fig. 2). Once ROS levels pass a certain threshold, MDR cells initiate apoptosis. It is unknown whether futile hydrolysis of ATP occurs in vivo, as cells are constantly affected by changes in drug availability as the circulatory system rapidly removes cellular wastes (in this case P-gp extruded substrates), versus in vitro conditions where cells remain under constant exposure to drugs in culture media.
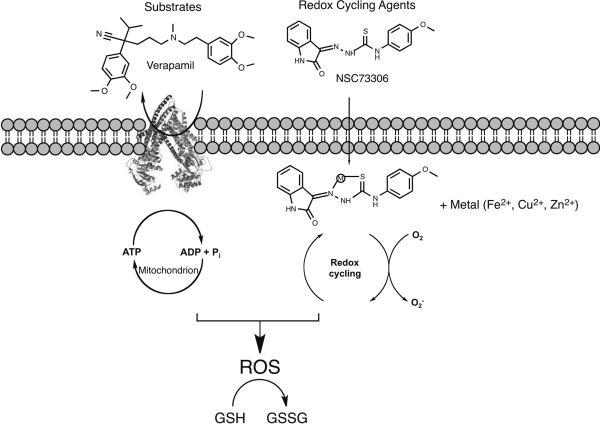
Fig. 2.
Scheme showing how substrates that stimulate P-gp ATPase activity and redox cycling agents can contribute to ROS production within cells. Substrates (left): The addition of a non-toxic substrate and subsequent futile cycling of the transporter consumes ATP, and its repletion results in ROS generation, while parental lines lacking P-gp are not exposed to ROS. The differential ROS generation results in preferential killing of MDR cells. Metal-mediated redox: Small molecule chelators that complex with endogenous iron or copper are known to allow redox-cycling and the resultant ROS generation would induce CS as put forward for organic redox cycling molecules. Increased levels of ROS converts glutathione (GSH) into its oxidized form glutathione disulfide (GSSH). Increased levels of GSSH is considered an indicator of cellular stress.
Studies supporting this mechanism show that ATP is depleted at twice the rate in MDR cells exposed to the potent ATPase stimulator verapamil, as compared to non-MDR parental cells (Broxterman et al., 1988). CS to verapamil is reversed by inhibitors of P-gp ATPase function, indicating that ATPase stimulation is necessary for cell killing. MDR cells exposed to verapamil showed elevated levels of ROS superoxide (O2−) and decreased levels of glutathione, both of which were abrogated by the P-gp inhibitor PSC883 (Karwatsky et al., 2003). Numerous CS agents show potency proportional to the amount of P-gp expressed, with cells expressing high levels of P-gp showing marked CS and cells with low levels of P-gp being relatively tolerant to the CS agent. This suggests that when P-gp levels are low, cells can cope with small increases in ROS production, and that levels of ROS must exceed a particular threshold to induce apoptosis.
A detailed examination of the changes to the cellular metabolic state after the P-gp ATPase has been stimulated has never been conducted, and to date there is no concrete evidence that CS agents increase oxidative phosphorylation, and only limited evidence that these agents significantly increase ROS. Additionally, cancer cells have historically been thought to rely primarily on glycolysis to meet energy needs, and have diminished rates of oxidative phosphorylation (the Warburg effect) (Cairns et al., 2011; Koppenol et al., 2011) The connection between ATPase stimulation and increases in oxidative phosphorylation to induce ROS remains unclear.
A second set of compounds support an alternative mechanism of ROS generation by CS agents, albeit in a more direct manner not associated with an interaction with P-gp. The CS agents NSC73306 and Dp44mt are both thiosemicarbazone metal chelators capable of complexing with intracellular metals that can cycle between two redox states, such as iron (II/III), through oxidation and reduction, yielding the formation of ROS (Fig. 2). (Kappus and Sies, 1981). Theoretically these agents generate ROS in both MDR and non-MDR cells, indicating a potential inherent hypersensitivity to increases in ROS in MDR cells. An in silico screening of 42,000 compounds against the NCI-60 cell line panel revealed an enriched set of CS agents capable of metal chelation, suggesting that redox cycling is able to increase ROS and initiate cell death (Turk et al., 2009).
2.2 Increased Sensitivity to Changes in Energy Levels
While the ROS generation hypothesis proposes that CS is mediated indirectly by overall levels of oxidative stress due to futile cycling of P-gp, several compounds that do not interact with P-gp, but rather interfere with cellular metabolic pathways, have been identified as MDR-selective. This has led to a more general hypothesis that P-gp-expressing cells are more sensitive to changes in energy utilization. Thus, agents that perturb pathways such as glycolysis or oxidative phosphorylation may preferentially kill MDR cells. This has been supported by findings that the glycolysis antimetabolite 2-deoxy-D-glucose, and the electron transport chain inhibitors rotenone and antimycin A preferentially kill P-gp-expressing cells. However, the ability of such compounds to significantly affect cellular ATP stores in MDR cells is yet to be examined.
2.3 Extrusion of an Endogenous Substrate
While P-gp is often considered a transporter of exogenous xenobiotics, it is equally important to consider the ability of P-gp to transport endogenous substrates. The extrusion hypothesis asserts that a CS agent does not mediate cytotoxicity directly, but rather indirectly by stimulating or facilitating the extrusion of an endogenous essential molecule or by sensitizing cells to the loss of an important metabolite which is a P-gp susbtrate. While there are no reports of this phenomenon occurring in P-gp-expressing cells, this may be the case for MRP1-mediated CS. MRP1 is involved in phase III metabolism of xenobiotics, transporting glutathione and glutathione conjugates, a process necessary for detoxification of harmful compounds (Cole and Deeley, 2006). As discussed in detail below, CS agents such as verapamil induce apoptosis through the stimulation of glutathione efflux by MRP1. Further investigation into whether this phenomenon occurs in P-gp-expressing cells would necessitate the identification of an endogenous essential molecule, a finding that has yet to be accomplished.
2.4 Membrane perturbation
Numerous CS agents, such as Triton X detergents, do not appear to have any direct interaction with P-gp, but rather alter membrane biophysical properties (Bech-Hansen et al., 1976). Some CS agents have been shown to induce membrane perturbation to a greater extent in P-gp-expressing cell lines, leading to a hypothesis that changes in membrane structure and fluidity contribute to CS (Callaghan and Riordan, 1995). The CS agents pentazocine and verapamil were shown to significantly reduce lipid structural order (i.e., membrane fluidity) in the colchicine-resistant B30 cell line. Additionally, verapamil, pentazocine, pethidine, and nalaxone were able to induce leakage of the polar florescent dye 6-CF through the cell membrane, perhaps due to the hydrophobic nature of these compounds and their ability to intercalate into the lipid bilayer (Callaghan and Riordan, 1995). While early reports found that expression of P-gp resulted in alterations of the biophysical properties of cell membranes, this is most likely the result of the drug selection process, as cells transfected with P-gp did not result in altered fluidity (Aleman et al., 2003). P-gp itself may not be responsible for alterations in membrane fluidity although P-gp may somehow render cells more sensitive to changes in fluidity after membrane perturbation by a CS agent. For many CS agents that kill P-gp expressing cells, such as tiopronin and austocystin D, P-gp is not sufficient to enable this killing, which suggests the existence of a more generalized mechanism of CS (Goldsborough et al., 2011; Marks et al., 2011).
3. Verapamil
3.1 Verapamil and P-gp
Verapamil is a phenylalkylamine L-type calcium antagonist, most commonly used for the clinical management of hypertension (Prisant, 2001). Verapamil shows a complex interaction with P-gp, being an avid substrate capable of stimulating P-gp ATPase activity at low concentrations, and an inhibitor that suppresses P-gp ATPase function at high concentrations (al-Shawi and Senior, 1993; Ambudkar et al., 1999; Laberge et al., 2009). Verapamil was first recognized as a chemosensitizing agent through observations that inhibitory concentrations of verapamil could reverse a MDR phenotype when co-cultured with a cytotoxic agent, increasing intracellular levels of the P-gp substrate vincristine 10-fold in the vincristine-resistant P388/VCR murine leukemia subline as compared to untreated controls (Tsuruo et al., 1981). Reversal of resistance to anthracyclines and vinca alkaloids by verapamil was shown in additional MDR animal and human cell lines (Helson, 1984). These studies highlight verapamils ability to antagonize P-gp at high concentrations (Ambudkar et al., 1999; Naito and Tsuruo, 1989). This inhibition allows for decreased efflux, and therefore, increased intracellular accumulation of cytotoxic P-gp substrates and overall reversal of the MDR phenotype.
Verapamil was first shown to be a CS agent when the vincristine-resistant CHO subline E29 vcr/a displayed “hypersensitivity” (i.e., collateral sensitivity) in the absence of additional cytotoxic agents (Warr et al., 1986). A revertant cell line derived from continuous culture of E29 vcr/a cells in drug-free media and subsequent reversion to a drug-sensitive phenotype, showed abrogation of verapamil hypersensitivity, thus exhibiting a correlation between a MDR phenotype and verapamil cytotoxicity. Further studies showed a marked decrease in accumulation of [3H]verapamil in E29 vcr/a as compared to the E29 drug sensitive parental cell line, consistent with verapamil's ability to be effluxed as an P-gp substrate. Accumulation studies of 45Ca2+ showed that verapamil did not affect calcium accumulation differentially between parental or MDR sublines, hence exerting CS through a calcium-independent mechanism (Cano-Gauci and Riordan, 1987; Warr et al., 1986). Other calcium channel antagonists such as nicardipine and diltiazem were also identified as CS agents, but in the same fashion as verapamil, did not alter 45Ca2+ accumulation (Naito and Tsuruo, 1989; Tsuruo et al., 1983; Warr et al., 1988)
Further studies have revealed complex interactions between verapamil and P-gp that have yet to be fully understood. Upon addition of sub-lethal concentrations of verapamil into culture medium, it has been shown to decrease P-gp expression 3-fold in MDR K562/ADR and CEM VBL100 human leukemia MDR cell lines (Muller et al., 1994). Northern Blot analysis reveals P-gp down-regulation at the transcriptional or post-transcriptional level (Muller et al., 1994). The P-gp inhibitor PSC883 abolished CS to verapamil, whereas the P-gp substrate ivermectin had no effect on verapamil mediated CS, highlighting the importance of the interaction between verapamil and P-gp and the requirement for functional P-gp for CS-mediated cell death (Karwatsky et al., 2003). Furthermore, down-regulation of P-gp by siRNA abrogates sensitivity to verapamil, demonstrating a direct relationship between P-gp expression and verapamil sensitivity (Laberge et al., 2009). Due to verapamil's ability to increase ATPase activity, it can be considered a prototype for the ROS mediated CS hypothesis. Verapamil has also been shown to affect membrane biophysical properties, and may be acting synergistically through combinations of cellular stresses induced by depletion of ATP stores along with increased membrane perturbation.
It is important to highlight that the abovementioned studies were carried out in Chinese hamster ovary (CHO) cells, and CS of verapamil towards P-gp-expressing human tumor cells has not been reported. Additionally, the potency of verapamil as a calcium channel blocker limits the clinical usability of verapamil at concentrations necessary to induce CS, as such concentrations would induce side effects such as hypotension and cardiac arrhythmia. Nevertheless, the hypersensitivity of P-gp-expressing cells to verapamil highlights an exploitable weakness of drug resistant cells, and initiated broader study into CS.
3.2 Verapamil and MRP1
Verapamil also exerts CS on MRP1-expressing cells, albeit in a drastically different manner as compared to P-gp expressing cells. In contrast to the high avidity of verapamil as a P-gp substrate, verapamil is not actively transported by MRP1 (Loe et al., 2000; Trompier et al., 2004). However, when MRP1-enriched membrane vesicles are incubated with both verapamil and glutathione (GSH), MRP1 is strongly inhibited (Trompier et al., 2004). MRP1 plays an important role in phase III metabolism of xenobiotics, actively effluxing glutathione-conjugated organic anions (Cole and Deeley, 2006). Studies with baby hamster kidney-21 (BHK-21) cells transfected with human MRP1 show selective apoptosis of MRP1-expressing cells as compared to inactive MRP1 mutant transfected control cells due to the active efflux of GSH in the presence of verapamil (Trompier et al., 2004). Verapamil and an iodinated verapamil analog have been shown to increase cellular efflux of GSH 3-fold and 9-fold, respectively (Trompier et al., 2004). Therefore, it is the efflux of GSH induced by verapamil, rather than verapamil itself, which can induce GSH-mediated apoptosis of cells. While these studies support the hypothesis that extrusion of an endogenous substrate, GSH in this case, can cause CS, the exact role of GSH efflux in apoptosis is debated and remains unclear (He et al., 2003).
While the above studies were carried out with racemic (±) verapamil, as used in clinical formulations, further investigation reveals the differential activity of (S)- and (R)- verapamil enantiomers against MRP1-expressing cells (Perrotton et al., 2007). Interestingly, only the S-isomer initiated CS against MRP1-transfected cells, exhibiting increased GSH efflux and subsequent apoptosis as compared to cells treated with racemic verapamil. In contrast, the R-enantiomer does not initiate GSH efflux or cell death, but rather inhibits MRP1, leading to reversal of a drug-resistant phenotype. Furthermore, both (R)- and (S)-verapamil were shown to bind to MRP1 with high affinity, each inducing a distinct MRP1 conformational change, indicating that it is conformational change upon verapamil binding to MRP1 that influences the differential activity of the (S)- and (R)-enantiomers (Perrotton et al., 2007). Due to cardiovascular side effects of the B phenyl ring of verapamil and the high concentrations needed to induce MRP1 CS in vivo, recent studies have synthesized iodinated verapamil analogs that display both decreased cardiotoxicity and a 10-fold increased potency (Barattin et al., 2010).
In an effort to find MRP1-selective CS agents that lack the undesirable cardiotoxicity of verapamil, xanthones were found to have similar potency to verapamil (Genoux-Bastide et al., 2011). Screening of 23 xanthones resulted in 5 which showed >50% MRP1-stimulated glutathione efflux (racemic verapamil resulted in 75% net glutathione efflux).
4. Other compounds that elicit collateral sensitivity
4.1 2-Deoxy-D-glucose
2-Deoxy-D-glucose (2-DG) is a glucose analog that decreases cellular ATP production by both competing with glucose for uptake by GLUT1 and subsequently inhibiting glycolysis by binding to hexokinase II, a crucial enzyme for the initial catabolism of glucose into glucose-6-phosphate (Bell et al., 1998; Xu et al., 2005). Cancer cells have been shown to exhibit an increased reliance on glycolysis for ATP generation (the Warburg effect), due to primary defects in mitochondrial oxidative phosphorylation, and/or anaerobic conditions resulting from hypoxia in the tumor microenvironment (Xu et al., 2005). Thus, MDR cells must sate the high-energy demands needed to support drug resistance mechanisms, such as ATPase drug efflux pumps, through the energetically inefficient glycolytic pathway, supporting the hypothesis that MDR cells may be hypersensitive to antimetabolites.
2-DG has been shown to initiate apoptosis and selectively kill numerous MDR sublines as compared to drug-sensitive parental lines (Bell et al., 1998; Kaplan et al., 1991; Kaplan et al., 1990). P-gp expressing human breast MCF7 ADR cells were shown to have a 3-fold increased rate of glycolysis as compared to parental drug-sensitive MCF7 cells, as well as a 15-fold sensitivity towards 2-DG, indicative of a correlation between the high energy requirement of MDR cells and their sensitivity towards glycolysis inhibition (Kaplan et al., 1990; Lyon et al., 1988). Further investigation showed that P-gp-expressing human cervical adenocarcinoma KB-V1 cells and the transfected MDCK-MDR cell lines both showed significant CS towards 2-DG (Kaplan et al., 1991). Tunicamycin, a N-linked glycosylation inhibitor that has been shown to decrease GLUT-1-mediated transport, was shown to potentiate effects of 2-DG on MDR cells, indicating that compounds that alter glucose availability may act synergistically to induce CS (Bentley et al., 1997). Uptake of [3H]2-DG was increased 3-fold and 9-fold in taxol- and melphalan-resistant cell lines, respectively, as compared to their parental cells (Keenan et al., 2004). As fluorodeoxyglucose is used in the clinic for PET tumor imaging, the above finding may be useful for the clinical imaging of MDR tumors in patients, potentially leading to improved treatment methods as clinicians could tailor chemotherapeutics regimes based on the presence or absence of MDR. Overall, the activity of 2-DG highlights important metabolic differences between drug-sensitive and MDR cells that could potentially be exploited for selective killing and clinical abolition of MDR tumors.
4.2 Desmosdumotin B
Flavonoids comprise a diverse family of plant-derived natural products which have numerous biological activities. They have been shown to inhibit P-gp ATPase, altering levels of cellular drug uptake through P-gp specific interactions (Tran et al., 2011). The flavonoid desmosdumotin B and its derivatives have recently been shown to selectively kill the P-gp-expressing cell lines KB-VIN and Hep3B-VIN (Nakagawa-Goto et al., 2008; Nakagawa-Goto et al., 2010). Most notably, the desmosdumotin B derivative 6,6,8-Triethyldesmosdumotin B and its 4′ -methyl and 4′ -ethyl analogs showed selectivity ratio values of >221, 460, and 320, respectively, with IC50 values against P-gp-expressing cells in the nanomolar range (see below for detailed explanation of selectivity ratio) (Nakagawa-Goto et al., 2010). Similar to other compounds that exert CS, the activity of desmosdumotion B seems to be dependant on functional P-gp, as the cytotoxic effect is partially reversed in the presence of verapamil and the P-gp allosteric inhibitor flupenthixol (Nakagawa-Goto et al., 2010). Further studies have shown a desmosdumotin B derivative may induce CS through the P-gp-dependent inhibition of the mTOR pathways in conjunction with the downregulation of the molecular chaperone GRP78. However, the connection between P-gp and mTOR as a downstream effector remains unclear (Kuo et al., 2011). Overall, due to both the high selectivity and potency desmosdumotin B analogs show towards P-gp expressing cells, they are promising compounds for further validation.
4.3 NSC73306 and analogs
NSC73306 was discovered using a bioinformatic approach that compared growth inhibitory profiles of 1,429 chemical compounds on the National Cancer Institute cell line panel (NCI-60) (Ludwig et al., 2006). While this approach was initially employed to unearth ABC transporter substrates, P-gp-expressing cells lines were found to be hypersensitive to NSC73306. NSC73306 is an isatin-β-thiosemicarbazone that has since been shown consistently to elicit CS towards numerous P-gp-expressing cells lines, including cell lines of different tissue origin, P-gp-transfected cell lines, and cell lines maintained in different selection agents (Hall et al., 2011; Hall et al., 2009b). CS of NSC73306 is dependent on levels of P-gp expression, as shown by a positive correlation between P-gp mRNA, protein expression, and selectivity, suggesting a P-gp-dependent mechanism of cell killing (Ludwig et al., 2006). Furthermore, activity of NCS73306 was abrogated by P-gp inhibition and siRNA knockdown.
The mechanism of NSC73306 cell killing may involve ROS generation due to its ability to complex with intracellular metals (Fe2+, Zn2+, Cu2+) causing cycling between two oxidative states, a process that yields superoxide anions. While P-gp-dependent selective compounds often show P-gp modulation, through either inhibitory or stimulatory effects, NSC73306 does not seem to interact directly with P-gp as shown by its poor ability to change ATPase levels in crude membranes isolated from insect cells expressing human P-gp (Handley and Gottesman, unpublished data; Ludwig et al., 2006). Taken together, this suggests that P-gp is necessary to create the hypersensitivity of MDR cells to changes in ROS. Long-term exposure experiments showed that HCT-15 P-gp-expressing cells exposed to NSC73306 for 21 days showed loss of resistance towards to the cytotoxic P-gp selection substrate doxorubicin, loss of hypersensitivity towards NSC73306, and complete loss of P-gp at the protein level. It is appears that NSC73306 inhibits growth of MDR cells (either by apoptosis or cell cycle arrest), selecting for a drug-sensitive population unharmed in long term culture and also somehow directly downregulates P-gp mRNA levels leading to resensitization (Handley and Gottesman, unpublished data).
Upon examination of interactions between NSC73306 and the ABC transporters BCRP, MRP1, MRP4 and MRP5, it was revealed that NSC73306 did not exert CS on these transporters. Rather, NCS73306 is a substrate of BCRP able to reverse resistance to the cytotoxic substrates mitoxantrone and topotecan through the ability to compete with BCRP for transport (Wu et al., 2007). This finding has implications for the clinical use of NSC73306 to act in a dual manner to both eliminate P-gp-expressing cells while competitively inhibiting efflux of BCRP cytotoxic substrates. Recently, analogs of NSC73306 with increased selectivity have been generated, with the most selective analog having a 15-fold selectivity ratio (Hall et al., 2011).
4.4 Dp44mT
The iron chelator di-2-pyridkylketone-4,4,-dimethyl-3-thiosemicarbazone (Dp44mT) was shown to exert broad antitumor killing as well as P-gp-mediated CS (Whitnall et al., 2006; Yuan et al., 2004). Iron deprivation can exert profound impacts on cancer cell growth, as the enzyme ribonucleotide reductase is the rate limiting step of DNA synthesis, thus causing G1/S cell cycle arrest and apoptosis of neoplastic cells upon chelation therapy (Le and Richardson, 2004, 2002). Preliminary studies have shown that Dp44mT exerted CS towards the P-gp-expressing human adenocarcinoma cell line KB-V1, while lacking CS towards the MRP1-expressing MCF7-VP16 breast cancer cell line (Whitnall et al., 2006). While the mechanism of CS against P-gp-expressing cells is unknown, Dp44mT is redox-active and induces ROS by the production of hydroxyl radical formation upon complexation with iron (Jansson et al., 2010). Thus, while the overall cytotoxicity of Dp44mT may be due to iron chelation, Dp44mT-mediated hypersensitivity may be related to ROS generation and the sensitivity of MDR cells towards oxidative stress. The structurally unrelated redox active iron chelate N-(2-hydroxy acetophenone) glycinate (FeNG) has also been shown to be MDR selective, generating ROS in a doxorubicin-resistant leukemia cell line, suggesting that a wide variety of agents capable of redox cycling may be potent CS agents (Ganguly et al., 2010).
4.5 Tiopronin
Tiopronin (N-(3-Mercapto-2-methylpropanoyl) glycine) is an orphan drug that has been used for over 30 years to treat a diverse range of pathophysiological conditions including cystinuria (Joly et al., 1999). It has recently been demonstrated that a series of drug-selected human adenocarcinoma cell lines (KB-A1, KB-8-5, KB-8-5-11 and KB-V1) expressing P-gp, and the breast cancer cell line MCF-7/ VP-16 expressing MRP1 are collaterally sensitive to tiopronin (Goldsborough et al., 2011). In the case of the MCF-7/VP-16 cell line pair, resistant cells were over 50-fold more sensitive to tiopronin than parental cells. However, the expression of P-gp is not sufficient to elicit collateral sensitivity by tiopronin as the transfected cell line Hela MDR Tet-off, which expresses high levels of P-gp, is not sensitive. Although tiopronin, like NSC73306, has been shown to reduce the stability of P-gp mRNA, the mechanism by which it works is largely unknown. Molecular studies have clearly shown that the thiol function is essential and that collateral sensitivity is exquisitely sensitive to the configuration of the thiol group (Goldsborough et al., 2011). Also, analogs of tiopronin replacing the Gly portion of the molecule with Ser, Val, Ala or Phe, have a smaller effect on activity. Rather than exploiting a single feature of MDR to sensitize cells, tiopronin may be acting via a broader mechanism that targets a common alteration in cells selected for MDR.
5. Quantitative and Experimental Evaluation of Collateral Sensitivity
Due to the clinical relevance of CS agents to potentially exploit MDR, it is important to identify and test potential agents in vitro for potency, selectivity, and the ability to interact with P-gp. At its simplest level, CS can be quantitatively evaluated as the ratio of a compound's cytotoxicity against parental, non-MDR cells divided by the cytotoxicity against P-gp-expressing cells (IC50 sensitive/IC50 MDR), a value termed the selectivity ratio (SR; Table 1). A SR value > 1 indicates that the compound kills P-gp-expressing cells more effectively than parental cells, i.e., that the compound exerts CS. Conversely, a SR value <1 indicates that the compound is a P-gp substrate, actively being effluxed from P-gp cells and exerting a greater cytotoxic effect on the parental line. It is important to note that the SR value gives a measure of selectivity rather than potency. In other words, a compound with a high SR value is highly selective in killing P-gp-expressing cells, but may have cytotoxicity values ranging anywhere from low nanomolar to high millimolar ranges. While SR values provide a way to compare CS between compounds, the drawbacks to this method must be realized, and overall potency values must also be taken into consideration upon comparison. Currently, there is no standardized terminology for the SR, and is sometimes denoted “Resistance Ratio” or “CS index.” While the focus of this review concerns SR values in the context of P-gp mediated cell killing, SR values can also be applied to the selective killing of MDR cells by non-transporter related mechanisms.
Table 1.
Representative cytotoxicity and selectivity ratio data of CS agents reported in the literature. The data shown has been compiled from reports that disclosed quantitative data. Due to differences in assay methodology and cell lines, selectivity ratios and absolute activity are not comparable between compounds.
| Compound | Parent cell line | Assay | (IC50 sensitive)/(IC50 resistant) | Selectivity Ratio | Source |
|---|---|---|---|---|---|
| Verapamil | CHO | Clonogenic* | (23 μgmL−1) / (0.2 μgmL−1)* | 115 | (Warr et al., 1986) |
| 2-DG | Human KB carcinoma | MTT | (2.7 mM) / (0.61 mM) | 4.4 | (Kaplan et al., 1991) |
| Desmosdumotin B analog | Human KB carcinoma | Suforhodamine B | (13.8 μgmL−1) / (0.03 μgmL−1) | 460 | (Nakagawa-Goto et al., 2008) |
| NSC73306 | Human KB carcinoma | MTT | (14.2 μM) / (3.3 μM) | 4.3 | (Hall et al., 2011) |
| NSC73306 analog | Human KB carcinoma | MTT | (15.9 μM) / (1.07 μM) | 15 | (Hall et al., 2011) |
| Tiopronin (P-gp) | Human KB carcinoma | MTT | (7.54 mM) / (0.15 mM) | 51 | (Goldsborough et al., 2011) |
| Tiopronin (MRP1) | Human breast carcinoma MCF7 | MTT | (12.27 mM) / (0.29 mM) | 41 | (Goldsborough et al., 2011) |
reported D10 Values
When identifying a potential CS agent, it is important to determine experimentally whether the agent requires P-gp to be present for selective MDR cell killing. While there are cases of both P-gp-mediated and non-P-gp-mediated CS, P-gp-mediated cell killing may be considered preferable, as it eliminates the chances that a CS agent is targeting a more general feature of MDR cells that has been acquired during drug selection of specific cell lines or tumors. If a CS agent is P-gp-dependent, CS can be abrogated by the addition of a P-gp inhibitor at non-toxic levels, such as the third generation P-gp inhibitor tariquidar (XR9576) (Fox and Bates, 2007). Additionally, abrogation of CS through P-gp knockdown by siRNA can be used to determine if CS is mediated in a P-gp-dependent manner. While these tests indicate whether P-gp is necessary for CS, only transfection experiments with P-gp, without MDR selection, can demonstrate that P-gp alone is sufficient for CS.
ATPase assays can be used to determine whether the CS agent inhibits or stimulates ATP hydrolysis (Ambudkar et al., 1999). Similarly, flow cytometry can help elucidate complex interactions between a CS agent and P-gp though assays in which the agent is allowed to compete with a fluorescent probe that is also a known P-gp substrate (Calcein AM or rhodamine 123) (Calcagno et al., 2007). Changes in overall fluorescence intensity indicate that the CS agent is interacting with P-gp in a manner that competes with the efflux of fluorescent probe. The effect that P-gp protein levels have on CS cytotoxicity can be determined by the use of isogenic cell lines that have been sequentially selected with increasing amounts of a cytotoxic agent to induce MDR. For example, the cervical adenocarcinoma drug-sensitive cell line KB-3-1 was selected through exposure to increasing concentrations of chemotherapeutic agents to yield KB-8-5, KB-8-5-11, and KB-V1 cells lines that display increasing amounts of P-gp mRNA and protein (Akiyama et al., 1985). A P-gp-dependent CS agent is expected to show increasing cytotoxicity correlating with the amount of P-gp expression. Variations of the abovementioned assays could be easily adapted to probe for interactions with other transporters including MRP1 and BCRP.
6. Conclusions
Nearly 35 years of MDR research has yielded a great body of basic knowledge, yet no successful P-gp-targeted therapeutics have been developed. The relatively understudied phenomenon of CS has been used successfully to exploit MDR in vitro. While a single CS mechanism has yet to be elucidated, there appear to be four main types of CS agents: agents that stimulate ATPase activity, agents that interfere with energy utilization, those that facilitate the extrusion of a vital molecule, and agents that affect biophysical membrane properties. While certain agents presented here act in ways that support these hypotheses, it is possible that CS agents work by alternative mechanisms, such as enhanced uptake by P-gp cells or altered intracellular drug trafficking, although these theories remain theoretical thus far. While a unifying hypothesis that can account for all cases of CS has historically been sought, it seems likely there is no one major mechanism that can account for all CS, and that mechanistic studies must be undertaken on a compound-by-compound basis.
While CS agents have undergone varying degrees of in vitro testing to quantify both selectivity and potency against MDR cells, the cancer-cell-specific activity of these compounds remains unclear. As P-gp is endogenously expressed in a number of organs to serve both protective and excretory roles, it will be necessary to investigate the effects of CS agents on normal cells that express P-gp (tested experimentally either by cells with endogenous or transfected P-gp expression). Unpublished data from our laboratory shows that NSC73306 appears to be non-toxic against organs that endogenously express P-gp upon i.v. and oral administration to dogs and upon i.p. administation to mice up to doses of 400 mg/kg. However, the ability to elicit P-gp-selective killing against MDR cells was not measured in this study (Handley and Gottesman). Additional in vivo models for testing efficacy must be established before the clinical relevance of CS can be fully realized.
Due to the fact that all CS compounds were uncovered by retrospective observations, compounds with increased potency and selectivity could be uncovered through high-throughput screening of compound libraries against P-gp over-expressing cells. This may eliminate off-target effects due to the primary pharmacological activity of the compound, a problem seen with the clinical use of verapamil. Alternatively, examination of structural commonalties of the abovementioned compounds may be used for combinatorial chemistry strategies for the development of novel CS agents with improved selectivity. Adjuvant administration of CS may help to prevent MDR during standard chemotherapeutic regimens. They may also or resensitize MDR tumors to commonly employed therapeutics through the selective killing of MDR cells in a heterogeneous tumor population. Given the devastating impact MDR often has on cancer treatment, exploitation of MDR by CS provides a novel strategy that warrants further investigation.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. We thank George Leiman for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1During tumor development, the cellular genome undergoes a multistep mutational process wherein cells acquire DNA lesions, so-called “hallmarks” of cancer, which together begin to account for a neoplastic phenotype (Hanahan and Weinberg, 2011). While these genetic lesions lead to cancer, they are also exploitable for development of therapeutics that selectively kill cancer cells. Two genes cause synthetic lethality when inhibition or mutation of either gene alone does not cause loss of viability, but loss of both genes in combination leads to cell death (Brough, et al., 2011). This concept is exemplified through the relationship between BRCA1/2 and hypersensitivity to inhibitors of the DNA repair enzyme Poly(ADP-Ribose) Polymerase 1 (PARP1) (Farmer, et al., 2005).
References
- Akiyama S, Fojo A, Hanover JA, Pastan I, Gottesman MM. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat. Cell Mol. Genet. 1985;11:117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- al-Shawi MK, Senior AE. Characterization of the adenosine triphosphatase activity of Chinese hamster P-glycoprotein. J. Biol. Chem. 1993;268:4197–4206. [PubMed] [Google Scholar]
- Aleman C, Annereau JP, Liang XJ, Cardarelli CO, Taylor B, Yin JJ, Aszalos A, Gottesman MM. P-glycoprotein, expressed in multidrug resistant cells, is not responsible for alterations in membrane fluidity or membrane potential. Cancer Res. 2003;63:3084–3091. [PubMed] [Google Scholar]
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- Barattin R, Perrotton T, Trompier D, Lorendeau D, Pietro AD, d'Hardemare Adu M, Baubichon-Cortay H. Iodination of verapamil for a stronger induction of death, through GSH efflux, of cancer cells overexpressing MRP1. Bioorg. Med. Chem. 2010;18:6265–6274. doi: 10.1016/j.bmc.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Till JE, Ling V. Pleiotropic phenotype of colchicine-resistant CHO cells: cross-resistance and collateral sensitivity. J. Cell. Physiol. 1976;88:23–31. doi: 10.1002/jcp.1040880104. [DOI] [PubMed] [Google Scholar]
- Bell SE, Quinn DM, Kellett GL, Warr JR. 2-Deoxy-D-glucose preferentially kills multidrug-resistant human KB carcinoma cell lines by apoptosis. Br. J. Cancer. 1998;78:1464–1470. doi: 10.1038/bjc.1998.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley J, Quinn DM, Pitman RS, Warr JR, Kellett GL. The human KB multidrug-resistant cell line KB-C1 is hypersensitive to inhibitors of glycosylation. Cancer Lett. 1997;115:221–227. doi: 10.1016/s0304-3835(97)04739-3. [DOI] [PubMed] [Google Scholar]
- Berger W, Setinek U, Hollaus P, Zidek T, Steiner E, Elbling L, Cantonati H, Attems J, Gsur A, Micksche M. Multidrug resistance markers P-glycoprotein, multidrug resistance protein 1, and lung resistance protein in non-small cell lung cancer: prognostic implications. J. Cancer Res. Clin. Oncol. 2005;131:355–363. doi: 10.1007/s00432-004-0653-9. [DOI] [PubMed] [Google Scholar]
- Brough R, Frankum JR, Costa-Cabral S, Lord CJ, Ashworth A. Searching for synthetic lethality in cancer. Curr. Opin. Genet. Dev. 2011;21:34–41. doi: 10.1016/j.gde.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Broxterman HJ, Pinedo HM, Kuiper CM, Kaptein LC, Schuurhuis GJ, Lankelma J. Induction by verapamil of a rapid increase in ATP consumption in multidrug-resistant tumor cells. FASEB J. 1988;2:2278–2282. doi: 10.1096/fasebj.2.7.3350243. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. ABC drug transporters as molecular targets for the prevention of multidrug resistance and drug-drug interactions. Curr. Drug Deliv. 2007;4:324–333. doi: 10.2174/156720107782151241. [DOI] [PubMed] [Google Scholar]
- Callaghan R, Riordan JR. Collateral sensitivity of multidrug resistant cells to narcotic analgesics is due to effects on the plasma membrane. Biochim. Biophys. Acta. 1995;1236:155–162. doi: 10.1016/0005-2736(95)00042-2. [DOI] [PubMed] [Google Scholar]
- Cano-Gauci DF, Riordan JR. Action of calcium antagonists on multidrug resistant cells. Specific cytotoxicity independent of increased cancer drug accumulation. Biochem. Pharmacol. 1987;36:2115–2123. doi: 10.1016/0006-2952(87)90139-0. [DOI] [PubMed] [Google Scholar]
- Cole SP, Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol. Sci. 2006;27:438–446. doi: 10.1016/j.tips.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Fox E, Bates SE. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev. Anticancer Ther. 2007;7:447–459. doi: 10.1586/14737140.7.4.447. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Basu S, Chakraborty P, Chatterjee S, Sarkar A, Chatterjee M, Choudhuri SK. Targeting mitochondrial cell death pathway to overcome drug resistance with a newly developed iron chelate. PLoS One. 2010;5:e11253. doi: 10.1371/journal.pone.0011253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux-Bastide E, Lorendeau D, Nicolle E, Yahiaoui S, Magnard S, Di Pietro A, Baubichon-Cortay H, Boumendjel A. Identification of Xanthones as Selective Killers of Cancer Cells Overexpressing the ABC Transporter MRP1. ChemMedChem. 2011;6:1478–1484. doi: 10.1002/cmdc.201100102. [DOI] [PubMed] [Google Scholar]
- Goldsborough AS, Handley MD, Dulcey AE, Pluchino KM, Kannan P, Brimacombe KR, Hall MD, Griffiths G, Gottesman MM. Collateral Sensitivity of Multidrug-Resistant Cells to the Orphan Drug Tiopronin. J. Med. Chem. 2011;54:4987–4997. doi: 10.1021/jm2001663. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gottesman MM, Ambudkar SV, Xia D. Structure of a multidrug transporter. Nat. Biotechnol. 2009;27:546–547. doi: 10.1038/nbt0609-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Hall MD, Brimacombe KR, Varonka MS, Pluchino KM, Monda JK, Li J, Walsh MJ, Boxer MB, Warren TH, Fales HM, Gottesman MM. Synthesis and structure-activity evaluation of isatin-beta-thiosemicarbazones with improved selective activity towards multidrug-resistant cells expressing P-glycoprotein. J. Med. Chem. 2011;54:5878–5889. doi: 10.1021/jm2006047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MD, Handley MD, Gottesman MM. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol. Sci. 2009a;30:546–556. doi: 10.1016/j.tips.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MD, Salam NK, Hellawell JL, Fales HM, Kensler CB, Ludwig JA, Szakacs G, Hibbs DE, Gottesman MM. Synthesis, activity, and pharmacophore development for isatin-beta-thiosemicarbazones with selective activity toward multidrug-resistant cells. J. Med. Chem. 2009b;52:3191–3204. doi: 10.1021/jm800861c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- He YY, Huang JL, Ramirez DC, Chignell CF. Role of reduced glutathione efflux in apoptosis of immortalized human keratinocytes induced by UVA. J. Biol. Chem. 2003;278:8058–8064. doi: 10.1074/jbc.M207781200. [DOI] [PubMed] [Google Scholar]
- Helson L. Calcium channel blocker enhancement of anticancer drug cytotoxicity--a review. Cancer Drug Deliv. 1984;1:353–361. doi: 10.1089/cdd.1984.1.353. [DOI] [PubMed] [Google Scholar]
- Jansson PJ, Hawkins CL, Lovejoy DB, Richardson DR. The iron complex of Dp44mT is redox-active and induces hydroxyl radical formation: an EPR study. J Inorg Biochem. 2010;104:1224–1228. doi: 10.1016/j.jinorgbio.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Joly D, Rieu P, Mejean A, Gagnadoux MF, Daudon M, Jungers P. Treatment of cystinuria. Pediatr. Nephrol. 1999;13:945–950. doi: 10.1007/s004670050736. [DOI] [PubMed] [Google Scholar]
- Kaplan O, Jaroszewski JW, Clarke R, Fairchild CR, Schoenlein P, Goldenberg S, Gottesman MM, Cohen JS. The multidrug resistance phenotype: 31P nuclear magnetic resonance characterization and 2-deoxyglucose toxicity. Cancer Res. 1991;51:1638–1644. [PubMed] [Google Scholar]
- Kaplan O, Navon G, Lyon RC, Faustino PJ, Straka EJ, Cohen JS. Effects of 2-deoxyglucose on drug-sensitive and drug-resistant human breast cancer cells: toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Res. 1990;50:544–551. [PubMed] [Google Scholar]
- Kappus H, Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981;37:1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Karwatsky J, Lincoln MC, Georges E. A mechanism for P-glycoprotein-mediated apoptosis as revealed by verapamil hypersensitivity. Biochemistry. 2003;42:12163–12173. doi: 10.1021/bi034149+. [DOI] [PubMed] [Google Scholar]
- Keenan J, Liang Y, Clynes M. Two-deoxyglucose as an anti-metabolite in human carcinoma cell line RPMI-2650 and drug-resistant variants. Anticancer Res. 2004;24:433–440. [PubMed] [Google Scholar]
- Kelly RJ, Draper D, Chen CC, Robey RW, Figg WD, Piekarz RL, Chen X, Gardner ER, Balis FM, Venkatesan AM, Steinberg SM, Fojo T, Bates SE. A pharmacodynamic study of docetaxel in combination with the P-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer. Clin. Cancer Res. 2010;17:569–580. doi: 10.1158/1078-0432.CCR-10-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- Kuo TC, Chiang PC, Yu CC, Nakagawa-Goto K, Bastow KF, Lee KH, Guh JH. A unique P-glycoprotein interacting agent displays anticancer activity against hepatocellular carcinoma through inhibition of GRP78 and mTOR pathways. Biochem. Pharmacol. 2011;81:1136–1144. doi: 10.1016/j.bcp.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Laberge RM, Ambadipudi R, Georges E. P-glycoprotein (ABCB1) modulates collateral sensitivity of a multidrug resistant cell line to verapamil. Arch. Biochem. Biophys. 2009;491:53–60. doi: 10.1016/j.abb.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Le NT, Richardson DR. Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: a link between iron metabolism and proliferation. Blood. 2004;104:2967–2975. doi: 10.1182/blood-2004-05-1866. [DOI] [PubMed] [Google Scholar]
- Le NT, Richardson DR. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim. Biophys. Acta. 2002;1603:31–46. doi: 10.1016/s0304-419x(02)00068-9. [DOI] [PubMed] [Google Scholar]
- Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- Loe DW, Deeley RG, Cole SP. Verapamil stimulates glutathione transport by the 190-kDa multidrug resistance protein 1 (MRP1) J. Pharmacol. Exp. Ther. 2000;293:530–538. [PubMed] [Google Scholar]
- Ludwig JA, Szakacs G, Martin SE, Chu BF, Cardarelli C, Sauna ZE, Caplen NJ, Fales HM, Ambudkar SV, Weinstein JN, Gottesman MM. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006;66:4808–4815. doi: 10.1158/0008-5472.CAN-05-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon RC, Cohen JS, Faustino PJ, Megnin F, Myers CE. Glucose metabolism in drug-sensitive and drug-resistant human breast cancer cells monitored by magnetic resonance spectroscopy. Cancer Res. 1988;48:870–877. [PubMed] [Google Scholar]
- Marks KM, Park ES, Arefolov A, Russo K, Ishihara K, Ring JE, Clardy J, Clarke AS, Pelish HE. The selectivity of austocystin D arises from cell-line-specific drug activation by cytochrome P450 enzymes. J. Nat. Prod. 2011;74:567–573. doi: 10.1021/np100429s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Bailly JD, Goubin F, Laredo J, Jaffrezou JP, Bordier C, Laurent G. Verapamil decreases P-glycoprotein expression in multidrug-resistant human leukemic cell lines. Int. J. Cancer. 1994;56:749–754. doi: 10.1002/ijc.2910560523. [DOI] [PubMed] [Google Scholar]
- Naito M, Tsuruo T. Competitive inhibition by verapamil of ATP-dependent high affinity vincristine binding to the plasma membrane of multidrug-resistant K562 cells without calcium ion involvement. Cancer Res. 1989;49:1452–1455. [PubMed] [Google Scholar]
- Nakagawa-Goto K, Bastow KF, Chen TH, Morris-Natschke SL, Lee KH. Antitumor agents 260. New desmosdumotin B analogues with improved in vitro anticancer activity. J. Med. Chem. 2008;51:3297–3303. doi: 10.1021/jm701208v. [DOI] [PubMed] [Google Scholar]
- Nakagawa-Goto K, Chang PC, Lai CY, Hung HY, Chen TH, Wu PC, Zhu H, Sedykh A, Bastow KF, Lee KH. Antitumor agents. 280. Multidrug resistance-selective desmosdumotin B analogues. J. Med. Chem. 2010;53:6699–6705. doi: 10.1021/jm100846r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili S, Landini I, Mazzei T, Mini E. Overcoming tumor multidrug resistance using drugs able to evade P-glycoprotein or to exploit its expression. Med. Res. Rev. 2011 doi: 10.1002/med.20239. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Perrotton T, Trompier D, Chang XB, Di Pietro A, Baubichon-Cortay H. (R)- and (S)-verapamil differentially modulate the multidrug-resistant protein MRP1. J. Biol. Chem. 2007;282:31542–31548. doi: 10.1074/jbc.M703964200. [DOI] [PubMed] [Google Scholar]
- Prisant LM. Verapamil revisited: a transition in novel drug delivery systems and outcomes. Heart Dis. 2001;3:55–62. doi: 10.1097/00132580-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Robey RW, Ierano C, Zhan Z, Bates SE. The challenge of exploiting ABCG2 in the clinic. Curr. Pharm. Biotechnol. 2010;12:595–608. doi: 10.2174/138920111795163913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Szybalski W, Bryson V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J. Bacteriol. 1952;64:489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran VH, Marks D, Duke RK, Bebawy M, Duke CC, Roufogalis BD. Modulation of P-glycoprotein-mediated anticancer drug accumulation, cytotoxicity, and ATPase activity by flavonoid interactions. Nutr. Cancer. 2011;63:435–443. doi: 10.1080/01635581.2011.535959. [DOI] [PubMed] [Google Scholar]
- Trompier D, Chang XB, Barattin R, du Moulinet D'Hardemare A, Di Pietro A, Baubichon-Cortay H. Verapamil and its derivative trigger apoptosis through glutathione extrusion by multidrug resistance protein MRP1. Cancer Res. 2004;64:4950–4956. doi: 10.1158/0008-5472.CAN-04-0143. [DOI] [PubMed] [Google Scholar]
- Tsuruo T, Iida H, Nojiri M, Tsukagoshi S, Sakurai Y. Circumvention of vincristine and Adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res. 1983;43:2905–2910. [PubMed] [Google Scholar]
- Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–1972. [PubMed] [Google Scholar]
- Turk D, Hall MD, Chu BF, Ludwig JA, Fales HM, Gottesman MM, Szakacs G. Identification of compounds selectively killing multidrug-resistant cancer cells. Cancer Res. 2009;69:8293–8301. doi: 10.1158/0008-5472.CAN-09-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr JR, Anderson M, Fergusson J. Properties of verapamil-hypersensitive multidrug-resistant Chinese hamster ovary cells. Cancer Res. 1988;48:4477–4483. [PubMed] [Google Scholar]
- Warr JR, Brewer F, Anderson M, Fergusson J. Verapamil hypersensitivity of vincristine resistant Chinese hamster ovary cell lines. Cell. Biol. Int. Rep. 1986;10:389–399. doi: 10.1016/0309-1651(86)90011-1. [DOI] [PubMed] [Google Scholar]
- Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc. Natl. Acad. Sci. U S A. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV. Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter. Mol. Cancer Ther. 2007;6:3287–3296. doi: 10.1158/1535-7163.MCT-07-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–621. [PubMed] [Google Scholar]
- Yuan J, Lovejoy DB, Richardson DR. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment. Blood. 2004;104:1450–1458. doi: 10.1182/blood-2004-03-0868. [DOI] [PubMed] [Google Scholar]
- Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38:802–832. doi: 10.1080/00498250701867889. [DOI] [PubMed] [Google Scholar]