Abstract
Background
Amputation has been the standard surgical treatment for distal tibia osteosarcoma. Advances in surgery and chemotherapy have made limb salvage possible. However, it is unclear whether limb salvage offers any improvement in function without compromising survival.
Questions/Purposes
We therefore compared the survival, local recurrence, function, and complications of patients with distal tibia osteosarcoma treated with limb salvage or amputation.
Methods
We retrospectively reviewed 42 patients with distal tibia osteosarcoma treated from 1985 to 2010. Nineteen patients had amputations and 23 had limb salvage and allograft reconstructions. We graded the histology using Broders classification, and staged patients using the Musculoskeletal Tumor Society (MSTS) and American Joint Committee on Cancer (AJCC) systems. The tumor grades tended to be higher in the group of patients who had amputations. We determined survival, local recurrence, MSTS function, and complications. The minimum followup was 8 months (median, 60 months; range, 8–288 months).
Results
The survival of patients who had limb salvage was similar to that of patients who had amputations: 84% at 120 and 240 months versus 74%, respectively. The incidence of local recurrence was similar: three of 23 patients who had limb salvage versus no patients who had amputations. The mean MSTS functional score tended to be higher in patients who had limb salvage compared with those who had amputations: 76% (range, 30%–93%) versus 71% (range, 50%–87%), respectively. The incidence of complications was similar.
Conclusion
Patients treated with either limb salvage or amputation experience similar survival, local recurrence, and complications, but better function is achievable for patients treated with limb salvage versus amputation. Local recurrence and complications are more common in patients with limb salvage.
Level of Evidence
Level III, retrospective comparative study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
After the femur, the tibia is the second most common site of osteosarcoma; osteosarcomas of the tibia account for 19% of all osteosarcomas, with 20% of them occurring in the distal tibia [33, 48]. The subcutaneous location and proximity of the distal tibia to the neurovascular bundle and tendons make microscopically negative margin resection of tumors in this location difficult. Therefore, some surgeons recommend below-knee amputation for tumors of the distal tibia, with predictable maintenance of function [14]. Currently, advances in surgery and chemotherapy have made limb salvage in this location possible without compromising the survival of the patients [28, 44]. However, the indications for limb salvage for patients with tumors of the distal tibia are limited. Abudu et al. [1] suggested these include Grade 3 benign tumors, malignant primary bone tumors confined to bone without soft tissue extension, and malignant tumors with extraosseous extension in patients who refuse amputation, whereas contraindications include tumor involvement of the neurovascular bundle, the ankle, or important tendons of the ankle and foot.
Conflicting findings have been reported regarding survival and function after limb salvage and amputation for patients with osteosarcoma of the distal tibia [1, 2, 8, 10, 14, 27, 28, 32, 36, 37, 39, 40, 44, 46]. Survival rates after limb salvage range from 100% to 50% at 6 to 288 months [1, 8, 14, 27, 28, 32, 36, 37, 39, 40, 44, 46], whereas those for amputation range from 100% to 84% at 36 to 60 months [6, 39, 40]. Musculoskeletal Tumor Society (MSTS) functional scores for patients [17] after limb salvage range from 50% to 100% [1, 8, 14, 27, 28, 32, 36, 37, 39, 40, 44, 46], whereas scores for patients who have had amputation range from 53% to 90% [2, 12, 15, 32, 39, 40, 47]. The best reconstruction option after limb salvage for osteosarcoma of the distal tibia remains unclear. Various megaprosthetic, allograft, and vascularized or nonvascularized autograft bone reconstruction techniques with varying survival, function, and complications rates have been reported [1, 10, 14, 20, 27, 28, 32, 36, 37, 39, 40, 44]. Survival, function, and complication rates for megaprosthetic reconstructions [18] range from 40% to 100%, 50% to 93%, and 17% to 60%, respectively [1, 28, 32, 40], whereas those for bone reconstructions range from 40% to 100%, 53% to 100%, and 12% to 92%, respectively [8, 14, 27, 36, 37, 39, 44].
To address these conflicting reports, we compared the survival, local recurrence, MSTS function, and complications of patients with osteosarcoma of the distal tibia treated with limb salvage or amputation.
Patients and Methods
We retrospectively reviewed the files of all 42 patients with osteosarcomas of the distal tibia diagnosed and treated at our institution from January 1985 to September 2010. There were 23 male and 19 female patients with a mean age of 26 years (range, 7–78 years). Limb salvage or amputation was decided after biopsy and staging. The decision regarding treatment was obtained with a high degree of consensus among the oncologic council that included orthopaedic oncology surgeons, medical oncologists, pathologists, and radiologists; decisions were made for limb salvage and allograft reconstruction for 23 patients (Table 1), and for amputation for 19 patients (Table 2). In contrast to a previous report [1], we considered limb salvage for patients with osteosarcomas of the distal tibia that did not involve the major vessels as determined by preoperative planning and intraoperative findings; if neither the posterior tibial artery nor the dorsalis pedis artery was salvageable, amputation was performed. In all cases, we aimed for microscopically negative margin resection. The minimum followup was 8 months (median, 60 months; range, 8–288 months); all patients were included in the postoperative followup and gave written informed consent for their data to be included in this study. No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. This study was approved by the Institutional Review Board/Ethics Committee of the authors’ institution.
Table 1.
Details of the patients with distal tibia osteosarcoma who had limb salvage
| Patient number/gender/age (years) | Histology/Broders grade [5] | MSTS stage [16] | AJCC stage [19] | Chemotherapy | Length of bone resection (mm)/bone margins# (proximal/distal) | Soft tissue margins# | Type of allograft reconstruction/osteosynthesis | Followup (months)/outcome | Complications (time of occurrence)/treatment | MSTS function score (%) [17] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/M/17 | Osteoblastic/4 | IIB | IIB | + | 125/wide/wide | Marginal | Arthrodesis/nail | 135/NED | – | 30 |
| 2/F/23 | Telangiectatic/4 | IIB | IIB | + | 130/wide/wide | Wide | Arthrodesis/nail | 118/NED | – | 30 |
| 3/F/18 | Chondroblastic/4 | IIA | IIA | + | 130/wide/wide | Wide | Intercalary resection/plate-screws | 48/NED | Delayed union (14 months)/iliac crest autograft | 67 |
| 4/M/12 | Osteoblastic/4 | IIB | IIA | + | 120/wide/marginal | Wide | Osteoarticular (medial malleolus preservation)/plate-screws | 8/NED | – | 70 |
| 5/F/13 | Osteoblastic/4 | IIB | IIB | + | 120/wide/wide | Wide | Arthrodesis/nail | 287/NED | – | 73 |
| 6/F/13 | Osteoblastic/4 | IIB | IIB | + | 115/wide/wide | Wide | Osteoarticular (complete)/plate-screws | 60/NED after lung metastases and metastasectomy | Allograft fracture (36 months)/arthrodesis | 77 |
| 7/F/15 | Osteoblastic/4 | IIB | IIA | + | 120/wide/wide | Wide | Osteoarticular (medial malleolus preservation)/plate-screws | 176/NED | – | 77 |
| 8/M/46 | Osteoblastic/2 | IB | IB | − | 120/wide/wide | Wide | Osteoarticular (medial malleolus preservation)/plate-screws | 12/NED | – | 77 |
| 9/M/35 | Osteoblastic/2 | IA | IB | − | 120/wide/wide | Marginal | Arthrodesis/plate-screws | 148/NED | – | 80 |
| 10/M/13 | Osteoblastic/4 | IIB | IIB | + | 150/wide/wide | Wide | Arthrodesis/plate-screws | 10/NED after local recurrence and amputation | – | 80 |
| 11/F/41 | Osteoblastic/4 | IIB | IIB | + | 150/wide/wide | Wide | Intercalary resection/plate-screws | 80/NED | – | 80 |
| 12/F/30 | Osteoblastic/2 | IB | IB | − | 160/wide/marginal | Marginal | Osteoarticular (medial malleolus preservation)/plate-screws | 8/NED | – | 80 |
| 13/M/18 | Osteoblastic/4 | IIB | IIB | + | 125/wide/wide | Wide | Arthrodesis/nail | 286/NED | – | 83 |
| 14/M/28 | Osteoblastic/4 | IIB | IIB | + | 145/wide/wide | Wide | Intercalary resection/plate-screws | 188/NED | Delayed union (18 months)/iliac crest autograft | 83 |
| 15/M/14 | Osteoblastic/4 | IIA | IIB | + | 140/wide/wide | Wide | Intercalary resection/plate-screws | 32/NED | Allograft fracture (28 months)/arthrodesis | 83 |
| 16/M/18 | Osteoblastic/2 | IB | IB | − | 155/wide/wide | Wide | Intercalary resection/plate-screws | 35/NED | – | 87 |
| 17/F/51 | Parosteal/1–2 | IB | IB | − | 130/wide/wide | Wide | Osteoarticular (medial malleolus preservation)/plate-screws | 105/NED after lung metastases and metastasectomy | Nonunion (22 months)/debridement, revision of fixation | 87 |
| 18/F/10 | Osteoblastic/4 | IIB | IIB | + | 135/wide/marginal | Marginal | Osteoarticular (medial malleolus preservation)/plate-screws | 38/NED | – | 90 |
| 19/M/18 | Parosteal/1–2 | IB | IB | − | 125/wide/wide | Intra-lesional | Arthrodesis/plate-screws | 288/NED after local recurrence and amputation | – | 80 |
| 20/F/34 | Osteoblastoma-like/4 | IIB | IIB | + | 130/wide/wide | Wide | Arthrodesis/plate-screws | 40/Dead of other disease | Infection (14 months)/debridement, revision of fixation | 83 |
| 21/M/43 | Fibroblastic/4 | IIIB* | IVA* | + | 150/wide/wide | Wide | Intercalary resection/plate-screws | 16/DWD | – | 87 |
| 22/F/12 | Osteoblastic/3 | IIB | IIB | + | 130/wide/wide | Wide | Intercalary resection/plate-screws | 22/DWD | – | 80 |
| 23/M/17 | Parosteal/2 | IIB | IIB | − | 150/wide/wide | Intra-lesional | Osteoarticular (medial malleolus preservation)/plate-screws | 60/NED after local recurrence and amputation | – | 93 |
* Lung metastases and metastasectomy at diagnosis and tumor size > 8 cm; #wide and marginal margins = microscopically negative, intralesional margins = microscopically positive; MSTS = Musculoskeletal Tumor Society; AJCC = American Joint Committee on Cancer; NED = no evidence of disease; DWD = dead with disease.
Table 2.
Details of the patients with distal tibia osteosarcoma who had amputations.
| Patient number/gender/age (years) | Histology/Broders grade [5] | MSTS stage [16] | AJCC stage [19] | Chemotherapy | Followup (months)/outcome | Complications/treatment | MSTS function score (%) [17] |
|---|---|---|---|---|---|---|---|
| 1/F/10 | Osteoblastic/4 | IIB | IIB | + | 268/NED | – | 70 |
| 2/F/11 | Osteoblastic/3 | IIB | IIB | + | 124/NED | – | 77 |
| 3/F/14 | Chondroblastic/4 | IIB | IIB | + | 175/NED | – | 63 |
| 4/M/19 | Osteoblastic/4 | IIB | IIB | + | 24/DWD | – | 80 |
| 5/M/18 | Osteoblastic/4 | IIB | IIB | + | 140/NED | – | 80 |
| 6/M/7 | Osteoblastic/4 | IIB | IIB | + | 42/DWD | – | 47 |
| 7/M/16 | Osteoblastic/4 | IIIB* | IVA* | + | 118/NED | – | 77 |
| 8/F/10 | Osteoblastic/4 | IIB | IIB | + | 130/NED | – | 70 |
| 9/M/10 | Chondroblastic/4 | IIB | IIB | + | 143/NED after lung metastases and metastasectomy | – | 60 |
| 10/F/24 | Osteoblastic/4 | IIIB* | IVA* | + | 58/DWD | – | 50 |
| 11/F/78 | Osteoblastic/4 | IIB | IIB | + | 10/DWD | – | 87 |
| 12/F/58 | Telangiectatic/4 | IIB | IIB | + | 84/NED | Wound dehiscence/débridement | 77 |
| 13/M/63 | Fibroblastic/4 | IIB | IIB | + | 67/NED | – | 83 |
| 14/M/18 | Osteoblastic/4 | IIB | IIB | + | 67/NED | – | 63 |
| 15/M/46 | Fibroblastic/3 | IIB | IIB | + | 50/NED after lung metastases and metastasectomy | – | 80 |
| 16/M/62 | Osteoblastic (Paget’s disease)/4 | IIB | IIB | + | 22/NED | – | 87 |
| 17/F/43 | Parosteal/2 | IIIB* | IVA* | + | 22/NED | – | 80 |
| 18/M/17 | Osteoblastic/4 | IB | IB | + | 20/NED | – | 60 |
| 19/M/25 | Osteoblastic/4 | IIB | IIA | + | 10/NED | – | 57 |
* Lung metastases and metastasectomy at diagnosis and tumor size > 8 cm; MSTS = Musculoskeletal Tumor Society; AJCC = American Joint Committee on Cancer; NED = no evidence of disease; DWD = dead with disease.
For the initial evaluation, all patients had plain orthogonal radiographs, CT scan, MRI, and bone scan. After imaging, we performed Tru-Cut® (Cardinal Health, Dublin, OH, USA) needle biopsy. In 15 patients, the needle biopsy was not diagnostic therefore we performed an incisional biopsy in these patients.
We graded the histologic sections based on the biopsy using the classification of Broders [5]. This classification defines four grades according to the rate of differentiation of the tumor cells: Grade 1 (well differentiated) includes less than 25% undifferentiated cells; Grade 2 (moderately differentiated) includes less than 50% undifferentiated cells; Grade 3 (poorly differentiated) includes less than 75% undifferentiated cells; and Grade 4 (anaplastic/pleomorphic) includes greater than 75% undifferentiated cells [5]. Seven of the 23 patients who had limb salvage had a Grade 1 or 2 tumor and 16 had a Grade 3 or 4 tumor. For the 19 patients who had amputations, one had a Grade 2 tumor and 18 had a Grade 3 or 4 tumor. All patients with Grades 3 and 4 osteosarcomas and one patient with a Grade 2 parosteal osteosarcoma with metastases at diagnosis (Patient 17; Table 2) had adjuvant and/or neoadjuvant chemotherapy.
We staged patients using the surgical staging systems of the MSTS [16] and the American Joint Committee on Cancer (AJCC) [19]. The tumor was Stage I in the MSTS and AJCC systems for six of the 23 patients who had limb salvage and in one of the 19 patients who had amputations; all remaining patients had tumors that were Stage II or greater. The tumor was contained in the bone (A in the MSTS system) in three of the 23 patients who had limb salvage; none of the patients who had amputations had the tumor contained in bone. The tumors were 8 cm or smaller (A in the AJCC system) in three of 23 patients who had limb salvage and in one of 19 patients who had amputations; all remaining patients had tumors that were larger than 8 cm (B in the AJCC system). Lung metastases (Stage III in the MSTS and Stage IV in the AJCC systems) were diagnosed at presentation and treated with metastasectomy in one of 23 patients who had limb salvage and in three of 19 patients who had amputations. The male to female ratio was 12:11 for the patients who had limb salvage and 11:8 for patients who had amputations. The mean age was 23 years (range, 10–51 years) for the patients who had limb salvage, and 29 years (range, 7–78 years) for patients who had amputations. The median followups were 67 months (range, 8–287 months) for the patients who had limb salvage, and 60 months (range, 10–268 months) for those who had amputations. The demographic and clinical details of the patients who had limb salvage and those who had amputations were evaluated for potential confounding variables at baseline; the tumor grades tended to be higher in the group of patients who had amputations (Mann-Whitney U test, p = 0.041) (Table 3).
Table 3.
Demographic and clinical variables at baseline and statistical analysis between the patients
| Variable | Limb salvage (n = 23 patients) | Amputation (n = 19 patients) | Statistical significance | Statistical test |
|---|---|---|---|---|
| Sex (male:female) | 12:11 | 11:8 | p = 0.711 | Chi-square |
| Age at diagnosis | Mean, 23 years (range, 10–51 years) | Mean, 29 years (range, 7–78 years) | p = 0.346 95% CI, −6.22–17.14 | Student’s t-test (normal distribution, Kolmogorov-Smirnov Z, p = 0.709) |
| Tumor grade [5] | ||||
| Grades 1 and 2 | 7 | 1 | *p = 0.041 | Mann-Whitney U |
| Grades 3 and 4 | 16 | 18 | ||
| Tumor stage [16, 19] | ||||
| Low | 6 | 1 | p = 0.075 | Mann-Whitney U |
| High | 17 | 18 | ||
| Tumor containment in bone [16] | ||||
| Contained | 3 | 0 | p = 0.239 | Fisher’s exact |
| Not contained | 20 | 19 | ||
| Tumor size [19] | ||||
| ≤ 8 cm | 3 | 1 | p = 0.398 | Mann-Whitney U |
| > 8 cm | 20 | 18 | ||
| Metastases at diagnosis | 1 | 3 | p = 0.313 | Fisher’s exact |
| Followup | Median, 67 months (range, 8–287 months) | Median, 60 months (range, 10–268 months) | p = 0.621 95% CI, −39.10–64.71 | Student’s t-test (normal distribution, Kolmogorov-Smirnov Z, p = 0.531) |
* Tumor grade tended to be higher in the group of patients who had amputations, indicating a potential confounding baseline variable between the two groups of patients.
Below-knee amputation was planned to (1) resect the bone 2 cm to 3 cm proximal to abnormal bone density and signal intensity as determined by CT and MRI, respectively, or increased radioisotope uptake as determined by bone scan, (2) obtain adequate length of the residual limb that was at least 3 cm below the tibial tubercle, and (3) achieve good soft tissue coverage with the residual fibula 2 cm to 3 cm shorter [29, 45]. Limb salvage was planned to (1) include the previous biopsy site, (2) resect the tumor with a normal muscle cuff in all directions, and (3) resect the bone 2 cm to 3 cm beyond abnormal uptake [29]. The mean length of bone resection in the patients who had limb salvage was 134 mm (range, 115–160 mm). Intraoperatively, the adequacy of bone resection was evaluated with frozen section biopsy of a tissue sample obtained from the medullary canal of the residual tibia. The margins of bone and soft tissue resections of the tumor specimens were defined as (1) intralesional (when the tumor is entered or cut into at any point during surgery; microscopically positive), (2) marginal (when a cuff of less than 2 cm to 3 cm of normal tissue is left on all sides of the tumor, or the surgical dissection extends into or through the abnormal, reactive tissues that surround the tumor but are not actually a part of the tumor, the so-called “reactive zone”; possibly microscopically positive), and (3) wide (when the reactive zone is not entered, but instead the dissection is through entirely normal tissues, and a cuff of 2 cm to 3 cm of normal tissue is left on all sides of the tumor; microscopically negative) [9, 16]. The margins of proximal bone resections were wide (microscopically negative) for all patients. The margins of distal bone resections were marginal (0.7 cm to 1 cm, microscopically negative) in three of the eight patients who had osteoarticular allograft reconstructions (Patients 4, 12, and 18; Table 1). Because we consider the articular cartilage as an anatomic barrier to tumor extension [41], in these patients we accepted microscopically negative marginal margins of bone resection from the articular cartilage. The margins of soft tissue resections were microscopically negative (wide) in 17 but less than 2 cm to 3 cm is required for the above definition of wide. The margins were microscopically negative and less than 2 cm to 3 cm in four (marginal), and microscopically positive (intralesional) in two of the 23 patients who had limb salvage. The margins were wide by the above definition and microscopically negative for all patients who had amputations.
In the case of limb salvage, after tumor resection we performed allograft reconstruction. A matched allograft was chosen and osteosynthesis was performed according to the surgeons’ preference with long plates and screws that spanned the total length of the allograft and extended to the proximal tibia for at least six to eight cortices (three to four screws), or with a calcaneal nail in cases with ankle arthrodesis. For patients with greater than 150 mm bone resection, to enhance union of the allograft with the residual tibia we performed osteotomy and transposition of the ipsilateral fibula; the fibular osteotomy was performed approximately 2 cm proximal to the level of the tibial osteotomy, and the fibula was approximated to the tibia and stabilized with screws (Fig. 1). Primary ankle arthrodesis was preferred for adults and for tumors involving the distal tibiofibular joint. The ankle was fused with either a calcaneal nail or tibiotalar screws in neutral dorsiplantar flexion and 0° to 5° valgus, with the foot shifted posteriorly and in external rotation so that the anatomic axis of the tibia is over the axis of the foot (middle of the ankle or second metatarsal) [7, 25, 26]. Intercalary resection and reconstruction were performed for tumors 3 cm or greater from the distal tibiofibular joint. Intraarticular resection and osteoarticular allograft reconstruction were performed for tumors less than 3 cm from the distal tibiofibular joint without joint involvement [41]. We aimed to preserve the deltoid ligament with part of the native medial malleolus for improved stability of the ankle; an oblique osteotomy was performed at the base of the medial malleolus, and the fragment with the medial ankle ligamentous complex intact was stabilized with one or two screws to the osteoarticular allograft after osteotomy of the medial malleolus of the allograft. For three patients (Patients 4, 12, and 18; Table 1), the osteotomy of the medial malleolus was performed closer to its apex aiming for microscopically negative, although marginal margins (0.7 cm to 1 cm) of bone resection. For one patient (Patient 6, Table 1), the medial malleolus was not salvageable and a complete osteoarticular allograft was used. If osteotomy of the lateral malleolus was necessary for resection or allograft reconstruction, osteosynthesis of the osteotomy was performed with a plate and screws or a long intramedullary screw. Primary wound closure was feasible without local or free muscle flaps in all cases. Postoperatively, for the patients who had limb salvage, a short cast was applied until wound healing was achieved, followed by a hinged brace and protective weightbearing until there was radiographic evidence of graft-host bone union.
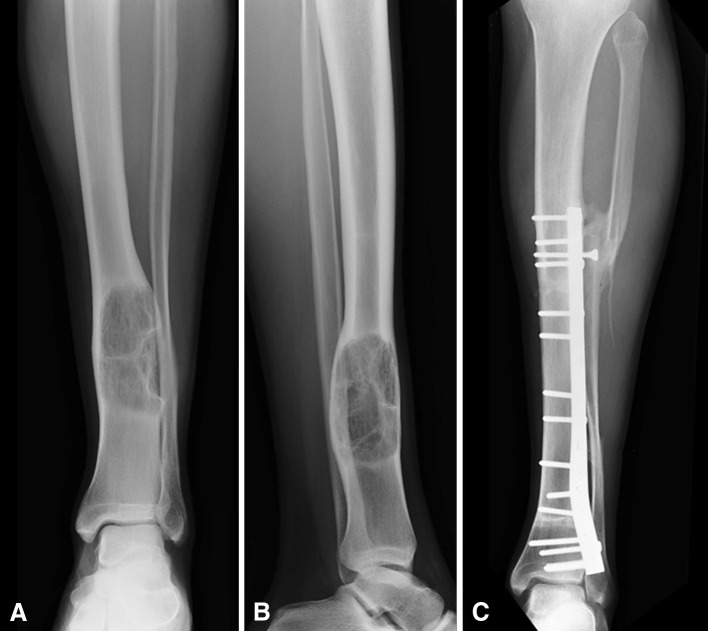
Fig. 1A–C.
(A) AP and (B) lateral radiographs show the left tibia and ankle of an 18-year-old male with a distal tibia osteosarcoma treated with intercalary resection and allograft reconstruction, in addition to osteotomy and transposition of the fibula to the tibia to enhance healing of the allograft-host bone junction (Patient 16). (C) Thirty-five months after diagnosis and surgical treatment, the AP radiograph shows complete healing of the allograft.
Routine followups including clinical examination and radiographs of the leg and chest were performed every 6 weeks for the first 6 months, every 6 months for the first 3 years, and then annually. Chest CT scan was performed every 6 months for the first 3 years, and then annually. Complications, local recurrence, metastasis, and death were recorded from the patients’ files. We classified patients as (1) having no evidence of disease (NED), and (2) dead with disease (DWD). We evaluated their function using the modified MSTS functional scoring system [17], and graded complications using the classification of surgical complications described by Dindo et al. [13]. Ankle instability was suspected on the basis of a patient’s feeling that his or her ankle was giving-way and having ankle pain when walking on even ground, and was assessed with clinical stress tests including the varus-valgus tilt test and anterior drawer test, which were compared with results of the contralateral side [21]. For patients with limb salvage, long AP and lateral radiographs of the tibia including the ankle were obtained at each followup. Two of us (AFM and PR) and one radiologist (ER) independently evaluated all radiographs. To reduce interobserver variability, we reviewed the last followup images on computer workstations using consistent radiographic criteria for union [23], ankle deformity [7, 25, 26], and degenerative changes [38]. We presumed union of the allograft was achieved when continuous external bony borders between the graft and the recipient bone in addition to obscured or absent osteotomy lines were observed, and union of the arthrodesis when trabeculation was seen across the site of the arthrodesis [23]. We evaluated deformity by equinus, varus, valgus, and rotational malalignment of the axis of the foot with respect to the anatomic axis of the tibia [7, 25, 26]. Degenerative changes of the ankle were evaluated by nonuniform joint space loss, osteophyte formation, cyst formation, and subchondral sclerosis [38]. There were no missing radiographs.
We compared demographic and preoperative clinical variables between the two groups of patients at baseline. Comparison with the parametric Student’s t-test was done for nominal variables such as age and followup after confirmation of the normality of the distribution with the Kolmogorov-Smirnov Z test. Comparison with nonparametric tests was done for ordinal variables such as gender (chi-square test), tumor grade (Mann-Whitney U test), stage (Mann-Whitney U test), containment in bone (Fisher’s exact test), size (Mann-Whitney U test), and metastases at diagnosis (Fisher’s exact test) (Table 3). Survival to death was evaluated using Kaplan-Meier analysis [24]. We compared survival between the two treatment groups using a log-rank test, MSTS function using the Mann-Whitney U test, and local recurrences and complications using Fisher’s exact test. The data were recorded in a Microsoft® Excel® 2003 spreadsheet (Microsoft Corporation, Redmond, WA, USA) and analyzed using MedCalc® Software Version 11.1 (MedCalc Software, Mariakerke, Belgium).
Results
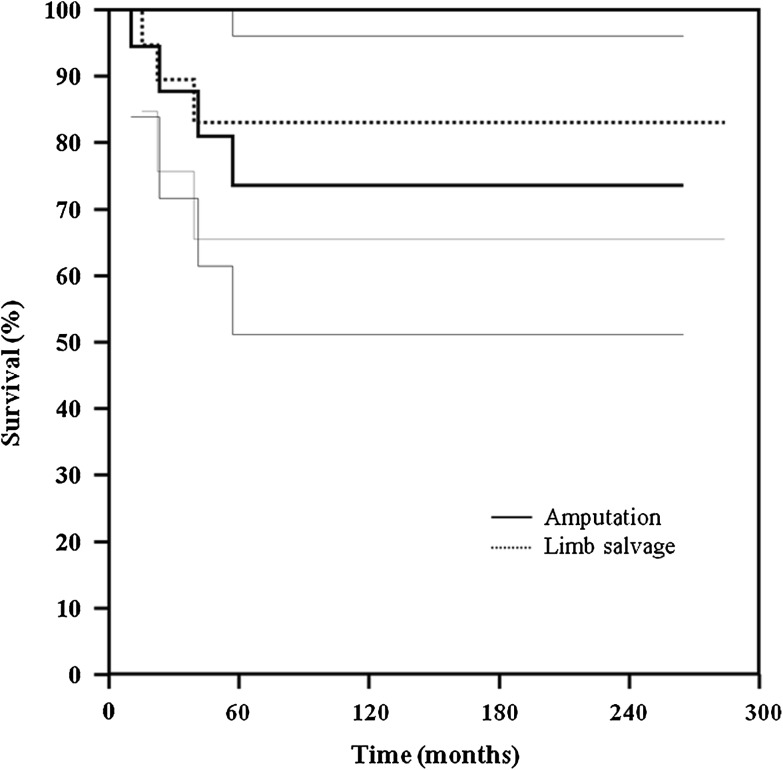
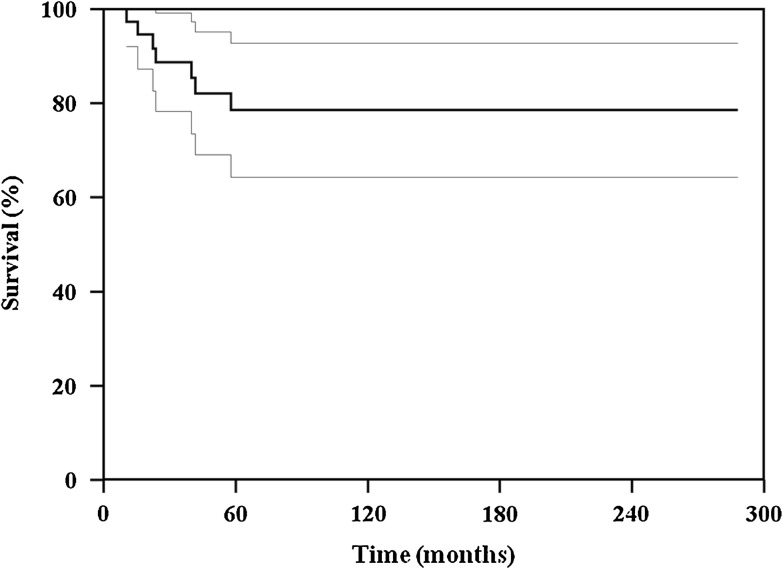
The survival of the patients who had limb salvage was similar (p = 0.599) to that of the patients who had amputations: 84% at 120 and 240 months versus 74%, respectively (Fig. 2). The overall survival for the patients with osteosarcoma of the distal tibia was 78% at 120 and 240 months (Fig. 3). At the last followup, 35 patients had NED and six were DWD; four patients had lung metastases develop and were treated with metastasectomy (Tables 1 and 2). One patient (Patient 20, Table 1) died from injuries sustained in a motor vehicle accident.
Fig. 2.
A graph shows similar (p = 0.599) survival for patients with distal tibia osteosarcomas treated with limb salvage and with amputation (95% CI, 0.3370–6.5701).
Fig. 3.
A graph shows the overall survival for patients with distal tibia osteosarcomas included in this series.
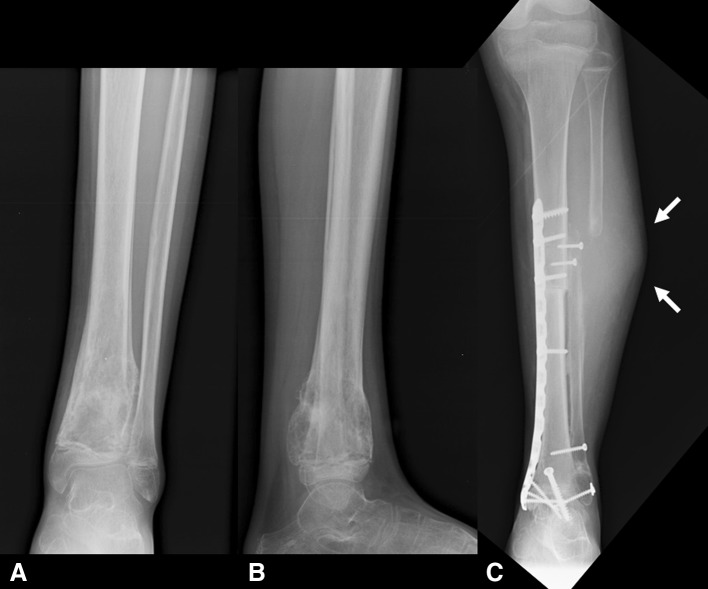
The incidence of local recurrence for the patients who had limb salvage was similar (p = 0.238) to the incidence for patients who had amputations: three of the 23 patients who had limb salvage (13%; Patients 10, 19, and 23, Table 1) experienced local recurrence and were treated with amputation (Fig. 4), whereas no patients with amputations experienced local recurrence.
Fig. 4A–C.
(A) AP and (B) lateral radiographs show the left tibia and ankle of a 13-year-old boy with a distal tibia osteosarcoma treated with arthrodesis (Patient 10). (C) An AP radiograph shows healing of the allograft and local recurrence at the proximal osteotomy (arrows) 10 months after diagnosis and surgical treatment; amputation was performed.
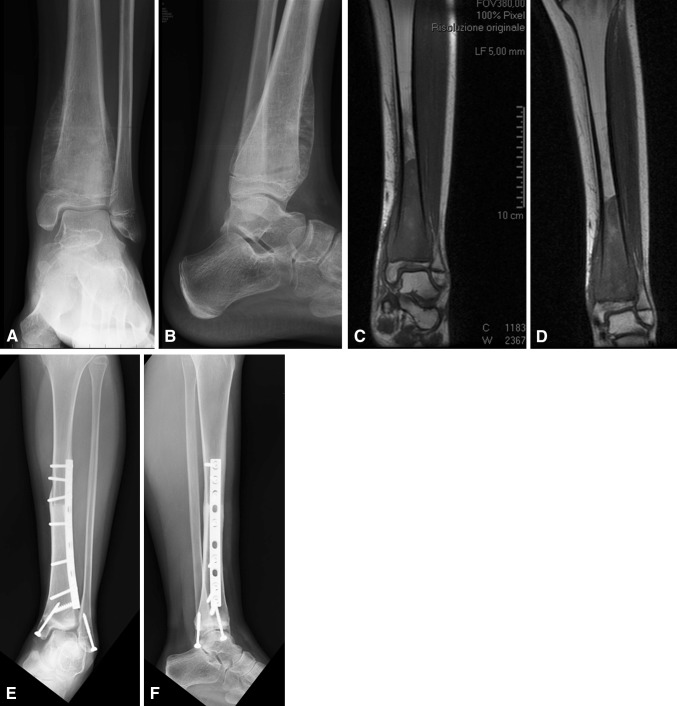
The mean MSTS functional score for the patients [17] who had limb salvage tended to be higher (p = 0.044) compared with the score for patients who had amputations: 76% (range, 30%–93%) versus 71% (range, 50%–87%), respectively. We observed no evidence of ankle instability, deformity, or radiographic evidence of degenerative changes of the ankle in any of the patients with osteoarticular allograft reconstructions who had retained their allografts as of the last followup (Fig. 5). All these patients were ambulatory with mild pain (Patients 4 and 8) or pain-free (Patients 6, 7, 12, 17, and 18).
Fig. 5A–F.
(A) AP and (B) lateral radiographs show the left tibia and ankle of a 10-year-old girl with a distal tibia osteosarcoma (Patient 18). Preoperative coronal T1-weighted MR images (C) before and (D) after neoadjuvant chemotherapy show a 10 cm distal tibia osteosarcoma that is not contained in bone. (E) AP and (F) lateral radiographs after limb salvage and osteoarticular allograft reconstruction with preservation of the medial malleolus show healing of the allograft 38 months after diagnosis and surgical treatment.
The incidence of complications for the patients who had limb salvage was similar (p = 0.100) to that for patients who had amputations. Six of the 23 patients who had limb salvage (Table 1) experienced complications. Two of the seven patients with intercalary allografts (Patients 3 and 14, Table 1) experienced delayed union and one (Patient 17, Table 1) experienced nonunion; these patients were treated with autologous iliac crest bone grafting and revision of the fixation and eventually achieved union. One of the eight patients with osteoarticular allografts (Patient 6, Table 1) and one of the seven patients with intercalary allografts (Patient 15, Table 1) experienced allograft fractures and were treated with arthrodesis. One of the eight patients who had arthrodesis (Patient 20, Table 1) experienced an infection and was treated with débridement and revision of the osteosynthesis without recurrence of the infection as of last followup. One of the 19 patients who had amputations (5.3%; Patient 12, Table 2) experienced wound dehiscence and was treated with wound debridement. All complications in both groups were Grade IIIb (not life-threatening but which required intervention under general anesthesia) [13].
Discussion
Although there is considerable literature regarding surgical treatment of osteosarcoma of the distal tibia [1, 8, 14, 27, 28, 32, 36, 37, 39, 40, 44, 46, 48], the data regarding survival and function of patients after limb salvage and amputation for osteosarcoma in this location have been conflicting [1, 2, 8, 10, 14, 27, 28, 32, 36, 37, 39, 40, 44, 46]. We therefore compared survival, local recurrence, function, and complications of patients with osteosarcoma of the distal tibia treated with limb salvage or amputation. Survival, local recurrence, and complications were similar, but function was better in the patients who had limb salvage versus those who had amputations; however, the conclusions are tempered by the small numbers and differences in confounding variables between the groups.
We acknowledge three limitations in this series. First, although it is the largest reported series of patients with osteosarcoma of the distal tibia, the problem is relatively infrequent and the sample size is still relatively small to draw definite conclusions regarding the treatments we studied because the study had inadequate power to control for potentially confounding variables. However, the consistency of treatment, inclusion of technical points, and complete followup allows us to draw limited conclusions we believe are valuable. Second, the osteosynthesis techniques of the reconstructions after limb salvage were not evaluated because they were not subjected to a treatment protocol but were performed according to the surgeons’ preference. However, the purpose of this study was not to evaluate the survival, function, and complications of reconstructions after limb salvage in the distal tibia. Third, the two groups of patients were not randomly selected. Therefore, we performed a statistical analysis to evaluate potentially confounding variables between the two groups at baseline (Table 3). Our analysis showed that the tumor grades tended to be higher in the group of patients who had amputations.
Previous studies reported similar survival for patients after limb salvage or amputation for osteosarcoma of the distal tibia (Table 4) [1, 8, 10, 14, 15, 28, 32, 34, 36, 39, 40, 46, 47]. However, these were small series that mostly evaluated different types of reconstructions after limb salvage for various benign and malignant tumors in the distal leg [27, 28, 32, 39, 40, 44, 46]. In addition, none of these series evaluated amputation as a primary treatment but rather as a secondary treatment for local tumor recurrence or complication of the limb salvage. By reviewing only patients with osteosarcoma of the distal tibia, the survival rates after limb salvage range from 100% to 50% at 6 to 288 months [1, 8, 14, 27, 28, 32, 36, 37, 39, 40, 44, 46], whereas those for amputation range from 100% to 84% at 36 to 60 months [39, 40]. In our series, the survival of the patients who had limb salvage was similar to that of the patients who had amputations.
Table 4.
Summary of published studies on limb salvage and amputation for tumors of the distal tibia
| Study | Patients | Tumor/MSTS stage [17] | Resection length (mm) | Reconstruction | Survival (patients) | Function/score | Local recurrence (patients, treatment) | Complications (patients, type) | Followup (months) | |
|---|---|---|---|---|---|---|---|---|---|---|
| LS | BKA | |||||||||
| Abudu et al. [1] | 5 | – | Variable/IIB | N/A | Total ankle megaprostheses | 2/4 | 65% (50%–90%)/MSTS | 1/5 (BKA) | 2/5 (infection, wound necrosis, implant loosening) | Mean, 74 (44–102) |
| Campanacci et al. [8] | 8 | – | Variable/N/A | 50–210 | Arthrodesis (allograft) | 8/8 | 80.4% (53%–93%)/MSTS | 1/8 (BKA) | 1/8 (infection) | Mean, 53.5 (23–113) |
| Ebeid et al. [14] | 13 | 6* | Osteosarcoma, Ewing’s sarcoma/IIB | 120 (80–170) | Arthrodesis (ipsilateral vascularized fibular graft) | 9/13 | 80% (73%–90%)/MSTS | 1/13 (BKA) | 12/13 (nonunion, infection, fracture, ankle deformity, LLD) | Mean, 27 (16–90) |
| Laitinen et al. [27] | 14 | 1* | Variable/variable | 135 (110–190) | Allografts, vascularized fibular graft, bone transport, fibula transfer, or megaprosthesis | 14/14 | Fair and poor/Casadei et al. score [10] | 0/14 | 11/14 (infection, wound healing problems, nonunion, fracture, deformity, LLD) | Mean, 84 (6–168) |
| Lee et al. [28] | 5 | – | Osteosarcoma/IIB | 120–170 | Total ankle megaprostheses | 5/5 | 80.5% (6%–90%)/MSTS | 0/5 | 2/5 (infection, talar collapse) | Mean, 65 (24–84) |
| Natarajan et al. [32] | 6 | – | Osteosarcoma/IIB | 163 | Total ankle megaprostheses | 6/6 | 80% (77%–87%)/MSTS | 2/6 (BKA) | 1/6 (wound necrosis, infection) | Mean, 40 (24–68) |
| Puri et al. [36] | 13 | – | Variable/N/A | 100–210 | Arthrodesis, intercalary (vascularized fibular graft) | 13/13 | 82% (53%–100%)/MSTS | 0/13 | 10/13 (nonunion, instability, LLD) | Mean, 29 (16–48) |
| Ramseier et al. [37] | 19 | – | Variable/N/A | N/A | Osteoarticular, intercalary (allograft) | 14/19 | N/A | 1/19 (BKA) | 17/19 (nonunion, wound healing problems, deformity, LLD, degenerative changes) | Mean, 66 (14–198) |
| Shalaby et al. [39] | 6 | – | Osteosarcoma/IIB | 170 (150–190) | Arthrodesis (autogenous fibular graft, Ilizarov) | 6/6 | 70% (63%–73%)/MSTS | 1/6 (BKA) | 2/6 (infection, fracture) | Mean, 35 (28–42) |
| Shekkeris et al. [40] | 6 | – | Variable/N/A | N/A | Total ankle megaprostheses | 6/6 | 70% (53%–80%)/MSTS | 0/6 | 3/6 (infection, LLD) | Mean, 11 (12–324) |
| 71% (57%–79%)/TESS | ||||||||||
| Stéphane et al. [44] | 13 | – | Variable | 120 (85–150) | Arthrodesis (autogenous tibia and fibula graft) | 10/13 | 82% (77%–87%)/MSTS | 2/13 (BKA) | 12/13 (infection, nonunion, fracture, deformity, LLD) | Mean, 106 (12–288) |
| Current study | 23 | 19 | Osteosarcoma/variable | 134 (115–160) | Arthrodesis, intercalary, osteoarticular (allograft) | LS, 20/23 | LS, 76% (30%–93%)/MSTS | LS, 3/23 (BKA) | LS, 6/23 (delayed/nonunion, fracture, infection) | Median, 60 (8–288) |
| BKA, 15/19 | BKA, 71% (50%–87%)/MSTS | BKA, 0/19 | BKA, 1/19 (wound dehiscence) | |||||||
* Not studied; LS = limb salvage; BKA = below-knee amputation; LLD = limb length discrepancy; N/A = not available; MSTS = Musculoskeletal Tumor Society score; TESS = Toronto extremity salvage score.
Limb salvage for tumors of the distal tibia is challenging owing to the difficulty in obtaining microscopically negative margin resection because of the proximity of nerves, vessels, and important tendons of the foot [12, 28]. Local recurrence rates after limb salvage for osteosarcoma of the distal tibia range from 10% to 33% [10, 27, 32, 39, 44]. To the best of our knowledge, local recurrence has not been reported after primary or secondary below-knee amputation for osteosarcoma of the distal tibia [10, 27, 32, 39, 40, 44, 46]. In our series, the incidence of local recurrence was similar between the groups; three of the 23 patients who had limb salvage experienced local recurrence, whereas no patients with amputations experienced local recurrence. An increased risk of local recurrence and distant metastases has been associated with poor response to chemotherapy as assessed by pain relief, change in tumor volume, and change in bone scan or positron emission tomography (PET) activity [39]. However, our decision for limb salvage or amputation is not based on the response to chemotherapy but on neurovascular involvement by the tumor. We believe that the response to chemotherapy should be related to the prognosis; excellent response to chemotherapy and microscopically negative margins obtained either with limb salvage or with amputation are important prognostic factors for local control of osteosarcoma [35].
Below-knee amputation has been the standard surgical treatment for patients with osteosarcoma of the distal tibia, achieving microscopically negative margins, predictable outcome, improved health-related quality of life, and best function with an appropriate prosthesis [3, 40]. Others reported similar results for function of patients after limb salvage or amputation for osteosarcoma in this location [1, 8, 10, 14, 15, 28, 32, 34, 36, 39, 40, 46, 47]. The MSTS function for patients [17] after limb salvage range from 50% to 100% [1, 8, 14, 27, 28, 32, 36, 37, 39, 40, 44, 46], whereas those for amputation range from 53% to 90% [2, 12, 15, 32, 39, 40, 47]. In our series, the mean MSTS functional scores for the patients tended to be better for those who had limb salvage versus those who had amputations. The strengths of the MSTS score are its ease of use, acceptance after extensive modifications and field trial, and provision of data from which it may be refined in the future. The major disadvantages are its ad hoc development with no formal item generation or reduction process, degree of subjectivity that allows slight interobserver variability, and inherent compromises of a system that is applicable to numerous different resections, reconstructions, and anatomic sites without becoming so complex that its usefulness would be sharply curtailed [18]. Although we acknowledge these limitations, we prefer to evaluate patients with the MSTS score because it emphasizes comprehensive evaluation of factors (pain, functional activities, and emotional acceptance) pertinent to the patient as a whole [17]. In addition, by using the MSTS score our results could be compared more easily with those in the literature, as it is commonly used in numerous series [1, 8, 14, 32, 36, 39, 40, 44].
The risk for complications should be considered when evaluating surgical treatment for tumors of the distal tibia. Complication rates after limb salvage for osteosarcoma of the distal tibia range from 17% to 92% [1, 8, 10, 14, 28, 32, 39, 40, 44, 46]. Many series reporting primary or secondary below-knee amputation have not reported complications [1, 10, 14, 28, 32, 39, 40, 44, 46]. In decreasing incidence, the most common complications after limb salvage and variable megaprosthetic and bone reconstructions at the distal tibia are infection [1, 8, 14, 27, 32, 37, 39, 44], allograft fractures [14, 27, 37, 39, 44], nonunion [14, 27, 36, 44], wound healing problems and flap necrosis [1, 27, 32, 37], ankle instability and deformity related to resection of the malleoli [14, 27, 34, 44], limb length discrepancy [14, 27, 40], degenerative changes of the ankle [37], and talar collapse [28]. Complications of total ankle megaprostheses including instability and loosening, soft tissue necrosis, infection, and deterioration of function with time range from 17% to 60% [1, 28, 32, 40]. Complications of vascularized fibula autograft including fracture, infection, nonunion, deformity, prolonged immobilization, and donor-site morbidity range from 33% to 92% [14, 23, 27, 36, 39]. Arthrodesis may be the best reconstructive procedure for the ankle because it provides for excellent stability [10, 34, 39, 44]. Complications of arthrodesis including nonunion, limb length discrepancy, infection, and fracture range from 12% to 92% [8, 10, 14, 27, 28, 36, 39, 44]. Complications of osteoarticular and intercalary allograft reconstructions including infection, fracture, nonunion, deformity, and degenerative changes with loss of ankle motion range from 12% to 89% [8, 27, 31, 34, 37]. In our series, the incidence of complications was similar between the groups; complications with limb salvage and allograft reconstructions included delayed union, nonunion, and fracture, none of which occurred in patients who had amputations. These complications were not life threatening but required an intervention under general anesthesia [13]. To avoid late ankle deformity or instability, repair of the soft tissues and reconstruction of the tibiofibular mortise are necessary [10]. Biomechanical and anatomic studies emphasized the role of ankle ligaments in the coupling mechanism between the foot and leg [4, 22, 30, 43], and showed that this mechanism substantially depends on the integrity of the deltoid ligament [11, 21, 22, 43]. When the deltoid ligament with part of the native medial malleolus can be saved and reattached to the osteoarticular distal tibia allograft, as in seven patients in our series, the biomechanics and stability of the ankle should be markedly improved [11, 21, 22, 43].
Similar survival, local recurrence and complications, but better function is achievable with limb salvage versus amputation for patients with osteosarcoma of the distal tibia. Although local recurrence and complications are more common in patients with limb salvage, as survival is not affected by the type of surgery it could be worthwhile to accept these higher risks, with strict followup of selected patients for whom limb salvage is feasible, and reserve amputation for patients with local recurrence.
Acknowledgment
We thank Eugenio Rimondi MD, radiologist, for evaluation of the imaging of the patients.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution has approved the reporting of these cases and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Istituto Ortopedico Rizzoli, Bologna, Italy.
References
- 1.Abudu A, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement of the distal tibia and ankle joint for aggressive bone tumours. Int Orthop. 1999;23:291–294. doi: 10.1007/s002640050374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aksnes LH, Bauer HC, Jebsen NL, Follerås G, Allert C, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br. 2008;90:786–794. doi: 10.1302/0301-620X.90B6.19805. [DOI] [PubMed] [Google Scholar]
- 3.Barrera M, Teall T, Barr R, Silva M, Greenberg M. Health related quality of life in adolescent and young adult survivors of lower extremity bone tumors. Pediatr Blood Cancer. 2012;58:265–273. doi: 10.1002/pbc.23017. [DOI] [PubMed] [Google Scholar]
- 4.Boardman DL, Liu SH. Contribution of the anterolateral joint capsule to the mechanical stability of the ankle. Clin Orthop Relat Res. 1997;341:224–232. doi: 10.1097/00003086-199708000-00033. [DOI] [PubMed] [Google Scholar]
- 5.Broders AC. Squamous-cell epithelioma of the skin: a study of 256 cases. Ann Surg. 1921;73:141–160. doi: 10.1097/00000658-192102000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchner M, Bernd L, Zahlten-Hinguranage A, Sabo D. [Bone and soft-tissue tumors of the foot and ankle][in German]. Chirurg. 2005;76:391–397. [DOI] [PubMed]
- 7.Buck P, Morrey BF, Chao EY. The optimum position of arthrodesis of the ankle: a gait study of the knee and ankle. J Bone Joint Surg Am. 1987;69:1052–1062. [PubMed] [Google Scholar]
- 8.Campanacci DA, Scoccianti G, Beltrami G, Mugnaini M, Capanna R. Ankle arthrodesis with bone graft after distal tibia resection for bone tumors. Foot Ankle Int. 2008;29:1031–1037. doi: 10.3113/FAI.2008.1031. [DOI] [PubMed] [Google Scholar]
- 9.Campanacci M. Bone and Soft Tissue Tumors: Clinical Features, Imaging, Pathology and Treatment. New York, NY: Springer Verlag; 1999. pp. 1–70. [Google Scholar]
- 10.Casadei R, Ruggieri P, Giuseppe T, Biagini R, Mercuri M. Ankle resection arthrodesis in patients with bone tumors. Foot Ankle Int. 1994;15:242–249. doi: 10.1177/107110079401500503. [DOI] [PubMed] [Google Scholar]
- 11.Cass JR, Settles H. Ankle instability: in vitro kinematics in response to axial load. Foot Ankle. 1994;15:134–140. doi: 10.1177/107110079401500308. [DOI] [PubMed] [Google Scholar]
- 12.Davis AM, Devlin M, Griffin AM, Wunder JS, Bell RS. Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: a matched case-control study. Arch Phys Med Rehabil. 1999;80:615–618. doi: 10.1016/S0003-9993(99)90161-2. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebeid W, Amin S, Abdelmegid A, Refaat Y, Ghoneimy A. Reconstruction of distal tibial defects following resection of malignant tumours by pedicled vascularised fibular grafts. Acta Orthop Belg. 2007;73:354–359. [PubMed] [Google Scholar]
- 15.Eiser C, Darlington AS, Stride CB, Grimer R. Quality of life implications as a consequence of surgery: limb salvage, primary and secondary amputation. Sarcoma. 2001;5:189–195. doi: 10.1080/13577140120099173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9–24. [PubMed] [Google Scholar]
- 17.Enneking WF. Modification of the system for the functional evaluation of surgical management of musculoskeletal tumors. In: Enneking WF, ed. Limb Salvage in Musculoskeletal Oncology (Bristol-Myers/Zimmer Orthopedic Symposium). London, England: Churchill Livingstone; 1987:626–639.
- 18.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6. New York, NY: Springer; 2002. pp. 221–228. [Google Scholar]
- 20.Hatori M, Ayoub KS, Grimer RJ, Carter SR, Tillman RM. The two-stage ipsilateral fibular transfer for tibial defect following tumour excision. Sarcoma. 2000;4:27–30. doi: 10.1155/S1357714X00000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8:723–738. doi: 10.1016/S1083-7515(03)00147-5. [DOI] [PubMed] [Google Scholar]
- 22.Hintermann B, Sommer C, Nigg BM. The influence of ligament transection on tibial and calcaneal rotation with loading and dorsi-plantarflexion. Foot Ankle Int. 1995;16:567–571. doi: 10.1177/107110079501600910. [DOI] [PubMed] [Google Scholar]
- 23.Hsu RW, Wood MB, Sim FH, Chao EY. Free vascularised fibular grafting for reconstruction after tumour resection. J Bone Joint Surg Br. 1997;79:36–42. doi: 10.1302/0301-620X.79B1.6818. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.King HA, Watkins TB, Jr, Samuelson KM. Analysis of foot position in ankle arthrodesis and its influence on gait. Foot Ankle. 1980;1:44–49. doi: 10.1177/107110078000100115. [DOI] [PubMed] [Google Scholar]
- 26.Kitaoka HB, Patzer GL. Arthrodesis for the treatment of arthrosis of the ankle and osteonecrosis of the talus. J Bone Joint Surg Am. 1998;80:370–379. doi: 10.1302/0301-620X.80B3.8383. [DOI] [PubMed] [Google Scholar]
- 27.Laitinen M, Hardes J, Ahrens H, Gebert C, Leidinger B, Langer M, Winkelmann W, Gosheger G. Treatment of primary malignant bone tumours of the distal tibia. Int Orthop. 2005;29:255–259. doi: 10.1007/s00264-005-0656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SH, Kim HS, Park YB, Rhie TY, Lee HK. Prosthetic reconstruction for tumours of the distal tibia and fibula. J Bone Joint Surg Br. 1999;81:803–807. doi: 10.1302/0301-620X.81B5.9588. [DOI] [PubMed] [Google Scholar]
- 29.Malawer MM, Helman LJ, O’Sullivan B. Sarcomas of bone. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. Vol 2, Ed 7. Philadelphia, PA: Lippincott Williams & Wilkins, 2004:1794–1893.
- 30.Milner CE, Soames RW. The medial collateral ligaments of the human ankle joint: anatomical variations. Foot Ankle Int. 1998;19:289–292. doi: 10.1177/107110079801900504. [DOI] [PubMed] [Google Scholar]
- 31.Musculo DL, Ayerza MA, Aponte-Tinao L, Ranalletta M, Abalo E. Intercalary femur and tibia segmental allografts provide an acceptable alternative in reconstructing tumor resections. Clin Orthop Relat Res. 2004;426:97–102. doi: 10.1097/01.blo.0000141652.93178.10. [DOI] [PubMed] [Google Scholar]
- 32.Natarajan MV, Annamalai K, Williams S, Selvaraj R, Rajagopal TS. Limb salvage in distal tibial osteosarcoma using a custom mega prosthesis. Int Orthop. 2000;24:282–284. doi: 10.1007/s002640000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 34.Papagelopoulos PJ, Savvidou OD, Mavrogenis AF, Galanis EC, Shaughnessy WJ, Unni KK, Sim FH. Lateral malleolus en bloc resection and ankle reconstruction for malignant tumors. Clin Orthop Relat Res. 2005;437:209–218. doi: 10.1097/01.blo.0000164356.99795.a2. [DOI] [PubMed] [Google Scholar]
- 35.Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 36.Puri A, Subin BS, Agarwal MG. Fibular centralisation for the reconstruction of defects of the tibial diaphysis and distal metaphysis after excision of bone tumours. J Bone Joint Surg Br. 2009;91:234–239. doi: 10.1302/0301-620X.91B2.21272. [DOI] [PubMed] [Google Scholar]
- 37.Ramseier LE, Malinin TI, Temple HT, Mnaymneh WA, Exner GU. Allograft reconstruction for bone sarcoma of the tibia in the growing child. J Bone Joint Surg Br. 2006;88:95–99. doi: 10.1302/0301-620X.88B1.16253. [DOI] [PubMed] [Google Scholar]
- 38.Resnick D, Kransdorf MJ. Bone and Joint Imaging. 3. Philadelphia, PA: Elsevier Saunders; 2005. [Google Scholar]
- 39.Shalaby S, Shalaby H, Bassiony A. Limb salvage for osteosarcoma of the distal tibia with resection arthrodesis, autogenous fibular graft and Ilizarov external fixator. J Bone Joint Surg Br. 2006;88:1642–1646. doi: 10.1302/0301-620X.88B12.17879. [DOI] [PubMed] [Google Scholar]
- 40.Shekkeris AS, Hanna SA, Sewell MD, Spiegelberg BG, Aston WJ, Blunn GW, Cannon SR, Briggs TW. Endoprosthetic reconstruction of the distal tibia and ankle joint after resection of primary bone tumours. J Bone Joint Surg Br. 2009;91:1378–1382. doi: 10.1302/0301-620X.91B10.22643. [DOI] [PubMed] [Google Scholar]
- 41.Shmookler B, Bickels J, Jelinek J, Sugarbaker P, Malawer MM. Bone and Soft-tissue Sarcomas: Epidemiology, Radiology, Pathology and Fundamentals of Surgical Treatment. In: Malawer MM, Sugarbaker PH, editors. Musculoskeletal Cancer Surgery: Treatment of Sarcomas and Allied Diseases. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. pp. 3–36. [Google Scholar]
- 42.Simon MA. Limb salvage for osteosarcoma in the 1980s. Clin Orthop Relat Res. 1991;270:264–270. [PubMed] [Google Scholar]
- 43.Sommer C, Hintermann B, Nigg BM, Bogert AJ. Influence of ankle ligaments on tibial rotation: an in vitro study. Foot Ankle Int. 1996;17:79–84. doi: 10.1177/107110079601700204. [DOI] [PubMed] [Google Scholar]
- 44.Stéphane S, Eric M, Philippe W, Félix DJ, Raphael S. Resection arthrodesis of the ankle for aggressive tumors of the distal tibia in children. J Pediatr Orthop. 2009;29:811–816. doi: 10.1097/BPO.0b013e3181b768ef. [DOI] [PubMed] [Google Scholar]
- 45.Sugarbaker P, Bickels J, Malawer MM. Below-knee Amputation. In: Malawer MM, Sugarbaker PH, editors. Musculoskeletal Cancer Surgery: Treatment of Sarcomas and Allied Diseases. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. pp. 363–370. [Google Scholar]
- 46.Yan TQ, Guo W, Yang RL, Sun X, Qu HY. [The survival and functional outcome of primary bone sarcomas in distal lower extremity][in Chinese]. Zhonghua Wai Ke Za Zhi. 2010;48:1550–1555. [PubMed]
- 47.Zahlten-Hinguranage A, Bernd L, Sabo D. Amputation or limb salvage? Assessing quality of life after tumor operations of the lower extremity][in German. Orthopade. 2003;32:1020–1027. doi: 10.1007/s00132-003-0548-5. [DOI] [PubMed] [Google Scholar]
- 48.Zeytoonjian T, Mankin HJ, Gebhardt MC, Hornicek FJ. Distal lower extremity sarcomas: frequency of occurrence and patient survival rate. Foot Ankle Int. 2004;25:325–330. doi: 10.1177/107110070402500509. [DOI] [PubMed] [Google Scholar]