Abstract
Objective
To evaluate the effects of aqueous extract of Carthamus tinctorius L., also named safflower, on mouse spermatogenesis.
Methods
Sixteen adult male NMRI mice were used. Experimental group received Carthamus tinctorius L. extract at the dose of 200 mg/kg for 35 consecutive days and control group received only distilled water. Testicular histopathology, morphometric analysis and spermatogenesis assessments were performed for evaluation of the Carthamus tinctorius L. extract effects on testis.
Results
Histopathological criteria such as epithelial vacuolization, sloughing of germ and detachment were significantly decreased in Carthamus tinctorius L. treated mice (p < 0.001). Carthamus tinctorius L. extract induced formation of multinucleated giant cells in the germinal epithelium. Carthamus tinctorius L. extract also caused a significant decrease in seminiferous tubule diameter, seminiferous epithelium height and maturation arrest (p < 0.001).
Conclusion
Carthamus tinctorius L. extract has toxic effects on mouse testicular tissue, and recommended to use it with caution if there is a reproductive problem.
Keywords: Spermatogenesis, Carthamus tinctorius L. (safflower) extract, Mice
Introduction
Spermatogenesis is an elaborate process of germ cell proliferation and differentiation which leads to the production and release of spermatozoa from the testis. This complex process is dependent upon hormonal stimulation as well as dynamic interactions between the Sertoli cells and the germ cells of the seminiferous epithelium [1, 2]. Sertoli cells secrete hormonal and nutritive factors into the adluminal compartment which create a specialized microenvironment that fosters the development and viability of resident germ cells. In addition, Sertoli cells form sites of attachment to germ cells that provide efficient paracrine signalling mechanism between these cells as well as physical support to developing germ cells [3]. The intricate regulation and cellular interactions that occur in the testis provide multiple distinct targets by which toxicants can disrupt spermatogenesis [2].
Herbal toxicity possess serious threats to human health and represents an important issue to be tackled [4]. It is necessary that health care professionals as well as patients are aware of potential herbal toxicity and researchers should strive to fill the numerous gaps in our present understanding of this problem.
Safflower, Carthamus tinctorius L. (CT), is a member of the family Compositae or Asteraceae, cultivated mainly for its seed, which is used as edible oil and as birdseed. Traditionally, the crop was grown for its flowers, used for colouring and flavouring foods and making dyes, and in medicines [5]. It is cheaper than saffron and therefore a water extract of this flower is used instead of saffron. CT has wide pharmacological and biological activities including cardioprotective [6], neuroprotective [7] and antitumor activity [8]. Previous studies demonstrate that CT is toxic and causes renal and brain tissue damage [9, 10]. However, the side effects of this plant have not been investigated in spermatogenesis.
Since CT is common used in food industry, it seems essential to study the eventual toxic effects of this plant on various tissues. This study was designed to investigate the eventual effects of this plant on the mouse testicular tissue.
Materials and methods
In this study, sixteen healthy and adult male NMRI (Naval Medical Research Institute) mice (6–8 weeks old, 25–30 g) were used. The animals were obtained from Ahvaz Jundishapur University of Medical Sciences, Experimental Research Center, and this study was approved by the ethics committee of Jundishapur University and carried out in an ethically proper way by following the guidelines provided. The animals were kept under standard laboratory conditions (12 h-dark and 12 h-light cycle, relative humidity of 50 ± 5% and 22 ± 3°C) for at least 1 week before the experiment and those conditions were preserved until the end of the experiment. Animal cages were kept clean, and commercial food (pellet) and water were provided ad libitum.
The mice were randomly divided into two groups, with eight animals in each group. CT extract was dissolved in distilled water and given orally (by gavage method) at the dose of 200 mg/kg for 35 consecutive days. The dose of CT was selected based on the previous studies that demonstrated the toxic action of CT [9]. Control group received only distilled water by gavage method for 35 consecutive days. One day after the last treatment, animals were killed by decapitation under ether anaesthesia and testes from each animal were fixed in 10% formalin. The samples were embedded in paraffin, sectioned (5 μm) and used for histopathology and morphometric studies. Two observers, blinded to the control and experimental groups, analyzed the sections independently.
Extract preparation
We selected an aqueous extract because the water extract of this flower is commonly used as a food additive. CT flos (5 g) was powdered, macerated in 100 ml distilled water for 1 h, and extracted by boiling for 60 min. After filtering, the filtrate (aqueous extract) was autoclaved (at 121°C for 20 min) and stored in a 4°C. All stages of the extraction procedure are the same as those used in local food preparations [10, 11]. Finally, the yield ratio of the extract was 8.9%.
Histopathology
Six slides per animal were examined for signs of germ cell degeneration including the following histopathological alterations: detachment (appearance of breaking off of cohorts of spermatocytes from the seminiferous epithelium); sloughing (release of clusters of germ cells into the lumen of the seminiferous tubule) and vacuolization (appearance of empty spaces in the seminiferous tubules). For each treatment, the average percentage of normal and regressed tubules was determined [12]. Average percentages were calculated for each sample by dividing the number of round tubules with a histopathologic index (vacuolization, detachment, sloughing) or normal tubules in a randomly microscopic field by the total number of round tubules in the same field and the result multiplied by 100. For each slide the mean of 3 fields was considered [13].
Morphometry
The diameters of the seminiferous tubules and the lumen diameter were measured by fitting a graticule of a calibrated linear scale in the 10× eyepiece of Leitz microscope at objective lens 40×. Only circular and near circular tubules were assessed. The height of the seminiferous epithelium was calculated by subtracting the lumen diameter from the tubule diameter [14, 15]. For each animal 150 tubules were analyzed.
Assessment of spermatogenesis
Maturity of the germinal epithelium was graded by using the Johnsen method [16]. Johnsen scoring is a simple method for assessment of spermatogenesis. By using a 40× magnification, 100 tubules were evaluated and each tubule was given a score ranging from 1 to 10. The tubules having complete inactivity scored as 1 and those with maximum activity (at least five or more spermatozoa in the lumen) scored as 10.
Statistical analysis
The data were analyzed using one-way ANOVA followed by Post hoc LSD test and were presented as the mean ± SD. p < 0.05 was considered significant. Data were analyzed using SPSS, version 15 (SPSS Inc, Chicago, Ill).
Results
Histopathology
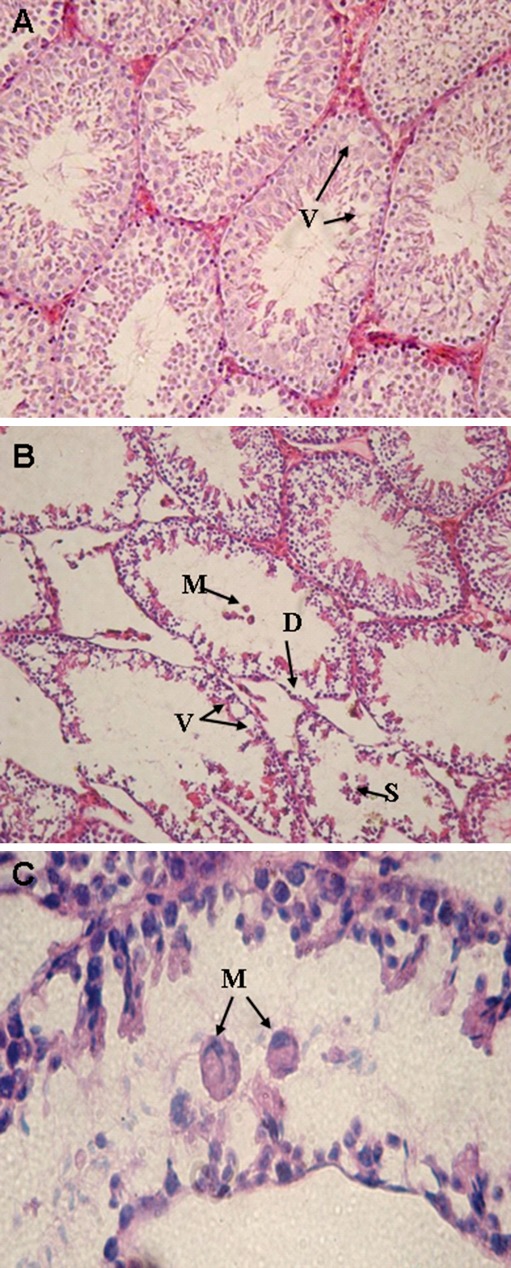
Testicular sections from control animals showed a low incidence of detached or vacuolized seminiferous tubules (Fig. 1a). In CT group, varying degrees of germ cell degenerative changes were occurred, ranging from loss of elongated spermatids, disorganization of germ cell layers, detachment and sloughing to vacuolization of the seminiferous tubules, contributing to eventual atrophy (Fig. 1b).
Fig. 1.
Light microscopy of cross sections of H&E stained testis from control and CT treated mice. a control group: small vacuoles (V) are observed in some tubules. b CT group: histopathological changes including disorganization of germ cell layers, detachment (D), sloughing (S), vacuolization (V) and multinucleated giant cells (M) are observed. c CT group: multinucleated giant cells (M) in the seminiferous tubules are observed. A (×250), B (×200) and C (×400)
In some tubules eosinophilic giant multinucleated cells were present in the lumen (Fig. 1c). Other tubules showed a loss of spermatid formation with spermatocyte degeneration or arrest. Spermatogonia did not show histopathological changes by light microscopy. Mainly the Sertoli cells showed large basal vacuoles in their cytoplasm. Leydig cells were not affected by CT. Blood vessels of the interstitial tissue appeared occasionally dilation and congestion. Percentages of abnormal tubules were significantly increased in CT–treated mice (p < 0.001). Table 1 shows the results obtained from the histopathological evaluations following CT treatment.
Table 1.
Histopathology assessments of the mouse testicular germ cells in control and CT groups
| Percentage of tubules | Control group | CT group | p-value |
|---|---|---|---|
| Normal (mean ± SD) | 95.3 ± 1.2 | 43.3 ± 0.8 | 0.000a |
| Detached (mean ± SD) | 2.2 ± 0.3 | 23.5 ± 1.5 | 0.000a |
| Sloughed (mean ± SD) | 1.7 ± 0.03 | 20.8 ± 1.2 | 0.000a |
| Vacuolized (mean ± SD) | 3.2 ± 0.4 | 34.6 ± 1.3 | 0.000a |
a statistically significant differences were found between the two groups
Morphometry
Diameters of the seminiferous tubules showed significantly decrease in CT–treated mice (105.6 ± 3.6 micrometer versus 208.6 ± 10.3 micrometer, p < 0.001). The height of seminiferous epithelium was significantly lower than those in the control group (86.8 ± 1.6 micrometer versus 166.3 ± 5.8 micrometer, p < 0.001).
Assessment of spermatogenesis
In control group, normal spermatogenesis was observed and the mean Johnsen score was 9.3 ± 0.7. In CT group, all sections of testes contain several tubules in which spermatogenesis were abnormal, and the mean Johnsen’s score was significantly lower than those in the control group (4.9 ± 0.3 versus 9.3 ± 0.7, p < 0.001).
Discussion
Herbs have a variety of complex chemical constituents that act on the body as a whole or on specific organ and systems. Some of the chemical constituents are mild and safe even in large doses while, some act more strongly or toxic in large doses or when taken continuously [4]. The present study demonstrated that 35 consecutive days treatment with 200 mg/kg aqueous extract of CT induces testicular damage in mice. CT is widely used in the food industry and associated hobbies in Iran. It is also added to traditional Iranian foods. Research reveals little or no information regarding dosage and effects of this plant on human. There are already a few reports about possible toxicological effects of this plant. Nobakht et al. [10] showed that safflower (CT) has teratogenic effects on the mouse central nervous system development. Liu et al. [9] reported that Hydroxysafflor yellow A, the main segment of the safflower yellow pigments, treatment induces a slight nephrotoxicity in rats. However, there was no evidence of nephrotoxicity in CT–treated mice in this study.
Takayama et al. [17] investigated the effects of 14 known testicular toxicants over varying time periods. They concluded that testicular histopathology is the most sensitive and reliable method for detecting effects on sperm production. Other studies have reached similar conclusions [18, 19]. Many studies have been performed by qualitative [20, 21] or quantitative [22, 23] examination to detect testicular toxicity. In this study, we performed qualitative examination of germ cell morphology by histopathology and quantitative examination using morphometry and Johnsen scoring.
With a histopathological observation, it was possible to demonstrate alterations in testis morphology such as epithelial vacuolizations, sloughing and atrophy in the mice exposed to CT. The sloughing of immature germ cells from the seminiferous tubules by CT indicates that this drug might affect Sertoli cell functions. The presence of vacuoles in the cytoplasm of the Sertoli cells shows direct damage to these cells. Russell and Griswold [24] have reported that these lesions are the early morphological sign of testicular injury and are considered as the main Sertoli cell response to many xenobiotics.
CT extract induced formation of multinucleated giant cells in the germinal epithelium. The presence of multinucleated giant cells is indicative of regressive changes in the germinal epithelium and is caused by destruction and loss of the intercellular bridges that are essential for the process of spermatogenesis, spermiogenesis and synchronization of germ cell maturation [25].
Morphometric studies showed that seminiferous diameter were significantly decrease in CT group. Previous studies demonstrated that increased seminiferous tubule diameter is indicative of fluid retention resulting from impaired emptying through the efferent ducts, whereas decreased seminiferous diameter may indicate germ cell loss [26]. Additionally, results of Johnsen’s scoring revealed poor spermatogenesis in CT group. Alterations in the Johnsen’s scoring relate to germ cells degenerations.
Within the testis, the three main target cells for toxicants that disrupt spermatogenesis are the somatic cells, the Leydig and Sertoli cells, and the germ cells themselves. In animal models of exposure, each of these cell types can be selectively targeted by specific c toxicants, resulting in germ cell death and spermatogenic failure [27]. The exact mechanism of CT effects on mouse testis is not obtained from this study. The multinucleated giant cell formation and sloughing of immature germ cells from the seminiferous tubules by CT indicates that this plant might affect Sertoli cell functions. Alterations in the Johnsen scoring and morphometric studies in CT–treated mice may relate to induction of apoptosis in testicular germ cells. Additionally, the reduction in the morphometrical alterations may have been a consequence of germ cell apoptosis. Thus, the spermatogenic defects might result not only from a direct effect of CT on germ cell death, but also from alterations of the Sertoli cells.
Vascular dilation and congestion found in the interstitial tissue have been described in CT–treated animals. These changes could be a consequence of the action of vasodilator substances like serotonin present in the CT extract [28]. Additionally, CT extract may induce production another vasodilator substances such as nitric oxide (NO).
Conclusion
CT extract can change the testes histological structure and lead to spermatogenesis failure. However, to state the mechanism by which CT exerts its effects needs more investigation. Although the effects of CT extract in human reproductive activities are unknown, regarding this study, it is better to recommend infertile men or men with reproductive disorders to use it with caution during treatment.
Acknowledgement
This research was supported by a Grant (88-S.126) from the research council of the Ahvaz Jundishapur University of Medical Sciences in 2011.
Footnotes
Capsule
Aqueous extracts of Safflower exert significant detrimental effects on spermatogenesis in a mouse exposure model.
References
- 1.Franca LR, Ghosh S, Ye SJ, Russell LD. Surface and surface to volume relationships of the Sertoli cells during the cycle of the seminiferous epithelium in the rat. Biol Reprod. 1993;49(6):1215–1228. doi: 10.1095/biolreprod49.6.1215. [DOI] [PubMed] [Google Scholar]
- 2.Boekelheide K, Fleming SL, Johnson KJ, Patel SR, Schoenfeld HA. Role of Sertoli cells in injury-associated testicular germ cell apoptosis. Exp Biol Med. 2000;225(2):105–115. doi: 10.1046/j.1525-1373.2000.22513.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli—germ cell interactions and male contraceptive development. Physiol Rev. 2002;82(4):825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 4.Chen XW, Serag ES, Sneed KB, Zhou SF. Herbal bioactivation, molecular targets and the toxicity relevance. Chem Biol Interact 2011;15:192(3):161–76. [DOI] [PubMed]
- 5.Emongor V. Safflower (Carthamus tinctorius L.) the underutilized and neglected crop: a review. Asian J Plant Sci. 2010;9:299–306. doi: 10.3923/ajps.2010.299.306. [DOI] [Google Scholar]
- 6.Han SY, Li HX, Ma X, Zhang K, Ma ZZ, Tu PF. Protective effects of purified safflower extract on myocardial ischemia in vivo and in vitro. Phytomedicine. 2009;16(8):694–702. doi: 10.1016/j.phymed.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu M, Takahashi T, Komatsu M, Kido T, Kasahara Y. Antioxidant and neuroprotective activities of Mogami-benibana (safflower, Carthamus tinctorius Linne) Neurochem Res. 2009;34(4):795–805. doi: 10.1007/s11064-008-9884-5. [DOI] [PubMed] [Google Scholar]
- 8.Loo WTY, Cheung MNB, Chow LWC. The inhibitory effect of an herbal formula comprising ginseng and carthamus tinctorius on breast cancer. Life Sci. 2004;76(2):191–200. doi: 10.1016/j.lfs.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Li C, Li M, Li D, Liu K. The subchronic toxicity of hydroxysafflor yellow A of 90 days repeatedly intraperitoneal injections in rats. Toxicology. 2004;203(1–3):139–43. doi: 10.1016/j.tox.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Nobakht M, Fattahi M, Hoormand M, Milanian I, Rahbar N, Mahmoudian M. A study on the teratogenic and cytotoxic effects of safflower extract. J Ethnopharmacol. 2000;73(3):453–459. doi: 10.1016/S0378-8741(00)00324-X. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi K, Demachi A, Takenaka A. Protective effects of hot water extract of safflower leaves and its component luteolin-7-O-glucoside on paraquat-induced oxidative stress in rats. Food Sci Technol Res. 2001;7:224–230. doi: 10.3136/fstr.7.224. [DOI] [Google Scholar]
- 12.D’Cruz OJ, Uckun FM. Vanadocene-mediated in vivo male germ cell apoptosis. Toxicol Appl Pharmacol. 2000;166(3):186–195. doi: 10.1006/taap.2000.8965. [DOI] [PubMed] [Google Scholar]
- 13.Oatley JM, Tibary A, de-Avila DM, Wheaton JE, McLean DJ, Reeves JJ. Changes in spermatogenesis and endocrine function in the ram testis due to irradiation and active immunization against luteinizing hormone-releasing hormone. J Anim Sci. 2005;83(3):604–612. doi: 10.2527/2005.833604x. [DOI] [PubMed] [Google Scholar]
- 14.Ma YH, Hu JH, Zhou XG, Mei ZT, Fei J, Guo LH. Gamma-aminobutyric acid transporter (GAT1) overexpression in mouse affects the testicular morphology. Cell Res. 2000;10(1):59–69. doi: 10.1038/sj.cr.7290036. [DOI] [PubMed] [Google Scholar]
- 15.Orazizadeh M, Khorsandi LS, Hashemitabar M. Toxic effects of dexamethasone on mouse testicular germ cells. Andrologia. 2010;42(4):247–253. doi: 10.1111/j.1439-0272.2009.00985.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnsen SG. Testicular biopsy score count: a method for registration of spermatogenesis in human testis. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 17.Takayama S, Akaike M, Kawashima K, Takahashi M, Kurokawa Y. A collaborative study in Japan on optimal treatment period and parameters for detection of male fertility disorders induced by drugs in rats. Int J Toxicol. 1995;14:266–292. doi: 10.3109/10915819509008702. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadian H, Ghanbari M, Moradi MR, Khaje-Dalouee M. Effect of cigarette smoke on spermatogenesis in rats. Urol J. 2007;4(3):159–63. [PubMed] [Google Scholar]
- 19.Hild SA, Reel JR, Dykstra MJ, Mann PC, Marshall GR. Acute adverse effects of the indenopyridine CDB-4022 on the ultrastructure of sertoli cells, spermatocytes, and spermatids in rat testes: comparison to the known sertoli cell toxicant Di-n-pentylphthalate (DPP) J Androl. 2007;28(4):621–9. doi: 10.2164/jandrol.106.002295. [DOI] [PubMed] [Google Scholar]
- 20.Lee KP, Frame SR, Sykes GP, Valentine R. Testicular degeneration and spermatid retention in young rats. Toxicol Pathol. 1993;21(3):292–302. doi: 10.1177/019262339302100305. [DOI] [PubMed] [Google Scholar]
- 21.Hew KW, Ericson WA, Michael JW. A single low cadmium dose causes failure of spermiation in the rat. Toxicol Appl Pharmacol. 1993;121(1):15–21. doi: 10.1006/taap.1993.1123. [DOI] [PubMed] [Google Scholar]
- 22.Souza Predes F, Diamante MA, Dolder H. Testis response to low doses of cadmium in Wistar rats. Int J Exp Pathol. 2010;91(2):125–131. doi: 10.1111/j.1365-2613.2009.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yari A, Asadi MH, Bahadoran H, Dashtnavard H, Imani H, Naghii MR. Cadmium toxicity in spermatogenesis and protective effects of l-Carnitine in adult male rats. Biol Trace Elem Res. 2010;137(2):216–225. doi: 10.1007/s12011-009-8577-5. [DOI] [PubMed] [Google Scholar]
- 24.Russell L, Griswold M. Sertoli cell toxicants. In: Russell L, Griswold M, editors. The Sertoli cell. Florida: Cache River; 1995. [Google Scholar]
- 25.Neumann F, Schenck B. Formal genesis of giant cells in the germinal epithelium in the rat thioglucose model. Andrologia. 1977;9(4):323–8. doi: 10.1111/j.1439-0272.1977.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 26.Moffit JS, Bryant BH, Hall SJ, Hall SJ. Dose-dependent effects of Sertoli cell toxicants 2, 5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol. 2007;35(5):719–727. doi: 10.1080/01926230701481931. [DOI] [PubMed] [Google Scholar]
- 27.Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr. 2005;34:6–8. doi: 10.1093/jncimonographs/lgi006. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Tsubaki S, Fujita M, Koyama N, Takabashi M, Takazawa K. Effects of safflower seed extract on arterial stiffness. Vasc Health Risk Manag. 2010;6:1007–1014. doi: 10.2147/VHRM.S13998. [DOI] [PMC free article] [PubMed] [Google Scholar]