Abstract
Background
Compared with conventional polyethylene, first-generation highly cross-linked polyethylenes have low wear, but controversy exists regarding their reduced mechanical strength and/or retained free radicals. Second-generation highly cross-linked polyethylenes have been developed to reduce wear, maintain mechanical strength, and have oxidative resistance, but it is unclear whether they do so.
Questions/purposes
The primary objective of this study therefore was to determine if a second-generation annealed material has low linear wear at 5 years followup. Secondary objectives were to evaluate for overall survivorship, implant fixation, osteolysis, and effect of socket inclination on wear.
Methods
In a multicenter prospective study, we radiographically evaluated 155 patients (167 hips) at 3 years, 124 patients (132 hips) at 4 years, and 46 patients (51 hips) at 5 years. The linear head penetration rate was measured at 6 weeks, 1 year, and yearly through 5 years.
Results
The head penetration per year after the first year of bedding-in was 0.024 mm per year at 3 years, 0.020 mm per year at 4 years, and 0.008 mm per year at 5 years. The average wear rate over 5 years was 0.015 mm per year and represents a 58% improvement over a first-generation annealed highly cross-linked polyethylene. The Kaplan-Meier survivorship (revision for any reason) was 97.8%. We revised no hip for bearing surface failure and observed no osteolysis. Socket inclination did not affect linear wear.
Conclusions
These data suggest the linear wear rate for a second-generation annealed highly cross-linked polyethylene is no greater than that for historic controls of first-generation highly cross-linked polyethylenes, and no untoward complications were encountered with this new material.
Level of Evidence
Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Increased wear rates of conventional polyethylene have been associated with the development of osteolysis and reduction in the overall survivorship of THA [9, 15]. Irradiating polyethylene to create cross-linking and reduce wear was pioneered by Grobbelaar et al. [13] in 1978 and Oonishi et al. [26]. To reduce polyethylene wear and wear debris, highly cross-linked polyethylenes (HXLPEs) became commercially available and FDA approved in 1998 [23]. The cross-linking process involves irradiation and heating to achieve stabilization. Heating may be done at a temperature lower than the melting point (annealing) or higher than the melting point (remelting). An advantage of remelting is that free radicals in the crystalline region are accessible for elimination [23, 25]. However, recrystallization of the molten state results in a change in the microstructure of the polyethylene and reduces crystallinity [19]. The consequence is reduction in the fatigue and ultimate tensile strength compared with conventional UHMWPE [4, 11, 23, 27]. Annealing has the advantage of causing little effect to the material microstructure and largely maintains the key mechanical properties [4, 8]. However, with a single annealing process, residual free radicals remain in the crystals of the material and the material has the potential for posttreatment oxidation [18, 30]. To further improve on HXLPEs to achieve oxidative resistance, maintain the mechanical strength of conventional polyethylene, and maintain low wear, two second-generation HXLPEs have been developed: sequentially irradiated and annealed (X3™; Stryker Orthopaedics; Mahwah, NJ, USA) [8] and E-poly [28]. The sequential irradiation and annealing process increases the amount of cross-linking. Simulator testing found 60% lower wear than for first-generation annealed material [8].
The clinical wear rates of the first-generation once annealed HXLPE at 5 and 10 years have been reported, showing reductions in linear wear of 72% and 78%, respectively, compared with conventional polyethylene gamma sterilized in an inert atmosphere [3, 5]. However, similar wear rates for second-generation HXLPEs have not been reported.
The primary objective of this study was to determine if a second-generation (sequentially) annealed material has low linear wear at 5 years followup. The secondary objectives were to evaluate for overall survivorship, implant fixation, osteolysis, and effect of socket inclination on linear wear.
Patients and Methods
In a prospective multicenter trial we enrolled 166 patients who underwent 178 THAs using a second-generation sequentially annealed polyethylene liner; all patients were enrolled from May 2005 to January 2007. Subjects were enrolled in the study provided they met all selection criteria for primary THA and agreed to participate in the study by signing a study-specific, IRB-approved, Informed Patient Consent Form. Inclusion requirements included the diagnosis of noninflammatory arthritis, age between 21 and 75 years, and agreement to follow scheduled clinical and radiographic evaluations. We excluded patients with a history of infection, morbid obesity (BMI > 45), chronic disabling neurologic or systemic disease, recent history of substance dependence, and immunologic suppression. Of the 166 patients, three had died, four (2.2%) had revision surgery, and four (2.2%) were lost to followup, leaving 155 patients (167 hips) with minimum 3 years followup. The demographics include 57.5% female and an average age of 62 years (Table 1). Of the 155 patients, 139 (149 hips; 84%) had adequate radiographs for evaluation at 3 years. Twelve patients (12 hips) had missing films and four patients (six hips) had poor-quality films. At the 4-year followup, 114 patients (121 hips) had adequate films, whereas eight patients (eight hips) had missing films and two patients (three hips) had poor-quality films. At 5 years followup, all 46 patients (51 hips) had adequate films for study. Of the 12 patients with missing films at 3 years, all were available at the 4- and 5- year followups. All of the eight patients with missing films at 4 years were available for the 5-year evaluation.
Table 1.
Demographics of patients with minimum 3 years followup
| Demographic | Value |
|---|---|
| Number of patients/hips | 155/167 |
| Male/female | 42.5%/57.5% |
| Mean age (years) | 62.3 (32–75) |
| Mean BMI | 29.7 (SD, 5.3) |
| Diagnosis | |
| Osteoarthritis | 154 (92.2%) |
| Avascular necrosis | 10 (6.0 %) |
| Slipped capital femoral epiphysis | 1 (0.6%) |
| Femoral fracture | 1 (0.6%) |
| Traumatic arthritis | 1 (0.6%) |
The sequentially annealed HXLPE (X3™) was produced using Hoechst GUR® 1020 UHMWPE (Hoechst AG, Oberhausen, Germany), consolidated by compression molding. Cross-linking for the specimens of the X3™ material was achieved in three cycles using a sequential irradiating and annealing process. Each cycle consisted of gamma irradiation to a dose of 3 Mrad followed by heating at 130°C for 8 hours. The total radiation dose was 9 Mrad. After machining, the specimens were sterilized using gas plasma. All patients received the same tapered titanium cementless femoral stem (Secur-Fit™ hydroxyapatite hip stem; Stryker Orthopaedics) and cementless titanium hemispherical acetabular shell (Stryker Orthopaedics) with a 32-mm CoCr head and a 0° liner (Trident® Acetabular System; Stryker Orthopaedics). This differs from the first-generation annealed HXLPE (Crossfire; Stryker Orthopaedics) which was produced using 75 kGy of gamma irradiation followed by annealing at 130°C for 8 hours and sterilized by 30 kGy of gamma irradiation in nitrogen [8].
The posterior surgical approach was used in 89.3% and the lateral approach in 10.7%.
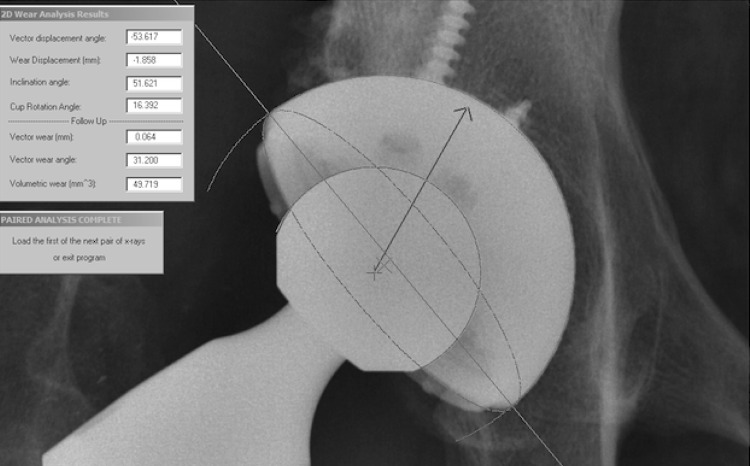
We followed patients at 6 to 7 weeks, 1 year, and yearly thereafter. At each visit we obtained an AP view radiograph of the pelvis centered over the symphysis pubis, an AP view of the hip centered over the femoral head, and a table-down lateral radiograph of the hip. The patient was in the supine position for all views. We digitized the AP radiographs of the pelvis with a high-resolution optical scanner (Vidar Corp, Herndon, VA, USA) and used Martell software (Hip Analysis Suite; University of Chicago, Chicago, IL, USA) [21, 22] to analyze linear head penetration from baseline (6-7 weeks) to each followup, cup inclination, and anteversion (Fig. 1). The underlying approach of the Martell method was to find the circles that best fit the prosthetic femoral head and the acetabular component using an edge-detection algorithm. The centers of the acetabular component and the femoral head then were found to calculate the magnitude and direction of the femoral head penetration. Femoral head penetration was measured between the 6-week radiograph and each followup radiograph. The 1-year radiographs were used to estimate initial bedding-in; by this time bedding-in is essentially complete [12, 21]. Penetration rates from 1 year to last evaluation then served as the basis for the yearly linear wear rates of the hips evaluated at 3, 4, and 5 years. Mean volumetric wear rate, V was calculated using the following geometric formula: V = Pl * R * R * W, where Pl = 3.1416, R = radius of the femoral head in mm; W = mean 2-D wear rate. The formula is based on cylindrical wear pattern perpendicular to the face of the cup.
Fig. 1.
The Martell method of linear wear measurement is shown in this radiograph. The circles that best fit the femoral head and acetabular cup are detected by the software. The direction of the head penetration is automatically calculated as shown.
The postoperative radiographs were reviewed by an orthopaedic surgeon (SZ) who was not part of the study group. Hips were evaluated radiographically for radiolucent lines and osteolysis in acetabular component zones as described by DeLee and Charnley [6] and in all femoral Gruen zones [14] on AP and mediolateral radiographs. Implant stability was evaluated according to criteria described by Engh et al. [10]. A stem was considered stable with osseous ingrowth if there were an absence of divergent radiodense lines and accretion of endosteal bone (cancellous condensation or spot welds) in hydroxyapatite-coated zones. A stem was considered stable with fibrous ingrowth if there were parallel radiodense lines involving the hydroxyapatite-coated zone but no subsidence, and a stem was considered unstable if it was surrounded by nonparallel radiodense lines or if it had subsided. A radiolucent line was defined as a lucent area in close proximity to the implant encompassing at least 50% of the zone and at least 1 mm in width. Implant migration was defined as prosthetic migration from fixed bony landmarks measured in 0.5-mm increments at each evaluation. Osteolysis was recorded for a specific area of bone loss (scalloping) without the presence of a reactive line for all implant zones.
We used Kaplan-Meier survivorship with revision for any reason as the end point [17]. The distribution of penetration data was examined for normality using histograms and statistically with the Shapiro-Wilk test. The median penetration rates were compared with the mean to show that the data are normally distributed. For the purpose of this article, the data are presented with mean and SD. A scatter plot of the femoral head penetration was plotted and the slope of the best-fit linear regression line was calculated to obtain the wear rate of X3™ polyethylene. We compared wear for hips with 40° to 50° inclination with that of hips with inclination less than 40° and greater than 50°. SAS statistical software package version 9.1 (SAS Institute Inc, Cary, NC, USA) was used for data analysis.
Results
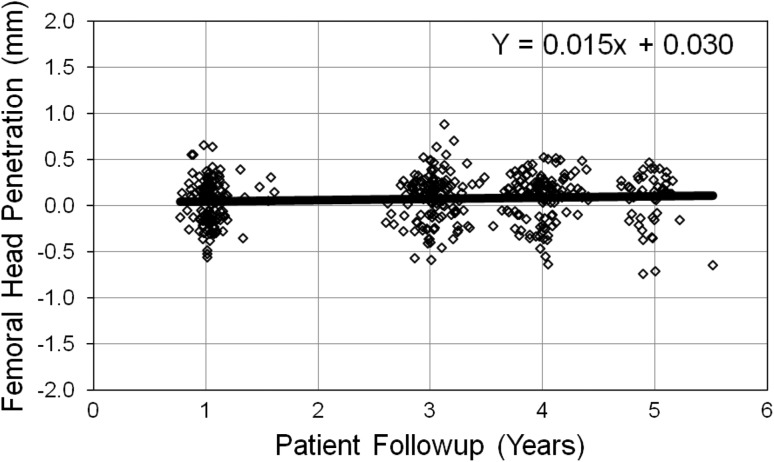
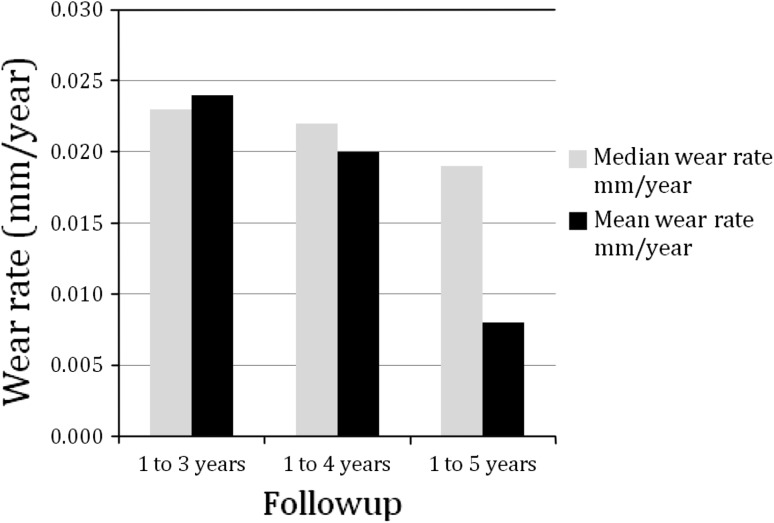
The mean linear head penetration ranged from 0.040 mm in the first year to 0.072 mm for 6 weeks to 5 years (Table 2). The polyethylene wear rate is represented by the slope of the best-fit linear regression line (Fig. 2). The total penetration at 1-year was found to be normally distributed (Shapiro-Wilk test, p = 0.096, Skewness = −0.032, and Kurtosis = −0.205). Data at other intervals are approximately normal. The 1- to 3-year linear penetration was 0.048 mm, yielding an estimated linear wear of 0.024 mm per year. Mean head penetration for 1 to 4 years was 0.061 mm, yielding an estimated linear wear of 0.020 mm per year. The 1- to 5-year penetration was 0.032 mm yielding an estimated wear of 0.008 mm per year. The slope of the best-fit regression line was 0.015 mm per year to 5 years, which represents the overall wear rate of the polyethylene and was 58% less than the reported wear of the first-generation annealed material at 5 years [5]. Median penetration rates were compared with the mean and the difference ranges from 0.001 mm at 3 years to 0.01 mm at 5 years (Fig. 3). Volumetric wear was calculated to be 12.1 cubic mm per year at 5 years followup.
Table 2.
Total linear head penetration at 6-month to 5-year followups
| Head penetration | 6 months–1 year | 6 months–3 years | 6 months–4 years | 6 months–5 years |
|---|---|---|---|---|
| Mean | 0.040 mm | 0.088 mm | 0.101 mm | 0.072 mm |
| SD | 0.231 mm | 0.254 mm | 0.241 mm | 0.286 mm |
Fig. 2.
The linear head penetration values at various followups are plotted here. The polyethylene wear rate is represented by the slope of the best-fit linear regression line. For our study, the wear rate is 0.015 mm per year.
Fig. 3.
Median penetration rates were compared with the mean and the difference ranges from 0.001 mm at 3 years to 0.01 mm at 5 years.
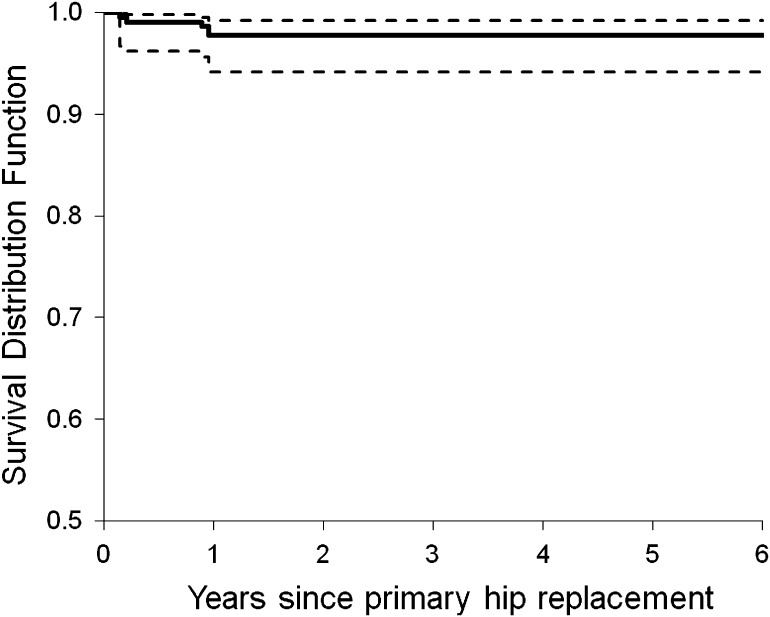
The Kaplan-Meier survivorship, with revision for any reason as the end point, was 97.8% at 5 years (Fig. 4). For the overall population of 166 patients (178 hips), four revisions (2.2%) occurred during the first year of followup (Table 3): one for acetabular component migration; one for recurrent dislocations; one for deep joint infection; and one for periprosthetic fracture. There were no failures of the bearing surface and there are no impending revisions.
Fig. 4.
The Kaplan-Meier survivorship plot with 95% confidence intervals shows 97.8% survivorship with revision for any reason as the end point.
Table 3.
Summary of reasons for revision surgery for four patients (four hips)
| Patient | Surgery date | Time to revision (months) | Reason for revision | Components revised |
|---|---|---|---|---|
| 1 | 3/20/06 | 2 | Acetabular component migration | Cup, liner, stem, head, bone screws |
| 2 | 6/28/06 | 3 | Multiple dislocations | Cup, liner, stem, head |
| 3 | 9/8/06 | 12 | Deep joint infection | Cup, liner, stem, head; antibiotic spacer insertion |
| 4 | 2/17/06 | 11 | Periprosthetic fracture, femoral component subsidence | Stem |
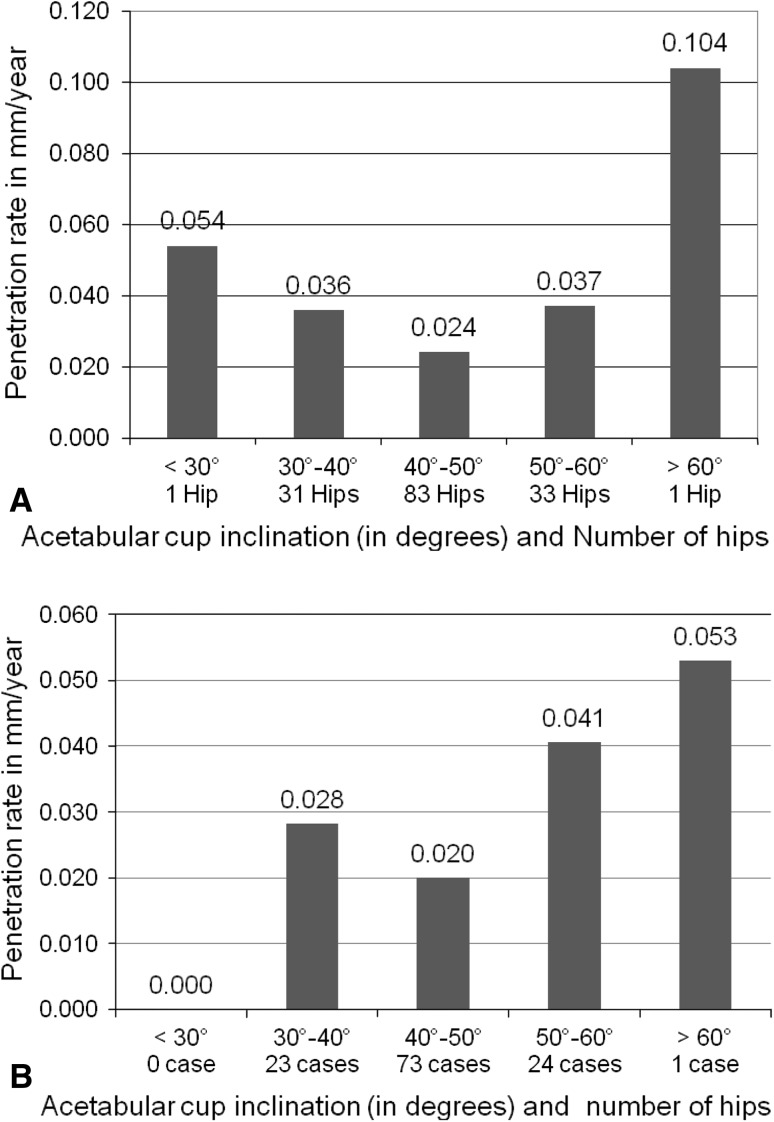
We observed no patients with osteolysis and all acetabular and femoral implants are well fixed. We observed no significant difference (p = 0.150) in penetration rates (6 weeks to 3 years and 4 years) for hips with inclination of 40° to 50° compared with those with inclinations less than 40° or greater than 50° (Fig. 5). The mean cup inclination was 45.1° (SD = 6.9°) and mean anteversion 16.1° (SD = 7.3°). The only high outlier had a penetration rate of 0.104 mm per year at 3 years, 0.053 mm per year at 4 years, and a cup inclination of 72° (Fig. 6).
Fig. 5A–B.
The femoral head penetration rates per year at (A) 3 years and (B) 4 years versus acetabular cup inclination are shown. No significant difference was observed (p = 0.150) for components in or outside the 40° to 50° cup inclination range.
Fig. 6.
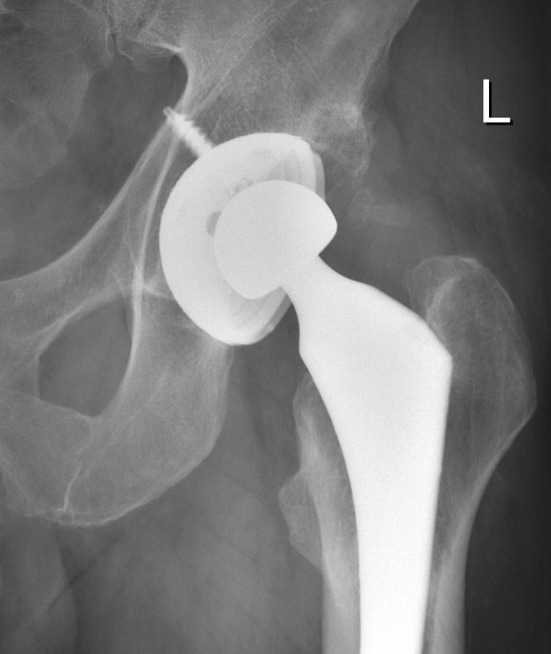
An AP radiograph of the only patient with wear greater than 0.06 mm per year and cup inclination of 72° is shown.
Discussion
Cross-links, bonds that interconnect polyethylene molecules, reduce wear in the hip by increasing molecular resistance to forces at the joint surface and were studied and described as early as 1978 [13, 26]. The cross-linking process involves irradiation and heating to achieve stabilization. Annealing (below melting temperature) is one such heating process. The first-generation annealed HXLPE was irradiated and annealed a single time, but to improve on this, a new second-generation material was sequentially irradiated and annealed. The primary objective of this study was to determine if a second-generation (sequentially) annealed material has low linear wear at 5 years followup. The secondary objectives were to evaluate for overall survivorship, implant fixation, osteolysis, and socket inclination versus wear. This is the first report of 5-year followup of linear wear for a second generation HXLPE.
We note limitations to our study. First, we had patients lost to followup (2.3%), and missing or unreadable radiographs at 3 years (10.7%) and 4 years (8.3%) that had the potential to miss outliers with high wear or implant failure. However, patients with the missing radiographs at 3 and 4 years had all available radiographs at 5 years. Second, we had no intraobserver and interobserver validation of radiographic readings but we believe the data are acceptable based on the Martell software. Repeatability of the measurements (on the basis of five measurements per patient) using Martell’s technique was reported by Hui et al. [16], with use of an intraclass correlation coefficient. The Hip Analysis Suite provided two-dimensional linear penetration estimates that had a good correlation with the data derived with the coordinate measuring machine, with r2 = 0.80 (p < 0.001). Third, this is a mid-term report and longer-term studies are important to show whether the use of this HXLPE continues to have low wear, reduce the incidence of osteolysis, and improve longevity of THA. Fourth, this was a nonconsecutive series that potentially could bias the selection of patients. Fifth, we found negative wear measurements that are inherent in the Martell technique, a common and acceptable method of measuring and reporting wear; these values reflect noise (negative values) at the lower limits of accuracy of the measurements. If we discard the negative values, the standard deviation would be artificially reduced whereas the mean wear values would increase. Sixth, we had no control study cohort and are relying on less than ideal historic reports. We believe that the strengths of this study include the prospective multicenter design, standardized radiographic protocol, and uniform implant system used for each study patient.
The 1- to 5-year head penetration for the 51 hips with 5 years followup was 0.008 mm per year and is similar to other 4- to 7-year reports of first-generation materials that range from 0.005 mm per year to 0.05 mm per year (Table 4) [1, 5, 20, 24, 29]. The overall linear wear rate (slope of the best-fit regression line) during the 5-year followup for this second-generation sequentially annealed HXLPE (X3™) was 0.015 mm per year. When comparing the wear rate of the HXLPE with a conventional polyethylene, reductions of 72% [5], 82% [1], 81% [24], and 86% [29] have been reported (Table 4). Different head size might influence linear and or volumetric wear. Lachiewicz et al. [20] found no difference in linear wear between 26 and 40 mm heads but did find higher volumetric wear with larger femoral heads when tested against HXLPE. In our earlier study [5] of a first-generation annealed HXLPE, we used a 28-mm head diameter, whereas in this study patients received a 32-mm head. Comparing the 5-year linear and volumetric wear of the first and second generation materials we found the linear wear was 0.036 mm per year versus 0.015 mm per year (58% reduction) and the volumetric wear also was reduced from 22.1 mm per year to 12.1 mm3 per year (45% reduction). If the two materials (first- and second-generation HXLPE) had similar wear characteristics, then one would have expected to possibly see an increase in volumetric wear with the larger head size. Instead we found the opposite, a decrease in volumetric wear suggesting that the second-generation material may be more wear resistant than the first-generation material.
Table 4.
Comparison of our data with published 4- to 7-year results of linear wear with HXLPE
| Study | Type of study | Femoral head size | Followup (years) | Number of hips | Method of cross-linking | Osteolysis | Wear measurement method | Two-dimensional penetration rate in mm/year: cross-linked | Two-dimensional penetration rate in mm/year: controls |
|---|---|---|---|---|---|---|---|---|---|
| Bragdon et al. [1] | Retrospective | 28 mm, 32 mm | 3.8 | 53 cross-linked, 58 controls | 10 Mrad remelted conventional | Not reported | Martell | 0.025 | 0.144 |
| Lachiewicz et al. [20] | Retrospective | 26 to 40 mm | 5.7 | 102 cross-linked | 10 Mrad remelted | 0% | Martell | 0.028 median 0.040 mean |
|
| Mutimer et al. [24] | Prospective, double-blinded RCT | 28 mm CoCr | 5.5 | 61 cross-linked, 61 controls | 5 MRad ir remelted conventional | 5% | PolyWare™-Devane | 0.05 | 0.26 |
| Thomas et al. [29] | Prospective, double-blinded RCT using RSA | 28 mm CoCr | 7.0 | 22 cross-linked, 22 controls | 10 Mrad remelted conventional | Not reported | Model-based RSA system | 0.005 | 0.037 |
| D’Antonio et al. [5] | Prospective multicenter | 28 mm | 4.8 | 56 cross-linked, 91 controls | 10 Mrad annealed conventional | 0% | Livermore-digital | 0.036 | 0.131 |
| Current study | Prospective, multicenter | 32 mm CoCr | 5.0 | 51 cross-linked | Sequentially irradiated (3×3 Mrad) annealed | 0% | Martell | 0.008 |
RCT = randomized controlled trial; RSA = radiostereometric analysis.
We found a survivorship with revision for any reason as the end point of 97.8%. All revisions occurred within the first year after surgery and were unrelated to the bearing surface. There was one revision for early acetabular component migration. This is similar to recent reports of 100% survivorship of the HXLPE bearing surface [20, 24, 29], and of those reports, only Mutimer et al. [24] reported aseptic loosening (2%).
We observed no osteolysis on routine radiographs at last followup. Lachiewicz et al. [20] also reported no osteolysis and Thomas et al. [29] reported a 5% incidence for patients receiving first-generation HXLPEs. Our previous study at 4.8 years with the first-generation annealed HXLPE also showed no evidence for osteolysis, but 17.4% of the patients who received the control (conventional polyethylene) had osteolytic changes in the proximal femur [5].
We found no difference in overall penetration rates for components in or outside the 40° to 50° cup inclination range. Our only outlier had a penetration rate of 0.104 mm per year at 3 years, 0.053 mm per year at 4 years, and had socket inclination of 72° (Fig. 5). Increased inclination of the acetabular component has not been associated with increased wear for gamma in air conventional polyethylene [7]. Longer-term followup with this study will further elucidate the relationship between wear of HXLPE and cup inclination.
Although the annealing process preserves crystallinity and the basic mechanical properties of conventional polyethylene [4, 8], the presence of free radicals in the first-generation material (Crossfire®; Stryker Orthopaedics) raised concerns regarding the potential for in vivo oxidation [18, 19, 30]. Although retrievals of Crossfire® implants have shown oxidation at the rim, no substantial oxidation at the bearing surface and/or locking mechanism has been observed and low wear has been reported for all retrieved implants [18].
The rationale for the second-generation annealed HXLPE was to minimize the presence of free radicals while maintaining the high wear resistance of the first-generation material without degradation of the mechanical strength properties of conventional polyethylene. By using a sequential irradiating and annealing process, preclinical testing showed the crystallinity, density, and tensile strength of the X3™ HXLPE was unchanged by oxidative challenge and the wear particles generated were the same size as those produced with conventional UHMWPE [8]. The wear rate for the Crossfire® at 5 years was 0.036 mm per year whereas the wear rate for the second-generation material (X3™) at 5 years was 0.015 mm per year, representing a 58% wear rate reduction. In a radiostereometric analysis by Campbell et al. [2], the annual wear rate of the X3™ polyethylene was reported as 0.015 mm per year, which is comparable to our findings.
Our clinical experience with annealed HXLPEs to date has shown low wear and maintenance of mechanical integrity of the bearing surface. The sequentially annealed HXLPE had lower wear than the first-generation annealed material at 5 years followup and offers the theoretical advantage of oxidative resistance. These new bearing surface materials offer hope for longevity of THA, but their clinical performance for a 15- to 20-year period will determine the true importance of this cross-linking process.
Acknowledgments
Twelve surgeon investigators contributed patients to and obtained IRB approval for this study: Benjamin Bierbaum MD (Boston, MA); Daniel Ward MD (Boston, MA); Peter Bonutti MD (Effingham, IL); Miguel Cabanela MD (Rochester, MN); Robert Trousdale MD (Rochester, MN); William Capello MD (Indianapolis, IN); J. Andrew Parr MD (Indianapolis, IN.) James D’Antonio MD (Moon Township, PA); J. Wesley Mesko MD (Lansing, MI); James Roberson MD (Atlanta, GA); Greg Erens MD (Atlanta, GA); and John Wright (Kearney, NE). We also thank Steven Zelicof MD (Harrison, NY), the independent radiographic reviewer for the study.
Footnotes
Each author certifies that he (JAD, WNC, RR), or a member of their immediate family, has or may receive payments or benefits, in any one year, an amount in excess of $10,000), from a commercial entity (Stryker Orthopaedics, Mahwah, NJ, USA) related to this work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his/her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
This work was performed at Sewickley Valley Hospital Sewickley, PA, USA, Indiana University School of Medicine, Indianapolis, IN, USA, and Stryker Orthopaedics, Mahwah, NJ, USA.
References
- 1.Bragdon CR, Barrett S, Martell JM, Greene ME, Malchau H, Harris WH. Steady-state penetration rates of electron beam-irradiated, highly cross-linked polyethylene at an average 45-month follow-up. J Arthroplasty. 2006;21:935–943. doi: 10.1016/j.arth.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Campbell DG, Field JR, Callary SA. Second-generation highly cross-linked X3™ polyethylene wear: a preliminary radiostereometric analysis study. Clin Orthop Relat Res. 2010;468:2704–2709. doi: 10.1007/s11999-010-1259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capello WN, D’Antonio JA, Ramakrishnan R, Naughton M. Continued improved wear with an annealed highly cross-linked polyethylene. Clin Orthop Relat Res. 2011;469:825–830. doi: 10.1007/s11999-010-1556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier JP, Currier BH, Kennedy FE, Currier JH, Timmins GS, Jackson SK, Brewer RL. Comparison of cross-linked polyethylene materials for orthopaedic applications. Clin Orthop Relat Res. 2003;414:289–304. doi: 10.1097/01.blo.0000073343.50837.03. [DOI] [PubMed] [Google Scholar]
- 5.D’Antonio JA, Manley MT, Capello WN, Bierbaum B, Ramakrishnan R, Naughton M, Sutton K. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:143–150. doi: 10.1097/00003086-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 6.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 7.Del Schutte H, Jr, Lipman AJ, Bannar SM, Livermore JT, Ilstrup D, Morrey BF. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621–626. doi: 10.1016/S0883-5403(98)80003-X. [DOI] [PubMed] [Google Scholar]
- 8.Dumbleton JH, D’Antonio JA, Manley MT, Capello WN, Wang A. The basis for a second-generation highly cross-linked UHMWPE. Clin Orthop Relat Res. 2006;453:265–271. doi: 10.1097/01.blo.0000238856.61862.7d. [DOI] [PubMed] [Google Scholar]
- 9.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 10.Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res. 1990;257:107–128. [PubMed] [Google Scholar]
- 11.Gencur SJ, Rimnac CM, Kurtz SM. Fatigue crack propagation resistance of virgin and highly crosslinked thermally treated ultra-high molecular weight polyethylene. Biomaterials. 2006;27:1550–1557. doi: 10.1016/j.biomaterials.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Glyn-Jones S, McLardy-Smith P, Gill HS, Murray DW. The creep and wear of highly cross-linked polyethylene: a three-year randomised, controlled trial using radiostereometric analysis. J Bone Joint Surg Br. 2008;90:556–561. doi: 10.1302/0301-620X.90B5.20545. [DOI] [PubMed] [Google Scholar]
- 13.Grobbelaar CJ, du Plessis TA, Marais F. The radiation improvement of polyethylene prostheses: a preliminary study. J Bone Joint Surg Br. 1978;60:370–374. doi: 10.1302/0301-620X.60B3.681412. [DOI] [PubMed] [Google Scholar]
- 14.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 15.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;311:46–53. [PubMed] [Google Scholar]
- 16.Hui AJ, McCalden RW, Martell JM, MacDonald SJ, Bourne RB, Rorabeck CH. Validation of two and three-dimensional radiographic techniques for measuring polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am. 2003;85:505–511. doi: 10.2106/00004623-200303000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Kurtz SM, Austin MS, Azzam K, Sharkey PF, MacDonald DW, Medel FJ, Hozack WJ. Mechanical properties, oxidation, and clinical performance of retrieved highly cross-linked Crossfire liners after intermediate-term implantation. J Arthroplasty. 2010;25:614–623.e1–2. [DOI] [PMC free article] [PubMed]
- 19.Kurtz SM, Ong K. Contemporary total hip arthroplasty: Hard-on-hard bearings and highly crosslinked UHMWPE. In: Kurtz SM, Ong K, editors. The UHMWPE Biomaterials Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacements and Medical Devices. 2. Burlington, MA: Elsevier Academic Press; 2009. pp. 72–75. [Google Scholar]
- 20.Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop Relat Res. 2009;467:3290–3296. doi: 10.1007/s11999-009-1038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martell JM, Berdia S. Determination of polyethylene wear in total hip replacements with use of digital radiographs. J Bone Joint Surg Am. 1997;79:1635–1641. doi: 10.2106/00004623-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Martell JM, Berkson E, Berger R, Jacobs J. Comparison of two and three-dimensional computerized polyethylene wear analysis after total hip arthroplasty. J Bone Joint Surg Am. 2003;85:1111–1117. doi: 10.2106/00004623-200306000-00020. [DOI] [PubMed] [Google Scholar]
- 23.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17:157–167. doi: 10.1002/jor.1100170203. [DOI] [PubMed] [Google Scholar]
- 24.Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop Relat Res. 2010;468:3228–3233. doi: 10.1007/s11999-010-1379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura K, Ogata S, Ikada Y. Assessment of heat and storage conditions on gamma-ray and electron beam irradiated UHMWPE be electron spin resonance. Biomaterials. 1998;19:2341–2346. doi: 10.1016/S0142-9612(98)00150-1. [DOI] [PubMed] [Google Scholar]
- 26.Oonishi H, Kuno M, Tsuji E, Fujisawa A. The optimum dose of gamma radiation-heavy doses to low wear polyethylene in total hip prostheses. J Mater Sci Mater Med. 1997;8:11–18. doi: 10.1023/A:1018582027349. [DOI] [PubMed] [Google Scholar]
- 27.Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27:917–925. doi: 10.1016/j.biomaterials.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oral E, Wannomae KK, Rowell S, Muratoglu OK. Diffusion of vitamin E in ultra-high molecular weight polyethylene. Biomaterials. 2007;28:5225–5237. doi: 10.1016/j.biomaterials.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas GER, Simpson DJ, Mehmood S, Taylor A, McLardy-Smith P, Gill HS, Murray DW, Glyn-Jones S. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93:716–722. doi: 10.2106/JBJS.J.00287. [DOI] [PubMed] [Google Scholar]
- 30.Wannomae K, Christensen SD, Freiberg AA, Bhattacharyya S, Harris WH, Muratoglu OK. The effect of real-time aging on the oxidation and wear of cross-linked UHMWPE acetabular liners. Biomaterials. 2006;27:1980–1987. doi: 10.1016/j.biomaterials.2005.10.002. [DOI] [PubMed] [Google Scholar]