Abstract
Objective
To examine whether late-life depression, including depressive symptoms and antidepressant use, was associated with smaller total brain volume, smaller hippocampal volume, and larger white matter hyperintensity volume in a large community-based cohort of old persons without dementia.
Methods
Within the Washington/Hamilton Height-Inwood Columbia Aging Project (WHICAP), a community-based cohort study in northern Manhattan, 630 persons without dementia (mean age 80 years, SD=5) had volumetric measures of the total brain, hippocampus, and white matter hyperintensities (WMH) at 1.5Tesla MRI and data on current depression, defined as a score of 4 or higher on the 10-item CES-D or use of antidepressants.
Results
Multiple linear regression analyses adjusted for age, sex, ethnicity, education, cardiovascular disease history, and MRI parameters showed that subjects with current depression had smaller relative total brain volume (B=−0.86%; 95%CI −1.68 to −0.05%; p<0.05), smaller relative hippocampal volume (B=−0.07 ml; 95%CI −0.14 to 0.00 ml; p=0.05), and larger relative WMH volume (natural logtransformed B=0.19 ml; 95%CI 0.02 to 0.35 ml; p<0.05). When examined separately, antidepressant use was significantly associated with smaller total brain, smaller hippocampal, and larger WMH volume, while high CES-D scores were not significantly associated with any of the brain measures, although the direction of association was similar as for antidepressant use.
Discussion
With the caveat that analyses were cross-sectional and we had no formal diagnosis of depression, our findings suggest that in this community-based sample of old persons without dementia, late-life depression is associated with more brain atrophy and more white matter lesions, which was mainly driven by antidepressant use.
Keywords: brain, hippocampus, white matter hyperintensity, depressive symptoms, antidepressants, elderly, population-based, cohort, MRI
INTRODUCTION
At older age, depressive symptoms are common[1] and often persist over years.[2] Dementia is also common at older age, and may affect 40% of people aged 85 years and over.[3] It has been estimated that 30–50% of patients with dementia also experience depression and this co-occurrence of depression and dementia results in more adverse consequences for both patient and caregivers.[4] A number of studies have shown that depression, and in particular depression early in life, may also increase the risk of developing dementia, including Alzheimer’s disease.[5–8] Results with respect to late life depression are less consistent although the majority of studies report an increased risk of dementia.[8] Still, the neurobiological substrate of this relation remains poorly understood. Several explanations have been put forward including depression being an early symptom of dementia, depression lowering the threshold of detecting dementia, neurodegenerative pathology causing both depression and dementia, and depression being a true risk factor for dementia.[9] One of the frequently proposed explanations for depression being a risk factor for AD is that hippocampal atrophy may occur as a result of hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis[10,11] although a number of studies indicate that this hypothesis is not correct.[12–14] However, late life depressive symptoms may also increase risk for dementia through its association with cerebral small vessel disease.[15–20] Compared to the number of studies that reported on the relation between late life depression and risk of dementia and on the relation between late life depression and cerebral small vessel disease, relatively few population-based studies examined the relation between late life depressive symptoms and volume of the hippocampus.[21–23] and these studies did not find a relation between depressive symptoms and hippocampal volume, although one prospective study found that depressive symptoms were related to a faster decline in hippocampal volume.[24]
The aim of this study was to examine the association of late life depression, including depressive symptoms and antidepressant use, with hippocampal volume, total brain volume, and white matter hyperintensity volume on MRI, in a large community-based cohort of nondemented persons with a mean age of 80 years, a group particularly at high risk for developing dementia. We hypothesize that late life depression is associated with smaller total brain and smaller hippocampal volumes and with larger white matter hyperintensity volume.
MATERIALS AND METHODS
Subjects
Data were included from 769 individuals participating in a prospective study of aging and dementia in Medicare-eligible northern Manhattan residents, age 65 years and older (Washington/Hamilton Heights-In wood Columbia Aging Project: WHICAP). [25] The population from which participants were drawn comprises individuals from three broadly defined ethnic categories (i.e., Hispanic, African American, and non-Hispanic White). Participants have been followed at approximately 18–24-month intervals with similar assessments at each interval. The imaging project was concurrent with the 2005–2007 follow-up of the WHICAP cohort. Details of the imaging project have been described previously.[26] In brief, participants were eligible for MRI if they did not meet criteria for dementia at the most recent visit before MRI assessment. Recruitment, informed consent and study procedures were approved by the Institutional Review Boards of Columbia Presbyterian Medical Center and Columbia University Health Sciences and the New York State Psychiatric Institute.
At each assessment, participants underwent an in-person interview of general health and functional ability followed by a semi-structured standardized assessment, including medical history, physical and neurological examination, and a neuropsychological test battery.[27] All participants received structured neurologic, medical and functional assessments. The diagnosis of dementia was based on standard research criteria [28] and was established using all available information (except the MRI results) gathered from the initial and follow-up assessments and medical records at a consensus conference. The diagnostic consensus conference comprises physicians and neuropsychologists, with extensive experience in diagnosis of dementia. It is typically attended by two neurologists and a neuropsychologist. The committee reviews all the available functional, medical, neurological, psychiatric, and neuropsychological data collected during the visit. Data from previous visits and from neuroimaging are not considered for diagnosis. First, the conference determines whether the participant meets criteria for dementia, based on DSM-IV criteria [28]. The diagnosis derived at the consensus conference is based on consistent operational definitions for dementia and neuropsychological paradigm requirements for cognitive deficit [27]. If a participant meets criteria for dementia, the putative etiology is determined using published research criteria.
MRI protocol
Scan acquisition was performed on a 1.5T Philips Intera scanner at Columbia University Medical Center and transferred electronically to the university of California at Davis for morphometric analysis in the Imaging of Dementia and Aging Laboratory. For measures of total brain volume and white matter lesion volume, axial fluid attenuated inverse recovery (FLAIR) weighted images (TR=11,000 ms, TE=144.0 ms, 2800 inversion time, flip angle=20, FOV 25 cm, 2 nex, 256×192 matrix with 3 mm slice thickness, no gap, voxel size 1.30×1.6 mm in plane) were acquired. T1-weighted images acquired in the axial plane and re-sectioned coronally were used to quantify hippocampal volumes (TR=20 ms, TE = 2.1 ms, flip angle=20; FOV 240 cm, 256×160 matrix with 1.3 mm slice thickness, no gap, voxel size 0.94×1.17 mm in plane).
Brain volume and white matter hyperintensities
Total brain and white matter hyperintensity (WMH) volumes were derived on FLAIR-weighted images following a two-step process, as previously described.[29] First, an operator manually traced the dura mater within the cranial vault, including the middle cranial fossa but not the posterior fossa and cerebellum. Intracranial volume was defined as the number of voxels contained within the manual tracings, multiplied by voxel dimensions and slice thickness. These manual tracings also defined the border between brain and non-brain elements and permitted for the removal of the latter. Nonuniformities in image intensity were removed [30] and two Gaussian probability functions, representing brain matter and cerebrospinal fluid (CSF), were fitted to the skull-stripped image.[29,30] WMH volume was calculated as the sum of voxels greater to or equal to 3.5 SD above the mean intensity value of the image and multiplied by voxel dimensions and slice thickness.[29–31] Similarly, total brain volume was the sum of voxels designated as brain volume from the segmentation process.
Hippocampus
Boundaries for the hippocampus were manually traced from the coronal 3D-T1 weighted images after reorientation along the axis of the left hippocampus.[32] The rostral end of the hippocampus was identified using the sagittal view to distinguish between amygdala and the head of the hippocampus. In anterior sections, the superior boundary of the hippocampus was the amygdala. In sections in which the uncus lies ventral to caudal amygdala, the uncus was included in the hippocampus. In more posterior sections that do not contain amygdala, the hippocampal (choroid) fissure and the superior portion of the inferior horn of the lateral ventricle formed the superior boundary. The fimbria were excluded from the superior boundary of the hippocampus. The inferior boundary of the hippocampus was the white matter of the parahippocampal gyrus. The lateral boundary was the inferior (temporal) horn of the lateral ventricle, taking care in posterior sections to exclude the tail of the caudate nucleus. The posterior boundary of the hippocampus was the first slice in which the fornices were completely distinct from any gray/white matter of the thalamus. Intra-rater reliability determined for both right and left hippocampus using this method was good with intraclass correlation coefficients of 0.98 for right hippocampus and 0.96 for left hippocampus.[32]
Depressive symptoms, antidepressant use, and depression
Current depressive symptoms were assessed with the 10-item version of the Center for Epidemiologic Studies-Depression (CES-D) scale.[33] The conventional cutoff score of 4 or higher was used to indicate presence of depressive symptoms.[33] Current use of antidepressants was assessed during the physician interview. The subject was asked to show all medication to the interviewer. Current depression was defined as having either depressive symptoms (CES-D score of 4 or higher) or taking antidepressants.[6,19]
Other variables
Ethnic group was determined by self-report using the format of the 2000 US Census. All individuals were first asked to report their race (American Indian/Alaska Native, Asian, Native Hawaiian or other Pacific Islander, Black or African American, or White), then, in a second question, were asked whether or not they were Hispanic. Other covariates included the number of years of formal education, the sums core of vascular disease history variables, and presence of infarcts on MRI. The sums core of vascular disease variables consisted of self-reported history of diabetes mellitus, hypertension, heart disease, and clinical stroke. History of heart disease included arrhythmias (eg, a trial fibrillation), coronary artery disease, and congestive heart failure. Stroke was defined according to the World Health Organization criteria based on self-report, supplemented by a neurologic examination. These 4 dichotomous variables were summed to create a vascular disease history variable (score range, 0–4).[32]Infarcts were determined as previously described.[34] Briefly, the presence or absence of infarct was determined visually on all available imaging modalities based on lesions that were greater than 3mm in its maximum diameter.
Study sample
Of the 769 participants with MRI data, 49 met diagnostic criteria for dementia at the clinical evaluation closest to the neuroimaging study. These individuals were excluded from the current analyses. Of the 720 participants without dementia, data on CES-D score or antidepressant medication were missing in 90 participants, leaving 630 participants for analysis. The 90 participants with missing data on depression were not significantly different on demographics or clinical variables from the 630 participants included in the analyses.
Data analysis
Multiple linear regression analysis and ANCOVA were performed to estimate the associations of current depression with total brain volume, hippocampal volume (left, right, and mean of left and right), and WMH volume (natural log transformed), as dependent variables. Total brain volume was expressed relative to intracranial volume, as percentage. Hippocampal and WMH volumes were normalized for intracranial volume and expressed as ml, by dividing the volumes by intracranial volume and multiplying this by the average intracranial volume of the study population (1132 ml) to obtain a relative measure in ml. All analyses were adjusted for age, gender, ethnicity, years of education, cardiovascular disease history sumscore, WMH volume, and presence of infarcts on MRI where brain volumes were the dependent variable, and total brain volume instead of WMH where WMH volume was the dependent variable. We repeated all analyses with CES-D score of 4 or higher and antidepressant use as separate variables to explore the relative contribution of these variables in the associations.
RESULTS
Of the total study sample (n=630) 13% had a CES-D score above cutoff and 10% used antidepressants; 125 persons (20%) had current depression according to our definition of a high CES-D score or use of antidepressants. The mean CES-D score in those with depression was 3.7 (SD 2.1) and 0.8 (SD 0.9) in those without depression. Persons with current depression had the same mean age (80 years) as those without depression (Table 1).
Table 1.
Characteristics of study sample (n=630) according to depression status*
| No depression N=505 |
Depression N=125 |
Total sample N=630 |
|
|---|---|---|---|
| Age, years (mean ± SD) | 80 ± 5 | 80 ± 6 | 80 ± 5 |
| Female (%) | 66 | 78 | 68 |
| Ethnicity (%) | |||
| White | 28 | 30 | 29 |
| Black | 38 | 19 | 34 |
| Hispanic | 32 | 50 | 36 |
| Other | 2 | 1 | 2 |
| Years of education (mean ± SD) | 11 ± 5 | 10 ± 5 | 11 ± 5 |
| Hypertension, % | 72 | 69 | 71 |
| Diabetes Mellitus, % | 24 | 26 | 24 |
| Heart disease, % | 23 | 26 | 23 |
| Stroke signs/symptoms, % | 10 | 14 | 11 |
| Intracranial volume (ml) | 1136 ± 125 | 1113 ± 111 | 1132 ± 123 |
| Total brain volume (ml) | 829 ± 96 | 808 ± 84 | 825 ± 94 |
| Total hippocampal volume (ml) | 3.3 ± 0.7 | 3.1 ± 0.7 | 3.3 ± 0.7 |
| Left hippocampal volume (ml) | 1.6 ± 0.3 | 1.5 ± 0.3 | 1.6 ± 0.3 |
| Right hippocampal volume (ml) | 1.7 ± 0.4 | 1.6 ± 0.4 | 1.6 ± 0.4 |
| Total white matter hyperintensity volume (ml), median (10–90%) | 9 (3 – 30) | 11 (3 – 36) | 9 (3 – 32) |
| Infarcts present on MRI | 31 | 34 | 32 |
| CES-D score (mean ± SD) | 0.8 ± 0.9 | 3.7 ± 2.1 | 1.3 ± 1.7 |
| CES-D 4 or higher, % | 0 | 66 | 13 |
| Antidepressant use, % | 0 | 53 | 10 |
Depression was defined as a score of 4 or higher on the short version of the CES-D or use of antidepressant medication.
Numbers are presented as mean ± SD or %, unless otherwise specified.
Hippocampal volume and white matter hyperintensity volume are presented as absolute volumes.
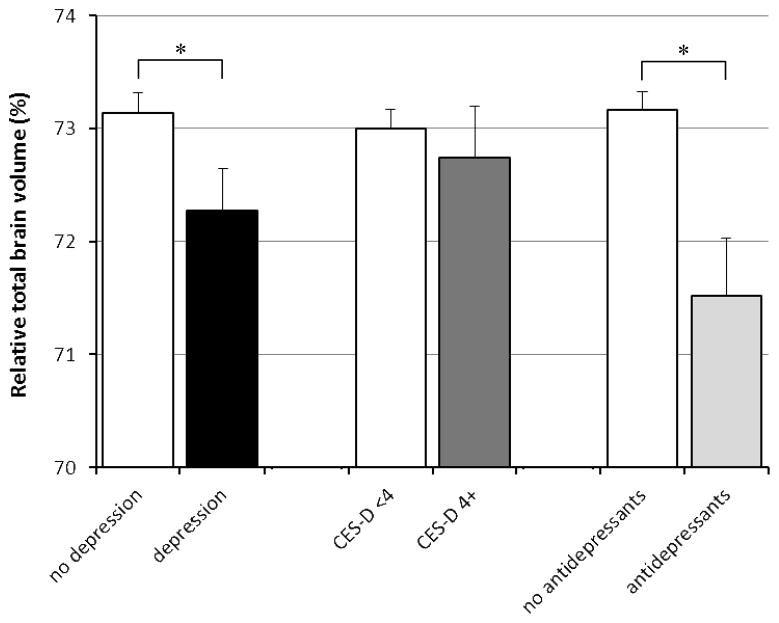
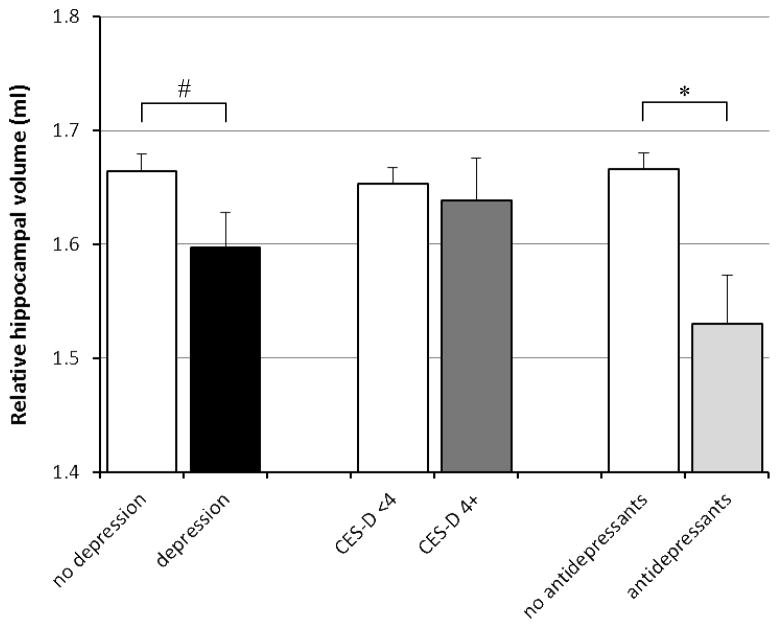
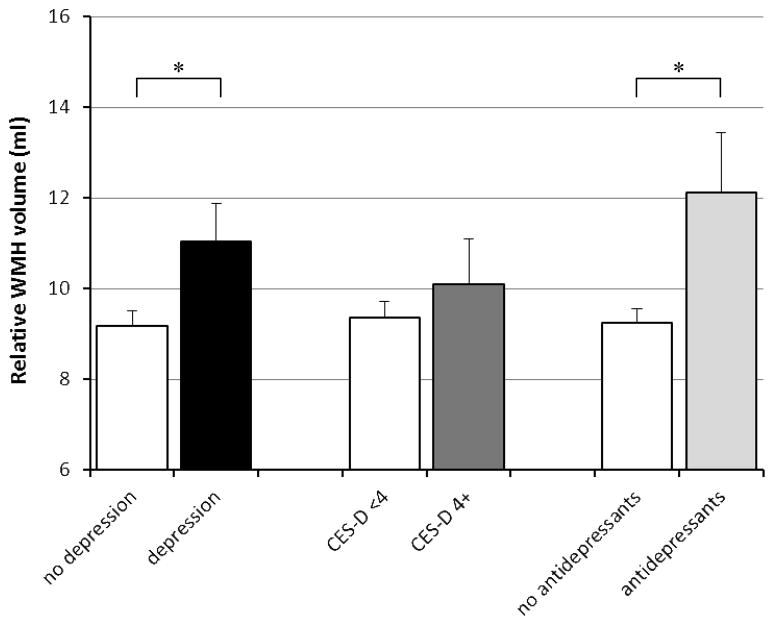
Linear regression analyses showed that compared with persons without depression, those with depression had a 0.86% (95% CI 0.05 to 1.68%) smaller relative total brain volume after adjusting for age, gender, ethnicity, years of education, cardiovascular disease history sumscore, WMH volume, and presence of infarcts on MRI (Table 2, Figure 1). Depression was also associated with smaller hippocampal volume after adjustments (Table 2, Figure 2). When we differentiated left and right hippocampus, depression was associated with 0.053 ml smaller left hippocampus (95% CI −0.12 to 0.016 ml) and with 0.081 ml smaller right hippocampus (95% CI −0.15 to −0.01 ml). Furthermore, depression was associated with larger white matter hyperintensity volume (Table 2, Figure 3).
Table 2.
Results from the linear regression analyses of the association of depression measures with brain atrophy measures and WML
| Relative total brain volume (%) B (95% CI) |
Relative hippocampal volume (mean of left and right) (ml) B (95% CI) |
Relative white matter lesion volume (log transformed) (ml) B (95% CI) |
|
|---|---|---|---|
| Depression (yes vs. no)* | −0.86 (−1.68 to −0.05) ¥ | −0.07 (−0.14 to 0.00)§ | 0.19 (0.02 to 0.35) ¥ |
| CES-D score (4+ vs. <4) | −0.27 (−1.24 to 0.70) | −0.02 (−0.10 to 0.07) | 0.08 (−0.12 to 0.27) |
| CES-D score (per point increase) | −0.13 (−0.32 to 0.06) | −0.003 (−0.02 to 0.01) | 0.025 (−0.01 to 0.06) |
| Antidepressant use (yes vs. no) | −1.64 (−2.71 to −0.57) ¥ | −0.14 (−0.23 to −0.05) ¥ | 0.27 (0.05 to 0.49) ¥ |
Depression was defined as a score of 4 or higher on the short version of the CES-D or use of antidepressant medication.
Models are adjusted for age, gender, ethnicity, years of education, sumscore of hypertension/diabetes/heart disease/stroke signs or symptoms, stroke on MRI, and white matter hyperintensity volume (when total brain and hippocampus are the dependent variable) or total relative brain volume (when white matter hyperintensity volume is the dependent variable).
p <0.05
p=0.05
Figure 1.
Relative total brain volume according to presence of depression, as defined as a high CES-D score or antidepressant use, CES-D score only, and antidepressant use only. Mean volumes are adjusted for age, gender, ethnicity, years of education, sumscore of hypertension/diabetes/heart disease/stroke signs or symptoms, stroke on MRI, and white matter hyperintensity volume. Error bars represent standard errors.
* indicates statistically significant difference (p<0.05).
Figure 2.
Mean hippocampal volume (mean of left and right hippocampus) according to presence of depression, as defined as a high CES-D score or antidepressant use, CES-D score only, and antidepressant use only. Mean volumes are adjusted for age, gender, ethnicity, years of education, sumscore of hypertension/diabetes/heart disease/stroke signs or symptoms, stroke on MRI, and white matter hyperintensity volume. Error bars represent standard errors.
* indicates statistically significant difference (p<0.05).
# p=0.05
Figure 3.
Mean relative white matter hyperintensity volume according to presence of depression, as defined as a high CES-D score or antidepressant use, CES-D score only, and antidepressant use only. Mean volumes are adjusted for age, gender, ethnicity, years of education, sumscore of hypertension/diabetes/heart disease/stroke signs or symptoms, stroke on MRI, and white matter hyperintensity volume. Error bars represent standard errors.
* indicates statistically significant difference (p<0.05).
When we analyzed CES-D score and antidepressant use separately, a high CES-D score (4 or higher) or CES-D score per point increase were not associated with total brain volume, but antidepressant use was associated with smaller relative total brain volume (B = −1.64%; 95% CI −2.71 to −0.57%; p<0.05) (Table 2, Figure 1). Also, antidepressant use was associated with smaller relative mean hippocampal volume (B= −0.14 ml; 95% CI −0.23 to −0.05 ml; p<0.05), left hippocampal volume (B=−0.12 ml; 95% CI −0.21 to −0.03 ml; p<0.05) and right hippocampal volume (B=−0.15 ml; 95% CI −0.24 to −0.06 ml; p<0.05), while CES-D score (analyzed as a dichotomous or as a continuous variable) was not (Table 2, Figure 2). Similarly, a high CES-D score (4 or higher vs. <4) or CES-D score per point increase were not associated with WMH volume, while antidepressant use was significantly associated with more WMH (Table 2, Figure 3).
DISCUSSION
In a large community-based cohort of elderly people without dementia we observed that current depression as defined as a high CES-D score or antidepressant use was associated with more global brain atrophy, more atrophy of the hippocampus and with more white matter hyperintensities. When examined separately, the associations between current depression and brain measures was explained by antidepressant use, while CES-D score itself was not significantly associated with the brain outcomes.
Our finding of a relation of antidepressant use with smaller brain volumes is inconsistent with findings from animal studies that suggest that antidepressants increase neurogenesis[35] although a recent study in old mice showed that fluoxetine did not enhance hippocampal neurogenesis.[36] An intuitively more likely explanation for our findings is that antidepressant use was a proxy for depressive symptoms’ severity, and that only more severe depression is associated with structural brain abnormalities. However, when we looked at severity of depressive symptoms using the CES-D score as a continuous variable, we found no association with any of the brain measures, although the direction of association was the same as for antidepressant use. Also, at older age, antidepressants are prescribed for other symptoms than depressive symptoms alone, and the association we observed may not reflect an association with depressive symptoms but with another psychological or physical complaint. A speculative hypothesis to explain our findings is that antidepressants were prescribed for elderly with depressive complaints or memory complaints associated with mild cognitive impairment or preclinical dementia, in the absence of clinical major depressive disorder. Antidepressants would then not lead to brain volume loss, but mild cognitive impairment associated with brain volume loss would lead to memory complaints and depressive symptoms, which may have been an indication for prescribing antidepressants if elderly presented with these complaints in primary care or memory clinic. More studies are needed with detailed data on type, dose, duration, and prescription indication of antidepressants to determine whether or not antidepressants are detrimental for the brain.
A relatively large number of studies examined the prospective association of late life depression/depressive symptoms with risk of developing dementia (for reviews see[5,8,9]) and the majority of studies, although not all, confirmed that depression increases the risk of developing dementia. Studies on early onset depression are less often performed, but these are more consistent in finding an increased for developing dementia.[8] The underlying mechanisms by which depression may increase risk for dementia are still not well understood however. One of the frequently proposed explanations is that prolonged exposure to glucocorticoids with repeated depressive episodes leads to hippocampal atrophy and subsequent dementia.[10,37] Although a recent large-scale cohort study found that higher basal cortisol levels and reduced suppression after dexamethasone were associated with smaller hippocampal volumes[11], this could not explain the observed relation between early onset depression and smaller hippocampal volumes in the same cohort.[14] Also, several cohort studies examining late life depressive symptoms and volume of the hippocampus did not find a relation between depressive symptoms and hippocampal volume, [21–23] although one prospective study found that depressive symptoms were related to a faster decline in hippocampal volume.[24] These studies did not examine antidepressant use separately however. In the present study, we observed an association of late life depression with smaller hippocampal volume, but this finding was not specific for hippocampal volume, because the association was also present for total brain volume. Morover, when we differentiated between high CES-D scores and antidepressant use, we also did not find an association between high CES-D scores and hippocampal volumes, which is consistent with these previous findings. Alternatively, late life depression may increase risk of dementia through cerebrovascular lesions.[15,18,19,38] The findings of our study are inconclusive because, similarly to the findings for hippocampal volume, antidepresant use and not CES-D score itself was associated with more white matter hyperintensities.
Strengths of this study are the community-based cohort design and the large number of participants included. Further, we used volumetric assessment of brain measures and WMH which provides more precise and objective estimates than visual rating scales[39] and we corrected for the co-occurrence of brain atrophy, WMH and cerebral infarcts.
A limitation of this study is the cross-sectional design, which does not allow to discern cause from consequence. Also, although we excluded persons with dementia, because of the old age of the study sample a proportion may have had preclinical dementia. Another limitation is that we did not have a DSM-IV diagnosis of major depressive disorder, although we tried to identify participants with clinically relevant depressive symptoms by including persons with high symptom scores and those taking antidepressants, irrespective of symptom level. Another limitation is that data on type, dosage, and duration of antidepressants was not available.
In conclusion, with the caveat that analyses were cross-sectional and we had no formal diagnosis of depression, our findings suggest that in this community-based sample of old persons without dementia, late-life depression is associated with more brain atrophy and more white matter lesions, which was mainly driven by antidepressant use.
Acknowledgments
Funding
This work was supported by grants PO1-AG07232, AG029949 and AG17761 from the National Institutes of Health. Dr Geerlings was supported by a grant from the University Medical Center Utrecht (Program Internationalisation) and the Netherlands Organization for Scientific Research (NWO: project No. 917-66-311).
We gratefully acknowledge Charles DeCarli, MD and coworkers from the Alzheimer’s Disease Center and Imaging of Dementia and Aging (IDeA) Laboratory, Department of Neurology and Center for Neuroscience, University of California at Davis, for their work on the MRI scans.
References
- 1.Beekman AT, Deeg DJ, van Tilburg T, Smit JH, Hooijer C, Van Tilburg W. Major and minor depression in later life: a study of prevalence and risk factors. J Affect Disord. 1995;36:65–75. doi: 10.1016/0165-0327(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 2.Beekman AT, Geerlings SW, Deeg DJ, Smit JH, Schoevers RS, de Beurs E, Braam AW, Penninx BW, Van Tilburg W. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry. 2002;59:605–611. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]
- 3.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 4.Starkstein SE, Jorge R, Mizrahi R, Robinson RG. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry. 2005;162:2086–2093. doi: 10.1176/appi.ajp.162.11.2086. [DOI] [PubMed] [Google Scholar]
- 5.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology. 2010;75:35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic- pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–34. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 11.Knoops AJ, Gerritsen L, van der Graaf Y, Mali WP, Geerlings MI. Basal hypothalamic pituitary adrenal axis activity and hippocampal volumes: the SMART-Medea study. Biol Psychiatry. 2010;67:1191–1198. doi: 10.1016/j.biopsych.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 13.Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJ, Penninx BW, Geerlings MI. Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes--the SMART Medea study. Biol Psychiatry. 2011;70:373–380. doi: 10.1016/j.biopsych.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 15.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MMB. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57:1071–6. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 16.Steffens DC, Krishnan KR, Crump C, Burke GL. Cerebrovascular disease and evolution of depressive symptoms in the cardiovascular health study. Stroke. 2002;33:1636–1644. doi: 10.1161/01.str.0000018405.59799.d5. [DOI] [PubMed] [Google Scholar]
- 17.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 18.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Godin O, Dufouil C, Maillard P, Delcroix N, Mazoyer B, Crivello F, Alperovitch A, Tzourio C. White matter lesions as a predictor of depression in the elderly: the 3C-Dijon study. Biol Psychiatry. 2008;63:663–669. doi: 10.1016/j.biopsych.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Herrmann LL, Le MM, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 21.Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70:1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 22.Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. 2009;34:367–375. [PMC free article] [PubMed] [Google Scholar]
- 23.Goveas JS, Espeland MA, Hogan P, Dotson V, Tarima S, Coker LH, Ockene J, Brunner R, Woods NF, Wassertheil-Smoller S, Kotchen JM, Resnick S. Depressive symptoms, brain volumes and subclinical cerebrovascular disease in postmenopausal women: the Women’s Health Initiative MRI Study. J Affect Disord. 2011;132:275–284. doi: 10.1016/j.jad.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.den Heijer T, Tiemeier H, Luijendijk HJ, van der Lijn F, Koudstaal PJ, Hofman A, Breteler MM. A study of the bidirectional association between hippocampal volume on magnetic resonance imaging and depression in the elderly. Biol Psychiatry. 2011;70:191–197. doi: 10.1016/j.biopsych.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 26.Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, Mayeux R. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49:453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16:274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 30.DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. J Magn Reson Imaging. 1996;6:519–528. doi: 10.1002/jmri.1880060316. [DOI] [PubMed] [Google Scholar]
- 31.DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 32.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch Intern Med. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 34.Reitz C, Schupf N, Luchsinger JA, Brickman AM, Manly JJ, Andrews H, Tang MX, DeCarli C, Brown TR, Mayeux R. Validity of self-reported stroke in elderly African Americans, Caribbean Hispanics, and Whites. Arch Neurol. 2009;66:834–840. doi: 10.1001/archneurol.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanis KQ, Newton SS, Duman RS. Targeting neurotrophic/growth factor expression and signaling for antidepressant drug development. CNS Neurol Disord Drug Targets. 2007;6:151–160. doi: 10.2174/187152707780363276. [DOI] [PubMed] [Google Scholar]
- 36.Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, Bogdahn U, Aigner L. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry. 2009;14:856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- 37.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan MS, O’Brien JT, Firbank MJ, Pantoni L, Carlucci G, Erkinjuntti T, Wallin A, Wahlund LO, Scheltens P, van Straaten EC, Inzitari D. Relationship between periventricular and deep white matter lesions and depressive symptoms in older people. The LADIS Study. Int J Geriatr Psychiatry. 2006;21:983–989. doi: 10.1002/gps.1596. [DOI] [PubMed] [Google Scholar]
- 39.van den Heuvel DMJ, ten Dam VH, de Craen AJM, Admiraal-Behloul F, van Es ACGM, Palm WM, Spilt A, Bollen ELEM, Blauw GJ, Launer L, Westendorp RGJ, van Buchem MA on behalf of the PROSPER Study Group. Measuring Longitudinal White Matter Changes: Comparison of a Visual Rating Scale with a Volumetric Measurement. AJNR Am J Neuroradiol. 2006;27:875–878. [PMC free article] [PubMed] [Google Scholar]