Abstract
Background
Postoperative spine infections cause considerable morbidity. Patients are subjected to long-term antibiotic regimens and may require further surgery. Delivery of electric current through instrumentation can detach biofilm, allowing better antibiotic penetration and assisting in eradicating infection.
Question/purposes
We asked (1) whether capacitive coupling treatment in combination with a single dose of antibiotics would reduce infection rates when compared with antibiotics alone in a rabbit spine infection model, (2) whether it would decrease the overall bacterial burden, and (3) whether there was a time-dependent response based on days treated with capacitive coupling.
Methods
Thirty rabbits were subjected to a well-established spine infection model with a single dose of intravenously administered systemic ceftriaxone (20 mg/kg of body weight) prophylaxis. Two noncontiguous rods were implanted inside dead space defects at L3 and L6 challenged with 106 colony-forming units of Staphylococcus aureus. Rabbits were randomly treated with a capacitive coupling or control device. Instrumentation and soft tissue bacterial growth were assessed after 7 days.
Results
Sites treated with capacitive coupling showed a decrease in the incidence of positive culture: 36% versus 81% in the control group. We observed no difference in the soft tissue’s infectious burden. Overall bacterial load was not decreased with capacitive coupling.
Conclusions
Capacitive coupling in conjunction with antibiotics reduced the instrumentation-related infection rate compared with antibiotics alone.
Clinical Relevance
Capacitive coupling noninvasively delivers an alternating current that may detach biofilm from instrumentation. Treatment of infection may be successful without removal of instrumentation, allowing for improved stability and overall decreased morbidity.
Introduction
Postoperative infection is the most common adverse event in orthopaedics. Postoperative antibiotic use reportedly decreases postoperative spine wound infections to a rate between 1% and 5% in healthy patients undergoing elective spine surgery [23]. In trauma patients, however, infection rates remain elevated and reportedly range from 6% to 10% [19, 22]. Surgical site infections have major implications for patients, their families, and, because of associated healthcare costs, society as a whole. The adverse events range from simple superficial infections to deep subfascial infections that involve implanted instrumentation. Patients have to endure long-term antibiotics and may be subject to revision surgery with removal of instrumentation [17]. Infection also negatively affects fusion beds and can decrease spine stability [29]. Average length of hospital stay and mortality risks are doubled in the setting of surgical site infection [16]. The costs of treating a single patient’s spine infection may reach $1,000,000 for all the healthcare providers involved [2, 11, 17]. In addition to decreased procedural reimbursement, new Medicare policies plan to withhold payment for the treatment of orthopaedic complications that result from infection [8].
The presence of instrumentation can make initial bacterial colonization difficult to eradicate. After colonizing an implanted device, bacteria form a layer of glycocalyx, or biofilm. This layer assists in changing the phenotypic properties of the organism and decreases the efficacy of antibiotics [14]. The self-produced layer of extracellular matrices, DNA, and polysaccharides attaches to inert material, preventing phagocytic action. Microorganisms can then proliferate. Ongoing infection can cause local damage and even lead to loosening of previously well-fixed instrumentation [28]. In selective circumstances, surgical removal of the implants may be necessary for treatment of the infection. Thus, innovative treatments are necessary in the face of modern-day challenges to treat postoperative spine infections.
Capacitive coupling technology is a means of delivering an alternating current in an egg-shaped electric field. Capacitive coupling and pulsed electromagnetic fields noninvasively deliver indirect current, potentially providing the same bioelectric benefits. A pulsed electromagnetic field induces a magnetic field placed outside the body and indirectly sustains a low current through the instrumentation that is placed in vivo. Substantial evidence indicates that pulsed electromagnetic fields assist in the treatment of long bone nonunions, and they have been used in clinical trials for decades [9, 18]. Electromagnetic fields also show promise in animal spine fusion models [10, 15]. Capacitive coupling also enhances both spinal fusion and long bone union through its alternating current generator [1, 24]. It upregulates osteoinductive factors such as bone morphogenetic protein 2 and bone morphogenetic protein 7 and activation of calmodulin with increased transmembrane calcium uptake [12].
The concept of using electricity as a means of treating infection has been described as the bioelectric effect [7]. When applied to instrumentation in vitro, small electric currents are able to detach biofilm [5, 6]. As a result, the bacteria are rendered more susceptible to antibiotics. In addition, electricity treatments can upregulate growth factors such as transforming growth factor beta, fibroblast growth factor 2, and vascular endothelial growth factor, which augment the immune response [7]. Electricity also has the ability to cause capillary extravasation, resulting in an increased influx of local immunomodulators [13].
We asked (1) whether capacitive coupling treatment in combination with a single dose of antibiotics would reduce infection rates when compared with antibiotics alone in a rabbit spine infection model, (2) whether it would decrease the overall bacterial burden, and (3) whether there was a time-dependent response based on days treated with capacitive coupling.
Materials and Methods
Thirty New Zealand White (NZW) rabbits with an average weight of 3.5 kg (weight range, 3.3–3.8 kg) were included in the study. Twenty NZW rabbits were randomly allocated to the capacitive coupling treatment group and 10 to the control group. The control group rabbits were fitted with matching capacitive coupling devices that were turned off. Each rabbit was challenged with 106 colony-forming units (CFU) of Staphylococcus aureus (ATCC 25923, sensitive to ceftriaxone) at L3 and L6 during surgery. Seven days after surgery, tissues were sampled using standard quantification techniques [3]. Only female rabbits were used, because they have a history of being more docile and less territorial. All rabbits went through 2 weeks of acclimation before surgery. The investigation was performed under the approval of the Institutional Animal Care and Use Committee (IACUC).
Our spine model consistently produces an 80% infection rate [26]. Presuming a 40% reduction of infection, a power analysis suggested that 20 was the minimum number of animals needed for the experimental group. We used SigmaStat (Version 2.03; Aspire Software International, Ashburn, VA, USA) for a power of 80% and a p value of 0.05.
Each rabbit was acclimated to a custom-fitted mesh jacket (Lomir Biomedical Inc, Malone, NY, USA) that would hold the capacitive coupling device. All rabbits wore this jacket for 1 week before surgery. Jackets were reapplied after surgery in addition to the capacitive coupling electrodes.
The experimental device (SpinalPak II Spine Fusion Stimulator; Biomet, Warsaw, IN, USA) was applied immediately postoperatively with two electrodes placed 3 inches apart on shaved rabbit skin cephalad and caudal to the incisions (Fig. 1). Both the L3 and L6 sites were subject to the egg-shaped field produced by the capacitive coupling device. The maximal sinusoidal current was 14 mA with an average current of 6 mA. Rabbits in the capacitive coupling group received treatment continuously during the next 7 days, whereas rabbits in the control group wore the device but with no battery. A test meter measured the amps, volts, and days applied. The values were recorded every other day for each experimental animal. Electrodes and leads were replaced as necessary. Because rabbits would occasionally remove their own leads, not all rabbits received the same treatment dose. The differences were measured with the test meter. The difference in volts received was based on the amount of time the capacitive coupling device remained in contact with the rabbit. We assessed the data for a correlation between the amount of electricity received (amps, volts, and days of treatment) and the bacterial burden present when the rabbits were euthanized.
Fig. 1.
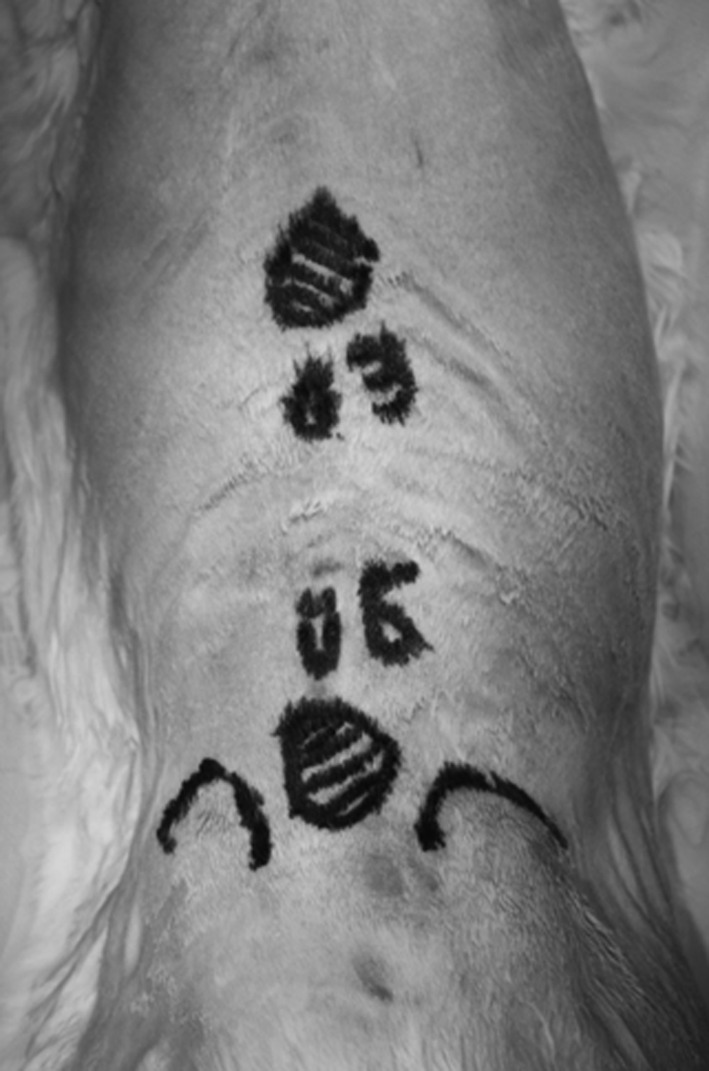
This photograph shows a rabbit back before surgery. Spinous processes of L3 and L6 are marked with electrodes cephalad and caudad to the incisions.
The NZW rabbit infection model with multiple surgical sites mimics posterior spinal surgery with instrumentation and is based on a previously described protocol [21, 26]. The creation of two implantation sites on each animal minimized the total number of animals used in the investigation.
On the day before surgery, a suspension of S aureus colonies in 5 mL trypticase soy broth was incubated at 37°C overnight for 16 hours. After the culture was centrifuged at 4000 revolutions per minute for 10 minutes, the supernatant was decanted and the pellet was diluted with 5 mL sterile saline. After repeat centrifuge, the washing process was repeated to ensure a stable nonmultiplying supply of microorganisms. Final concentrations of bacteria were obtained with further dilutions in sterile saline. The concentration was standardized with a densitometric turbidity meter (LaMotte 2020e; LaMotte Company, Chestertown, MD, USA), which correlated with CFU/100 μL plated on trypticase soy agar plates with 10% sheep blood (Fisher Scientific, Pittsburgh, PA, USA).
General anesthesia was induced with ketamine and xylazine and maintained with isoflurane inhalation through a nose cone mask for all rabbits in both groups. Ceftriaxone (20 mg/kg of body weight) was intravenously administered to all rabbits before surgery to mimic preoperative prophylaxis. The goal of the study was to evaluate the capacitive coupling effect with minimal dependence on antibiotics. Rabbits were positioned prone, shaved, prepped, and draped in a sterile fashion, exposing the superior end of the sacrum to the middle of the thoracic spine. The surgical approach was identical for both the L3 and L6 sites. Accurate anatomic incision was based on palpation of the sacrum, the seventh lumbar spinous process, and the sixth lumbar spinous process in succession.
A 2-cm longitudinal midline skin incision over the spinous process was followed by a linear incision through the fascia. The entire spinous process was removed from its base with a small rongeur. A dead space was formed with removal of attached muscle and fascia. Further decompression was not performed considering the dura was not exposed. A 1-cm Ti90/Al6/V4 rod (2-mm diameter, Item TI017905; Goodfellow Corporation, Oakdale, PA, USA) was placed into the created envelope. Fascia was then closed with a 3–0 Vicryl suture (Ethicon, Inc, Somerville, NJ, USA). The site was inoculated with 106 CFU/100 μL of S aureus with use of a sterile syringe and 27-gauge needle. Skin was closed with a 2–0 nylon suture (Ethicon, Inc). The procedure was repeated at the second site (Fig. 2).
Fig. 2.
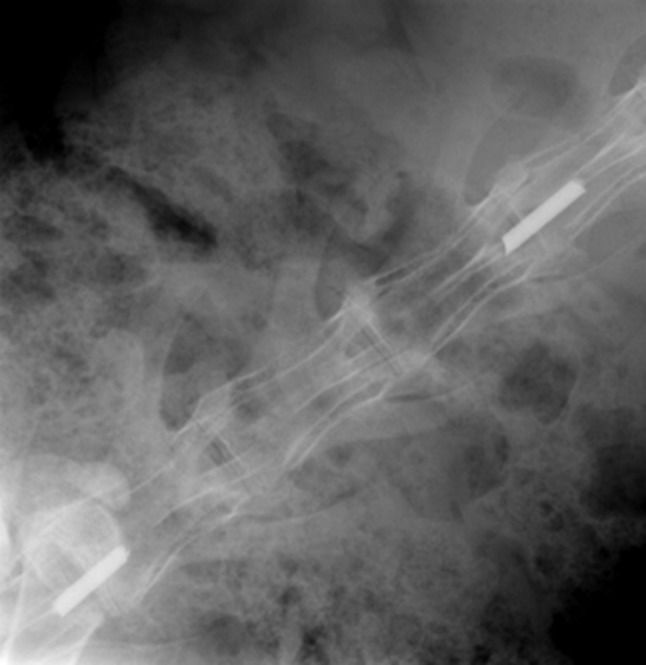
This radiograph shows instrumentation implanted in dead space defects at L3 and L6.
Postoperative analgesia was provided based on a standard IACUC protocol, and rabbits were permitted full cage activity and nutrition ad libitum. Wound healing, body weight, and signs of distress were monitored daily. Overall, rabbits showed little to no signs of discomfort after surgery and maintained their weight throughout the postoperative period. One rabbit had a prolapsed vagina postoperatively and was excluded from the study. The remaining 29 rabbits completed the entire treatment protocol. In the end, 19 rabbits remained in the capacitive coupling group and 10 rabbits in the control group. None had systemic infection as determined by a lack of bacterial growth in blood samples obtained when the rabbits were euthanized.
Seven days after surgery, the rabbits were euthanized with administration of phenobarbital (100 mg/kg of body weight). Arterial blood samples were obtained at that time to ensure the rabbits were not systemically infected. Capacitive coupling devices continued to run during culturing. One gram each of fascia, bone, and hematoma was harvested from the surgical site using a sterile technique. Harvested materials were weighed and immediately homogenized (PowerGen Model 35 Handheld Homogenizer; Fisher Scientific). Implanted instrumentation was sonicated (UBATH-Y; World Precision Instruments, Inc, Sarasota, FL, USA) in cold saline to obtain a sensitive culture. Culture samples were serially diluted and plated on blood agar plates. After 24-hour incubation at 37°C, final CFU count was calculated on a per-gram basis. Severity of infection was determined based on the serial plating techniques and was measured as CFU. CFU counting was evaluated by an observer who was blinded to the treatment group. Infection incidences, or presence of bacteria, were calculated separately for fascia, hematoma, bone, and instrumentation. The incidence of infection was defined as a culture positive for S aureus exclusively and independently in the fascia, bone, hematoma, and instrumentation [21, 26, 27].
Chi-square calculations were assessed to determine differences in proportions of infection between the two groups. We used a two-way analysis of variance with a post hoc Student’s t-test to identify differences in bacterial burden between the two groups. A nonparametric calculation with a Spearman rho correlation coefficient was used to link the amount of capacitive coupling treatment received (amps, volts, days) with the bacterial burden severity.
Results
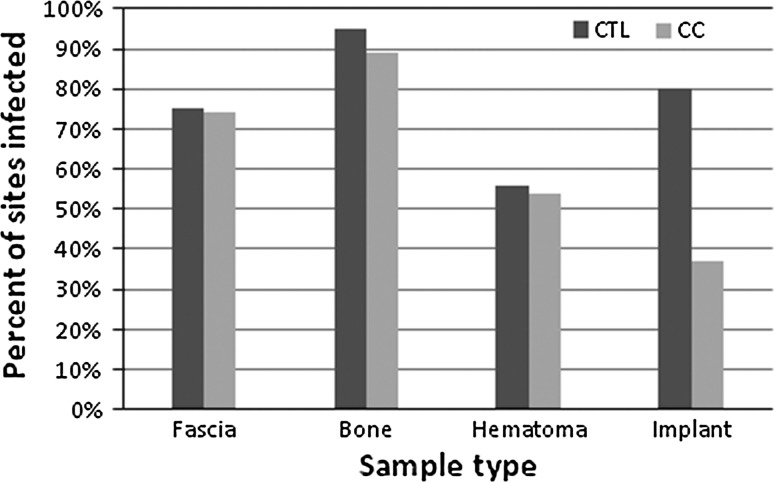
Capacitive coupling had the strongest positive treatment effect on the instrumentation: the presence of S aureus on the implant was reduced (p = 0.0011) with capacitive coupling compared with control sites. We observed no difference in the incidence of bacteria presence in the soft tissues (Fig. 3).
Fig. 3.
Bar graph shows percentages of surgical sites with bacterial growth on fascia, bone, hematoma, and implants in the capacitive coupling (CC) and control (CTL) groups. Only the implant-related infection was statistically significant.
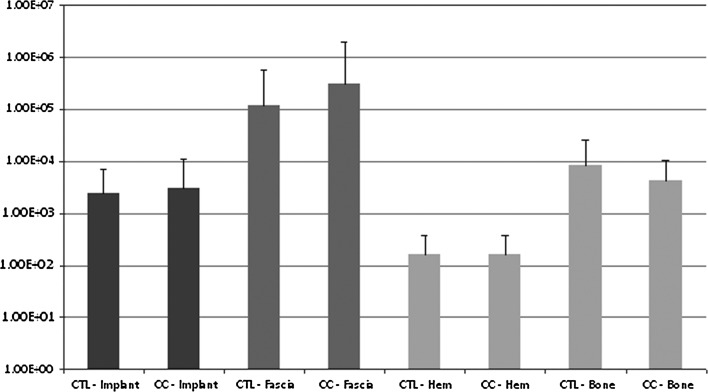
We observed no difference (p = 0.81) in the overall average bacterial counts when comparing the capacitive coupling group with the control group. In addition, we found no difference when comparing the average counts split among fascia, bone, hematoma, and implant (Fig. 4).
Fig. 4.
Bar graph shows the average bacterial counts (in colony-forming units) for fascia, bone, hematoma, and implants in the capacitive coupling (CC) and control (CTL) groups. No difference was shown in the overall bacterial burden.
We observed a correlation between the amount of volt-days received and the bacterial burden of the fascia (r = 0.567, p < 0.001) and bone (r = 0.569, p < 0.001) when the rabbits were euthanized. We did not control for this variable, and this analysis was conducted purely retrospectively.
Discussion
Multiple interventions for prevention and treatment of instrumentation-related infections have been investigated. The evolution of antibiotic prophylaxis has been the single most important advance in preventing surgical site infection [25]. With this study, we explored the effect of electricity as an adjunct in the treatment of instrumentation-related infection. We found the application of a capacitive coupling current reduced the presence of S aureus on the spinal implant but had no effect on the soft tissue. In addition, there was no effect on the overall bacterial burden. There was a time-dependent response in that increased treatment days led to decreased infectious burden.
We acknowledge limitations to our study. First, we used nonfixed instrumentation because this was the established model. We do not know whether fixed instrumentation would have influenced the bioelectric effect. It is possible that with fixed instrumentation, there is increased impedance and the required voltage would be increased to maintain a threshold current. This may or may not be at a clinically safe level. A second limitation was that our intent was for a full 7 days of capacitive coupling treatment and we therefore did not control for the varying days of treatment. A third limitation was not using electron microscopy to prove that the proposed mechanism of action was indeed biofilm detachment.
The bioelectric effect has shown some promise in vivo in other studies as well. van der Borden et al. [27] studied the treatment of external fixation pin tract infection by using direct electric current in a goat model. An 80% drop in infection rate was associated with a minimal 100-μA electric current. Del Pozo et al. [4] more recently studied the bioelectric effect on implanted instrumentation. The authors applied a 200-μA direct current to a rabbit tibial intramedullary rod that had Staphylococcus epidermidis present. Infection rates and overall bacterial burden decreased, even when compared with use of an antibiotic alone. Despite the proposed bioelectric theory of an increased local immune response, no treatment effect was noted in the bone or fascia in our model. The biofilm-centered theory proved dominant because the instrumentation-related infection rate decreased from 81% to 36%. This decrease in infection rate is even more impressive considering the high culture sensitivity of the sonicated instrumentation. The lack of soft tissue effect may be secondary to its increased impedance. An increase in the voltage applied may improve the soft tissue infection rate considering the time-related response shown by regression analysis.
The effect of alternating current on infection rates has been less extensively tested in an in vivo model [20]. The overall bacterial burden in our study was unchanged, however. This may mean that electric current can help sterilize the instrumentation but without a bactericidal effect and the microorganisms return to an active state in the soft tissue. Further studies are warranted to examine the exact mechanism of action.
We also attempted to evaluate for a time-dependent response of electricity. It seemed that more volt-days of electricity led to a more predictable treatment effect. Both a dose-response and time-dependent bactericidal effect have been proven in vitro [6]. Seven-day exposure to electricity at 20, 200, and 2000 microamperes was examined by del Pozo et al. [6]. Dose-dependent and time-dependent bactericidal activity was observed in the presence of Staphylococcus and Pseudomonas biofilms.
The use of noninvasive capacitive coupling electric current is an enticing option as an addition to the prophylaxis of postoperative spinal wound infection. Its effect on biofilm has been proven in vitro and in vivo. When applied in the setting of a rabbit spine model infected with S aureus, capacitive coupling in combination with ceftriaxone decreased the instrumentation-related infection rate compared with ceftriaxone alone. A time-dependent response was noticed, but there was no effect on the soft tissue infection rate or on the overall bacterial burden. Reversing the protection that biofilms presumably confer to the microorganisms, capacitive coupling may effectively render the bacteria more susceptible to intravenously administered antibiotics. Future considerations include testing the bioelectric theory with different modes of electric current such as direct current.
Acknowledgments
We thank Hyunchul Kim, MS, who analyzed and ensured the accuracy of the data, and Daniel Gelb, MD, and Eugene Koh, MD, who oversaw the project and contributed to the writing. We also thank Senior Editor and Writer Dori Kelly, MA, for invaluable assistance editing the manuscript.
Footnotes
The institution of one or more of the authors (University of Maryland) has received, in any 1 year, grant funding from Biomet, EBI Medical Systems, Inc, and the Orthopaedic Research and Education Foundation. The institution also receives fellowship support from Synthes Spine. Each author certifies that he or she and members of his or her immediate family have no commercial associations that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of Maryland, Baltimore, MD, USA.
References
- 1.Benazzo F, Mosconi M, Beccarisi G, Galli U. Use of capacitive coupled electric fields in stress fractures in athletes. Clin Orthop Relat Res. 1995;310:145–149. [PubMed] [Google Scholar]
- 2.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27:171–182. [PubMed] [Google Scholar]
- 3.Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TE. A novel total knee arthroplasty infection model in rabbits. J Orthop Res. 2005;23:1100–1104. doi: 10.1016/j.orthres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Del Pozo JL, Rouse MS, Euba G, Kang CI, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrob Agents Chemother. 2009;53:4064–4068. doi: 10.1128/AAC.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, Patel R. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2009;53:35–40. doi: 10.1128/AAC.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of Staphylococcus and pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob Agents Chemother. 2009;53:41–45. doi: 10.1128/AAC.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Pozo JL, Rouse MS, Patel R. Bioelectric effect and bacterial biofilms: a systematic review. Int J Artif Organs. 2008;31:786–795. doi: 10.1177/039139880803100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Health and Human Services. HHS Action Plan to Prevent Healthcare-associated Infections. Washington, DC, USA: US Government Printing Office; 2009.
- 9.Gan JC, Glazer PA. Electrical stimulation therapies for spinal fusions: current concepts. Eur Spine J. 2006;15:1301–1311. doi: 10.1007/s00586-006-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glazer PA, Heilmann MR, Lotz JC, Bradford DS. Use of electromagnetic fields in a spinal fusion: a rabbit model. Spine (Phila Pa 1976) 1997;22:2351–2356. doi: 10.1097/00007632-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 11.Gold HS, Moellering RC., Jr Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199609263351304. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine (Phila Pa 1976) 1999;24:1349–1356. doi: 10.1097/00007632-199907010-00013. [DOI] [PubMed] [Google Scholar]
- 13.Hodges SD, Eck JC, Humphreys SC. Use of electrical bone stimulation in spinal fusion. J Am Acad Orthop Surg. 2003;11:81–88. doi: 10.5435/00124635-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hosman AH, Mei HC, Bulstra SK, Busscher HJ, Neut D. Metal-on-metal bearings in total hip arthroplasties: influence of cobalt and chromium ions on bacterial growth and biofilm formation. J Biomed Mater Res A. 2009;88:711–716. doi: 10.1002/jbm.a.31922. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Fay LA, Ito Y, Yuan MR, Edwards WT, Yuan HA. The effect of pulsed electromagnetic fields on instrumented posterolateral spinal fusion and device-related stress shielding. Spine (Phila Pa 1976) 1997;22:382–388. doi: 10.1097/00007632-199702150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 17.Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86:975–980. doi: 10.3171/jns.1997.86.6.0975. [DOI] [PubMed] [Google Scholar]
- 18.Marcer M, Musatti G, Bassett CA. Results of pulsed electromagnetic fields (PEMFs) in ununited fractures after external skeletal fixation. Clin Orthop Relat Res. 1984;190:260–265. [PubMed] [Google Scholar]
- 19.Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99–108. [PubMed] [Google Scholar]
- 20.Pickering SA, Bayston R, Scammell BE. Electromagnetic augmentation of antibiotic efficacy in infection of orthopaedic implants. J Bone Joint Surg Br. 2003;85:588–593. doi: 10.1302/0301-620X.85B4.12644. [DOI] [PubMed] [Google Scholar]
- 21.Poelstra KA, Barekzi NA, Grainger DW, Gristina AG, Schuler TC. A novel spinal implant infection model in rabbits. Spine (Phila Pa 1976) 2000;25:406–410. doi: 10.1097/00007632-200002150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rechtine GR, Bono PL, Cahill D, Bolesta MJ, Chrin AM. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma. 2001;15:566–569. doi: 10.1097/00005131-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Sasso RC, Garrido BJ. Postoperative spinal wound infections. J Am Acad Orthop Surg. 2008;16:330–337. doi: 10.5435/00124635-200806000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Scott G, King JB. A prospective, double-blind trial of electrical capacitive coupling in the treatment of non-union of long bones. J Bone Joint Surg Am. 1994;76:820–826. doi: 10.2106/00004623-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Slobogean GP, Kennedy SA, Davidson D, O’Brien PJ. Single- versus multiple-dose antibiotic prophylaxis in the surgical treatment of closed fractures: a meta-analysis. J Orthop Trauma. 2008;22:264–269. doi: 10.1097/BOT.0b013e31816b7880. [DOI] [PubMed] [Google Scholar]
- 26.Stall AC, Becker E, Ludwig SC, Gelb D, Poelstra KA. Reduction of postoperative spinal implant infection using gentamicin microspheres. Spine (Phila Pa 1976). 2009;34:479–483. doi: 10.1097/BRS.0b013e318197e96c. [DOI] [PubMed] [Google Scholar]
- 27.Borden AJ, Maathuis PG, Engels E, Rakhorst G, Mei HC, Busscher HJ, Sharma PK. Prevention of pin tract infection in external stainless steel fixator frames using electric current in a goat model. Biomaterials. 2007;28:2122–2126. doi: 10.1016/j.biomaterials.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Vinh DC, Embil JM. Device-related infections: a review. J Long Term Eff Med Implants. 2005;15:467–488. doi: 10.1615/JLongTermEffMedImplants.v15.i5.20. [DOI] [PubMed] [Google Scholar]
- 29.Young PM, Berquist TH, Bancroft LW, Peterson JJ. Complications of spinal instrumentation. Radiographics. 2007;27:775–789. doi: 10.1148/rg.273065055. [DOI] [PubMed] [Google Scholar]