Abstract
An informed choice about health-related direct-to-consumer genetic testing (DTCGT) requires knowledge of potential benefits, risks, and limitations. To understand the information that potential consumers of DTCGT services are exposed to on company websites, we conducted a content analysis of 23 health-related DTCGT websites. Results revealed that benefit statements outweighed risk and limitation statements 6 to 1. The most frequently described benefits were 1) disease prevention, 2) consumer education, 3) personalized medical recommendations, and 4) the ability to make health decisions. Thirty-five percent of websites also presented at least one risk of testing. Seventy-eight percent of websites mentioned at least one limitation of testing. Based on this information, potential consumers might get an inaccurate picture of genetic testing which could impact their ability to make an informed decision. Practices that enhance the presentation of balanced information on DTCGT company websites should be encouraged.
Keywords: Direct-to-consumer, genetic testing, Internet, informed choice, persuasion, content analysis
INTRODUCTION
In the new millennium, direct-to-consumer genetic testing (DTCGT) services have become widely available; this, in conjunction with the internet being used as a mass communication tool, has facilitated the creation of several web-based genetic testing services. A systematic search of the World Wide Web in 2002 found that 14 sites offered health-related genetic tests. Less than half of these websites discussed risks of testing; the benefits and limitations of testing were not assessed (Gollust et al, 2003). Since that time, the number of sites offering health-related genetic tests has more than doubled, some offering susceptibility testing for complex, multi-factorial diseases (Lachance et al, 2010). Research is now needed to assess in depth the benefit, risk, and limitation information to which a consumer is exposed on these sites.
Opponents of direct-to-consumer (DTC) marketing of genetic tests assert that companies fail to adequately inform potential consumers by deemphasizing the involvement of a health care professional in assessing their personal risk and in deciding whether or not to undergo genetic testing. Rather, company advertisements often suggest that consumers contact the company directly (Hull and Prasad, 2001; Mouchawar et al, 2005). A central question raised, particularly in the absence of direct health care provider involvement, is whether the consumer can sufficiently understand the benefits, risks, and limitations of undergoing genetic testing as presented in DTCGT websites in order to make an informed decision about purchasing a genetic test. Although proponents have suggested that DTCGT may increase consumers’ awareness, usage, and access to genetic tests, enhance consumer privacy, and promote healthy lifestyle changes (Mouchawar et al, 2005; Wade and Wilfond, 2006), many concerns have also been raised. An informed consumer should arguably be able to balance these possible benefits with the knowledge that some experts believe that services are being offered prematurely, calling into question the current clinical validity and utility, and, to a lesser extent, the analytic validity of some types of DTCGT (Hunter et al, 2008). Moreover, consumers who are not able to make informed decisions prior to purchasing DTCGT may be at risk for misinterpretation of results, leading to confusion, false reassurance or unwarranted anxiety (Gollust et al, 2002; Gollust et al, 2003; Wade and Wilfond, 2006; Wasson et al, 2006).
The few studies of DTCGT information conducted by the Government Accountability Office and the Evaluation of Genomic Applications in Practice and Prevention working group have shown that companies provided misinformation and used vague wording, with the potential for confusion amongst both consumers and physicians (FTC 2006; Katsanis et al, 2008). Additionally, oversight of DTCGT is complex and contains many gaps and ambiguities. It is shared by multiple government and non-governmental bodies and there has been no comprehensive system to assess analytical or clinical validity before tests are released to the public, or to develop standards for information (Hudson et al, 2007; Teutch and Tuckson 2008; Bell et al, 2000). However, U.S. consumers generally assume that the government assesses the safety and effectiveness of medical products before they are made available commercially (Bell et al, 2000). In order to support the development of regulatory policies that maximize the educational quality of consumer information and informed decision-making by consumers with respect to obtaining genetic testing, a first step is to investigate what information is currently being presented by DTCGT websites and how it is being presented.
To address this issue, we conducted a research study using theories of informed choice that are based on clinical encounters where health-related decisions are involved. We have applied the same standard to the purchase of genetic testing because consumers are making decisions about whether or not to obtain permanent health information. These frameworks are based on two core components of an informed choice: (1) the decision-maker has relevant, high-quality information which presents the various alternatives and outcomes; and 2) it is consistent with the decision-maker’s values (Marteau and Dormandy, 2001). In the context of genetic testing, relevant, high-quality information should include a presentation of the risks, benefits, and limitations of undergoing testing (van den Berg et al, 2006). We systematically assessed the information presented on websites about DTCGT according to categories of benefits, risks, and limitations of testing.
METHODS
Website selection
This study was a descriptive content analysis of 23 English-language DTCGT websites offering health-related genetic tests between June 15 and July 1, 2009. Websites were identified initially through a list of 39 health-related DTCGT websites available on the John Hopkins University Genetics and Public Policy Center’s website (www.dnapolicy.org; version date 5/27/2009). In conjunction with a parallel study simultaneously being conducted by two of the study authors, extensive additional keyword searches adapted from Gollust and colleagues (Gollust et al, 2003) were used in four internet search engines (Google.com, Ask.com, Altavista.com, and MSN Live Search) and one meta-search engine (Metacrawler.com). The comprehensive web search identified one additional health-related DTC genetic testing website (Lachance et al, 2010). Seventeen websites were excluded from this list for one of three reasons: 1- the website did not have a working homepage at the time of analysis; 2- the website displayed a message that they were no longer offering health-related genetic testing; or 3- they did not test for health conditions (e.g., testing for athletic performance). This strategy yielded a total of 23 websites for our analysis, including sites that sold whole genome single nucleotide polymorphism (SNP) scans, sites that offered more limited sets of SNP-based tests, and sites that offered tests for known mutations in single genes. (Table I).
Table I.
Sample of 23 health-related DTCGT websites
| 6 companies offering whole genome analysis either by SNP scan (~100,000 SNPs) or sequencing | ||
| 1 | 23andMe | https://www.23andme.com |
| 2 | Biomarker_gene essence™ | http://www.biomarkerinc.com |
| 3 | DeCODEme™ | http://www.decodeme.com |
| 4 | Inneova™ | http://www.inneova.com |
| 5 | Knome | http://www.knome.com |
| 6 | Navigenics | http://www.navigenics.com |
| 10 companies offering SNP-based tests (one or more SNPs) | ||
| 1 | Consumer Genetics | http://www.consumergenetics.com |
| 2 | DNA Dimensions | http://www.detroitdna.com |
| 3 | EnteroLab | https://www.enterolab.com |
| 4 | gnostics_NicoTest™ | http://www.g-nostics.com |
| 5 | Genelex_HealthandDNA | http://www.healthanddna.com |
| 6 | GeneLink Biosciences, Inc. | http://www.genelinkbio.com |
| 7 | GRACEFUL EARTH, INC. | http://www.gracefulearth.com |
| 8 | Mygenome | http://www.mygenome.com |
| 9 | new hope medical | http://www.newhopemedical.org |
| 10 | Amway_quixtar_Interleukin_Gensona™ | http://www.quixtar.com |
| 7 companies offering tests for single gene, known mutations, or clinically available chromosome testing (traditional tests offered in clinical settings) | ||
| 1 | CyGene DIRECT™ | https://cygenedirect.com/default.html |
| 2 | DNA CARDIOCHECK, INC. | http://www.bebedna.com |
| 3 | DNAdirect | http://www.dnadirect.com |
| 4 | DNA Traits | http://www.dnatraits.com |
| 5 | HealthCheckUSA | http://www.healthcheckusa.com |
| 6 | Matrix | http://www.matrixgenomics.com |
| 7 | Medichecks | http://www.medichecks.com |
Instrumentation: Codebook development
The three major domains of coding included benefits (related to potential positive outcomes of undergoing genetic testing); risks (related to potential adverse outcomes of genetic testing on the individual or their family); and limitations (related to scientific, clinical, and/or technical limits of the laboratory, test, or test interpretation) (Marteau and Dormandy, 2001; van den Berg et al, 2006). A preliminary codebook was initially developed based on these broad domains and pretested. In addition, new themes that emerged during coding were added to the codebook and applied to all websites.
Coding and analysis
Using the codebook, a content analysis was carried out on the main pages of all websites. These pages included all consumer-focused content excluding pages labeled as terms and conditions and/or the website’s privacy statement. When a site had separate information for health professionals and the general public, only the latter was analyzed. Any hyperlinks that led out of the original website as indicated by a change in the root website address were not evaluated.
The website content was imported into the software package QSR NVivo Version 2.0 (QSR International Pty. Ltd., 1999–2002) for thematic coding and analysis. In addition to the qualitative coding, the number of benefits, risks, and limitations statements on each site was quantified. After pretesting the codebook with websites excluded from this study (e.g., websites offering nutrigenomic testing), the main sample was coded independently by two coders (AS and KF); AS coded all websites, and KF double-coded 11 out of 23 (48%). Percent agreement was greater than 80% for all codes; consequently, all variables presented in the next section had high levels of agreement between coders.
Because websites were classified as selling one or more of the three different categories of genetic tests (Table I), we also looked for differences in emerging themes across websites selling different categories of tests.
RESULTS
Benefits
We will describe the types of information presented in the websites according to the categories of benefits, risks, and limitations (Table 2). The average number of benefit statements made per website was 26.3 (range 3–155). The benefits most often described by the websites were prevention, consumer education, personalized medicine, and the ability to make informed decisions based on genetic testing results. The most common type of benefit (96% of websites) was the potential for the genetic test to prevent the onset of a disease or reduce the burden of morbidity of a disease or health condition: When you understand your DNA and how it affects your health, you have valuable information that can help you detect health conditions early, reduce their effects or even prevent them entirely (Company21). On average, there were seven statements per website regarding prevention. The second most common category of benefits (83% of websites) was increased consumer knowledge as a stand-alone benefit resulting from undergoing genetic testing: The gift of knowledge…to give students, academics, physicians and other professionals with an interest in genetics a chance to get a more in-depth view of their code and genome (Company5). An average of five statements per website involved increasing consumer knowledge. Seventy-four percent of websites included statements related to the potential for the genetic test to personalize a customer’s healthcare recommendations: All of the SNPs analyzed in these panels are actionable ones for which we can provide personalized interventions (Company22). On average, there were ten statements per website regarding the potential for personalized healthcare recommendations. Fifty-two percent of websites described a consumer’s ability to use the results to make informed decisions, with an average of only one statement per website:. … genetic information can help many people make informed choices about family, career, and finances that may positively affect their quality of life (Company20). Less frequently mentioned benefits included altruism, participation in research, being part of a community of individuals who have undergone genetic testing, and enhanced privacy.
Table II.
Types of information presented in the websites according to the categories of benefits, risks, and limitations
| % of websites (n) |
||
|---|---|---|
| Benefits | Prevention | 96 (22) |
| Consumer education | 83 (19) | |
| Personalized medicine | 74 (17) | |
| Informed decisions | 52 (12) | |
| Altruism | 30 (7) | |
| Research participation | 26 (6) | |
| Enhanced privacy | 22 (5) | |
| Community | 13 (3) | |
| Risks | Worry/Anxiety | 26 (6) |
| Genetic discrimination | 17 (4) | |
| No risks | 9 (2) | |
| Negative impact on family members | 4 (1) | |
| Limitations | Clinical usage | 74 (17) |
| Multi-factorial nature of disease | 30 (7) | |
| Scientific understanding | 30 (7) | |
Risks
The average number of risk statements per website was 1.04 (range 0–7). Thirty-five percent of the websites had at least one mention of a risk, while 65% had no references to any type of risk associated with genetic testing. Two websites mentioned risks only by stating explicitly that there are no risks involved in genetic testing. The most common risk (26% of websites) referred to the potential for worry and anxiety associated with undergoing genetic testing with an average of only one statement per website: Some reasons why people decide not to have testing are: They have a personal history of depression or anxiety and are concerned about how they would cope with test results — whether positive or negative (Company8). Another type of risk (17% of websites) was the potential for genetic discrimination or threats to privacy. On average there were three statements per website regarding this risk. However, the websites generally presented this type of risk as unlikely: Although confirmed cases of genetic discrimination are thankfully rare, the fear of discrimination by insurance companies is one of the main reasons people hesitate to pursue access to their genetic information (Company1). Two of the four websites that mentioned the potential for genetic discrimination as a risk of undergoing testing also presented the Genetic Information Nondiscrimination Act (GINA) as a source of protection from this discrimination. Myth: My health insurance rates can go up based on my genetic test and employers will discriminate against me. Truth: A federal law – called GINA – protects you from those types of discrimination (Company21).
Limitations
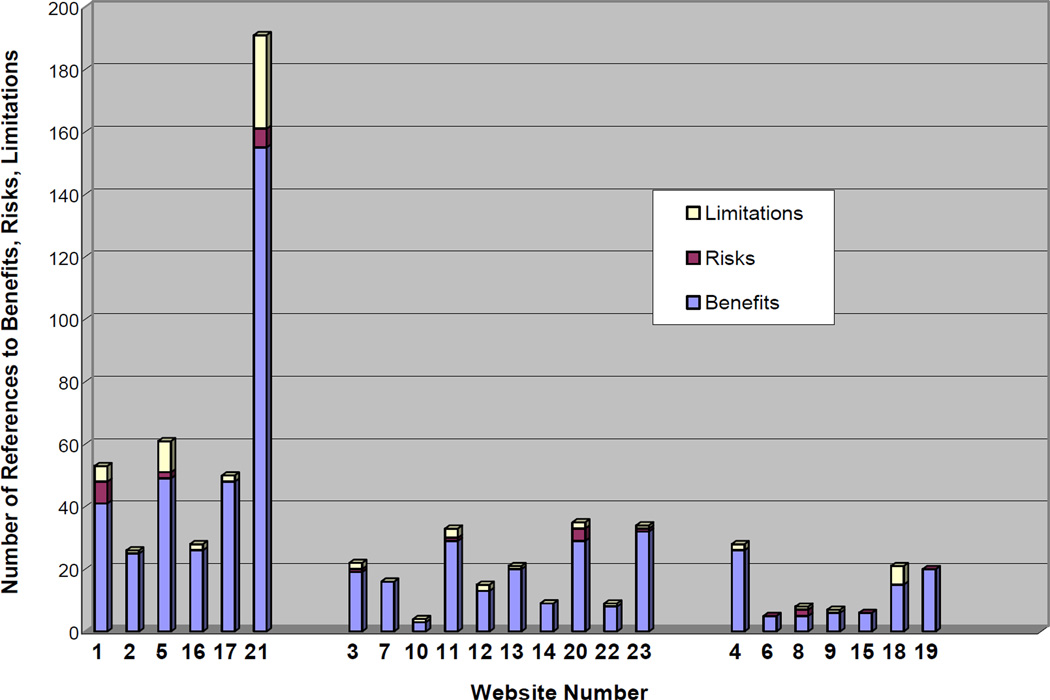
The average number of limitations statements per website was 3.17 (range 0–30) (Figure 1). Seventy-eight percent of websites mentioned at least one limitation of testing. Seventy-four percent of websites included disclaimers about clinical usage of the genetic test result with an average of three statements per website. These disclaimers seemed to be regulatory statements intended to specify or delimit the scope of use that might be exercised by the consumer: Company17 does not provide medical advice, diagnosis or treatment (Company17); These products are not intended to diagnose, treat, cure or prevent any disease (Company 22). Seventy percent of the websites stated that consumers should involve their physician in decision-making about health-related concerns based on test results with an average of five statements per website: Your test results report is a guide to help you partner with your physician to implement appropriate lifestyle changes (Company7). Thirty percent of websites discussed that in addition to genetic predispositions or causes of disease, there are other factors that contribute to whether or not a person develops a disease with an average of one statement per website: Your lifetime experiences - as well as changes you may make in your lifestyle, nutrition, environment, or health care – also determine whether or not you get the disease (Company20). Finally, 30% of websites discussed limitations that exist in regard to the current predictive ability of genetic tests. Fifty percent of the 16 websites offering any SNP-based testing or SNP-based whole genome scanning mentioned this as a limitation: At this time many of the one million SNPs in the XXX scan have no scientifically valid study results associating them with any information relevant to you or your health (Company5).
Figure 1.
Ratio of Benefits, Risks, and Limitations in each website grouped according to company type (1st set: whole genome 2nd set: individual/few SNPs 3rd set: single gene/chromosome testing)
DISCUSSION
The primary goal of this study was to better understand the benefit, risk, and limitation information consumers are exposed to on DTCGT websites. Based on the ethical principle of informed choice, consumers should have unambiguous, consistent information about the potential benefits, risks, and limitations of testing (van der Berg et al, 2006). This project revealed that in the main pages of these websites, consumers are exposed to an average of 6 times as many benefits as risks and limitations. Therefore, consumers who only read the main web pages may be getting a skewed picture of the benefits, risks, and limitations of testing.
Based on research and commentary, a potential benefit of genetic testing is consumer education about genetics and disease in a general sense. Although consumer education was presented as a benefit by many companies, this benefit was presented alongside the possibilities of prevention and personalized medicine in all instances. These benefits are contradictory to recent studies which indicate that, currently, genomic profiling is not useful for risk assessment in common disease or in making personalized health recommendations (Janssens, Gwinn, et al, 2008; Janssens, van Duijn et al, 2008). In addition, websites often gave inconsistent information by stating that benefits of testing would include prevention while simultaneously stating that “these products are not intended to diagnose, treat, cure or prevent any disease.”
Although some opponents of DTC marketing of genetic tests have criticized companies for bypassing the involvement of health care professionals (Hull and Prasad, 2001; Mouchawar et al, 2005), we found that 70% of DTCGT websites did recommend that consumers contact a healthcare professional in decisions related to genetic testing. This recommendation might be problematic however if consumers approach their general practitioner with questions about DTCGT; physicians who are not genetics specialists may not have sufficient knowledge to inform decision making or to provide appropriate interpretations of test results (Kolor et al, 2008; Shirts and Parker, 2008) These results are particularly concerning for those consumers who undergo testing at those companies which do not provide professional genetic counseling, do not provide information on how to contact a genetic specialist, and do not recommend involvement of a healthcare professional in the genetic testing process (Lachance, 2010). In the absence of any kind of genetic counseling, the consumer of a DTC genetic test is potentially left vulnerable to misinterpretation of results, such as false reassurance, with limited or no benefits (Wasson et al, 2006).
Given that science is in the early days of elucidating the complete etiology of many diseases, one limitation that we expected to find on most websites offering SNP-based testing is the evolving nature of consumer disease-risk status. As more genome-wide association studies are published, some SNPs will inevitably be found to confer a different risk than previously reported (Mihaescu et al, 2009). Depending on the change in magnitude of the risk, a person’s risk status may be reclassified. However, our results showed only half of the companies offering SNP-based testing presented any information about how customers would get updated risk information.
At the time of this study, most company websites did not provide a balanced representation of benefits, risks, and limitations, and in fact, often presented conflicting information. The absence of the requirement for a healthcare intermediary to be involved in most DTCGT puts an even bigger burden on information sources to provide the highest quality information. The results of our analysis suggest that the content on many websites is weighted toward motivating consumers to purchase a test. This is perhaps not surprising given that these companies have an interest and even a commitment to their shareholders to sell their product and make a profit (Wade and Wilfond, 2006). However, this fact only emphasizes the need for balance in presentation of benefits, risks and limitations to facilitate informed choices amongst potential consumers of these tests.
Study Limitations
There are several limitations to this study. First, the authors only assessed information presented directly on the company websites; actual company practices and information given privately to consumers might be different from what was analyzed in this study. Materials excluded from analysis (e.g., hyperlinks) were also not evaluated; these may have contained information related to benefits, risks, and limitations of testing. Thirdly, this analysis was limited to a snapshot of content included in English-language DTCGT company websites between June 15 and July 1, 2009. Giving the ever-evolving nature of genetic information, and the ease with which companies can change their website content, the interpretations we offer are specific to a limited time-period of analysis.
Practice Implications
Even given these limitations, our results suggest the importance of developing regulations concerning the content of DTCGT websites. One standard that could be applied is FDA’s “fair balance” requirement regarding DTC prescription drug advertising (Baylor-Henry and Drezin, 1998). Drug companies are required to follow specific guidelines regarding the balanced representation of risks and benefits of a drug in consumer advertisements. Ideally, DTCGT companies might be required to not only provide information about risks, benefits, and limitations of the offered tests, but also to be sure that this information is presented in a balanced fashion. More specifically, companies offering SNP-based testing might be required to discuss the limited ability of any one SNP to predict disease, and the changing nature of an individual’s disease risk according to an evolving understanding of susceptibility variants. The U.S. Secretary’s Advisory Committee on Genetics, Health and Society (SACGHS) has proposed the development of a registry of DTCGT companies as one step towards establishing criteria to evaluate validity and utility of DTC genetic tests. However, the existence of such a registry, while offering a powerful information tool to the public, would not directly address the content of the DTCGT company websites themselves as potential vehicles of informed decision-making (DHHS 2008).
Companies should consider revising their messages to place more emphasis on consumer education and awareness about genetics and disease through genetic testing. Websites should also be clear that although personalized medicine is possible and in fact likely in the future, giving healthcare recommendations based on results from current genetic testing capabilities is premature.
Additionally, companies might consider more frequent employment of genetic counselors to help consumers make informed choices and interpret their genetic information. This may be particularly important in light of what has been recently uncovered about the limited ability of general practitioners to interpret these tests (Castle and Ries, 2007). The genetic counselor would also be responsible for updating consumers on their risk status, if and when their status changes due to additional reports in the scientific literature. Genetic counselors are also uniquely qualified to understand issues of privacy regarding genetic information and could be instrumental in helping companies to obtain certificates of confidentiality so that if asked, these companies could refuse to release any identifying information about their clients to civil authorities. Although hiring client-centered genetic counselors will give a company more resources with which to disseminate accurate information to the consumer, it is unlikely this information will be completely unbiased until legislation for fair and balanced advertising of DTCGT is in place. Genetic counselors who are not employed by DTCGT companies would serve their existing clients well by becoming familiar with the information available to consumers on the internet about available testing.
Research Recommendations
Future research should focus on consumer values regarding DTCGT and emotional reactions to DTCGT websites since decisions are influenced by both cognitive and emotional responses to information. We know an individual’s decision making is not only based on technical and probabilistic information about the risks, benefits and limitations of testing, but also their own emotional and attitudinal reactions to this information. Further understanding of not only what the consumer understands about DTCGT, but also how the consumer feels about DTCGT could help elucidate whether consumer decisions about undergoing genetic testing through DTC avenues are truly informed. This understanding could also help companies to better tailor their messages to consumer concerns. Furthermore, a comparison between consumer decision-making about DTCGT mediated through company-offered genetic counseling versus decision-making without company-mediated genetic counseling will help us to understand how genetic counseling can best facilitate consumer choices through DTC avenues.
CONCLUSIONS
Since most company websites did not provide a balanced representation of benefits, risks, and limitations, and in fact, often presented conflicting information, there is a great burden on other information sources such as the news media to provide high quality information. Companies should consider revising their messages to place more emphasis on consumer education and awareness about genetics and disease through genetic testing. Websites should also be clear that although personalized medicine is possible and in fact likely in the future, giving healthcare recommendations based on results from current genetic testing capabilities is premature. In the absence of regulations to support more balanced information, genetic counselors and other healthcare providers need to be prepared to assist in the decision-making process, to provide support once results become available, and perhaps most importantly, to engage in public education efforts to create a more informed public.
ACKNOWLEDGEMENTS
This design and conduct of this study was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health in partial fulfillment of the requirements of the first author’s ScM degree at the Johns Hopkins Bloomberg School of Public Health. All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
We would like to acknowledge the following individuals for their review of this manuscript: Joan Scott, M.S., C.G.C. (Director, Genetics and Public Policy Center at Johns Hopkins University); Gail Geller, Sc.D. (Professor, Department of Pediatrics and the Bioethics Institute, McKusick-Nathans Institute of Genetic Medicine at Johns Hopkins University); Debra Roter, DrPH (Professor, Department of Health, Behavior, and Society, Johns Hopkins Bloomberg School of Public Health); Dani Fallin, Ph.D., (Associate Professor, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health); and Barbara B. Biesecker, M.S., C.G.C., (Associate Investigator, Social and Behavioral Research Branch and Director, National Human Genome Research Institute). No individual listed above received any compensation for their contributions.
Footnotes
No authors had any conflicts of interest, including financial interests or affiliations relevant to the subject of this manuscript. These results were presented in part at the National Society of Genetic Counselors Annual Education Conference, Dallas, TX, 2010.
REFERENCES
- Baylor-Henry M, Drezin NA. Regulation of prescription drug promotion: direct-to-consumer advertising. Clin Ther. 1998;20(Suppl C):C86–C95. doi: 10.1016/s0149-2918(98)80012-x. [DOI] [PubMed] [Google Scholar]
- Bell RA, Wilkes MS, Kravitz RL. The educational value of consumer-targeted prescription drug print advertising. J Fam Pract. 2000 Dec;49(12):1092–1098. [PubMed] [Google Scholar]
- Castle D, Ries NM. Ethical, legal and social issues in nutrigenomics: the challenges of regulating service delivery and building health professional capacity. Mutat Res. 2007;622(1–2):138–143. doi: 10.1016/j.mrfmmm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. U.S. System of Oversight of Genetic Testing: A Response to the Charge of the Secretary of Health and Human Services Report of the Secretary's Advisory Committee on Genetics, Health, and Society. Washington, DC: DHHS; 2008. [Google Scholar]
- Federal Trade Commission(FTC) FTC Facts for Consumers: At-Home Genetic Tests: A Healthy Dose of Skepticism May Be the Best Prescription. Washington, DC: FTC; 2006. [Google Scholar]
- Gollust SE, Hull SC, Wilfond BS. Limitations of direct-to-consumer advertising for clinical genetic testing. JAMA. 2002 Oct 9;288(14):1762–1767. doi: 10.1001/jama.288.14.1762. [DOI] [PubMed] [Google Scholar]
- Gollust SE, Wilfond BS, Hull SC. Direct-to-consumer sales of genetic services on the Internet. Genet Med. 2003 Jul-Aug;5(4):332–337. doi: 10.1097/01.GIM.0000076972.83711.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson K, Javitt G, Burke W, Byers P. ASHG Statement* on direct-to-consumer genetic testing in the United States. Obstet Gynecol. 2007 Dec;110(6):1392–1395. doi: 10.1097/01.AOG.0000292086.98514.8b. [DOI] [PubMed] [Google Scholar]
- Hull SC, Prasad K. Reading between the lines: direct-to-consumer advertising of genetic testing in the USA. Reprod Health Matters. 2001 Nov;9(18):44–48. doi: 10.1016/s0968-8080(01)90089-8. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle--will we get our wish? N Engl J Med. 2008 Jan 10;358(2):105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- Janssens AC, Gwinn M, Gwinn M, et al. A critical appraisal of the scientific basis of commercial genomic profiles used to assess health risks and personalize health interventions. Am J Hum Genet. 2008;82(3):593–599. doi: 10.1016/j.ajhg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens AC, van Duijn CM. Genome-based prediction of common diseases: advances and prospects. Hum Mol Genet. 2008;17(R2):R166–R173. doi: 10.1093/hmg/ddn250. [DOI] [PubMed] [Google Scholar]
- Kolor K, Liu T, St Pierre J, Khoury MJ. Health care provider and consumer awareness, perceptions, and use of direct-to-consumer personal genomic tests, United States, 2008. Genet Med. 2009;11(8):595. doi: 10.1097/GIM.0b013e3181b1cc2c. [DOI] [PubMed] [Google Scholar]
- Katsanis SH, Javitt G, Hudson K. Public health. A case study of personalized medicine. Science. 2008 Apr 4;320(5872):53–54. doi: 10.1126/science.1156604. [DOI] [PubMed] [Google Scholar]
- Lachance CR, Erby LA, Ford BM, Allen VC, Jr, Kaphingst KA. Informational content, literacy demands, and usability of websites offering health-related genetic tests directly to consumers. Genet Med. 2010;12(5):304–312. doi: 10.1097/GIM.0b013e3181dbd8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Dormandy E. Facilitating informed choice in prenatal testing: how well are we doing? Am J Med Genet. 2001;106(3):185–190. doi: 10.1002/ajmg.10006. [DOI] [PubMed] [Google Scholar]
- Mihaescu R, van Hoek M, Sijbrands EJ, et al. Evaluation of risk prediction updates from commercial genome-wide scans. Genet Med. 2009;11(8):588–594. doi: 10.1097/GIM.0b013e3181b13a4f. [DOI] [PubMed] [Google Scholar]
- Mouchawar J, Hensley-Alford S, Laurion S, et al. Impact of direct-to-consumer advertising for hereditary breast cancer testing on genetic services at a managed care organization: a naturally-occurring experiment. Genet Med. 2005 Mar;7(3):191–197. doi: 10.1097/01.gim.0000156526.16967.7a. [DOI] [PubMed] [Google Scholar]
- Shirts BH, Parker LS. Changing interpretations, stable genes: responsibilities of patients, professionals, and policy makers in the clinical interpretation of complex genetic information. Genet Med. 2008;10(11):778–783. doi: 10.1097/GIM.0b013e31818bb38f. [DOI] [PubMed] [Google Scholar]
- Teutch S, Tuckson RV. U.S System of Oversight of Genetic Testing. A Response to the Charge of the Secretary of Health and Human Services. 2008 Apr;2008:205–207. [Google Scholar]
- van den Berg M, Timmermans DR, ten Kate LP, van Vugt JM, van der Wal G. Informed decision making in the context of prenatal screening. Patient Educ Couns. 2006 Oct;63(1–2):110–117. doi: 10.1016/j.pec.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Wade CH, Wilfond BS. Ethical and clinical practice considerations for genetic counselors related to direct-to-consumer marketing of genetic tests. Am J Med Genet C Semin Med Genet. 2006 Nov 15;142C(4):284–292. doi: 10.1002/ajmg.c.30110. discussion 293. [DOI] [PubMed] [Google Scholar]
- Wasson K, Cook ED, Helzlsouer K. Direct-to-consumer online genetic testing and the four principles: an analysis of the ethical issues. Ethics Med. 2006 Summer;22(2):83–91. [PubMed] [Google Scholar]