Abstract
The ability to reproduce relies in most eukaryotes on specialized cells called gametes. Gametes are formed by the process of meiosis in which, after a single round of replication, two successive cell divisions reduce the ploidy of the genome. Fusion of gametes at fertilization reconstitutes diploidy. In most animal species, chromosome segregation during female meiosis occurs on spindles assembled in the absence of the major microtubule-organizing center, the centrosome. In mammals, oocyte meiosis is error-prone and underlies the majority of birth aneuploidies. Here, we review recent work on acentrosomal spindle formation and chromosome alignment/separation during oocyte meiosis in different animal models.
Oocyte meiosis is acentrosomal
In the majority of eukaryotic organisms, sexual reproduction relies on a specialized type of cell division termed meiosis that generates a reproduction-specific cell population called gametes. A hallmark of these specialized cells is that they contain a haploid number of chromosomes. This feature is crucial to maintaining the ploidy of sexually reproducing organisms upon fertilization, when the paternal gamete (sperm) fuses with the maternal one (oocyte). In order to halve the amount of genetic material, a single phase of genome replication precedes two successive cell divisions termed meiosis I and II (Figure 1). If the meiotic divisions are not properly executed, fertilization can result in aneuploid embryos carrying an incorrect number of chromosomes, an outcome that typically causes spontaneous abortion or birth defects in humans [1].
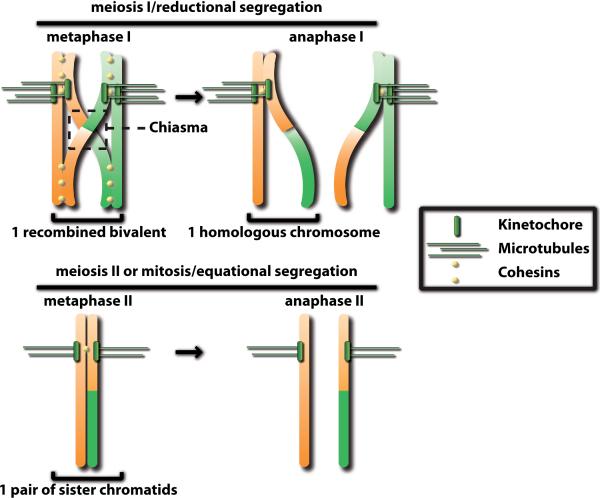
Figure 1. Chromosome segregation during meiosis.
Recombined homologous chromosomes are held together by sister chromatid cohesion after exchange of genetic material during meiotic recombination at the chiasma. During anaphase I, dissolution of chromosome arm cohesion leads to reductional segregation of homologs. Sister chromatids are equationally segregated following dissolution of centromeric cohesion during anaphase II.
Chromosome alignment and segregation occur on a spindle-shaped structure that is built from microtubules, 25 nm diameter tubes composed of α/β-tubulin dimers. In somatic animal cells and spermatocytes, microtubules are primarily assembled from the centrosome, the major microtubule organizing center (MTOC) that is comprised of two orthogonal tubulin cylinders – the centrioles – embedded in a cloud of pericentriolar material (PCM). Microtubule nucleating and anchoring activities are provided by the PCM while centrioles are essential to maintain the integrity of the centrosomal structure [2]. In dividing cells, centrosome duplication occurs once per cell cycle in G1/S phase. At mitosis onset the two centrosomes separate to form the two opposite poles of the mitotic spindle (Figure 2).
Figure 2. Models of centrosomal and acentrosomal spindle assembly.
Centrosomal spindles assemble in somatic cells, in spermatocytes and in echinoderm oocytes. Microtubules nucleated from the centrosomes (yellow) oscillate between phases of growth and shrinkage `searching' for chromosomes. End-on contact with a kinetochore results in `capture' of a chromosome and stabilization of the microtubule to form a kinetochore fiber (orange).
In mouse oocytes, multiple MTOCs (yellow) assemble microtubule aster-like structures in the vicinity of chromosomes that subsequently are organized into a bipolar spindle. Kinetochore fibers are periodically destabilized and re-established to maintain oscillating chromosomes at the spindle equator during the extended prometaphase.
In Drosophila oocytes, microtubule asters first assemble at NEBD away from chromosomes in the absence of discrete PCM-containing MTOCs. These acentrosomal asters are subsequently remodeled and incorporated into the forming spindle, which is assembled by sorting and focusing of microtubule (−)-ends at the spindle poles.
In cell-free Xenopus extracts, microtubules are nucleated in the vicinity of chromatin-coated beads in random orientation and are subsequently sorted into an antiparallel microtubule array that surrounds chromosomes. Microtubule (−)-ends are focused away from chromosomes to form the spindle poles.
During female meiosis in most animal species, however, chromosome segregation is preceded by an earlier step of centrosome elimination [3]. Electron microscopy indicates that oocyte meiotic spindles lack conventional centriole-containing centrosomes in Drosophila, Caenorhabditis elegans, Xenopus, chicken, rodents, humans and all other mammals analyzed [4, 5]. In contrast, male gametes typically retain their centrosomes (although there are exceptions; [3]) enabling reconstitution of correct centrosome number after fertilization. Female meiotic spindles are thus assembled in a centrosome-independent manner. Although the molecular details of the centrosome elimination process are still unclear, mechanisms underlying assembly of acentrosomal spindles in oocytes, and the segregation of recombined homologous chromosomes (during meiosis I) and sister chromatids (during meiosis II) on these acentrosomal spindles are starting to be elucidated. Here, we review recent work on acentrosomal spindle formation and chromosome alignment/separation on these spindles. For reviews discussing chromosome dynamics (homolog pairing, recombination and synapsis) during meiotic prophase and the regulation of cohesion removal that controls homolog versus chromatid segregation during the two meiotic divisions, see [6–9].
Generation of spindle microtubules in the absence of centrosomes
Chromatin can act as an acentrosomal MTOC
The study of acentrosomal spindles has led to significant progress in understanding how and where microtubules are assembled in the absence of centrosomes. In a seminal experiment, DNA-coated beads were shown to support formation of bipolar spindles in cell-free Xenopus egg extracts [10] indicating that chromatin can act as a localized origin for spindle microtubules (Figure 2).
In contrast to frog egg extracts, spindle microtubules in intact vertebrate oocytes do not emanate directly from chromatin but instead originate from non-centrosomal MTOCs that contain proteins normally present at centrosomes [4] (Figure 2). The exact composition and structure of these non-centrosomal MTOCs is unclear [5]. In Xenopus oocytes, a single lamellar MTOC assembles a transient microtubule array (TMA) at the base of the nucleus, which is subsequently remodeled into a bipolar spindle [11, 12]. In mouse oocytes, up to 80 MTOCs originate in the cytoplasm from the splitting of 2 to 3 larger aggregates; MTOCs can also form de novo after NEBD [13, 14]. These MTOCs assemble microtubule aster-like structures around chromatin that are subsequently organized into a bipolar spindle [14, 15]. In Drosophila oocytes, microtubule asters first assemble at NEBD away from chromosomes in the absence of discrete PCM-containing MTOCs. These acentrosomal asters are subsequently remodeled and incorporated into the forming spindle [16]. The site of microtubule assembly in vivo during meiosis II has not been examined carefully in any system. Thus, the difference in the origin of spindle microtubules in cell-free Xenopus extract and in vivo could reflect the division being analyzed (e.g. meiosis I in vivo versus meiosis II in vitro).
The RanGTP pathway
The first molecular activity implicated in microtubule formation around meiotic chromatin is the small GTPase Ran, whose active GTP-bound state is generated by the chromatin-localized exchange factor RCC1 (Regulator of Chromosome Condensation 1) [17](Figure 3). Compartmentalization of RanGTP is well studied in the context of nuclear transport, where the high concentration of RanGTP in the nucleus controls transport polarity. Direct observations with a FRET-based biosensor (Glossary) in mouse oocytes demonstrated the presence of similar RanGTP compartmentalization forming a gradient of active Ran centered on chromosomes after NEBD [15]. This chromatin-proximal increase in RanGTP has been proposed to promote activation of a number of spindle assembly factors (SAFs) specifically around chromosomes ([18], Table 1). SAFs display microtubule nucleation, interaction or motor activities that are regulated by the binding of nuclear import receptors to their nuclear localization sequences (NLS; Glossary) [18]. High concentrations of RanGTP around chromatin is proposed to cause local release of SAFs from import receptors and activation of SAFs to nucleate microtubules and control their organization. In support of this idea, inhibiting RanGTP prevents spindle assembly in frog egg extracts, reduces microtubule density around chromosomes and delays spindle assembly in mouse oocytes, and affects spindle pole organization in Drosophila oocytes [19].
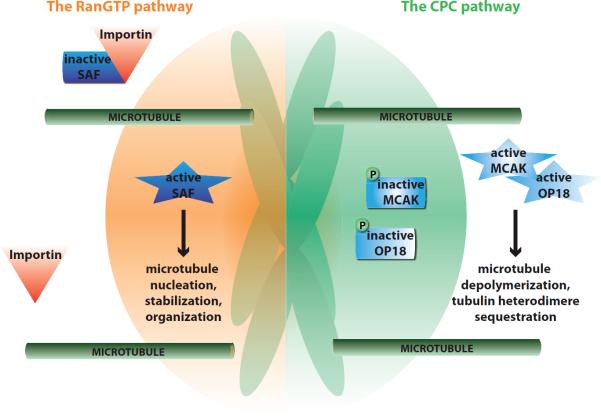
Figure 3. Chromatin-dependent pathways of microtubule assembly.
During M-Phase, spindle assembly factors (SAFs) are sequestered by Importins that bind to their NLS. A gradient of active Ran (RanGTP; orange) centered on chromosomes locally releases spindle assembly factors (SAFs) from the inhibitory effect of Importins. In parallel, Aurora B, which is activated by clustering at the inner centromere or on microtubules, phosphorylates and inhibits the microtubule depolymerase MCAK, and Oncoprotein 18 (OP18) to reduce its ability to sequester tubulin heterodimers. The Ran and CPC-dependent pathways create a locally favorable environment for microtubule and spindle assembly.
Table 1.
Ran-regulated spindle assembly factors
| Spindle Assembly Factor | Protein type | Function in oocyte |
|---|---|---|
| TPX2 (Targeting Protein for Xklp2) | MAP | MT assembly and spindle pole integrity [88] |
| NuMA (Nuclear Mitotic Apparatus) | MAP | Unknown |
| Kid (Kinesin-like DNA binding protein) | Chromokinesin | Anaphase chromosome compaction [53] |
| XCTK2 (Xenopus C-Terminal Kinesin 2) | Kinesin | Unknown |
| RAE1 (RNA export 1 protein) | MAP | Unknown |
| NuSAP (Nuclear and Spindle Associated Protein) | MAP | Unknown |
| HURP (Hepatoma Up Regulated Protein) | MAP | Metaphase central MT array formation [42] |
| Lamin B | Spindle matrix | Unknown |
| CDK11 (Cyclin-Dependent Kinase 11) | MAP | Unknown |
| ISWI (Imitation Switch) | MAP | Unknown |
| APC (Adenomatous Polyposis Coli) | MAP | Unknown |
MAP: microtubule associated protein; MT: microtubule
The Chromosomal Passenger Complex pathway
While there is significant evidence supporting the involvement of RanGTP in centrosome-independent spindle microtubule assembly, an alternate pathway also appears important. In mouse, frog and Drosophila oocytes, for example, inhibiting RanGTP production during meiosis I affects, but does not prevent, acentrosomal spindle assembly [15, 19]. Studies initiated in frog egg extracts and extended to other systems provide strong evidence that acentrosomal spindle assembly also requires the conserved Chromosomal Passenger Complex (CPC) – comprised of the kinase Aurora B (in vertebrates there is a meiosis-specific Aurora C that acts in parallel or substitutes for Aurora B [20]), the inner centromere protein (Incenp), and the chromatin-targeting subunits Survivin and Borealin/Dasra [21]. The CPC exhibits a complex localization pattern. During mitosis, soon after nuclear envelope breakdown, the CPC is broadly localized to chromatin; subsequently, its localization is restricted to the inner centromere region of chromosomes until anaphase onset when it concentrates on the anaphase central spindle. While the CPC also localizes to centromeric regions of chromosomes in most species during metaphase of meiosis, species-specific localizations have been described. In mouse oocytes, Aurora B/C remain broadly localized to chromosome arms and centromeres until anaphase onset [22, 23]. In Drosophila, the CPC concentrates on the equatorial region of the acentrosomal meiosis I spindle [24]. In C. elegans oocytes, CPC components form small ring-like structures between homologous chromosomes and sister chromatids during metaphase of meiosis I and II, respectively [25].
CPC inhibition prevents spindle assembly in Xenopus egg extracts [26] and identification of a Drosophila female-sterile Incenp mutant with impaired meiotic spindle assembly bolstered the idea that a CPC-dependent pathway contributes to acentrosomal spindle assembly in oocytes in vivo [24]. Further evidence was provided in mouse oocytes, where inhibiting Aurora kinase function significantly perturbs spindle formation [22, 23, 27, 28].
A mechanistic model for CPC-dependent microtubule assembly duringoocyte meiosis was suggested by recent experiments performed in Xenopus egg extracts [26, 29, 30]. The key concept emerging from these studies is that clustering of the kinase, either on chromatin or on microtubules, leads to its activation (Figure 3). The requirements for chromatin-based versus microtubule-based activation are distinct – the former requires the chromatin-targeting subunits of the CPC (Survivin and Borealin/Dasra) [26], whereas the latter requires a microtubule-binding region of the activating scaffold protein Incenp [30]. One model emerging from these studies is that microtubule-dependent activation of Aurora B creates a positive feedback loop that rapidly promotes microtubule assembly around chromatin soon after NEBD [30].
The two downstream targets of the CPC implicated to date in acentrosomal spindle assembly are negative regulators of microtubule polymerization [29, 30]. The first is the kinesin-13 depolymerase MCAK, which is phosphorylated and inactivated by Aurora B. In Xenopus egg extracts, depletion of MCAK partially restores spindle assembly in the absence of the CPC, indicating it is a functionally critical substrate [26]. The down-regulation of MCAK activity critical for spindle assembly involves xSgo2, a Shugoshin family member required for protection of centromeric cohesion during meiosis [31, 32]. xSgo2 interacts with the CPC and modulates its activity to promote phosphorylation of MCAK at serine 196, a modification that inhibits its microtubule depolymerase activity [33]. The second target is Oncoprotein 18 (OP18), whose phosphorylation by Aurora B reduces its ability to sequester tubulin heterodimers [29, 34]. These actions of xSgo2 generate a gradient of phosphorylated inactive OP18 (and potentially also MCAK) centered on chromatin, creating a locally favorable environment for microtubule polymerization [35]. Additional proteins with microtubule-directed activities are likely to be regulated by the CPC. In particular, positive regulators implicated in acentrosomal spindle assembly and activated by the CPC remain to be identified.
A key future challenge will be to understand the relative contributions of the RanGTP and CPC-based chromosomal pathways, as well as potential crosstalk between them, in acentrosomal spindle assembly. An additional challenge specific to vertebrate oocytes will be to understand the pathways that control formation of non-centrosomal MTOCs; while microtubules formed around chromatin are affected by Ran or CPC inhibition, the mechanism underlying formation of these protein clusters remains unresolved.
Organization of spindle microtubules without centrosomes
The spindle self-organization process
Concomitant with their assembly, microtubules must organize into a bipolar spindle-shaped structure. During mitosis, the two centrosomes mature, split and move toward opposite sides of the nucleus via antiparallel microtubule sliding and/or cortical forces. This mechanism ensures bipolarity of the spindle and imposes a spindle axis prior or during rupture of the nuclear envelope. During female meiosis, in the absence of pre-imposed bipolarity, the spindle axis is progressively aligned as microtubules assemble [36]. Studies of meiotic spindle assembly in cell-free Xenopus egg extracts led to the development of a self-organization model based on the ability of microtubule motors to move microtubules relative to each other [10]. In this model, microtubules in random orientation that are assembled near the chromatin are sorted through the activity of a tetrameric (+)-end-directed microtubule motor of the kinesin-5 family, leading to the formation of an antiparallel microtubule array that surrounds chromosomes. The (−)-end-directed motor Dynein focuses microtubule ends away from chromosomes to form the spindle poles. This model is consistent with the requirement of (+)-end directed motors to sort microtubules into a bipolar array, and of (−)-end directed motors to focus spindle poles in the absence of centrosomes [24, 37–41].
The central microtubule array
In mouse oocytes, the non-centrosomal MTOCs assemble microtubule aster-like structures in the vicinity of chromosomes that subsequently are organized into a bipolar spindle [14, 15]. MTOCs are sorted on the forming bipolar spindle and accumulate away from the chromosomes to form spindle poles [42]. MTOC sorting involves assembly of a central microtubule array, which acts as a scaffold for establishing spindle bipolarity and is essential for its maintenance. Formation of this central array requires the protein HURP (Hepatoma Up-Regulated Protein), which is regulated by RanGTP and accumulates on microtubules in the vicinity of chromosomes in a kinesin-5-dependent manner [42]. How HURP stabilizes the central array is unclear, however, it probably depends on its microtubule binding activity [43]. In Drosophila oocytes, the kinesin-6 family member Subito and the CPC member Incenp are required to assemble a similar structure, which is termed the metaphase central spindle [24]. Interestingly, the meiotic central array is reminiscent of the structure that assembles between segregating chromosomes in mitotic cells, termed the anaphase central spindle [44]. An in silico modeling approach demonstrated that formation of this mitotic structure, which is comprised of bundled antiparallel microtubules, only requires the activity of a (+)-end directed motor and a microtubule bundling protein [45]. It is tempting to speculate that the primary function of HURP in frog/mouse oocytes, or Incenp in Drosophila oocytes, is to bundle or crosslink microtubules organized by the (+)-end directed motor of the kinesin-5 or kinesin-6 family, respectively. Thus, formation of a central microtubule array that acts as a scaffold for bipolarity establishment and maintenance is likely a common theme in acentrosomal spindle formation that may have mechanistic parallels with formation of the mitotic anaphase central spindle.
Chromosome alignment and segregation on acentrosomal spindles
Although acentrosomal spindle assembly has been the subject of numerous studies, how these spindles interact with chromosomes is less clear. Spindle microtubules can interact either with motors localized on chromosome arms termed chromokinesins (Glossary), or with the centromere regions via the kinetochore (Glossary) [46, 47]. During mitosis in animal cells, microtubules assembled from the centrosomes grow toward the chromosomes where they interact with chromosome arms and kinetochores [47]. Whether the same model applies to meiotic acentrosomal spindles is the subject of recent work.
Chromosome congression and alignment on acentrosomal spindles
Studies in starfish oocytes revealed an unusual actin contractile network that aids in chromosome congression [48, 49]. However, actin polymerization inhibitors do not appear to affect congression in other species [50, 51].
In mouse oocytes, chromosome congression and alignment seems to be a multi-step process. Soon after NEBD, initial congression of chromosomes toward the spindle equator occurs in the absence of stable end-on microtubule kinetochore attachments. Consistent with this view, electron microscopy revealed multiple microtubules that project directly inside the chromosome arms during prometaphase [36]. Based on this, a model for chromosome congression in mouse oocytes is that chromokinesins interacting with these microtubules `push' chromosomes toward the spindle equator to promote initial congression events [36, 52]. However, the exact nature of this chromokinesin remains elusive because genetic loss-of-function of the chromokinesin Kid in mouse oocytes does not prevent congression [53]. Once chromosomes have reached the spindle equator, they oscillate along the spindle axis while attempting bi-orientation. Oscillatory movements of chromosomes observed during this phase reflect lateral kinetochore-microtubule interactions as well as CPC-dependent control of end-on kinetochore-microtubule attachments [52]. In mitosis, the CPC destabilizes incorrect end-on attachments due to lack of tension across kinetochores and promotes re-orientation [54]. The same CPC-dependent mechanism is at play during meiosis in mouse oocytes and explains why end-on kinetochore-spindle microtubule attachments established soon after NEBD are periodically destabilized and re-established to maintain oscillating chromosomes at the spindle equator until the last hour preceding anaphase [22, 23, 27, 28, 52].
In C. elegans, the chromokinesin KLP-19 contributes to proper chromosome congression when oocytes are artificially arrested in metaphase I, leading to a chromokisesin-dependent model of chromosome congression. KLP-19-mediated lateral interaction between microtubule bundles and chromosomes could push the chromosomes towards the spindle equator and promote their congression [55]. However, depletion of KLP-19 does not lead to a significant congression defect in normally progressing C. elegans oocytes [25]. Instead, chromosome alignment in these oocytes seems to primarily depend on kinetochore function and may be facilitated by an unusual spindle shrinkage that occurs during late metaphase and requires microtubule-severing activity; this shrinkage could collect dispersed chromosomes [25, 56].
Chromosome segregation on acentrosomal spindles
After removal of cohesion, aligned chromosomes are physically separated via forces exerted by spindle microtubules. During mitosis these forces are transmitted to chromosomes via kinetochores [47]; in the absence of kinetochores, mitotic chromosomes do not align and fail to segregate in anaphase. Both kinetochore-generated forces and poleward flux of the kinetochore microtubules contribute to the separation process [57]. The function of core kinetochore components during female meiosis has primarily been studied in the worm C. elegans [25, 58]. In this system, kinetochores are assembled by a centromeric-chromatin independent pathway on the chromosome surface, coincident with NEBD in meiosis I [59]. Kinetochore assembly is required for proper chromosome orientation on the pre-anaphase spindle and thus for accuracy of chromosome segregation; similarly, in mouse oocytes inaccurate chromosome segregation is observed upon perturbation of kinetochore function [60, 61]. Surprisingly, kinetochores are not essential for anaphase chromosome physical separation in C. elegans [25]. In this system, significant shrinkage of the spindle occurs prior to anaphase so that spindle poles are almost directly apposed to the poleward chromosomal surface [56]. Anaphase chromosome separation is accompanied by microtubule assembly between the separating chromosomes. This meiotic anaphase central spindle is proposed to promote physical separation of chromosomes in an `inside-out' pushing mechanism that functionally substitutes for the classical mitotic kinetochore-mediated pulling mechanism (Figure 4). Functional analysis implicated the microtubule dynamics regulator CLASP (Cytoplasmic Linker Associated Protein) in kinetochore-independent segregation, which, together with a set of proteins downstream of the CPC, forms ring-like structures between homologous chromosomes [62, 63]. Consistent with this, depletion of CLASP in Xenopus egg extracts leads to abrupt loss of spindle microtubules following anaphase onset [64]. Whether kinetochore-independent anaphase separation represents a general adaptation required for chromosome segregation on acentrosomal spindles is unclear. In mouse oocytes, residual kinetochore components following siRNA-mediated depletion may account for the physical separation of chromosomes observed following kinetochore inhibition [60, 61]. However, as DNA-coated beads or chromatin injected into mouse oocytes separate into distinct masses without functional kinetochores [65], an alternative possibility is that anaphase chromosome separation in vertebrate oocytes is also kinetochore-independent.
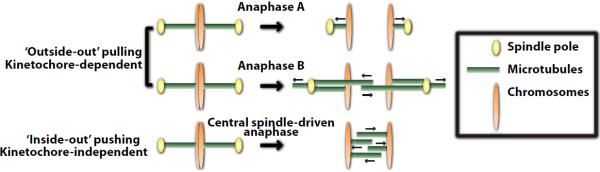
Figure 4. Mechanisms of anaphase chromosome physical separation.
During anaphase A, shortening of kinetochore-attached microtubules moves chromosomes toward the spindle poles without increase in the pole-to-pole distance. Anaphase B involves separation of the spindle poles while chromosome-to-pole distance remains constant. Spindle pole separation relies on sliding of antiparallel interpolar microtubules and/or cortical pulling forces exerted on astral microtubules. Both anaphase A and B chromosome physical separation requires functional kinetochores with end-on attached microtubules. Central spindle-driven anaphase relies on microtubule assembly between the separating chromosomes and is kinetochore-independent. The nature of the link between microtubules and chromosomes, and whether microtubule assembly and/or sliding promote chromosome physical separation in this mechanism are still unclear.
Acentrosomal Spindles and Polar Body Formation
In most animal species, with the notable exception of Drosophila [66], meiosis I and II end with highly asymmetric divisions leading to the extrusion of half the segregated chromosome complement into non-dividing polar bodies. These asymmetric divisions depend on positioning the acentrosomal spindle in close proximity to the oocyte cortex, which, depending on the species, occurs prior to or after meiosis I spindle assembly [67].
Positioning of the acentrosomal spindle
In frog eggs, the transient microtubule array (TMA) that assembles on one side (the vegetal surface) of the nucleus is thought to act as a `lift' that brings chromosomes close to the cortex [12]. The mechanisms at play and the cytoskeletal components involved remain unclear, however. In C. elegans, prior to NEBD, a microtubule- and kinesin-1-dependent process moves the centrally positioned oocyte nucleus towards one side, the future anterior cortex [68]. At NEBD, the assembling spindle is thus already off-center. In contrast, mouse oocyte spindles assemble near the center of the cell during meiosis I. In this system, an F-actin meshwork nucleated by Spire 1 and 2 and Formin 2 proteins is interconnected with spindle microtubules and promotes metaphase spindle translocation toward the oocyte cortex [51, 69–72].
Defining the site of polar body extrusion
Spindle translocation promotes local differentiation of the oocyte cortex, defining the site of polar body extrusion and restricting its size [67]. In many species, the oocyte external surface is covered by multiple microvilli except in the region directly adjacent to the translocated spindle. This cortical change alters the surface of this region, blocking sperm penetration to avoid meiotic spindle damage and preventing extrusion of the sperm genome into the polar body. The microvilli-free zone correlates with a special cortical actin-rich domain. In Xenopus oocytes, cortical differentiation is triggered by spindle pole attachment to the oocyte cortex at anaphase onset [73]. In mouse oocytes, chromosomes on the acentrosomal spindles, via their generation of RanGTP, trigger cortical differentiation [74–76]. Downstream targets of RanGTP involved in this process have not yet been identified but this finding indicates a link between chromosomes and cortical differentiation. The differentiated cortex is the site of recruitment of the small GTPase Cdc42 [77], which promotes cortical relaxation and polar body `outpocketing' through assembly of a highly dynamic F-actin network [73, 78, 79]. Polar body cytokinesis relies on a ring of active RhoA that circumscribes the region of active Cdc42 and directs assembly of an actomyosin contractile ring that extends into a tube before abscission [77, 80]. Active Rac1 also is observed in the cortical area adjacent to oocyte spindles after their translocation [81], but the functional significance of this is unclear as Rac1 inhibition does not impair polar body formation in Xenopus oocytes [82].
This brief summary highlights recent work linking chromosome alignment and segregation on acentrosomal spindles to the highly asymmetric division of oocytes that generates the mature female gamete.
Concluding remarks
Nearly a century after the pioneering work of Van Beneden on meiosis the molecular mechanisms underlying this specialized cell division are beginning to be elucidated. Here, we have focused on the acentrosomal oocyte meiotic spindle, whose assembly and function is critical to the formation of the female gamete in animal cells. A key question that remains unanswered is why are centrosomes eliminated in oocytes of most animal species? A simple answer would be that centrosome elimination is essential to prevent multipolar spindle assembly after fertilization and introduction of the sperm centrosome. However, in many species, including rodents and some primates, the sperm does not contribute a centrosome at fertilization [3]. Instead centrosomes are formed de novo in the dividing embryo. An alternative hypothesis for maternal centrosome elimination function would be to prevent parthenogenesis. Indeed, in Xenopus oocyte, an exogenously introduced centrosome can induce activation of the unfertilized oocyte and successful parthenogenesis [83]. Whether this holds true in other species, however, remains to be tested. Clearly the functional significance of maternal centrosome elimination is far from being understood and will require further work.
As errors in female meiotic chromosome segregation are highly prevalent in the human population, insights into the mechanism of this division have the potential to influence reproductive medicine [1]. Although explanations for this sexual dimorphism have recently been proposed, and implicate age-related chromosome cohesion loss, our understanding of the processes that control the genetic integrity of female gametes is far from complete [84–87]. In particular, the mechanisms that lead to haploidization of the oocyte genome are just beginning to be elucidated. Studies on oocyte spindles have shed light on fundamental mechanisms controlling microtubule assembly, self-organization and chromosome segregation in the absence of centrosomes. The development of dynamic imaging systems and new approaches to investigate this specialized division in vivo will continue to advance our understanding of this fascinating process, whose proper execution is critical for sexual reproduction.
ACKNOWLEDGMENTS
This work was supported by grants from the ANR (ANR-09-RPDOC-005-01) and the FRM (Fondation pour la Recherche Médicale) to J.D., funding from the Ludwig Institute for Cancer Research and a grant from the NIH to A.D. (GM074215). We apologize to our colleagues whose work could not be cited owing to space limitations.
Glossary
- NLS
Short amino-acid sequence that binds strongly to nuclear import proteins Importin α and/or β.
- FRET
Fluorescence Resonance Energy Transfer. Useful light microscopy tool to detect and quantify protein-protein interactions or conformational changes.
- Flux
Continuous poleward movement of microtubules due to constant addition of tubulin subunits at microtubule plus ends and their corresponding removal from microtubule minus-ends at spindle poles.
- Chromokinesin
Kinesin that displays a classical microtubule motor domain and a DNA-binding domain.
- Kinetochore
Multiprotein complex that assembles at the centromere and provides a site of attachment for microtubules.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21:703–708. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Manandhar G, et al. Centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13. doi: 10.1095/biolreprod.104.031245. [DOI] [PubMed] [Google Scholar]
- 4.Szöllösi D, et al. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci. 1972;11:521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- 5.Dumont J, Brunet S. Meiotic Spindle Assembly and Chromosome Segregation in Oocytes. In: Verlhac MH, Villeneuve AM, editors. Oogenesis: The Universal Process. Wiley-Blackwell; 2010. pp. 269–290. [Google Scholar]
- 6.Cromie GA, Smith GR. Branching out: meiotic recombination and its regulation. Trends Cell Biol. 2007;17:448–455. doi: 10.1016/j.tcb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Zickler D. From early homologue recognition to synaptonemal complex formation. Chromosoma. 2006;115:158–174. doi: 10.1007/s00412-006-0048-6. [DOI] [PubMed] [Google Scholar]
- 8.Schvarzstein M, et al. Coordinating cohesion, co-orientation, and congression during meiosis: lessons from holocentric chromosomes. Genes Dev. 2010;24:219–228. doi: 10.1101/gad.1863610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishiguro K, Watanabe Y. Chromosome cohesion in mitosis and meiosis. J Cell Sci. 2007;120:367–369. doi: 10.1242/jcs.03324. [DOI] [PubMed] [Google Scholar]
- 10.Heald R, et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 11.Huchon D, et al. Germinal vesicle breakdown in the Xenopus laevis oocyte: description of a transient microtubular structure. Reprod Nutr Dev. 1981;21:135–148. doi: 10.1051/rnd:19810112. [DOI] [PubMed] [Google Scholar]
- 12.Gard DL. Microtubule organization during maturation of Xenopus oocytes: assembly and rotation of the meiotic spindles. Dev Biol. 1992;151:516–530. doi: 10.1016/0012-1606(92)90190-r. [DOI] [PubMed] [Google Scholar]
- 13.Calarco PG. Centrosome precursors in the acentriolar mouse oocyte. Microsc Res Tech. 2000;49:428–434. doi: 10.1002/(SICI)1097-0029(20000601)49:5<428::AID-JEMT4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Dumont J, et al. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol. 2007;176:295–305. doi: 10.1083/jcb.200605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skold HN, et al. Assembly pathway of the anastral Drosophila oocyte meiosis I spindle. J Cell Sci. 2005;118:1745–1755. doi: 10.1242/jcs.02304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalab P, et al. The ran GTPase regulates mitotic spindle assembly. Curr Biol. 1999;9:481–484. doi: 10.1016/s0960-9822(99)80213-9. [DOI] [PubMed] [Google Scholar]
- 18.Clarke PR, Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol. 2008;9:464–477. doi: 10.1038/nrm2410. [DOI] [PubMed] [Google Scholar]
- 19.Cesario J, McKim KS. RanGTP is required for meiotic spindle organization and the initiation of embryonic development in Drosophila. J Cell Sci. 2011;124:3797–3810. doi: 10.1242/jcs.084855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang CJ, et al. Dynamic localization and functional implications of Aurora-C kinase during male mouse meiosis. Dev Biol. 2006;290:398–410. doi: 10.1016/j.ydbio.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Ruchaud S, et al. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 22.Shuda K, et al. Aurora kinase B modulates chromosome alignment in mouse oocytes. Mol Reprod Dev. 2009;76:1094–1105. doi: 10.1002/mrd.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharif B, et al. The chromosome passenger complex is required for fidelity of chromosome transmission and cytokinesis in meiosis of mouse oocytes. J Cell Sci. 2010;123:4292–4300. doi: 10.1242/jcs.067447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombie N, et al. Dual roles of Incenp crucial to the assembly of the acentrosomal metaphase spindle in female meiosis. Development. 2008;135:3239–3246. doi: 10.1242/dev.022624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumont J, et al. A kinetochore-independent mechanism drives anaphase chromosome separation during acentrosomal meiosis. Nat Cell Biol. 2010;12:894–901. doi: 10.1038/ncb2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampath SC, et al. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Swain JE, et al. Regulation of spindle and chromatin dynamics during early and late stages of oocyte maturation by aurora kinases. Mol Hum Reprod. 2008;14:291–299. doi: 10.1093/molehr/gan015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang KT, et al. Aurora-C kinase deficiency causes cytokinesis failure in meiosis I and production of large polyploid oocytes in mice. Mol Biol Cell. 2010;21:2371–2383. doi: 10.1091/mbc.E10-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly AE, et al. Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell. 2007;12:31–43. doi: 10.1016/j.devcel.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng BS, et al. Dual detection of chromosomes and microtubules by the chromosomal passenger complex drives spindle assembly. Dev Cell. 2010;18:903–912. doi: 10.1016/j.devcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, et al. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol. 2008;10:42–52. doi: 10.1038/ncb1667. [DOI] [PubMed] [Google Scholar]
- 32.Llano E, et al. Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 2008;22:2400–2413. doi: 10.1101/gad.475308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera T, et al. Xenopus Shugoshin 2 regulates the spindle assembly pathway mediated by the chromosomal passenger complex. Embo J. 2012 doi: 10.1038/emboj.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budde PP, et al. Regulation of Op18 during spindle assembly in Xenopus egg extracts. J Cell Biol. 2001;153:149–158. doi: 10.1083/jcb.153.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niethammer P, et al. Stathmin-tubulin interaction gradients in motile and mitotic cells. Science. 2004;303:1862–1866. doi: 10.1126/science.1094108. [DOI] [PubMed] [Google Scholar]
- 36.Brunet S, et al. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J Cell Biol. 1999;146:1–12. doi: 10.1083/jcb.146.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mailhes JB, et al. Transient exposure to the Eg5 kinesin inhibitor monastrol leads to syntelic orientation of chromosomes and aneuploidy in mouse oocytes. Mutat Res. 2004;559:153–167. doi: 10.1016/j.mrgentox.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Zhang D, et al. Cytoplasmic dynein participates in meiotic checkpoint inactivation in mouse oocytes by transporting cytoplasmic mitotic arrest-deficient (Mad) proteins from kinetochores to spindle poles. Reproduction. 2007;133:685–695. doi: 10.1530/rep.1.01167. [DOI] [PubMed] [Google Scholar]
- 39.Fitzharris G. A shift from kinesin 5-dependent metaphase spindle function during preimplantation development in mouse. Development. 2009;136:2111–2119. doi: 10.1242/dev.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang JK, et al. The kinesinlike protein Subito contributes to central spindle assembly and organization of the meiotic spindle in Drosophila oocytes. Mol Biol Cell. 2005;16:4684–4694. doi: 10.1091/mbc.E04-11-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang JK, et al. Misregulation of the kinesin-like protein Subito induces meiotic spindle formation in the absence of chromosomes and centrosomes. Genetics. 2007;177:267–280. doi: 10.1534/genetics.107.076091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breuer M, et al. HURP permits MTOC sorting for robust meiotic spindle bipolarity, similar to extra centrosome clustering in cancer cells. J Cell Biol. 2010;191:1251–1260. doi: 10.1083/jcb.201005065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sillje HH, et al. HURP is a Ran-importin beta-regulated protein that stabilizes kinetochore microtubules in the vicinity of chromosomes. Curr Biol. 2006;16:731–742. doi: 10.1016/j.cub.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 44.Douglas ME, Mishima M. Still entangled: assembly of the central spindle by multiple microtubule modulators. Semin Cell Dev Biol. 2010;21:899–908. doi: 10.1016/j.semcdb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Janson ME, et al. Crosslinkers and motors organize dynamic microtubules to form stable bipolar arrays in fission yeast. Cell. 2007;128:357–368. doi: 10.1016/j.cell.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 46.Mazumdar M, Misteli T. Chromokinesins: multitalented players in mitosis. Trends Cell Biol. 2005;15:349–355. doi: 10.1016/j.tcb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 48.Mori M, et al. Intracellular transport by an anchored homogeneously contracting F-actin meshwork. Curr Biol. 2011;21:606–611. doi: 10.1016/j.cub.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Lenart P, et al. A contractile nuclear actin network drives chromosome congression in oocytes. Nature. 2005;436:812–818. doi: 10.1038/nature03810. [DOI] [PubMed] [Google Scholar]
- 50.Yang HY, et al. MEI-1/katanin is required for translocation of the meiosis I spindle to the oocyte cortex in C elegans. Dev Biol. 2003;260:245–259. doi: 10.1016/s0012-1606(03)00216-1. [DOI] [PubMed] [Google Scholar]
- 51.Dumont J, Million K, Sunderland K, Rassinier P, Lim H, Leader B, Verlhac MH. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev Biol. 2007;301:254–265. doi: 10.1016/j.ydbio.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 52.Kitajima TS, et al. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell. 2011;146:568–581. doi: 10.1016/j.cell.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 53.Ohsugi M, et al. Kid-mediated chromosome compaction ensures proper nuclear envelope formation. Cell. 2008;132:771–782. doi: 10.1016/j.cell.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 54.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–140. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wignall SM, Villeneuve AM. Lateral microtubule bundles promote chromosome alignment during acentrosomal oocyte meiosis. Nat Cell Biol. 2009;11:839–844. doi: 10.1038/ncb1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNally K, et al. Katanin controls mitotic and meiotic spindle length. J Cell Biol. 2006;175:881–891. doi: 10.1083/jcb.200608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desai A, et al. Anaphase A chromosome movement and poleward spindle microtubule flux occur At similar rates in Xenopus extract spindles. J Cell Biol. 1998;141:703–713. doi: 10.1083/jcb.141.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howe M, et al. HIM-10 is required for kinetochore structure and function on Caenorhabditis elegans holocentric chromosomes. J Cell Biol. 2001;153:1227–1238. doi: 10.1083/jcb.153.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monen J, et al. Differential role of CENP-A in the segregation of holocentric C. elegans chromosomes during meiosis and mitosis. Nat Cell Biol. 2005;7:1248–1255. doi: 10.1038/ncb1331. [DOI] [PubMed] [Google Scholar]
- 60.Sun SC, et al. Ndc80 regulates meiotic spindle organization, chromosome alignment, and cell cycle progression in mouse oocytes. Microsc Microanal. 2011;17:431–439. doi: 10.1017/S1431927611000274. [DOI] [PubMed] [Google Scholar]
- 61.Sun SC, et al. Perturbation of Spc25 expression affects meiotic spindle organization, chromosome alignment and spindle assembly checkpoint in mouse oocytes. Cell Cycle. 2010;9:4552–4559. doi: 10.4161/cc.9.22.13815. [DOI] [PubMed] [Google Scholar]
- 62.Maiato H, et al. Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell. 2003;113:891–904. doi: 10.1016/s0092-8674(03)00465-3. [DOI] [PubMed] [Google Scholar]
- 63.Cheeseman IM, et al. The CENP-F-like proteins HCP-1 and HCP-2 target CLASP to kinetochores to mediate chromosome segregation. Curr Biol. 2005;15:771–777. doi: 10.1016/j.cub.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 64.Hannak E, Heald R. Xorbit/CLASP links dynamic microtubules to chromosomes in the Xenopus meiotic spindle. J Cell Biol. 2006;172:19–25. doi: 10.1083/jcb.200508180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng M, et al. Kinetochore-independent chromosome poleward movement during anaphase of meiosis II in mouse eggs. PLoS One. 2009;4:e5249. doi: 10.1371/journal.pone.0005249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Theurkauf WE, Hawley RS. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J Cell Biol. 1992;116:1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verlhac MH, Lee KW. Mechanisms of asymmetric division in metazoan meiosis. In: Verlhac MH, Villeneuve AM, editors. Oogenesis: The Universal Process. Wiley-Blackwell; 2010. pp. 291–310. [Google Scholar]
- 68.McNally KL, et al. Kinesin-dependent transport results in polarized migration of the nucleus in oocytes and inward movement of yolk granules in meiotic embryos. Dev Biol. 2010;339:126–140. doi: 10.1016/j.ydbio.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schuh M, Ellenberg J. A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol. 2008;18:1986–1992. doi: 10.1016/j.cub.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 70.Azoury J, et al. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol. 2008;18:1514–1519. doi: 10.1016/j.cub.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 71.Azoury J, et al. Actin filaments: key players in the control of asymmetric divisions in mouse oocytes. Biol Cell. 2009;101:69–76. doi: 10.1042/BC20080003. [DOI] [PubMed] [Google Scholar]
- 72.Pfender S, et al. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr Biol. 2011;21:955–960. doi: 10.1016/j.cub.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, et al. Polar body emission requires a RhoA contractile ring and Cdc42-mediated membrane protrusion. Dev Cell. 2008;15:386–400. doi: 10.1016/j.devcel.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maro B, et al. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton and the plasma membrane. J Embryol Exp Morphol. 1986;92:11–32. [PubMed] [Google Scholar]
- 75.Deng M, et al. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev Cell. 2007;12:301–308. doi: 10.1016/j.devcel.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 76.Verlhac MH, Dumont J. Interactions between chromosomes, microfilaments and microtubules revealed by the study of small GTPases in a big cell, the vertebrate oocyte. Mol Cell Endocrinol. 2008;282:12–17. doi: 10.1016/j.mce.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 77.Ma C, et al. Cdc42 activation couples spindle positioning to first polar body formation in oocyte maturation. Curr Biol. 2006;16:214–220. doi: 10.1016/j.cub.2005.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leblanc J, et al. The Small GTPase Cdc42 Promotes Membrane Protrusion during Polar Body Emission via ARP2-Nucleated Actin Polymerization. Mol Hum Reprod. 2011 doi: 10.1093/molehr/gar026. [DOI] [PubMed] [Google Scholar]
- 79.Sun SC, et al. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS One. 2011;6:e18392. doi: 10.1371/journal.pone.0018392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dorn JF, et al. Actomyosin tube formation in polar body cytokinesis requires Anillin in C. elegans. Curr Biol. 2010;20:2046–2051. doi: 10.1016/j.cub.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 81.Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell. 2007;12:309–317. doi: 10.1016/j.devcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 82.Na J, Zernicka-Goetz M. Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr Biol. 2006;16:1249–1254. doi: 10.1016/j.cub.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 83.Maller J, et al. Spindle formation and cleavage in Xenopus eggs injected with centriole-containing fractions from sperm. Exp Cell Res. 1976;99:285–294. doi: 10.1016/0014-4827(76)90585-1. [DOI] [PubMed] [Google Scholar]
- 84.Revenkova E, et al. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol. 2010;20:1529–1533. doi: 10.1016/j.cub.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiang T, et al. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–1528. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lister LM, et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–1521. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 87.Tachibana-Konwalski K, et al. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010;24:2505–2516. doi: 10.1101/gad.605910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brunet S, Dumont J, Lee KW, Kinoshita K, Hikal P, Gruss OJ, Maro B, Verlhac MH. Meiotic regulation of TPX2 protein levels governs cell cycle progression in mouse oocytes. PLoS One. 2008;3:e3338. doi: 10.1371/journal.pone.0003338. [DOI] [PMC free article] [PubMed] [Google Scholar]