Abstract
The persistent environmental contaminant, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is an ovarian toxicant. These studies were designed to characterize the actions of TCDD on steroidogenesis and growth of intact mouse antral follicles in vitro. Specifically, these studies tested the hypothesis that TCDD exposure leads to decreased sex hormone production/secretion by antral follicles as well as decreased growth of antral follicles in vitro. Since TCDD acts through binding to the aryl hydrocarbon receptor (AHR), and the AHR has been identified as an important factor in ovarian function, we also conducted experiments to confirm the presence and activation of the AHR in our tissue culture system. To do so, we exposed mouse antral follicles for 96 hours to a series of TCDD doses previously shown to have effects on ovarian tissues and cells in culture, which also encompass environmentally relevant and pharmacological exposures (0.1-100nM), to determine a dose response for TCDD in our culture system for growth, hormone production, expression of the Ahr and Cyp1b1. The results indicate that TCDD decreases progesterone, androstenedione, testosterone, and estradiol levels in a non-monotonic dose response manner without altering growth of antral follicles. The addition of pregnenolone substrate (10μM) restores hormone levels to control levels. Additionally, Cyp1b1 levels were increased by 3-4 fold regardless of the dose of TCDD exposure, evidence of AHR activation. Overall, these data indicate that TCDD may act prior to pregnenolone formation and through AHR transcriptional control of Cyp1b1, leading to decreased hormone levels without affecting growth of antral follicles.
Keywords: TCDD, antral follicle, proliferation, ovary, steroidogenesis, aryl hydrocarbon receptor
Introduction
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a persistent environmental contaminant inadvertently produced as a by-product of herbicide and pesticide manufacturing (Hites, 2011). TCDD is also released during the bleaching process at tree pulp and paper mills, and during burning of municipal solid waste (Frakes et al., 1993;Tuppurainen et al., 2003). TCDD is the most toxic member of a class of chemicals called dioxins. Dioxins, like TCDD, have a long environmental half-life, bioaccumulate in the food chain, and can be found in human fat tissue, blood serum, breast milk and ovarian follicular fluid (Birnbaum and DeVito, 1995;Humblet et al., 2011;Schecter et al., 2006;Tsutsumi et al., 2011;Ulaszewska et al., 2011).
Studies of human populations accidentally exposed to high levels of TCDD and controlled studies using various animal models exposed to TCDD have shown that TCDD is a potent endocrine disruptor. TCDD exposures have been linked to delayed puberty and early onset of menopause in women (Eskenazi et al., 2005;Warner et al., 2007). Similarly, TCDD exposures lead to early puberty, irregular estrous cycles, reduced or blocked ovulation, decreased circulating estradiol levels (E2), and early reproductive senescence in female rodents (Gray and Ostby, 1995; Li et al., 1995 Chaffin et al., 1996;Franczak et al., 2006;Jablonska et al., 2010;Myllymäki et al., 2005;Shi et al., 2007).
Though it has been shown that TCDD can affect the development and functioning of the rat hypothalamus and pituitary, there is convincing evidence that it has direct effects on the ovary as well (Cao et al., 2011;Gao et al., 2001;Mizuyachi et al., 2002;Petroff et al., 2001;Petroff et al., 2003). Further, it has been demonstrated that TCDD can accumulate in ovarian follicles following controlled dosing experiments in animals and in culture (Baldridge and Hutz, 2007;Grochowalski et al., 2001).
Antral follicles are the main functional unit of the ovary, housing the gametes and serving as the primary source of sex steroid hormones, such as 17β-estradiol (E2). Follicles grow from the primordial to the antral stage in a process called folliculogenesis. In the process of folliculogenesis, follicles increase in size mainly due to proliferation of granulosa cells. It has been proposed that TCDD decreases circulating E2 levels in part by inhibiting the growth of follicles from the preantral to antral stage and by disrupting the synthesis and metabolism of sex steroid hormones (Dasmahapatra et al., 2000;Gregoraszczuk et al., 2001;Grochowalski et al., 2001;Heimler et al., 1998b;Heimler et al., 1998a; Morán et al., 2003a;Morán et al., 2000;Morán et al., 2003b;Myllymäki et al., 2005;Pesonen et al., 2006). In the ovary, E2 synthesis requires both the thecal and granulosa cell compartments. In the rodent, the thecal cells are responsible for producing progesterone (P4) and androstenedione (A4). A4 then diffuses to nearby granulosa cells where it is converted to testosterone (T) and E2. In antral follicles, the rate limiting steps in steroid hormone production are the mobilization of cholesterol from the cytoplasm to the inner mitochondrial membrane via steroidogenic acute regulatory protein (StAR) and the conversion of cholesterol to pregnenolone by the mitochondrial enzyme cytochrome P450, family 11, subfamily a, polypeptide 1 (P450scc) (Edson et al., 2009). Pregnenolone is then converted to P4, and then by a multistep process to the androgens, and finally to E2.
To date, the majority of studies conducted with the purpose of understanding the mechanism underlying disrupted follicle growth and female sex steroid hormone production have been conducted using isolated granulosa or thecal cells in vitro. Additionally, the results reported vary widely and sometimes are conflicting depending on the cell line, culture system, animal model, dose of TCDD, length of exposure, and age of the animals being used. Thus, in this study, we set out to determine the effect of various doses of TCDD on intact adult mouse antral follicles on growth and steroid hormone production/secretion. We specifically tested the hypothesis that TCDD exposure leads to decreased sex hormone production/secretion by antral follicles as well as decreased growth of antral follicles in vitro. Since TCDD is known to act through binding to the transcription factor, the aryl hydrocarbon receptor (AHR), and the AHR has been identified as an important factor in ovarian function (Hernández-Ochoa et al., 2009;Rowlands and Gustafsson, 1997), we also conducted experiments to confirm the presence and activation of the AHR in our tissue culture system. Since our results indicate that TCDD inhibits steroidogenesis in antral follicles, we further investigated whether we could restore steroid production/secretion in TCDD exposed follicles by co-treating with pregnenolone, an early substrate in steroidogenesis.
Material and Methods
Chemicals
2,3,7,8-tetrachlorodibenzo-p-dioxin dissolved in dimethyl sulphoxide (DMSO) at 50μg/mL (#ED-901-B) was purchased from Cambridge Isotope Laboratories, Inc., Andover, MA. Pregnenolone (P9129), DMSO (D2650), 100X insulin, transferrin, and selenium (ITS), penicillin and streptomycin were purchased from Sigma-Aldrich (St. Louis, MO). Alpha minimal essential media (α-MEM) was purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals, Lawrenceville, GA. Human recombinant follicle-stimulating hormone (hFSH) was obtained from Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA.
Animals
CD-1 mice were purchased from Charles River and maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the University of Illinois Animal Care and Use Committee. Upon arrival to the University of Illinois animal care facility, mice were allowed to acclimate for a minimum of 48 hours prior to tissue collection. All mice were housed under strict 12L:12D lighting and temperature was maintained at 22±1°C. Food and water were provided ad libitum.
In vitro mouse antral follicle culture
Female mice were sacriced by CO2 asphyxiation followed by cervical dislocation on postnatal day (PND) 33 and their ovaries removed. Small antral follicles were isolated mechanically from the ovaries of 2-4 mice for each experiment based on relative size (200-350μm) and cleaned of interstitial tissue using ne #5 watchmaker forceps in unsupplemented α-MEM. Approximately 20–30 antral follicles were isolated from each mouse. Follicles were then placed randomly one per individual well in a 96-well culture plate with α-MEM prior to treatment. For each experiment, a minimum of eight follicles were plated per treatment group. Supplemented α-MEM was prepared with 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium), 100 U/ml penicillin, 100 μg/ml streptomycin, 5 IU/ml hFSH, and 5% FBS. To determine a dose response for TCDD in our culture system (0.1nM-100nM), 10 fold serial dilutions of TCDD were prepared from the original stock with vehicle DMSO (133μM, 13.3 μM, 1.33μM, and 0.133μM). The individual dilutions or vehicle alone were added to the supplemented α-MEM at an equal volume (0.75μL/mL media) to maintain the vehicle concentration at a constant of 0.075% for each treatment. For treatment, unsupplemented α-MEM was removed from each well and replaced with 150μL of supplemented α-MEM containing vehicle or TCDD. Follicles were then incubated for 96 h at 37°C in 95% air and 5% CO2.
Similarly, for the cultures with TCDD and pregnenolone co-treatment, each chemical was added to the supplemented α-MEM such that each treatment contained the same volume of chemicals in combination or alone. Specifically, pregnenolone was solubilized in DMSO at 26.7mM and some of this stock was diluted two fold to 13.3mM. Two stock solutions of TCDD were made: 2.66μM and 1.33μM. Pregnenolone was added to the media for a final concentration of 10μM and TCDD was added to the media for a final concentration of 1nM. Finally, follicles were incubated with vehicle, pregnenolone (10μM), TCDD (1nM), or pregnenolone (10 μM) and TCDD (1nM) for 96 h at 37°C in 95% air and 5% CO2. At least 3 individual experiments were performed for each treatment.
Follicle growth analysis
Antral follicles were cultured as described above for 96 h. Follicle growth was examined at 24 hour intervals by measuring follicle diameter on perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. Small antral follicles were considered as those having diameters of 200-350μm (Smitz and Cortvrindt, 2002). At least three separate culture experiments were performed for each chemical treatment. Follicle diameter measurements were averaged among treatment groups and plotted as percent change in diameter from time zero to compare the effects of chemical treatments on growth over time.
Measurement of sex steroid hormones by ELISA
Media were collected after the 96 hours culture and frozen at -80°C until they were subjected to enzyme-linked immunosorbent assays (ELISAs) for P4, A4, T, and E2 levels. ELISA kits were purchased from DRG Diagnostics (USA) and procedures were followed using the manufacturer’s protocols. The media were randomly selected from 8-23 wells and were assayed individually for each treatment group from 3-6 experiments. The intra-assay and inter-assay variabilities were less than 10%.
Quantitative real time PCR analysis of Ahr and Cyp1b1 transcript levels
At the end of each of the 96 hour cultures, 8-16 follicles from each treatment group were pooled and immediately snap frozen in liquid nitrogen and stored at -80°C until RT-qPCR analysis. Total RNA was extracted using the RNeasy Micro Kit with DNase treatment to eliminate potential genomic DNA contamination according to the manufacturer’s instructions (Qiagen, Valencia, CA). Concentration, purity, and quality of the RNA were determined using the NanoDrop ND-1000 UV-Vis spectrophotometer at 260 nm and 280 nm (Nanodrop Technologies, Wilmington, DE). Total RNA (250ng) was reverse transcribed using an iScript cDNA synthesis kit according to manufacturer’s instructions (Bio-Rad, Hercules, CA). Negative controls included omission of reverse transcriptase and omission of template. Each of the final cDNA samples were then diluted 1:4 for real time qPCR analysis.
All qPCR reactions were performed in triplicate using the CFX96 Real-time System C1000 Thermal Cycler (Bio-Rad). Each qPCR reaction contained 5μL of 2X SsoFast™ Evagreen® supermix, 0.6μL of gene specific primers, 2.4μL of nuclease free water, and 2μL of diluted cDNA template. The final concentration for each gene specific primer in the qPCR reactions was 0.3pmoles/ μL. A BLASTN search was performed in GenBank to ensure that all primers were unique to the gene of interest. To avoid amplification from genomic DNA contamination, all primer sets spanned a large exon-intron-exon junction. Primer sequences were as follows: cytochrome P450, family 1, subfamily b, polypeptide 1 (Cyp1b1) Forward 5’-GCG ACG ATT CCT CCG GGC TG- 3’, Cyp1b1 Reverse 5’ –TGC ACG CGG GCC TGA ACA TC- 3’, aryl hydrocarbon receptor (Ahr) Forward 5’-TTC TTA GGC TCA GCG TCA GCT A- 3’, Ahr Reverse 5’ -GCA AAT CCT GCC AGT CTC TGA T -3’, beta actin (Actb) Forward 5’ –GGG CAC AGT GTG GGT GAC -3’, Actb Reverse 5’ –CTG GCA CCA CAC CTT CTA C -3’. Initial denaturation of the cDNA and enzyme activation occurred at 95°C for 1 min, followed by 40 cycles of (10 seconds at 95°C, 10 seconds at 60°C, and a fluorescent absorbance reading), and one final annealing/elongation step for 5 minutes at 72°C. A heat dissociation curve (from 65°C-95°C with a fluorescent absorbance reading after each 0.5°C increment) was performed at the end of every run to assure specificity of each primer pair for the chosen transcript of interest.
For relative quantification of transcript levels, standard curves were generated using a six step serial dilution of cDNA generated from pooled RNA samples for each primer set being used (Actb, Ahr, and Cyp1b1). These standard curves were used to calculate the amplification efficiencies for each primer pair. Relative transcript amount was then calculated by a mathematical model developed by Pfaffl (Pfaffl, 2001). Briefly, the method involves calculating the relative expression ratio of the target gene based on the amplification efficiency of each amplicon and the ΔCt of the treated samples versus the vehicle control. These ratios were then compared to the expression of the reference gene Actb. Actb was verified as a good internal control because its levels were unchanged with treatment (data not shown). The data were reported as mean transcript expression ratios relative to Actb from 3-4 separate follicle culture experiments.
Statistical Analysis
Least squares one-way analysis of variance (ANOVA) was used to analyze the data followed by the Tukey’s post hoc test. If the data did not pass the assumption of homogeneity of variance, the non-parametric Kruskal-Wallis ANOVA was performed followed by the Mann-Whitney test. Statistical significance was assigned at p≤0.05 for all comparisons. All data were analyzed using SPSS 11.0 statistical software (SPSS Inc., Chicago, IL).
Results
Effect of TCDD exposure on antral follicle growth
Antral follicle growth is mainly characterized by proliferation of granulosa cells and accumulation of follicular fluid in the antral space leading to an increase in follicle volume over time (Hirshfield, 1991). To determine whether TCDD has an effect on follicle growth in vitro, follicle diameter was measured on a perpendicular axis every 24 hours for a period of 96 hours. TCDD had no effect on follicle growth at any of the four doses (0.1, 1, 10, and 100nM) when compared to vehicle alone (Fig 1). Additionally, follicles exposed to TCDD or vehicle had a similar gross morphology at the beginning and at the end of the 96 hour cultures. All follicles consisted of a single oocyte, an antrum, multiple layers of healthy granulosa cells, and a thecal layer surrounding the oocyte (Fig 2).
Fig 1. Effect of TCDD on mouse antral follicle growth in vitro.
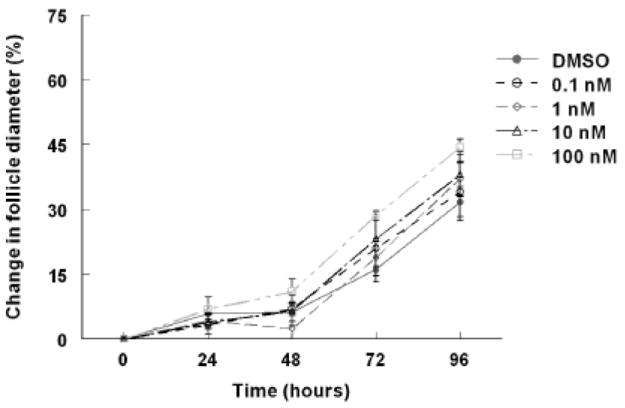
Antral follicles were mechanically isolated from young cycling CD1 mice and exposed for 96 hours in culture to TCDD (0.1nM-100nM) or vehicle alone. The follicle diameters were measured every 24 hours for a period of 96 hours. Follicle growth was plotted as percent change in diameter over time from the beginning of the culture. The graph represents the means ±SEM from at least three individual experiments (n=8-16 follicles per treatment group per individual experiment).
Fig 2. Effect of TCDD on the gross morphology of mouse antral follicles in vitro.
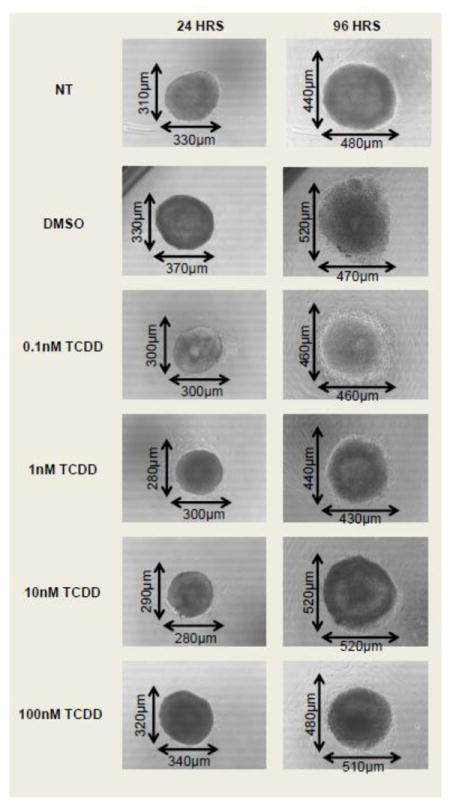
Antral follicles were mechanically isolated from young cycling CD1 mice and exposed for 96 hours to TCDD (0.1nM-100nM) or vehicle alone. Digital images were taken of mouse antral follicles at 24 hours and at 96 hours of culture using a digital camera (ProgRes CT3, Jenoptik, Germany) attached to an inverted microscope using the 10X objective (Olympus CK40). NT= non-treated.
Effect of TCDD exposure on antral follicle steroid secretion
Antral follicles are the primary source of female sex steroid hormone production (Edson et al., 2009). To determine whether TCDD has an effect on antral follicle steroid hormone production, follicles were treated with TCDD and the media were collected at the end of the 96 hour culture and assayed for levels of P4, A4, T, and E2. TCDD exposure for 96 hours resulted in U-shaped dose response curves for the amount of P4, A4, T, and E2 secreted into the media by the antral follicles in vitro when compared to vehicle alone (Fig 3). Specifically, the level of P4 was significantly decreased only at the 1nM dose of TCDD and not at the lowest (0.1nM) and two highest (10 and 100nM) doses of TCDD (Fig 3 a). The levels of androgens (A4 and T) were significantly decreased at the two lowest doses of TCDD (0.1 and 1nM), but not at the two highest doses (10 and 100nM) of TCDD (Fig 3 b and c). E2 levels were decreased after exposure to 0.1, 1, and 10nM, but not after exposure to the highest dose of TCDD (100nM) (Fig 3 d). Interestingly, only the 1nM dose of TCDD significantly decreased the level of all four hormones simultaneously.
Fig 3. Effect of TCDD on mouse antral follicle sex steroid hormone secretion in vitro.
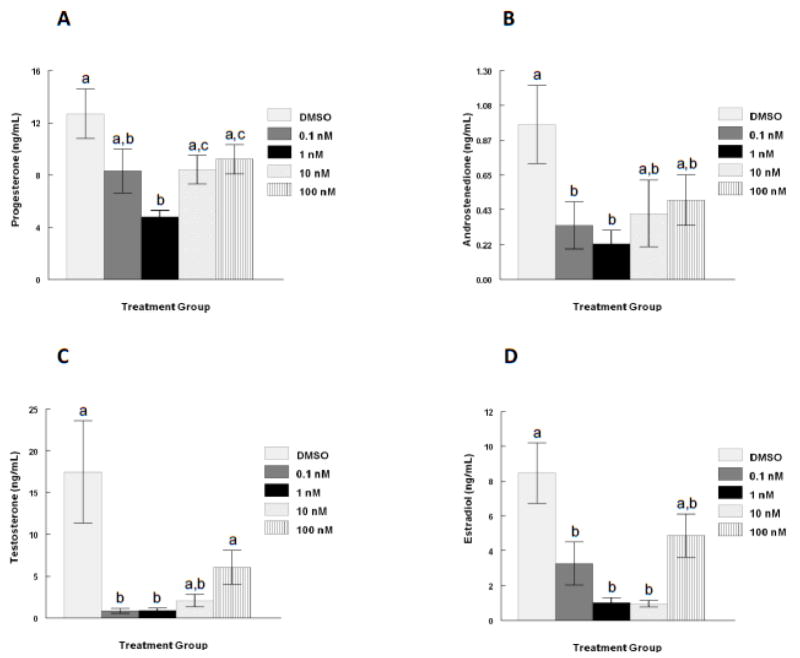
Antral follicles were mechanically isolated from young cycling CD1 mice and exposed for 96 hours in culture to TCDD (0.1nM-100nM) or vehicle alone. After the 96 hour culture period, media were collected and subjected to hormone assays for (A) progesterone, (B) androstenedione, (C) testosterone, and (D) estradiol levels. The graphs represent the mean hormone levels ±SEM from 8-23 culture wells from a minimum of three separate follicle culture experiments. Bars with different letters are significantly different from each other (p≤0.05).
Effect of co-treatment of TCDD and pregnenolone on antral follicle growth and steroid secretion
To determine whether the decrease in hormone levels observed in the follicle culture media after 96 hours TCDD exposure was due to impaired StAR or P450scc function, pregnenolone was supplemented in the media with or without 1nM TCDD. The 1nM dose was chosen because this dose impairs the production or secretion of all four of the steroid hormones of interest (Fig 3). Antral follicle growth was monitored every 24 hours and the media were collected at the end of the 96 hour period for hormone assays. As demonstrated in the dose response experiments (Fig 3), exposure to 1nM TCDD for 96 hours again resulted in decreased production/ secretion of P4, A4, T, and E2 by the antral follicles in culture when compared to vehicle alone (Fig 4 a-d). Pregnenolone co-treatment restored hormone levels to control levels, suggesting a block in the steroidogenic pathway prior to pregnenolone formation, possibly via a StAR or P450scc impairment (Fig 4 a-d). Although pregnenolone co-treatment restored sex steroid hormone levels in the media, it did not alter antral follicle growth (Fig 5).
Fig 4. Effect of pregnenolone substrate co-treatment with TCDD on mouse antral follicle steroid hormone secretion in vitro.
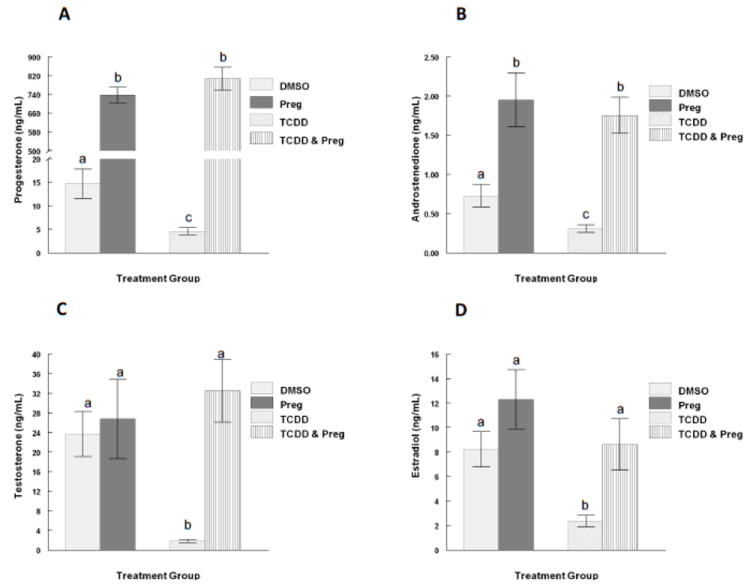
Mouse antral follicles were treated with vehicle (DMSO), pregnenolone (10μM), TCDD (1nM), or TCDD (1nM) and pregnenolone (10 μM) for 96 hours in culture. After the 96 hour culture, media were collected and subjected to hormone assays for (A) progesterone, (B) androstenedione, (C) testosterone, and (D) estradiol levels. The graphs represent the mean hormone levels ±SEM from 8-23 culture wells from a minimum of three separate follicle culture experiments. Bars with different letters are significantly different from each other (p≤0.05). Preg = pregnenolone.
Fig 5. Effect of pregnenolone substrate co-treatment with TCDD on mouse antral follicle growth in vitro.
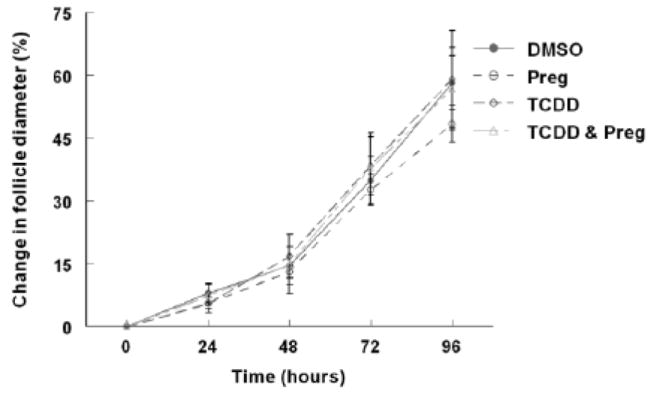
Mouse antral follicles were treated with vehicle (DMSO), pregnenolone (10μM), TCDD (1nM), or TCDD (1nM) and pregnenolone (10 μM) for 96 hours in culture. The follicle diameters were measured every 24 hours for a period of 96 hours. Follicle growth was plotted as percent change in diameter over time from the beginning of the culture. The graph represents the means ±SEM from at least three individual experiments (n=8-16 follicles per treatment group per individual experiment).
Effect of TCDD exposure on the expression of Ahr and Cyp1b1 in antral follicles
AHR activation by TCDD has been shown to increase metabolism of E2 by inducing the cytochrome P450 monooxygenases, such as CYP1B1 (Hayes et al., 1996; Spink et al., 2003). Thus, follicles were subjected to real time qPCR for levels of Ahr and Cyp1b1 mRNA. TCDD exposure resulted in a three to four-fold induction of Cyp1b1 expression in antral follicles regardless of the dose after 96 hours of culture, evidence of AHR pathway activation (Fig 6 a). Additionally, TCDD did not affect the expression level of Ahr mRNA in cultured antral follicles regardless of the dose (Fig 6 b).
Fig 6. Effect of TCDD exposure on the expression of Cyp1b1 and Ahr transcripts in antral follicles in vitro.
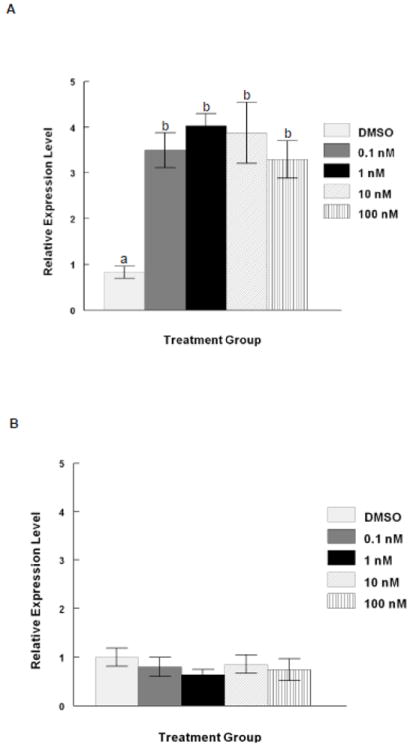
At the end of each of the 96 hour cultures, 8-16 follicles from each treatment group were pooled and immediately snap frozen in liquid nitrogen and assayed by real time qPCR for levels of (A) Cyp1b1 and (B) Ahr. Levels of Cyp1b1 and Ahr were normalized to beta actin (Actb). Data are expressed as mean relative expression ratios ± SEM calculated from 3 - 4 separate culture experiments. Bars with different letters are significantly different from each other (p≤0.05).
Discussion
To our knowledge, studies have not reported the direct effects of TCDD on mouse antral follicles in vitro. In fact, only one published study has reported on direct effects of TCDD on steroidogenesis and growth in follicles isolated from cycling animals in vitro and this study was conducted using pig follicles (Grochowalski et al., 2000). The follicle culture system we used in this study has been well characterized and has been proven to be an excellent model for in vitro testing of ovarian function in reproductive toxicology studies (Cortvrindt and Smitz, 2002;Gupta et al., 2006). The cultured follicles maintain much of their steroidogenic and proliferative capacity throughout the 96 hour culture period; maintaining the presence of granulosa cells, theca cells, a basement membrane and oocytes in meiotic arrest (Cortvrindt et al., 1997). Thus, unlike studies utilizing primary granulosa cells or human luteinized granulosa cells in culture, all the functional compartments of the follicle are present in our system. In addition, we also chose a series of TCDD doses previously shown to have effects on ovarian tissues and cells in culture, which also encompass environmentally relevant (0.1 and 1nM) as well as pharmacological exposures (10nM and 100nM), so that we could determine a dose response for TCDD in our culture system (Birnbaum and DeVito, 1995;Gregoraszczuk, 2002).
Proliferation of follicular cells contributes to the overall growth of follicles (Hirshfield, 1991). Our data indicate that TCDD does not affect growth of follicles, suggesting that it does not affect proliferation of granulosa cells in mice. These data differ from those obtained using pig follicles and from in vivo dosing experiments in rats. Specifically, Grochowalski et al. found that TCDD reduced the percentage of proliferating cells in porcine follicles over time and that this was attributed to increasing accumulation of TCDD in the tissue over time (Grochowalski et al., 2000). Heimler et al. found a decrease in the number of antral follicles without an increase in atresia in the ovaries of TCDD exposed rats suggesting an anti-proliferative effect (Heimler et al., 1998b). While it is unclear why our results differ from others, it is likely due to species differences or differing experimental conditions.
We also found that TCDD alters steroidogenesis in mouse follicles in a non-monotonic/ U-shaped dose response manner. These data are consistent with studies in other species and culture systems (Dasmahapatra et al., 2000;Gregoraszczuk, 2002;Grochowalski et al., 2000;Heimler et al., 1998a). Specifically, in studies utilizing isolated porcine thecal and granulosa cell co-cultures, TCDD exposures of 0.1nM or 10nM resulted in reduced E2 accumulated in the media, while only the 10nM exposure resulted in a significant reduction in progesterone in the media after 96 hour cultures (Grochowalski et al., 2001). Further, unlike our results for E2 levels in the media after TCDD exposure, Gregoraszczuk et al. observed a dose response decrease in E2 levels with increasing TCDD concentration up to 100nM in the media after only 48 hours of culture (Gregoraszczuk, 2002). We did not observe a significant decrease after exposure to the highest dose of TCDD (100nM) after 96 hours. This difference could be due to time dependent effects of TCDD on steroidogenesis in follicles. Studies comparing single versus prolonged exposures to TCDD on steroidogenesis in pig follicles have revealed a varying response with a decrease and an increase in testosterone levels, respectively (Grochowalski et al., 2000). It is also possible that at pharmacological doses of TCDD, steroidogenesis is able to recover after prolonged TCDD exposure such as was demonstrated in porcine luteal cells and in human luteinized granulosa cells in culture (Gregoraszczuk et al., 2000;Heimler et al., 1998a). This could be an explanation for the lack of effect we observed on the levels of P4, A4, T and E2 assayed from the mouse follicles exposed to the highest pharmacological dose of TCDD (100nM) in the media after 96 hours in culture. Interestingly, only the 1nM dose of TCDD significantly inhibited the accumulation of P4 in the media after 96 hours culture. These results suggest that like hormones and other environmental contaminants that act as endocrine disruptors, TCDD may have multiple modes of action in mouse antral follicles depending on the dose (Gierthy, 2002;Gore et al., 2006).
Since P4 accumulation in the media was decreased after TCDD exposure and P4 synthesis is early in the steroidogenic pathway, we investigated whether we could by-pass this effect with pregnenolone substrate co-treatment with the 1nM dose of TCDD. Addition of pregnenolone substrate restored P4 in the media to control levels, suggesting that TCDD could be acting on StAR or P450scc function in mouse antral follicles. Similar to our findings, Gregoraszczuk et al. were able to restore P4 levels after co-treatment with pregnenolone and TCDD in porcine granulosa thecal cell co-cultures (Gregoraszczuk, 2002). Supportive of this observation, TCDD has been shown to activate the AHR, leading to transcriptional regulation of the promoter activities of P450scc, the mitochondrial enzyme responsible for cleaving cholesterol to pregnenolone in the steroidogenenic pathway (Dasmahapatra et al., 2000). Specifically, Dasmahapatra et al. found a reduction in the level of P450scc mRNAs in isolated rat granulosa cells exposed to TCDD (Dasmahapatra et al., 2000). Contrary to this finding, studies investigating the effects of TCDD on steroidogenesis in rat testes and adrenal glands in vivo suggest that TCDD may not directly act on inhibiting P450scc activity or levels, but may inhibit the mobilization of cholesterol to P450scc; ultimately leading to a decrease in hormone levels (DiBartolomeis et al., 1987;Kleeman et al., 1990;Moore et al., 1991). Though it was not directly tested in this study, it is likely that at the 1nM dose, TCDD does not affect downstream enzymes in the steroidogenic pathway since pregnenolone co-treatment also restored A4, T, and E2 accumulation in the media.
Since androgen and E2 levels were also decreased at doses of TCDD when P4 levels were not affected, it is likely that TCDD acts downstream of pregnenolone formation in the steroidogenic pathway in the follicle as well. This is supported by several studies utilizing porcine granulosa thecal cell co-cultures, rat granulosa cell cultures and human luteinized granulosa cell cultures. Gregoraszczuk found evidence of reduced aromatase activity in porcine granulosa and thecal cell co-cultures after TCDD exposure (Gregoraszczuk, 2002). Similarly, studies conducted using FSH-stimulated rat granulosa cells found that the addition of A4 partially restored E2 levels. Upon further investigation, they found that TCDD inhibited the FSH-stimulated increase in aromatase activity usually exhibited by these cells, indicating possible actions on this enzyme as well as the follicle stimulating hormone receptor (Dasmahapatra et al., 2000). Studies exposing human luteinized granulosa cells (hLGCs) to TCDD in culture also found a decrease in E2 accumulation in the media after TCDD exposure, but contrary to the studies using rat granulosa cells, Morán et al. did not find a decrease in aromatase levels or activity (Morán et al., 2000). Instead, they demonstrated that the reduced E2 was the result of an impairment in the supply of aromatizable androgens due to a decrease in the lyase activity of 17α hydroxylase/ 17,20 lyase cytochrome P450 (CYP17A1) enzyme as well as decreased levels of CYP17A1 protein levels in hLGCs (Morán et al., 2003a;Morán et al., 2003b).
Other mechanisms by which TCDD could be affecting hormone levels in the follicle are through an AHR pathway that drives transcriptional activation of genes responsible for E2 catabolism. AHR transcriptional activation via TCDD ligand binding has been shown to increase catabolism of E2 by inducing the cytochrome P450 monooxygenases such as CYP1B1 (Badawi et al., 2001;Hayes et al., 1996). CYP1B1 is responsible for converting E2 to the catecholestrogen 4-hydroxyestradiol (Badawi et al., 2001). CYP1B1 is expressed in the ovary in the absence of exogenous ligands and it is hormonally regulated and inducible by TCDD (Bhattacharyya et al., 1995;Brake and Jefcoate, 1995;Dasmahapatra et al., 2002;Otto et al., 1992;Savas et al., 1994;Shen et al., 1993;Shen et al., 1994;Sutter et al., 1994). Additionally, it has been shown in porcine granulosa cells that the AHR shuttles from the cytoplasm into the nucleus in the presence of TCDD, evidence of transcriptional activity in the ovary (Wojtowicz et al., 2005). Thus, we tested the hypothesis that TCDD exposure transcriptionally activates the AHR and induces Cyp1b1 leading to increased levels of Ahr and Cyp1b1 mRNA in our cultured antral follicles. Interestingly, we did not observe an increase in Ahr transcript levels after TCDD exposure similar to what was observed in rat and porcine granulosa cells in culture (Dasmahapatra et al., 2001;Wojtowicz et al., 2005). This could be due to the longer exposure we employed, or because the presence of thecal cells may conceal the effects on granulosa cells in antral follicle cultures. Currently, very little is known about how TCDD regulates AHR levels in the ovary, though it has been suggested that the aryl hydrocarbon receptor repressor (AHRR) plays a role in the absence of exogenous ligands, and this regulation might be at the protein level since Ahr mRNA levels remain unchanged in ovaries from gonadotropin primed mice (Baba et al., 2005).
Although we did not find any significant changes in Ahr transcript levels, we did observe a 3-4 fold increase in Cyp1b1 levels following 96 hours of TCDD exposure (0.1-100nM), which is slightly higher than was observed in isolated rat granulosa cells exposed to TCDD for only 6 hours (3.1nM) (Dasmahapatra et al., 2002). These findings strongly suggest and agree with the majority of published data that in addition to the effects of TCDD on the steroidogenic pathway, TCDD exposure may also lead to increased catabolism of E2 to catecholestrogens in the ovary.
This idea is further supported by studies that found that acute exposure to TCDD in the ovariectomized rat decreases the elimination constant and clearance of E2 (Petroff and Mizinga, 2003). This could be one explanation for why contrary to our findings in vitro, circulating E2 levels were unaltered following in vivo TCDD exposure in CD-1 mice (DeVito et al., 1992). It could be that TCDD exposure decreases E2 synthesis and increases E2 catabolism in the mouse ovary, while it does not change circulating levels due to a slower clearance rate. The lack of effect on E2 levels after the in vivo TCDD exposure in CD-1 mice could also be related to the route of administration, the age of the animals and the stage of the reproductive cycle. Studies conducted using rats show that circulating E2 levels do not change in a gonadotropin primed immature hypophysectomized model with a very high single exposure to TCDD, while chronic exposure to low doses in utero lead to lower levels of circulating E2 in pubertal life (Son et al., 1999; Franczak et al., 2006). Though TCDD metabolites have not been measured from follicles exposed to TCDD in vitro, ovaries express a battery of enzymes known to metabolize TCDD such as CYP1B1 (Bengtsson AND Rydstrom; 1983; Dasmahapatra et al. 2002). Thus, it is likely that some of the local effects of TCDD on the ovary could be due to actions of its metabolites on steroidogenesis as well.
In conclusion, we have demonstrated that TCDD disrupts sex steroid hormone production in a non-monotonic dose response manner without altering growth of mouse antral follicles. We were able to by-pass the disrupted steroid hormone production with pregnenolone co-treatment, suggesting that TCDD may be involved in inhibiting the mobilization of cholesterol to the inner mitochondrial membrane and/or in the conversion of cholesterol to pregnenolone in antral follicles. These actions of TCDD on steroid hormone metabolism are likely through an AHR pathway, as demonstrated by induction of the Cyp1b1 transcript and constitutive expression of the Ahr in our culture system. Overall, the experimental results reported here and by others demonstrate complex actions of TCDD toxicity in the ovary. Since humans and animals are exposed to a mixture of dioxins and other environmental toxicants, it will be important in the future to study the toxicity of these environmentally relevant mixtures.
Highlights.
TCDD disrupts sex steroid hormone levels, but not growth of antral follicles.
Pregnenolone co-treatment by-passes TCDD-induced steroid hormone disruption.
TCDD affects steroid hormone levels through an AHR pathway in antral follicles.
Acknowledgments
This work was supported by NIH R01 HD 047275 (JAF), NIEHS ES07326 Research training program in endocrine, developmental and reproductive toxicology (BNK), NIH R01 ES 019178 and ES 019178S1 (JAF), and an Environmental Toxicology Predoctoral Fellowship (MSB). The authors would also like to thank Dr. Wei Wang for her technical assistance.
Footnotes
Conflict of Interest Statement
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bethany N. Karman, Email: bklement@illinois.edu.
Mallikarjuna S. Basavarajappa, Email: mbshivapur@gmail.com.
Zelieann R. Craig, Email: zelieann@illinois.edu.
Jodi A. Flaws, Email: jflaws@illinois.edu.
References
- Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K, Fujii-Kuriyama Y. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol. 2005;25:10040–10051. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16[alpha]-hydroxylation of 17[beta]-estradiol. Metabolism. 2001;50:1001–1003. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- Baldridge MG, Hutz RJ. Autoradiographic localization of aromatic hydrocarbon receptor (AHR) in rhesus monkey ovary. Am J Primatol. 2007;69:681–691. doi: 10.1002/ajp.20381. [DOI] [PubMed] [Google Scholar]
- Bengtsson M, Rydstrom J. Regulation of carcinogenic metabolism in the rat ovary by the estrous cycle and gonadotropin. Science. 1983;219:1437–1438. doi: 10.1126/science.6681915. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya KK, Brake PB, Eltom SE, Otto SA, Jefcoate CR. Identification of a rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. Demonstration of a unique tissue-specific pattern of hormonal and aryl hydrocarbon receptor-linked regulation. J Biol Chem. 1995;270:11595–11602. doi: 10.1074/jbc.270.19.11595. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, DeVito MJ. Use of toxic equivalency factors for risk assessment for dioxins and related compounds. Toxicology. 1995;105:391–401. doi: 10.1016/0300-483x(95)03237-a. [DOI] [PubMed] [Google Scholar]
- Brake PB, Jefcoate CR. Regulation of cytochrome P4501B1 in cultured rat adrenocortical cells by cyclic adenosine 3’,5’-monophosphate and 2,3,7,8-tetrachlorodibenzo-p-dioxin. Endocrinology. 1995;136:5034–5041. doi: 10.1210/endo.136.11.7588239. [DOI] [PubMed] [Google Scholar]
- Cao J, Patisaul HB, Petersen SL. Aryl hydrocarbon receptor activation in lactotropes and gonadotropes interferes with estradiol-dependent and - independent preprolactin, glycoprotein alpha and luteinizing hormone beta gene expression. Mol Cell Endocrinol. 2011;333:151–159. doi: 10.1016/j.mce.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin CL, Peterson RE, Hutz RJ. In utero and lactational exposure of female Holtzman rats to 2,3,7,8- tetrachlorodibenzo-p-dioxin: modulation of the estrogen signal. Biology of Reproduction. 1996;55:62–67. doi: 10.1095/biolreprod55.1.62. [DOI] [PubMed] [Google Scholar]
- Cortvrindt R, Smitz J, Van Steirteghem AC. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod. 1997;12:759–768. doi: 10.1093/humrep/12.4.759. [DOI] [PubMed] [Google Scholar]
- Cortvrindt RG, Smitz JE. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Human Reproduction Update. 2002;8:243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra AK, Trewin AL, Hutz RJ. Estrous cycle-regulated expression of CYP1B1 mRNA in the rat ovary. Comp Biochem Physiol B Biochem Mol Biol. 2002;133:127–134. doi: 10.1016/s1096-4959(02)00119-7. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra AK, Wimpee BA, Trewin AL, Hutz RJ. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases steady-state estrogen receptor-beta mRNA levels after CYP1A1 and CYP1B1 induction in rat granulosa cells in vitro. Mol Cell Endocrinol. 2001;182:39–48. doi: 10.1016/s0303-7207(01)00545-7. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra AK, Wimpee BA, Trewin AL, Wimpee CF, Ghorai JK, Hutz RJ. Demonstration of 2,3,7,8-tetrachlorodibenzo-p-dioxin attenuation of P450 steroidogenic enzyme mRNAs in rat granulosa cell in vitro by competitive reverse transcriptase-polymerase chain reaction assay. Mol Cell Endocrinol. 2000;164:5–18. doi: 10.1016/s0303-7207(00)00245-8. [DOI] [PubMed] [Google Scholar]
- DeVito MJ, Thomas T, Martin E, Umbreit TH, Gallo Antiestrogenic action of 2,3,7,8-tetrachlorodibenzo-p-dioxin: tissue specific regulation of estrogen receptor in CD1 mice. Toxicol Appl Pharmacol. 1992;113:284–292. doi: 10.1016/0041-008x(92)90126-d. [DOI] [PubMed] [Google Scholar]
- DiBartolomeis MJ, Moore RW, Peterson RE, Christian BJ, Jefcoate CR. Altered regulation of adrenal steroidogenesis in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats. Biochem Pharmacol. 1987;36:59–67. doi: 10.1016/0006-2952(87)90382-0. [DOI] [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM. The Mammalian ovary from genesis to revelation. Endocrine Reviews. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Marks AR, Samuels S, Gerthoux PM, Vercellini P, Olive DL, Needham L, Patterson D., Jr Serum dioxin concentrations and age at menopause. Environ Health Perspect. 2005;113:858–862. doi: 10.1289/ehp.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frakes RA, Zeeman CQ, Mower B. Bioaccumulation of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) by fish downstream of pulp and paper mills in Maine. Ecotoxicol Environ Saf. 1993;25:244–252. doi: 10.1006/eesa.1993.1023. [DOI] [PubMed] [Google Scholar]
- Franczak A, Nynca A, Valdez KE, Mizinga KM, Petroff BK. Effects of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescence in female sprague-dawley rats. Biology of Reproduction. 2006;74:125–130. doi: 10.1095/biolreprod.105.044396. [DOI] [PubMed] [Google Scholar]
- Gao X, Mizuyachi K, Terranova PF, Rozman KK. 2,3,7,8-tetrachlorodibenzo-p-dioxin decreases responsiveness of the hypothalamus to estradiol as a feedback inducer of preovulatory gonadotropin secretion in the immature gonadotropin-primed rat. Toxicol Appl Pharmacol. 2001;170:181–190. doi: 10.1006/taap.2000.9099. [DOI] [PubMed] [Google Scholar]
- Gierthy JF. Testing for endocrine disruption: how much is enough? Toxicol Sci. 2002;68:1–3. doi: 10.1093/toxsci/68.1.1. [DOI] [PubMed] [Google Scholar]
- Gore AC, Heindel JJ, Zoeller RT. Endocrine disruption for endocrinologists (and others) Endocrinology. 2006;147:S1–3. doi: 10.1210/en.2005-1367. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JS. In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring. Toxicol Appl Pharmacol. 1995;133:285–294. doi: 10.1006/taap.1995.1153. [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk EL. Dioxin exposure and porcine reproductive hormonal activity. Cad Saude Publica. 2002;18:453–462. doi: 10.1590/s0102-311x2002000200010. [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk EL, Wójtowicz AK, Zabielny E, Grochowalski A. Dose- and-time dependent effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on progesterone secretion by porcine luteal cells cultured in vitro. J Physiol Pharmacol. 2000;51:127–135. [PubMed] [Google Scholar]
- Gregoraszczuk EL, Zabielny E, Ochwat D. Aryl hydrocarbon receptor (AhR)-linked inhibition of luteal cell progesterone secretion in 2,3,7,8-tetrachlorodibenzo-p-dioxin treated cells. J Physiol Pharmacol. 2001;52:303–311. [PubMed] [Google Scholar]
- Grochowalski A, Chrzaszcz R, Pieklo R, Gregoraszczuk EL. Estrogenic and antiestrogenic effect of in vitro treatment of follicular cells with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chemosphere. 2001;43:823–827. doi: 10.1016/s0045-6535(00)00440-9. [DOI] [PubMed] [Google Scholar]
- Grochowalski A, Pieklo R, Gasinska A, Chrzaszcz R, Gregoraszczuk EL. Accumulation of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in porcine preovulatory follicles after in vitro exposure to TCDD: effects on steroid secretion and cell proliferation. Cytobios. 2000;102:21–31. [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicological Sciences. 2006;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad of Sci U S A. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimler I, Rawlins RG, Owen H, Hutz RJ. Dioxin perturbs, in a dose- and time-dependent fashion, steroid secretion, and induces apoptosis of human luteinized granulosa cells. Endocrinology. 1998a;139:4373–4379. doi: 10.1210/endo.139.10.6264. [DOI] [PubMed] [Google Scholar]
- Heimler I, Trewin AL, Chaffin CL, Rawlins RG, Hutz RJ. Modulation of ovarian follicle maturation and effects on apoptotic cell death in holtzman rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin(TCDD) in utero and lactationally. Reprod Toxicol. 1998b;12:69–73. doi: 10.1016/s0890-6238(97)00101-9. [DOI] [PubMed] [Google Scholar]
- Hernández-Ochoa I, Karman BN, Flaws JA. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77:547–59. doi: 10.1016/j.bcp.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hites RA. Dioxins: an overview and history. Environ Sci Technol. 2011;45:16–20. doi: 10.1021/es1013664. [DOI] [PubMed] [Google Scholar]
- Humblet O, Williams PL, Korrick SA, Sergeyev O, Emond C, Birnbaum LS, Burns JS, Altshul L, Patterson DG, Jr, Turner WE, Lee MM, Revich B, Hauser R. Dioxin and polychlorinated biphenyl concentrations in mother’s serum and the timing of pubertal onset in sons. Epidemiology. 2011;22:827–835. doi: 10.1097/EDE.0b013e318230b0d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska O, Shi Z, Valdez KE, Ting AY, Petroff BK. Temporal and anatomical sensitivities to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin leading to premature acyclicity with age in rats. Int J Androl. 2010;33:405–412. doi: 10.1111/j.1365-2605.2009.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeman JM, Moore RW, Peterson RE. Inhibition of testicular steroidogenesis in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats: evidence that the key lesion occurs prior to or during pregnenolone formation. Toxicol Appl Pharmacol. 1990;106:112–125. doi: 10.1016/0041-008x(90)90111-7. [DOI] [PubMed] [Google Scholar]
- Li XL, Johnson DC, Rozman KK. Reproductive Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in Female Rats: Ovulation, Hormonal Regulation, and Possible Mechanism(s) Toxicol Appl Pharmacol. 1995;133:321–327. doi: 10.1006/taap.1995.1157. [DOI] [PubMed] [Google Scholar]
- Mizuyachi K, Son DS, Rozman KK, Terranova PF. Alteration in ovarian gene expression in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin: reduction of cyclooxygenase-2 in the blockage of ovulation. Reprod Toxicol. 2002;16:299–307. doi: 10.1016/s0890-6238(02)00024-2. [DOI] [PubMed] [Google Scholar]
- Moore RW, Jefcoate CR, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits steroidogenesis in the rat testis by inhibiting the mobilization of cholesterol to cytochrome P450scc. Toxicol Appl Pharmacol. 1991;109:85–97. doi: 10.1016/0041-008x(91)90193-i. [DOI] [PubMed] [Google Scholar]
- Morán FM, Vandevoort CA, Overstreet JW, Lasley BL, Conley AJ. Molecular target of endocrine disruption in human luteinizing granulosa cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin: inhibition of estradiol secretion due to decreased 17 alpha-hydroxylase/17,20-lyase cytochrome P450 expression. Endocrinology. 2003b;144:467–473. doi: 10.1210/en.2002-220813. [DOI] [PubMed] [Google Scholar]
- Morán FM, Conley AJ, Corbin CJ, Enan E, VandeVoort C, Overstreet JW, Lasley BL. 2,3,7,8-tetrachlorodibenzo-p-dioxin decreases estradiol production without altering the enzyme activity of cytochrome P450 aromatase of human luteinized granulosa cells in vitro. Biology of Reproduction. 2000;62:1102–1108. doi: 10.1095/biolreprod62.4.1102. [DOI] [PubMed] [Google Scholar]
- Morán FM, Lohstroh P, Vandevoort CA, Chen J, Overstreet JW, Conley AJ, Lasley BL. Exogenous steroid substrate modifies the effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estradiol production of human luteinized granulosa cells in vitro. Biology of Reproduction. 2003a;68:244–251. doi: 10.1095/biolreprod.102.007161. [DOI] [PubMed] [Google Scholar]
- Myllymäki SA, Haavisto TE, Brokken LJ, Viluksela M, Toppari J, Paranko J. In utero and lactational exposure to TCDD; steroidogenic outcomes differ in male and female rat pups. Toxicol Sci. 2005;88:534–544. doi: 10.1093/toxsci/kfi308. [DOI] [PubMed] [Google Scholar]
- Otto S, Bhattacharyya KK, Jefcoate CR. Polycyclic aromatic hydrocarbon metabolism in rat adrenal, ovary, and testis microsomes is catalyzed by the same novel cytochrome P450 (P450RAP) Endocrinology. 1992;131:3067–3076. doi: 10.1210/endo.131.6.1332854. [DOI] [PubMed] [Google Scholar]
- Pesonen SA, Haavisto TE, Viluksela M, Toppari J, Paranko J. Effects of in utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on rat follicular steroidogenesis. Reprod Toxicol. 2006;22:521–528. doi: 10.1016/j.reprotox.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Petroff BK, Croutch CR, Hunter DM, Wierman ME, Gao X. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) stimulates gonadotropin secretion in the immature female sprague-dawley rat through a pentobarbital- and estradiol-sensitive mechanism but does not alter gonadotropin-releasing hormone (GnRH) secretion by immortalized GnRH neurons in vitro. Biology of Reproduction. 2003;68:2100–2106. doi: 10.1095/biolreprod.102.010439. [DOI] [PubMed] [Google Scholar]
- Petroff BK, Mizinga KM. Pharmacokinetics of ovarian steroids in sprague-dawley rats after acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Reprod Biol. 2003;3:131–141. [PubMed] [Google Scholar]
- Petroff BK, Roby KF, Gao X, Son DS, Williamsn S, Johnson D, Rozman KK, Terranova PF. A review of mechanisms controlling ovulation with implications for the anovulatory effects of polychlorinated dibenzo-p-dioxins in rodents. Toxicology. 2001;158:91–107. doi: 10.1016/s0300-483x(00)00367-x. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- Savas U, Bhattacharyya KK, Christou M, Alexander DL, Jefcoate CR. Mouse cytochrome P-450EF, representative of a new 1B subfamily of cytochrome P-450s. Cloning, sequence determination, and tissue expression. J Biol Chem. 1994;269:14905–14911. [PubMed] [Google Scholar]
- Schecter A, Birnbaum L, Ryan JJ, Constable JD. Dioxins: an overview. Environ Res. 2006;101:419–428. doi: 10.1016/j.envres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Shen Z, Liu J, Wells RL, Elkind MM. cDNA cloning, sequence analysis, and induction by aryl hydrocarbons of a murine cytochrome P450 gene, Cyp1b1. DNA Cell Biol. 1994;13:763–769. doi: 10.1089/dna.1994.13.763. [DOI] [PubMed] [Google Scholar]
- Shen Z, Wells RL, Liu J, Elkind MM. Identification of a cytochrome P450 gene by reverse transcription--PCR using degenerate primers containing inosine. Proc Natl Acad Sci U S A. 1993;90:11483–11487. doi: 10.1073/pnas.90.24.11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Valdez KE, Ting AY, Franczak A, Gum SL, Petroff BK. Ovarian endocrine disruption underlies premature reproductive senescence following environmentally relevant chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biology of Reproduction. 2007;76:198–202. doi: 10.1095/biolreprod.106.053991. [DOI] [PubMed] [Google Scholar]
- Smitz JE, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction. 2002;123:185–202. doi: 10.1530/rep.0.1230185. [DOI] [PubMed] [Google Scholar]
- Son DS, Ushinohama K, Gao X, Taylor CC, Roby KF, Rozman KK, Terranova PF. 2,3,7,8-Tetrachlorodibenzo-p-dioxin(TCDD) blocks ovulation by a direct effect on the ovary without alteration of ovarian steroidogenesis: lack of a direct effect on ovarian granulosa and thecal-interstitial cell steroidogenesis in vitro. Reprod Toxicol. 1999;13:521–530. doi: 10.1016/s0890-6238(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Spink DC, Katz BH, Hussain MM, Pentecost BT, Cao Z, Spink BC. Estrogen regulates Ah responsiveness in MCF-7 breast cancer cells. Carcinogenesis. 2003;24:1941–1950. doi: 10.1093/carcin/bgg162. [DOI] [PubMed] [Google Scholar]
- Sutter TR, Tang YM, Hayes CL, Wo YY, Jabs EW, Li X, Yin H, Cody CW, Greenlee WF. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem. 1994;269:13092–13099. [PubMed] [Google Scholar]
- Tsutsumi O, Uechi H, Sone H, Yonemoto J, Takai Y, Momoeda M, Tohyama C, Hashimoto S, Morita M, Taketani Y. Presence of dioxins in human follicular fluid: their possible stage-specific action on the development of preimplantation mouse embryos. Biochem Biophys Res Commun. 2011;250:498–501. doi: 10.1006/bbrc.1998.9340. [DOI] [PubMed] [Google Scholar]
- Tuppurainen K, Asikainen A, Ruokojärvi P, Ruuskanen J. Perspectives on the formation of polychlorinated dibenzo-p-dioxins and dibenzofurans during municipal solid waste (MSW) incineration and other combustion processes. Acc Chem Res. 2003;36:652–658. doi: 10.1021/ar020104+. [DOI] [PubMed] [Google Scholar]
- Ulaszewska MM, Zuccato E, Davoli E. PCDD/Fs and dioxin-like PCBs in human milk and estimation of infants’ daily intake: a review. Chemosphere. 2011;83:774–782. doi: 10.1016/j.chemosphere.2011.02.066. [DOI] [PubMed] [Google Scholar]
- Warner M, Eskenazi B, Olive DL, Samuels S, Quick-Miles S, Vercellini P, Mocarelli P. Serum dioxin concentrations and quality of ovarian function in women of Seveso. Environ Health Perspect. 2007;115:336–340. doi: 10.1289/ehp.9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz A, Tomanek M, Augustowska K, Gregoraszczuk EL. Aromatic hydrocarbon receptor (AhR) in the porcine theca and granulosa cells: effect of TCDD, PCB 126 and PCB 153 on the expression of AhR. Endocr Regul. 2005;39:109–118. [PubMed] [Google Scholar]


