Abstract
Aims
Most but not all epidemiological studies suggest a cardioprotective association for low to moderate average alcohol consumption. The objective was to quantify the dose-response relationship between average alcohol consumption and ischaemic heart disease (IHD) stratified by sex and IHD end point (mortality vs. morbidity).
Methods
A systematic search of published studies using electronic databases (1980–2010) identified 44 observational studies (case-control or cohort) reporting a relative risk measure for average alcohol intake in relation to IHD risk. Generalized least-squares trend models were used to derive the best-fitting dose-response curves in stratified continuous meta-analyses. Categorical meta-analyses were used to verify uncertainty for low to moderate levels of consumption in comparison to long-term abstainers.
Results
The analyses used 38,627 IHD events (mortality or morbidity) among 957,684 participants. Differential risk curves were found by sex and end point. Although some form of a cardioprotective association was confirmed in all strata, substantial heterogeneity across studies remained unexplained and confidence intervals were relatively wide, in particular for average consumption of 1–2 drinks/day.
Conclusions
A cardioprotective association between alcohol use and ischaemic heart disease cannot be assumed for all drinkers, even at low levels of intake. More evidence on the overall benefit-risk ratio of average alcohol consumption in relation to ischaemic heart disease and other diseases is needed in order to inform the general public or physicians about safe or low-risk drinking levels.
Keywords: alcohol drinking, alcoholic beverages, case-control studies, cohort studies, coronary artery disease, coronary disease, meta-analysis
INTRODUCTION
The health effects of alcohol consumption are manifold, some beneficial, but most detrimental. While the influence on injuries, whether intentional or unintentional, and on several cancers has been shown to be negative with substantial public health impact, the effect on some health outcomes, such as ischaemic stroke, possibly diabetes, but most strongly ischaemic heart disease (IHD), seems to be beneficial when drinking is not heavy on average (1). Many drinkers cite health benefits, mostly for cardioprotection, as a reason for drinking alcohol (2), despite often raised concern in the scientific literature about the causality of a cardioprotective effect.
Oftentimes referred to as a J-shaped curve, several meta-analyses of observational studies seem to show relatively strong evidence for a cardioprotective association of average alcohol intake on IHD risk (3–6). However, there has been a consistent debate on the limitations of current observational evidence, much of which relates to questions of exposure assessment (7–9), the choice of the reference group (sick-quitter effect) (10, 11), and residual confounding and/or over-adjustment for intermediate risk factors for IHD (1, 12–14). These limitations make clinical and public health recommendations for low levels almost impossible at this point, and concern about assuming a causal relationship between alcohol consumption and IHD incidence seems to be well justified.
The risk curve seems to decline sharply with a slow turn up with higher average alcohol consumption. The two most recent meta-analyses showed that a detrimental risk for heart disease is not reached until average consumption exceeds 72 g/day (3) and > 60 g/day (6). The lowest consumption levels are of particular interest because this sharply declining risk curve suggests that cardioprotection is already achieved at very low doses of alcohol intake and the risk of other diseases shows a strong positive and linear association with increasing alcohol intake. However, results from meta-analyses suggest that the risk from average alcohol consumption is differential for men and women, and for the investigated heart health outcome (mortality versus morbidity). Furthermore, the shape of the risk curve has been shown to depend on the reference group, that is, whether the comparison group comprised current non-drinkers or long-term abstainers. Thus, relative risk estimates of low or moderate drinkers are typically biased, depending on which reference group was used.
Ronksley et al. focused on the question of whether any alcohol consumption is beneficial compared with non-drinkers (6). While they found strong evidence for a protective effect of alcohol consumption on several heart disease outcomes, they did not stratify average alcohol consumption by sex, or report the risk of IHD by levels of alcohol consumption in relation to long-term abstainers. They reported a pooled statistically significant protective effect for both mortality and incidence for up to 60 g/day in comparison to current non-drinkers at baseline, thus ignoring the effect of former drinkers.
In this meta-analysis we used strict inclusion criteria to identify high quality observational studies reporting analyses stratified by sex and end point suitable for an investigation of a curvilinear relationship (i.e., identification of a cardioprotective or detrimental association at different levels of alcohol intake), as well as consideration of bias in reported effect estimates because of differentially defined reference groups. Furthermore, we conducted meta-analyses using a categorical approach in addition to a continuous dose-response approach, thus reflecting a more realistic assessment of uncertainty around the curvilinear relationship, in particular at low levels of alcohol intake.
METHODS
Search strategy
This meta-analysis followed the guidelines set by the MOOSE statement (15). We systematically searched the following electronic databases from January 1980 to the second week of April 2010: MEDLINE, EMBASE, Web of Science (Science Citation Index Expanded, Social Sciences Citation Index, Arts & Humanities Citation Index). In addition we scrutinized relevant reviews (16–24), meta-analyses (3, 25–29), and references of identified papers. Excluding letters, editorials, conference abstracts, reviews, and comments, the following free-text keywords and subject headings were used to identify relevant articles in electronic databases: (alcohol drinking OR alcoholic beverages OR beverages OR (alcohol AND (drinking or intake or consumption) OR (ethanol AND drinking or intake or consumption)) AND (myocardial ischemia OR myocardial infarct* OR coronary disease OR heart diseases OR coronary artery disease OR coronary heart disease OR angina OR cardiac death* OR ischaemic heart disease OR ischaemic heart disease OR cardiac event* OR coronary event*) AND (cohort studies OR epidemiologic studies OR follow-up studies OR longitudinal studies OR prospective studies OR case-control studies OR retrospective studies) AND (ratio* OR risk*). No language restrictions were applied. Inclusion criteria were: 1) case-control or cohort study, 2) a measure of risk and its corresponding measure of variability was reported (or sufficient data to calculate these), 3) IHD analyzed as a separate outcome (ICD-9: 410–414, ICD-10: I20–25), 4) exposure measurement had to: (a) have at least three categories of alcohol consumption reported among current drinkers to allow for finding a curvilinear relationship, (b) cover a reference period of more than 2 weeks for average alcohol consumption at baseline (or before incident case for case-control studies), (c) average consumption had to be determined by at least a combination of usual frequency and usual volume or the number of drinks in the specified reference period, 5) estimates were at least age-adjusted.
Because the focus of this meta-analysis was epidemiologic quality of selected studies, including measurement of alcohol consumption, we excluded studies where a semi-quantitative food frequency questionnaire with an ambiguous combination of frequency and volume in a single question was used to assess average consumption, as well as qualitative characterizations of alcohol exposure, such as “problem drinkers” or “social drinkers”. Self-reported IHD morbidity, or cardiovascular outcomes combined (i.e., including stroke), and samples containing only high risk populations were also excluded. We preferred estimates stratified by sex, endpoint (morbidity and mortality), and race (black and white). Where possible, we avoided estimates that were adjusted for blood pressure or cholesterol level or treatment/history for these conditions because these represent mediators rather than confounders in the relationship between alcohol consumption and IHD (30, 31), but accepted these if other estimates were not available. One author performed the search and excluded studies at the first exclusion pass based on title and abstract. Studies identified for a more detailed assessment were discussed and agreed upon by both authors without blinding of study characteristics.
Data extraction and synthesis
We abstracted information on RR estimates and their corresponding variances, number of cases and controls or persons at risk for each reported category of average alcohol intake (if not directly reported, we estimated these based on standard formulas) (32, 33), study design, end point, sex, country, age at baseline, length of follow-up, first year of baseline assessment, and specific adjustment for covariates. We converted alcohol intake into g/day using the midpoints (mean) of reported categories. For open-ended categories we added ¾ of the previous category to the lower bound. We used reported conversion factors when standard drinks were the unit of measurement, or standard conversion factors (34). If necessary, multiple reported analyses per stratum were combined using fixed-effects models, so that each article contributed at most one dose-response curve per stratum(35). If the reference category was not a corresponding abstainer group but, for example light drinkers, we re-calculated the effect size measure to reflect abstainers as the reference category. Former drinkers were excluded from all analyses; when current non-drinkers were the reference group, we adjusted mortality estimates for the effect of former drinking compared to lifetime abstention based on a previous meta-analysis (36) to avoid the sick-quitter effect. Based on a previous meta-analysis on former drinking compared with lifetime abstainer were used to correct the RRs of current drinkers in primary studies in our analysis where current non-drinkers were the reference group (i.e., the reference group included former drinkers). In men, a pooled RR = 1.25 was multiplied by the mean fraction of former drinkers among all current non-drinkers (0.32) and added to the respective RRs of current drinking groups from primary studies used in our analysis when current non-drinking was the reference group. In women, the correction factors were RR = 1.54 with 0.08 fraction former drinkers among all current non-drinkers (36). These corrections were done on the log scale. The analyses with morbidity as the health outcome were not adjusted because the risk of former drinking was not statistically significant from that of lifetime abstainers. Those consuming > 72 g/day were excluded from all analyses because of scarcity of data.
Statistical analysis
Hazard ratios, relative risks and odds ratios were treated as measures of relative risk. Expecting a curvilinear relationship between alcohol and IHD risk, we used fractional polynomials (37) to derive the best fitting function for average alcohol consumption in g/day within each stratum of end point and sex using the ‘pool-first’ approach described by Greenland and Longnecker (35) and Orsini et al. (38). Linear, first- and second-degree models were estimated using the following range of powers for the fractional polynomial meta-analysis: −2, −1, 0, 1, 2, 3 (37). Significant gain in deviance by first- and second-order models was determined by likelihood ratio tests with 1 and 2 degrees of freedom, respectively. Goodness-of-fit statistics were used to choose the best-fitting model (39) with one turning point to avoid local maxima or minima. Many functional forms can be estimated with this approach, among those J-, L-, and U-shaped functions. We investigated sources of heterogeneity across studies in meta-regression models (40–42). A significant effect modification was determined by a likelihood ratio test with 2 d. f. and sub-group analyses were conducted in these cases. Study characteristics included in these interaction analyses were: age at time of IHD event (<65 years, ≥65 years), dummy variables for age-only adjustment, and adjustment for blood pressure or cholesterol in reported relative risk estimates. We further tested the impact of study design (cohort vs. case-control) on the results of the analysis involving morbidity in men, which was the only stratum where this was possible due to the number of primary case-control studies.
For the categorical analysis, alcohol intake was classified as follows: 1) lifetime abstainer, 2) occasional drinker (less than weekly drinking or 0.1–2.49 g/day), 3) average amount of alcohol consumed during the reference period (categorization 2.5–11.99 g/day, 12–23.99, 24–35.99). The classification of average alcohol intake corresponds to about 1 standard drink (12g pure alcohol content) (34). When more than one estimate from primary studies was assigned to these categories, we pooled those using fixed-effects and then pooled across studies using DerSimonian-Laird random-effect models to account for between-study heterogeneity (43). We quantified between-study heterogeneity using Cochrane’s Q (44) and the I2 statistic (45). I2 can be interpreted as the proportion of the total variation in the estimated slopes for each study that is due to heterogeneity between studies. Potential publication bias was examined using Peter’s regression-based test (46), and sensitivity analyses for the influence of single studies on the pooled relative risk were conducted. All meta-analytical analyses were conducted on the natural log scale in Stata statistical software, version 10.1 (47), and P<.05 (two-sided) was considered statistically significant.
RESULTS
Out of 1,538 unique citations identified in the search, 392 full papers were retrieved and scanned for inclusion (Figure 1). After removal of studies because of exclusion criteria and duplicate analyses, we selected 44 unique articles for our quantitative analysis (Table 1).
Figure 1.
Study selection process
Table 1.
Characteristics of 43 studies selected for quantitative analysis of the association between average alcohol consumption and risk of ischaemic heart disease, 1980–2010
| Study | Provided at least one stratified data set | End Point | Sex | Study Design | No. of Cases | Total Sample Size, No. | Country | Adjustment |
|---|---|---|---|---|---|---|---|---|
| Dyer et al, (65) 1980 | Yes | Mortality | M | Cohort | 149 | 1832 | US | Age, diastolic BP, smoking, serum cholesterol |
| Kagan et al, (66) 1981 | Yes | Morbidity | M | Cohort | 113 | 7591 | US | Age |
| Gordon et al, (67) 1983 | Yes | Mortality | W, M | Cohort | 906 | 4625 | US | Age, systolic BP, smoking, relative weight, Sf 0–20, Sf 20–400 lipoproteins |
| Kaufman et al, (68) 1985 | Yes | Morbidity | M | Case-control | 1095 | 1596 | US | Age, smoking |
| Colditz et al, (69) 1985 | No | Mortality | Combined | Cohort | 42 | 1161 | US | Age |
| Camacho et al, (70) 1987 | Yes | Mortality | W, M | Cohort | 421 | 4590 | US | 65 years or older, male, black, disabled, health fair or poor, less than adequate income, less than 12 years schooling, never smoked, relatively inactive, not married, no organization membership, depressed, uncertain |
| Scragg et al, (71) 1987 | Yes | Mortality, Morbidity | W, M | Case-control | 594 | 2321 | New Zealand | Age |
| Kono et al, (72) 1991 | No | Morbidity | Combined | Case-control | 83 | 340 | Japan | Age, smoking, strenuous exercise, BMI, systemic hypertension, diabetes, parental heart disease, job class |
| Jackson et al, (73) 1991 | Yes | Morbidity | W, M | Case-control | 283 | 1035 | New Zealand | Age, smoking, BP, social class, exercise, recent change in drinking |
| Goldberg et al, (74) 1994 | Yes | Mortality | M | Cohort | 132 | 3793 | US | Age, systolic BP, serum cholesterol, serum triglycerides, serum uric acid, smoking, coffee intake, total caloric intake |
| Doll et al, (75) 1994 | Yes | Mortality | M | Cohort | 1100 | 10 604 | UK | Age, smoking, year of death, history of previous disease |
| Shaper at al, (76) 1994 | Yes | Mortality, morbidity | M | Cohort | 512 | 7729 | UK | Age, social class, BMI, smoking |
| Iso et al, (77) 1995 | No | Combined | M | Cohort | 34 | 2890 | Japan | Age |
| Rehm et al, (78) 1997 | Yes | Combined, mortality, morbidity | W, M | Cohort | 2112 | 6788 | US | Age, smoking |
| McElduff et al, (79) 1997 | No | Combined | W, M | Case-control | 2483 | 3964 | Australia | Age, smoking, BP, high cholesterol, angina, stroke, previous MI, diabetes |
| Kitamura et al, (80) 1998 | No | Combined | M | Cohort | 80 | 8476 | Japan | Age, serum total cholesterol, smoking, BMI, left ventricular hypertrophy, history of diabetes |
| Maskarinec et al, (81) 1998 | Yes | Mortality | W, M | Cohort | 1100 | 27 678 | US | Age, race, education, BMI, smoking |
| Romelsjö et al, (82) 1999 | No | Combined | M | Cohort | 279 | 49 618 | Sweden | BP (cont.), BMI, father’s social class, running away from home, poor schooling wellbeing, parental divorce, poor emotional control, 0–1 friends, unemployment for >3 months during lifetime, poor health, smoking |
| Hippe et al, (83) 1999 | No | Combined | W, M | Cohort | 1763 | 24 664 | Denmark | Age, population of origin |
| Grønbæk et al, (84) 2000 | No | Mortality | Combined | Cohort | 1075 | 19 006 | Denmark | Age, sex, smoking, education, physical activity, BMI, |
| Liao et al, (85) 2000 | Yes | Mortality | W, M | Cohort | 1378 | 43 695 | US | Age, race, smoking, history of hypertension, diabetes, heart disease, marital status, education, self-perceived health status |
| Genchev et al, (86) 2001 | No | Morbidity | Combined | Case-control | 155 | 309 | Bulgaria | Age, sex |
| Tavani et al, (87) 2001 | No | Morbidity | Combined | Case-control | 507 | 985 | Italy | Age, sex, education, physical activity, BMI, cholesterol, coffee, smoking, hyperlipidemia, diabetes, hypertension, family history of MI |
| Sempos et al, (49) 2002 | No | Combined | W, M | Cohort | 244 | 1158 | US | Age |
| Romelsjö et al, (88) 2003 | Yes | Morbidity | W, M | Case-control | 1300 | 3534 | Sweden | Age, hospital, marital status, smoking, physical activity, cardioartherosclerotic disease, job strain, social anchorage, life control |
| Marques-Vidal et al, (89)2004 | Yes | Combined, morbidity | M | Cohort | 318 | 9750 | France, Northern Ireland | Age, marital status, education, vigorous exercise, BMI, systolic BP, diastolic BP, total cholesterol, triglycerides (Ln), smoking, anti-hypertensive drug treatment, hypolipidaemic treatment, centre |
| Wells et al, (90) 2004 | No | Combined | W, M | Case-control | 1164 | 2935 | New Zealand | Age |
| Fuchs et al, (48) 2004 | No | Combined | M | Cohort | 449 | 6276 | US | Age, smoking, BMI, LDL-level, WHR, education, income, sport index, diabetes |
| Tavani et al, (91) 2004 | Yes | Morbidity | W | Case-control | 558 | 1602 | Italy | Age, cohort, education, BMI, smoking, coffee, diabetes, hyperlipedemia, BP, family history of AMI |
| Mäkelä et al., (92) 2005 | No | Combined | W, M | Cohort | 854 | 6392 | Finland | Age, cohort period, marital status, education, smoking |
| Kabagambe et al, (93) 2005 | No | Morbidity | Combined | Case-control | 1465 | 3234 | Costa Rica | Age, smoking |
| Mukamal et al, (94) 2006 | No | Combined | Combined | Cohort | 675 | 4410 | US | Age, race, sex, education, marital status, smoking, exercise intensity, depression score, frequent aspirin use, BMI, diabetes |
| Tolstrup et al, (95) 2006 | No | Combined | W, M | Cohort | 2032 | 53 500 | Denmark | Age, education, smoking, physical activity, BMI, total intake of vegetables, fruits, fish, and saturated fat |
| Gun et al., (96) 2006 | Yes | Mortality | M | Cohort | 295 | 16 547 | Australia | Age, calendar period, smoking |
| Harriss et al, (97) 2007 | Yes | Mortality | W, M | Cohort | 249 | 38 248 | Australia | Age, country of birth, smoking, total daily energy intake and fruit intake, saturated fat intake |
| Dorn et al, (98) 2007 | Yes | Morbidity | W | Case-control | 159 | 1190 | US | Age, BMI, education, race, smoking, menopausal status |
| Henderson et al, (99) 2007 | Yes | Mortality | M | Cohort | 572 | 70 739 | US | Age |
| Hart et al, (100) 2008 | Yes | Mortality, morbidity | M | Cohort | 2534 | 6000 | UK | Age |
| Ikehara et al, (101) 2008 | Yes | Mortality | W, M | Cohort | 736 | 83 682 | Japan | Age |
| Ikehara et al, (102) 2009 | Yes | Mortality | M | Cohort | 183 | 18 595 | Japan | Age |
| Bazzano et al, (103) 2009 | Yes | Combined, mortality | M | Cohort | 1564 | 64 597 | China | Age |
| Key et al, (104) 2009 | No | Mortality | Combined | Cohort | 213 | 47 254 | UK | Age, sex, smoking |
| Mukamal et al, (105) 2010 | No | Mortality | Combined | Cohort | 6135 | 245 207 | US | Age, sex, race, smoking, marital status, education, region, urbanization, BMI, general health status |
| Arriola et al, (106) 2010 | No | Combined | W, M | Cohort | 532 | 37 544 | Spain | Age, centre, smoking, height, education, physical activity, WHR, vitamin E, antithrombotic and antihaemorrhagic drugs, energy intake |
Abbreviations: BMI, body mass index; IHD, ischaemic heart disease; LDL, low density cholesterol level; M, men; W, women; WHR, waist-hip-ratio.
A total of 9,846 IHD events with 13,199 controls among case-control studies, and 6,942 IHD events with end points combined (mortality or morbidity) and 21,839 IHD events stratified by end point among 934,639 persons at risk among cohort studies contributed to this analysis. The number of cases per study ranged from 34 to 6,135, and the total sample size from 309 to 245,207. The majority of selected articles originated in the US (n = 16), Japan (n = 5), and the UK (n = 4), but a wide range of countries were included (Table 1). Only two studies (48, 49) provided stratified estimates for race other than white. We therefore refrained from analyzing those separately and included each estimate into the respective sex and endpoint strata.
Among the articles selected for a quantitative analysis (Table 1), 20 articles reported only estimates for endpoint or sex combined. These estimates were used in any of the respective analyses labeled as ‘combined’ (Table 3 and 4, Figure 3), whereas 24 articles reporting sex- and endpoint-specific estimates were used in our main analyses.
Table 3.
Model-based functional form and key features of the association between average alcohol intake and risk of ischaemic heart disease, by sex and end point, 1980–2010
| Sex | Stratum | Functional form (x = average alcohol intake, g/day) | No. of Studies | Age at Time of IHD Event, years | Nadir, g/day | Reversion Point, g/daya | P Value for Heterogeneity | I2, % (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Stratified only (n = 24 studies) | ||||||||
| Men | Mortality | log RR = x0.5 + x3 | 17 | 65 | 32 | 63 | <0.001 | 51 (35–63) |
| Morbidity | log RR = x0.5 + ln(x)* x0.5 | 9 | 63 | 69 | - | 0.001 | 46 (21–63) | |
| Women | Mortality | log RR = x + ln(x)* x | 8 | 67 | 11 | 31 | 0.014 | 56 (45–64) |
| Morbidity | log RR = x0.5 + x | 5 | 63 | 14 | 57 | <0.001 | 58 (31–74) | |
| All estimates (n = 44 studies) | ||||||||
| Men | Mortality | log RR = x0.5 + x3 | 34 | 57 | 37 | - | <0.001 | 54 (44–63) |
| Morbidity | log RR = x0.5 + ln(x)* x0.5 | 28 | 56 | 72 | - | <0.001 | 46 (32–58) | |
| Women | Mortality | log RR = x + ln(x)* x | 18 | 58 | 23 | 62 | <0.001 | 51 (34–64) |
| Morbidity | log RR = x0.5 + x | 17 | 63 | 29 | - | <0.001 | 59 (44–69) | |
Abbreviations: CI, confidence interval.
Point where the risk function for average alcohol intake turns into a detrimental association with ischaemic heart disease
Table 4.
Categorical analysis of the association between average alcohol intake and risk of ischaemic heart disease, by sex and end pointa (n = 44 studies), 1980–2010
| Sex | End point | Average alcohol intake, g/day | No. of Studies | No. of Cases | Total sample size | Relative Risk (95% CI) | P Value for Heterogeneity | I2, % (95% CI) | P Value for Publication bias |
|---|---|---|---|---|---|---|---|---|---|
| Men | Mortality | Lifetime abstainer | 34 | 8347 | 233 360 | 1.0 | |||
| Occasionalb | 10 | 606 | 8720 | 0.90 (0.76–1.08) | 0.16 | 32 (0–67) | 0.62 | ||
| 2.5–11.99 | 34 | 5006 | 238 530 | 0.81 (0.74–0.90) | <0.001 | 70 (58–79) | 0.65 | ||
| 12–23.99 | 26 | 2816 | 588 135 | 0.74 (0.66–0.84) | <0.001 | 68 (53–79) | 0.56 | ||
| 24–35.99 | 20 | 1377 | 45 259 | 0.74 (0.63–0.86) | <0.001 | 74 (60–83) | 0.26 | ||
| Morbidity | Lifetime abstainer | 28 | 3595 | 60 666 | 1.0 | ||||
| Occasionalb | 9 | 847 | 6586 | 0.87 (0.75–1.01) | 0.23 | 24 (0–64) | 0.63 | ||
| 2.5–11.99 | 27 | 4445 | 74 562 | 0.77 (0.69–0.86) | <0.001 | 67 (51–78) | 0.61 | ||
| 12–23.99 | 22 | 2852 | 30 136 | 0.70 (0.63–0.77) | .006 | 48 (15–68) | 0.99 | ||
| 24–35.99 | 16 | 1231 | 13 297 | 0.66 (0.57–0.76) | .004 | 55 (21–74) | 0.89 | ||
| Women | Mortality | Lifetime abstainer | 18 | 7043 | 204 285 | 1.0 | |||
| Occasionalb | 6 | 410 | 6731 | 0.97 (0.84–1.11) | 0.34 | 12 (0–55) | 0.19 | ||
| 2.5–11.99 | 18 | 3103 | 194 512 | 0.77 (0.70–0.84) | 0.037 | 41 (0–66) | 0.77 | ||
| 12–23.99 | 15 | 702 | 38 696 | 0.74 (0.60–0.90) | 0.002 | 60 (29–77) | 0.61 | ||
| 24–35.99 | 10 | 416 | 12 575 | 0.67 (0.56–0.80) | 0.15 | 32 (43–67) | 0.67 | ||
| Morbidity | Lifetime abstainer | 17 | 2630 | 21 704 | 1.0 | ||||
| Occasionalb | 6 | 635 | 4749 | 0.92 (0.83–1.02) | 0.59 | 0 (0–64) | 0.16 | ||
| 2.5–11.99 | 16 | 2055 | 31 254 | 0.70 (0.62–0.78) | 0.043 | 41 (0–68) | 0.54 | ||
| 12–23.99 | 13 | 848 | 13 987 | 0.69 (0.56–0.84) | <0.001 | 69 (45–82) | 0.57 | ||
| 24–35.99 | 11 | 382 | 6291 | 0.62 (0.50–0.77) | 0.022 | 52 (5–76) | 0.19 |
Note: Former drinkers were excluded.
Abbreviations: CI, confidence interval.
Combined end point or sex included.
Occasional = less than 1 drink/week or <2.5 g/day average alcohol intake.
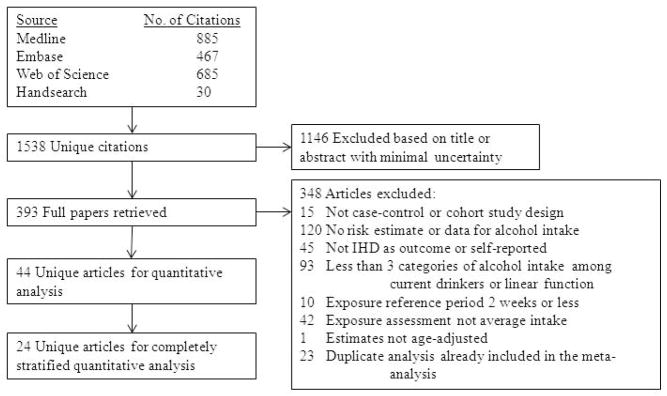
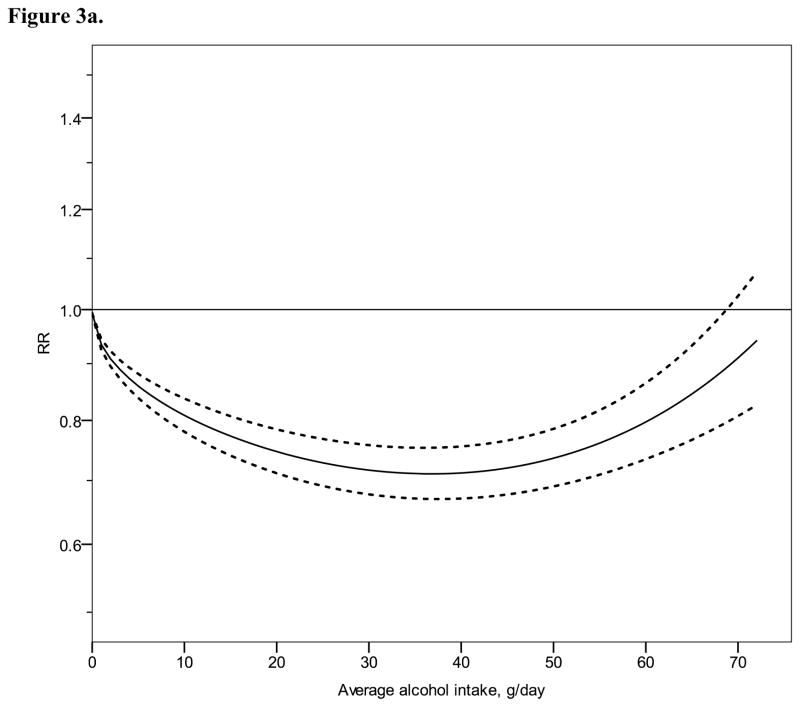
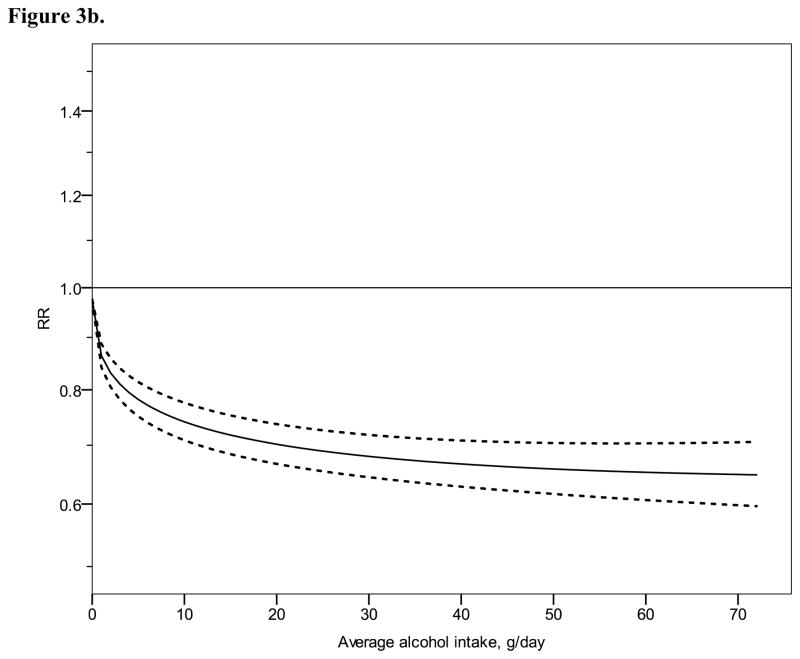
Figure 3.
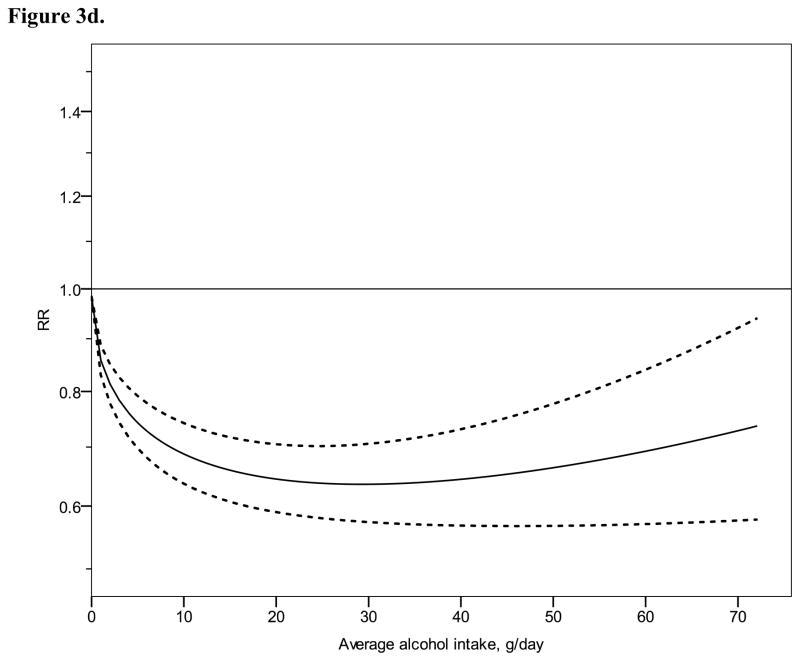
Relative risk functions (solid lines, on the natural log scale) and corresponding 95% confidence intervals (dashed lines) for the dose-response relationship between average alcohol intake and risk of ischaemic heart disease (IHD), using also studies with combined sex or endpoint, 1980–2010. 3a) IHD mortality in men, 3b) IHD morbidity in men, 3c) IHD mortality in women, 3d) IHD morbidity in women
Continuous dose-response meta-analysis
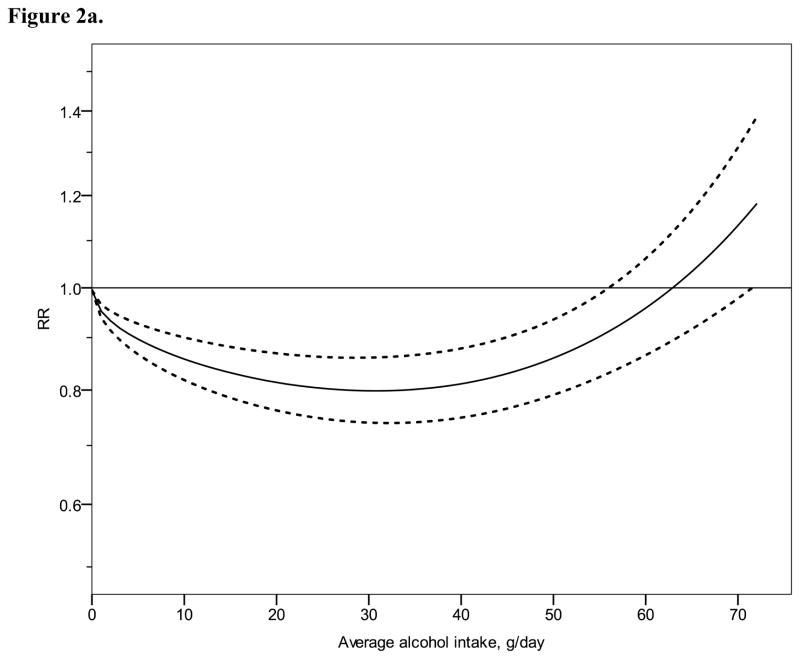
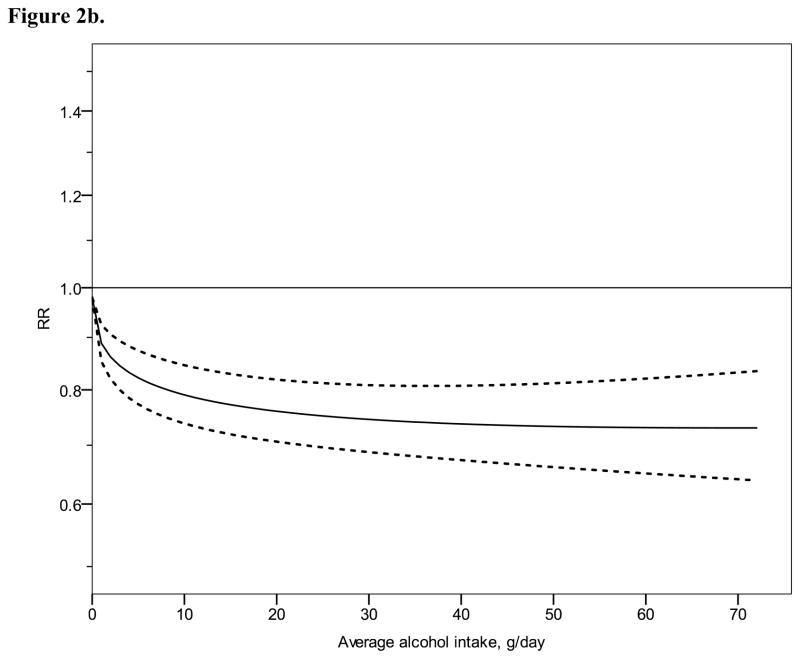
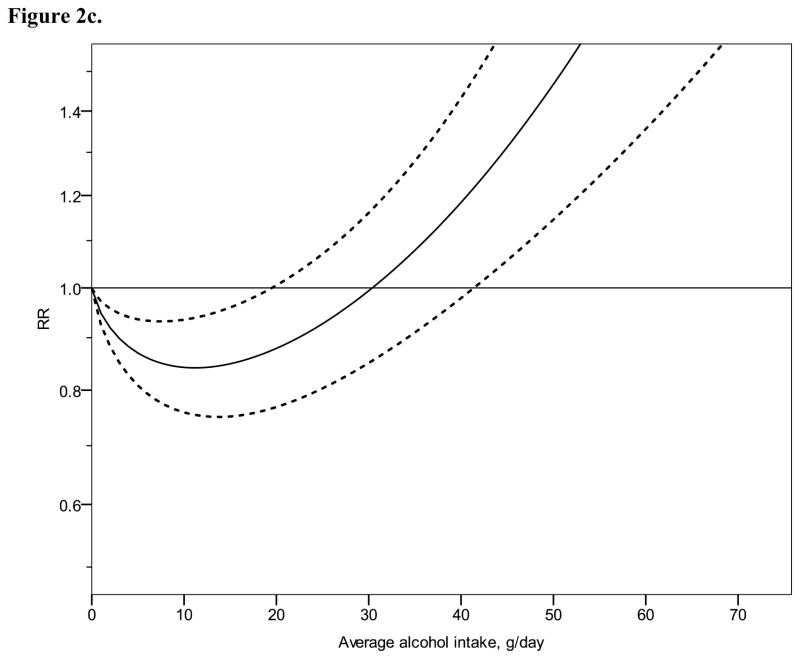
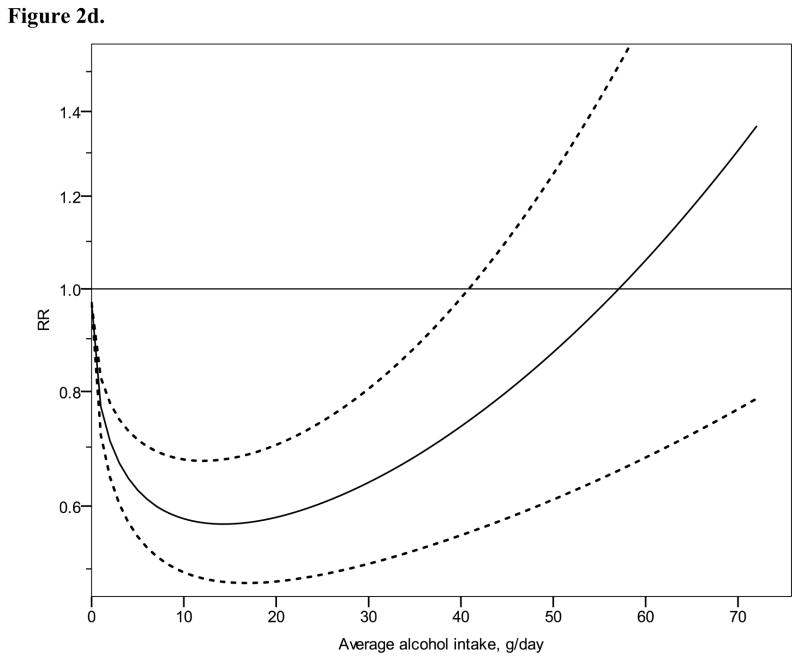
Figure 2 shows derived continuous dose-response curves for IHD mortality and morbidity stratified by sex. In men, the risk function follows a J-curve with a nadir (lowest point of the curve, i.e., lowest IHD risk) at 31 g/day for IHD mortality (Figure 2a). The reversion point, where no statistical evidence for a cardioprotective effect exists, was reached at 63 g/day. Regarding morbidity in men, a declining curve leveled off for stratified only estimates (Figure 2b) with the nadir at 69 g/day. Analyses using estimates that combined sex or end point (Figures 3a, b), showed similar curves and nadirs. In women a steep J-curve was observed for IHD mortality and morbidity (Figures 2c, d). The nadir and reversion points were substantially lower for both IHD mortality and morbidity in women (11 g/day and 14 g/day, respectively) compared with men. In both sexes, heterogeneity was substantial and highly statistically significant in most models, with I2 between 46 and 59% (Table 3).
Figure 2.
Relative risk functions (solid lines, on the natural log scale) and corresponding 95% confidence intervals (dashed lines) for the dose-response relationship between average alcohol intake and risk of ischaemic heart disease (IHD), using only studies completely stratified by sex and endpoint, 1980–2010. 2a) IHD mortality in men, 2b) IHD morbidity in men, 2c) IHD mortality in women, 2d) IHD morbidity in women
Categorical meta-analysis
The categorical analysis (Table 2) shows the relationship between average alcohol intake and risk of IHD for 1, 2, and 3 standard drinks in comparison to lifetime abstainers. Although the general form of the dose-response relationship derived from the fractional polynomial analyses was confirmed in each stratum, confidence intervals were markedly wider, in particular for 1 or 2 drinks on average. For male mortality, a statistically significant cardioprotective association was detected for 3 standard drinks (RR = 0.78, 95% CI: 0.63–0.97), but not for 1 or 2 drinks of average alcohol consumption (RR = 0.89, 95% CI: 0.79–1.00 for 1 drink/day and RR = 0.86, 95% CI: 0.73–1.02 for 2 drinks/day, Table 2). Except for the category with 3 drinks/day, a statistically significant cardioprotective association was found for male morbidity, regardless of whether only stratified estimates were used (Table 2) or also estimates using combined sex or end points (Table 4); however, there were only 3 studies available for a fully stratified analysis for 3 drinks of average alcohol intake. In women, using only completely stratified studies, a statistically significant association was found only for up to 1 standard drink on average for mortality, and for up to 2 drinks considering IHD morbidity. The number of studies reporting drinking levels of 3 or more drinks/day on average was very low (n = 3).
Table 2.
Categorical analysis of the association between average alcohol intake and risk of ischaemic heart disease, stratified by sex and end point (n = 24 studies), 1980–2010
| Sex | End Point | Average alcohol intake, g/day | No. of Studies | No. of Cases | Total sample size | Relative Risk (95% CI) | P Value for Heterogeneity | I2, % (95% CI) | P Value for Publication bias |
|---|---|---|---|---|---|---|---|---|---|
| Men | Mortality | Lifetime abstainer | 16 | 2460 | 98 797 | 1.0 | |||
| Occasionala | 5 | 248 | 7376 | 0.94 (0.74–1.21) | 0.18 | 37 (0–76) | 0.90 | ||
| 2.5–11.99 | 17 | 1792 | 68 249 | 0.89 (0.79–1.00) | <0.001 | 65 (42–79) | 0.92 | ||
| 12–23.99 | 12 | 1113 | 23 994 | 0.86 (0.73–1.02) | <0.001 | 72 (49–84) | 0.42 | ||
| 24–35.99 | 11 | 617 | 34 821 | 0.78 (0.63–0.97) | <0.001 | 76 (58–87) | 0.40 | ||
| Morbidity | Lifetime abstainer | 9 | 1644 | 48 270 | 1.0 | ||||
| Occasionala | 3 | 412 | 3856 | 0.82 (0.65–1.02) | 0.31 | 15 (0–59) | 0.80 | ||
| 2.5–11.99 | 9 | 1540 | 14 313 | 0.77 (0.65–0.92) | 0.001 | 68 (39–83) | 0.13 | ||
| 12–23.99 | 8 | 1112 | 6601 | 0.75 (0.64–0.88) | 0.084 | 42 (0–73) | 0.95 | ||
| 24–35.99 | 3 | 264 | 2840 | 0.74 (0.53–1.02) | 0.057 | 65 (0–90) | 0.60 | ||
| Women | Mortality | Lifetime abstainer | 8 | 1333 | 72 808 | 1.0 | |||
| Occasionala | 3 | 252 | 8884 | 0.98 (0.74–1.30) | 0.10 | 58 (0–88) | 0.24 | ||
| 2.5–11.99 | 8 | 427 | 23 569 | 0.84 (0.74–0.96) | 0.24 | 23 (0–65) | 0.81 | ||
| 12–23.99 | 7 | 75 | 5442 | 1.03 (0.84–1.27) | 0.40 | 3 (0–29) | 0.01 | ||
| 24–35.99 | 5 | 37 | 4188 | 0.89 (0.57–1.40) | 0.10 | 48 (0–81) | 0.57 | ||
| Morbidity | Lifetime abstainer | 5 | 650 | 2377 | 1.0 | ||||
| Occasionala | 2 | 325 | 1972 | 0.91 (0.78–1.07) | 0.49 | 0 | NA | ||
| 2.5–11.99 | 5 | 429 | 2325 | 0.54 (0.45–0.65) | 0.95 | 0 (0–44) | 0.92 | ||
| 12–23.99 | 5 | 222 | 912 | 0.61 (0.38–0.99) | 0.009 | 70 (25–88) | 0.41 | ||
| 24–35.99 | 3 | 132 | 421 | 0.40 (0.14–1.13) | 0.002 | 84 (50–95) | 0.25 |
Note: Former drinkers were excluded.
Occasional = less than 1 drink/week to <2.5 g/day average alcohol intake.
Only one of the models displayed in Table 2 and 4 showed evidence of publication bias. Sensitivity analyses omitting studies one by one and re-estimating the pooled RR did not reveal any substantial influence of a particular study on the pooled effect estimates. Heterogeneity across studies was substantial in most analyses and highly statistically significant in all continuous dose-response curve models (Table 3) and most categorical models (Table 2 and 4). This was in part expected due to different study design and populations under study; however, power was relatively low in any attempts to identify sources of this heterogeneity. None of the interaction terms investigated were significant, except for age at the time of the IHD event (<65 years, ≥65 years of age) in women for IHD mortality (sub-group analyses presented in Table 5). We found no evidence for a study design effect in the analysis with IHD morbidity as the outcome measure in men (likelihood ratio test p = 0.57, 2 d. f.).
Table 5.
Subgroup analysis for ischaemic heart disease mortality in women (stratified estimates only), 1980–2010
| Subgroup | Average alcohol intake, g/day | Studies, No. | Relative Risk (95% CI) | P Value for Heterogeneity | I2, % (95% CI) |
|---|---|---|---|---|---|
| Age at time of event < 65 years | |||||
| Lifetime abstainer | 4 | 1.00 | |||
| 2.5–11.99 | 4 | 0.78 (0.60–1.00) | 0.47 | 0(0–78) | |
| 12–23.99 | 3 | 0.93 (0.59–1.46) | 0.63 | 40 (0–66) | |
| 24–35.99 | 3 | 0.69 (0.20–2.42) | 0.036 | 70 (0–91) | |
| Age at time of event ≥ 65 years | |||||
| Lifetime abstainer | 4 | 1.00 | |||
| 2.5–11.99 | 4 | 0.87 (0.72–1.05) | 0.11 | 51 (0–84) | |
| 12–23.99 | 4 | 1.00 (0.68–1.47) | 0.17 | 0 (0–80) | |
| 24–35.99 | 2 | 0.95 (0.68–1.32) | 0.29 | 10 (0–41) | |
Abbreviations: CI, confidence interval.
DISCUSSION
Many epidemiological studies have reported a cardioprotective association for low to moderate alcohol intake in the last three decades; however, the number of published studies alone certainly is not an indicator of the strength of the evidence for a cardioprotective association, let alone a causal effect. This meta-analysis separated former drinkers from the reference group and presents the respective risk curves for average alcohol consumption stratified by sex and IHD endpoint with lifetime abstainers as the comparison group. The results indicate that, given current epidemiological evidence, some form of a cardioprotective association seems plausible for both sexes and end points.
The strength of the cardioprotective association, at low levels of average alcohol consumption in particular, differed by sex and outcome. Furthermore, the upturn of the risk function, indicating a turn into a detrimental association, was differential by sex and outcome. With regard to the difference in risk curves for mortality and morbidity, one potential explanation might be the younger age at the time of the event in morbidity studies. Although the difference is relatively small, risk curves are typically attenuated with increasing age because age is one of the strongest risk factors for chronic diseases (50). Regarding levels of average alcohol consumption, in analyses completely stratified by sex and end point, we detected less cardioprotection for mortality as an outcome compared to previous meta-analyses, in particular for low levels of alcohol intake (1–2 drinks per day). The exception was morbidity in women, which showed stronger effects compared with other meta-analyses, but relatively few studies were available for such an evaluation. The risk estimates for current occasional drinkers did not reach statistical significance (comparable to those by Ronksley et al. (6)), nor was the potential cardioprotective association substantial. However, the difference for IHD endpoints was already apparent at such low consumption levels with stronger protective effects for morbidity outcomes.
The shape of the risk curves in each stratum supports a cardioprotective association. However, although we stratified by sex and end point and focused on the quality of exposure and outcome assessment, except for mortality in women, all models showed substantial unexplained heterogeneity, which makes it likely that more factors play a substantial role than we were able to incorporate in our analysis. This heterogeneity is better reflected in CIs from the categorical analysis because it takes into account all data points in a given category, unlike the CIs from the continuous dose-response analysis, which were derived from the functional form and the distance from the origin (0 g/day alcohol and lifetime abstention). CIs from the continuous analysis thus overestimate precision around the curves at low levels of consumption (1–3 drinks on average per day), as we have shown. Considering the categorical meta-analysis, evidence of a cardioprotective association for IHD mortality among both sexes was borderline for 1–3 drinks/day as upper confidence limits for pooled relative risk estimates were close to or above 1, indicating no statistically difference in IHD risk compared to lifetime abstainer.
Limitations
Several limitations apply to this analysis. Although results were robust in several sensitivity analyses examining study-specific aspects including assessment of adjustment for several IHD risk factors, we cannot exclude the possibility of residual confounding because our meta-analysis was subject to bias, which might be present in the primary studies. Potential residual confounding could bias the results in both ways, a more pronounced cardioprotective effect or a less pronounced effect. We did, however, include many quality characteristics in our eligibility criteria, stratified by sex and end point, adjusted for the sick-quitter effect, and used individual study characteristics in meta-regression models to examine detected heterogeneity across studies. Nevertheless, although we used strict inclusion and exclusion criteria, these were not optimal from a pure evidence point of view. For example, strict control for smoking, health status at baseline, or longer reference periods for alcohol assessment could be important factors to consider, but would have resulted in very few studies for analysis. Thus, our inclusion and exclusion criteria were somewhat driven by practicality. The list of confounders adjusted for in the individual studies varied widely, and a substantial number only included age (sometimes to avoid inclusion of blood pressure or cholesterol level as intermediate factors). However, confounding other than age on the alcohol-heart relationship seems to be usually small (6). Our results were confirmed when only studies were considered that did not adjust for intermediate factors, such as blood pressure or cholesterol level. Problems of residual confounding apply equally to all other risk factors for IHD examined in observational studies. Many risk factors for IHD have been identified, of which many potentially interact with alcohol, enhancing or diminishing the effect of alcohol. However, the number of cases in cohort studies is usually too small to thoroughly investigate such interaction effects. Nevertheless, alcohol is one of the most investigated dietary risk factors for IHD (51).
Although self-reported alcohol consumption seems to be reasonably valid (8, 52), some drinking and non-drinking groups change their alcohol consumption over time (53, 54). Thus, all drinking groups we have identified were subject to misclassification bias. It should be noted that sensitivity analyses investigating potential effect modification by study characteristics were subject to low power because of the small number of studies in several subgroups. Furthermore, we cannot derive meaningful conclusions on the shape of the curve beyond 72 g/day because of scarcity of data.
Implications
Based on our meta-analysis, some form of a cardioprotective association for IHD morbidity and mortality is hard to deny given epidemiological evidence. However, one needs to consider sex and a specific endpoint as a reference point for any risk-benefit relationship. An important issue at low levels of alcohol intake, where a cardioprotective effect can be a substantial part of the overall risk-benefit relationship (28). While the nadir (maximum cardioprotective association) for mortality and morbidity in men was located at average intake between 33 and 69 g/day, showing a significant effect in both the fractional polynomial and categorical analysis, these levels are by no means safe from a clinical and public health perspective as they have been shown to be detrimentally associated with many other disease outcomes (55). However, for low average intake, such as 1 to 2 drinks per day, we have shown that a cardioprotective association cannot be readily assumed for all populations at such drinking levels. Attenuation of IHD risk with higher age at the time of the event in women for IHD mortality in our study warrants caution in assuming the cardioprotective effect is most important or pronounced in the elderly because of higher prevalence of IHD. Nevertheless, the low number of studies to investigate this issue warrants cautious interpretation.
A substantial part of the unexplained heterogeneity might have been caused by irregular heavy drinking occasions, which we were unable to investigate in this report. A previous meta-analysis found a relative risk of 1.45 (95% CI: 1.24–1.70) for participants with such drinking occasions vs. no such drinking occasions, excluding abstainers, former drinkers where possible, occasional drinkers, and regular heavy drinkers (29). Other effect modifiers are certainly plausible. However, given the shape of the derived function, if a strong effect modification by study characteristics would be found, the curve would be divided into a stronger cardioprotective association and an attenuated one. This means that identification of a strong effect modifier would also identify a group with stronger cardioprotection compared to our results, given no bias due to other factors occurred. Nevertheless, heterogeneity suggests that a potential cardioprotective association cannot be generally assumed, even at low levels of intake. The reasons for this heterogeneity of effect need to be investigated before alcohol consumption for health reasons can be advocated in general. Moreover, for any particular individual, the alcohol-IHD relationship cannot be seen in isolation from other disease outcomes because even at low levels of alcohol intake the effect on many other disease outcomes is detrimental (1, 56).
Physicians are faced with numerous problems regarding advice on alcohol intake for individual patients because of the complex potentially beneficial or detrimental effects of alcohol on IHD, although patients seem to be open to advice on change of alcohol consumption from their physician (57). Due to ethical and logistical reasons resulting in a lack of long-term randomized trials providing important experimental evidence, it is of utmost importance to carefully examine the available epidemiological evidence. Regarding causality of effects, a potential cardioprotective association is supported by short-term experimental evidence on surrogate biomarkers, such as increasing HDL cholesterol, reducing fibrinogen levels, and inhibition of platelet activation (58–60). Indeed, this might be the strongest argument for causality given that observational findings are always prone to residual confounding and bias due to study design.
Forming clinical advice for individuals to start drinking for health purposes based on epidemiological evidence alone cannot be advocated here because too many questions on confounding or effect modification from other heart disease risk factors, such as education, income, physical activity, or smoking cannot be accurately answered at this time (12, 61, 62). Substantial heterogeneity even at low levels of alcohol intake we found in our analysis strengthens this conclusion. One or two drinks per day of averaged intake should not be seen as a safe level of drinking because problem drinking behaviour, which is not limited to a specific average daily alcohol intake, can already be seen at these levels (63). It seems that neither taking up drinking because of health reasons, nor abstinence for low level drinkers who have shown themselves able to control their drinking should be promoted. Moreover, the number of drinkers with 1 or 2 drinks per day as a steady daily amount of drinking have shown to be very small even in populations with overall low abstention rates (64).
Findings from this study support current low-risk drinking guidelines, if these recognize lower drinking limits for women. If one only takes into account average volume, this study showed that most of the cardioprotective effect can be already achieved with 1–2 drinks/day for men and 1 drink/day for women. Higher average consumption should be discouraged because of the negative effects on many other disease outcomes (1). Furthermore, very low consumption levels, such as below 1–2 drinks per week do not seem to confer substantial cardioprotective effects. However, at the same time it seems that this does not apply to all drinkers and that other determinants of the alcohol effect on heart disease that were not captured by average consumption as an exposure measurement, such as drinking patterns (29), might play an important role. Given the negative impact of heavy drinking occasions on heart disease and injuries (1), low-risk drinking guidelines should also include limits of drinks per occasion.
Acknowledgments
This work was financially supported by a small contribution from the Global Burden of Disease (GBD) Study and by the grant “Drinking Patterns & Ethnicity: Impact on Mortality Risks” (NIAAA: 1R01AA016644-01A1) to the second author. J. Rehm has participated in scientific meetings organized or sponsored by the alcohol industry and received financial support for this participation. He has also received financial support for his research from governments, various national scientific research funds, the National Institutes of Health, the Canadian Institutes for Health Research, other public health oriented agencies in Australia, Austria, Canada, Germany, the United States as well as from international organizations.
Abbreviations
- CI
confidence interval
- IHD
ischaemic heart disease
- RR
relative risk
Footnotes
Declarations of interest:
All other authors have no interests to declare.
Reference List
- 1.Rehm J, Baliunas D, Borges G, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukamal KJ, Phillips RS, Mittleman MA. Beliefs, motivations, and opinions about moderate drinking: A cross-sectional survey. Fam Med. 2008;40:188–195. [PubMed] [Google Scholar]
- 3.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95:1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 4.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2010;55:1339–1347. doi: 10.1016/j.jacc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Maclure M. Demonstration of deductive metaanalysis - ethanol intake and risk of myocardial infarction. Epidemiol Rev. 1993;15:328–351. doi: 10.1093/oxfordjournals.epirev.a036124. [DOI] [PubMed] [Google Scholar]
- 6.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson DA. Volume of ethanol consumption: Effects of different approaches to measurement. J Stud Alcohol. 1998;59:191–197. doi: 10.15288/jsa.1998.59.191. [DOI] [PubMed] [Google Scholar]
- 8.Greenfield TK. Ways of measuring drinking patterns and the difference they make: experience with graduated frequencies. J Subst Abuse. 2000;12:33–49. doi: 10.1016/s0899-3289(00)00039-0. [DOI] [PubMed] [Google Scholar]
- 9.Midanik LT. Comparing usual quantity frequency and graduated frequency scales to assess yearly alcohol consumption - results from the 1990 United States National Alcohol Survey. Addiction. 1994;89:407–412. doi: 10.1111/j.1360-0443.1994.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaper AG, Phillips AN, Pocock SJ, Walker M. Alcohol and ischemic heart disease in middle-aged British men. BMJ. 1987;294:733–737. doi: 10.1136/bmj.294.6574.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men -explaining the U-shaped curve. Lancet. 1988;2:1267–1273. doi: 10.1016/s0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- 12.Jackson R, Broad J, Connor J, Wells S. Alcohol and ischaemic heart disease: probably no free lunch. Lancet. 2005;366:1911–1912. doi: 10.1016/S0140-6736(05)67770-7. [DOI] [PubMed] [Google Scholar]
- 13.Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation. 2007;116:1306–1317. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- 14.O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health - The razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50:1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Britton A. Alcohol and heart disease. Eur J Public Health. 2004;14:217–218. [Google Scholar]
- 17.Grønbæk M. Alcohol, type of alcohol, and all-cause and coronary heart disease mortality. Alcohol and Wine in Health and Disease. 2002;957:16–20. doi: 10.1111/j.1749-6632.2002.tb02902.x. [DOI] [PubMed] [Google Scholar]
- 18.Grønbæk M. Epidemiologic evidence for the cardiprotective effects associated with consumption of alcoholic beverages. Pathophysiology. 2004;10:83–92. doi: 10.1016/j.pathophys.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Marmot MG. Alcohol and coronary heart disease. Int J Epidemiol. 2001;30:724–729. doi: 10.1093/ije/30.4.724. [DOI] [PubMed] [Google Scholar]
- 20.Rehm J, Sempos CT, Trevisan M. Average volume of alcohol consumption, patterns of drinking and risk of coronary heart disease - a review. J Cardiovasc Risk. 2003;10:15–20. doi: 10.1097/01.hjr.0000051961.68260.30. [DOI] [PubMed] [Google Scholar]
- 21.Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos CT. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98:1209–1228. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 22.Rehm J, Sempos CT, Trevisan M. Alcohol and cardiovascular disease--more than one paradox to consider. Average volume of alcohol consumption, patterns of drinking and risk of coronary heart disease--a review. J Cardiovasc Risk. 2003;10:15–20. doi: 10.1097/01.hjr.0000051961.68260.30. [DOI] [PubMed] [Google Scholar]
- 23.Rimm EB, Klatsky A, Grobbee D, Stampfer MJ. Review of moderate alcohol consumption and reduced risk of coronary heart disease: Is the effect due to beer, wine, or spirits? BMJ. 1996;312:731–736. doi: 10.1136/bmj.312.7033.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaper AG. Alcohol and mortality - a review of prospective studies. Br J Addict. 1990;85:837–847. doi: 10.1111/j.1360-0443.1990.tb03710.x. [DOI] [PubMed] [Google Scholar]
- 25.Di Castelnuovo A, Iacoviello L, de Gaetano G. Alcohol and coronary heart disease. N Engl J Med. 2003;348:1720–1721. [PubMed] [Google Scholar]
- 26.English D, Holman C, Milne E, Winter M, Hulse G, Codde G. The Quantification of Drug-caused Morbidity and Mortality in Australia, 1995. Canberra, Australia: Commonwealth Department of Human Services and Health; 1995. [Google Scholar]
- 27.Fillmore KM, Kerr WC, Stockwell T, Chikritzhs T, Bostrom A. Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies. Addict Res Theory. 2006;14:101–132. doi: 10.1016/j.annepidem.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Gmel G, Gutjahr E, Rehm J. How stable is the risk curve between alcohol and all-cause mortality and what factors influence the shape? A precision-weighted hierarchical meta-analysis. Eur J Epidemiol. 2003;18:631–642. doi: 10.1023/a:1024805021504. [DOI] [PubMed] [Google Scholar]
- 29.Roerecke M, Rehm J. Irregular heavy drinking occasions and risk of ischemic heart disease; a systematic review and meta-analysis. Am J Epidemiol. 2010;171:633–644. doi: 10.1093/aje/kwp451. [DOI] [PubMed] [Google Scholar]
- 30.Mckee M, Britton A. The positive relationship between alcohol and heart disease in eastern Europe: potential physiological mechanisms. J R Soc Med. 1998;91:402–407. doi: 10.1177/014107689809100802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rehm J. Alcohol consumption and mortality. What do we know and where should we go? Addiction. 2000;95:989–995. doi: 10.1046/j.1360-0443.2000.9579891.x. [DOI] [PubMed] [Google Scholar]
- 32.Bland JM, Altman DG. Statistics notes - The odds ratio. BMJ. 2000;320:1468. doi: 10.1136/bmj.320.7247.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenland S, Rothman KJ. Introduction to Categorical Statistics. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3. Philadelphia, US: Lippincott Williams & Wilkens; 2008. pp. 238–257. [Google Scholar]
- 34.World Health Organization. International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva: World Health Organization, Department of Mental Health and Substance Dependence, Noncommunicable Diseases and Mental Health Cluster; 2000. [Google Scholar]
- 35.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 36.Roerecke M, Rehm J. Ischemic heart disease mortality and morbidity in former drinkers: a meta-analysis. Am J Epidemiol. 2011;173:245–258. doi: 10.1093/aje/kwq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royston P, Sauerbrei W. Multivariable Model-building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, UK: Jon Wiley & Sons; 2008. [Google Scholar]
- 38.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 39.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 40.Altman DG, Matthews JNS. Statistics notes 24. Heterogeneity of effects. BMJ. 1996;313:486. doi: 10.1136/bmj.313.7055.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Royston P, Sauerbrei W. A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med. 2004;23:2509–2525. doi: 10.1002/sim.1815. [DOI] [PubMed] [Google Scholar]
- 42.Royston P, Sauerbrei W. Two techniques for investigating interactions between treatment and continuous covariates in clinical trials. Stata J. 2009;9:230–251. [Google Scholar]
- 43.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 44.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 45.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 46.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 47.Stata Statistical Software, Release 10.1 [computer program] College Station, TX: StataCorp, LP; 2008. [Google Scholar]
- 48.Fuchs FD, Chambless LE, Folsom AR, Eigenbrodt ML, Duncan BB, Gilbert A, et al. Association between alcoholic beverage consumption and incidence of coronary heart disease in whites and blacks - The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2004;160:466–474. doi: 10.1093/aje/kwh229. [DOI] [PubMed] [Google Scholar]
- 49.Sempos C, Rehm J, Crespo CJ, Trevisan M. No protective effect of alcohol consumption on coronary heart disease (CHD) in African Americans: Average volume of drinking over the life course and CHD morbidity and mortality in a U.S. national cohort. Contemp Drug Probl. 2002;29:805–820. [Google Scholar]
- 50.Rehm J, Gutjahr E, Gmel G. Alcohol and all-cause mortality: A pooled analysis. Contemp Drug Probl. 2001;28:337–361. [Google Scholar]
- 51.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 52.Midanik L. Perspectives on the validity of self-reported alcohol use. Br J Addict. 1989;84:1419–1424. doi: 10.1111/j.1360-0443.1989.tb03920.x. [DOI] [PubMed] [Google Scholar]
- 53.Kerr WC, Fillmore KM, Bostrom A. Stability of alcohol consumption over time: Evidence from three longitudinal surveys from the United States. J Stud Alcohol. 2002;63:325–333. doi: 10.15288/jsa.2002.63.325. [DOI] [PubMed] [Google Scholar]
- 54.Rehm J, Irving H, Ye Y, Kerr WC, Bond J, Greenfield TK. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am J Epidemiol. 2008;168:866–871. doi: 10.1093/aje/kwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–619. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 56.Lauer MS, Sorlie P. Alcohol, cardiovascular disease, and cancer: Treat with caution. J Natl Cancer Inst. 2009;101:282–283. doi: 10.1093/jnci/djp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukamal KJ. A 42-year old man considering whether to drink alcohol for his health. JAMA. 2010;303:2065–2073. doi: 10.1001/jama.2010.550. [DOI] [PubMed] [Google Scholar]
- 58.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zakhari S. Alcohol and the cardiovascular system: molecular mechanisms for beneficial and harmful action. Alcohol Health Res World. 1997;21:21–29. [PMC free article] [PubMed] [Google Scholar]
- 60.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lieber CS. Alcohol and health: A drink a day won’t keep the doctor away. Cleve Clin J Med. 2003;70:945. doi: 10.3949/ccjm.70.11.945. [DOI] [PubMed] [Google Scholar]
- 62.Naimi TS, Brown DW, Brewer RD, Giles WH, Mensah G, Serdula MK, et al. Cardiovascular risk factors and confounders among nondrinking and moderate-drinking US adults. Am J Prev Med. 2005;28:369–373. doi: 10.1016/j.amepre.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Ammar KA, Samee S, Colligan R, Morse R, Faheem O, Shapiro M, et al. Is self-reported “moderate” drinking in the cardiovascular benefit range associated with alcoholic behavior? A population based study. J Addict Dis. 2009;28:243–249. doi: 10.1080/10550880903014205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knupfer G. Drinking for health: the daily light drinker fiction. Br J Addict. 1987;82:547–555. doi: 10.1111/j.1360-0443.1987.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 65.Dyer AR, Stamler J, Paul O, Lepper M, Shekelle RB, Mckean H, et al. Alcohol consumption and 17-year mortality in the Chicago Western Electric Company study. Prev Med. 1980;9:78–90. doi: 10.1016/0091-7435(80)90060-2. [DOI] [PubMed] [Google Scholar]
- 66.Kagan A, Yano K, Rhoads GG, McGee DL. Alcohol and cardiovascular disease: The Hawaiian experience. Circulation. 1981;64:27–31. [PubMed] [Google Scholar]
- 67.Gordon T, Kannel WB. Drinking habits and cardiovascular disease - The Framingham Study. Am Heart J. 1983;105:667–673. doi: 10.1016/0002-8703(83)90492-1. [DOI] [PubMed] [Google Scholar]
- 68.Kaufman DW, Rosenberg L, Helmrich SP, Shapiro S. Alcoholic beverages and myocardial infarction in young men. Am J Epidemiol. 1985;121:548–554. doi: 10.1093/oxfordjournals.aje.a114032. [DOI] [PubMed] [Google Scholar]
- 69.Colditz GA, Branch LG, Lipnick RJ, Willett WC, Rosner B, Posner B, et al. Moderate alcohol and decreased cardiovascular mortality in an elderly cohort. Am Heart J. 1985;109:886–889. doi: 10.1016/0002-8703(85)90654-4. [DOI] [PubMed] [Google Scholar]
- 70.Camacho TC, Kaplan GA, Cohen RD. Alcohol consumption and mortality in Alameda County. J Chronic Dis. 1987;40:229–236. doi: 10.1016/0021-9681(87)90158-5. [DOI] [PubMed] [Google Scholar]
- 71.Scragg R, Stewart A, Jackson R, Beaglehole R. Alcohol and exercise in myocardial infarction and sudden coronary death in men and women. Am J Epidemiol. 1987;126:77–85. doi: 10.1093/oxfordjournals.aje.a114664. [DOI] [PubMed] [Google Scholar]
- 72.Kono S, Handa K, Kawano T, Hiroki T, Ishihara Y, Arakawa K. Alcohol intake and nonfatal acute myocardial infarction in Japan. Am J Cardiol. 1991;68:1011–1014. doi: 10.1016/0002-9149(91)90487-6. [DOI] [PubMed] [Google Scholar]
- 73.Jackson R, Scragg R, Beaglehole R. Alcohol consumption and risk of coronary heart disease. BMJ. 1991;303:211–216. doi: 10.1136/bmj.303.6796.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldberg RJ, Burchfiel CM, Reed DM, Wergowske G, Chiu D. A prospective study of the health effects of alcohol consumption in middle-aged and elderly men. The Honolulu Heart Program. Circulation. 1994;89:651–659. doi: 10.1161/01.cir.89.2.651. [DOI] [PubMed] [Google Scholar]
- 75.Doll R, Peto R, Hall E, Wheatley K, Gray R. Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. BMJ. 1994;309:911–918. doi: 10.1136/bmj.309.6959.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaper AG, Wannamethee G, Walker M. Alcohol and coronary heart disease: A perspective from the British Regional Heart Study. Int J Epidemiol. 1994;23:482–494. doi: 10.1093/ije/23.3.482. [DOI] [PubMed] [Google Scholar]
- 77.Iso H, Kitamura A, Shimamoto T, Sankai T, Naito Y, Sato S, et al. Alcohol intake and the risk of cardiovascular disease in middle-aged Japanese men. Stroke. 1995;26:767–773. doi: 10.1161/01.str.26.5.767. [DOI] [PubMed] [Google Scholar]
- 78.Rehm JT, Bondy SJ, Sempos CT, Vuong CV. Alcohol consumption and coronary heart disease morbidity and mortality. Am J Epidemiol. 1997;146:495–501. doi: 10.1093/oxfordjournals.aje.a009303. [DOI] [PubMed] [Google Scholar]
- 79.McElduff P, Dobson A. How much alcohol and how often? Population based case-control study of alcohol consumption and risk of a major coronary event. BMJ. 1997;314:1159–1164. doi: 10.1136/bmj.314.7088.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kitamura A, Iso H, Sankai T, Naito Y, Sato S, Kiyama M, et al. Alcohol intake and premature coronary heart disease in urban Japanese men. Am J Epidemiol. 1998;147:59–65. doi: 10.1093/oxfordjournals.aje.a009367. [DOI] [PubMed] [Google Scholar]
- 81.Maskarinec G, Meng L, Kolonel LN. Alcohol intake, body weight, and mortality in a multiethnic prospective cohort. Epidemiology. 1998;9:654–661. [PubMed] [Google Scholar]
- 82.Romelsjö A, Leifman A. Association between alcohol consumption and mortality, myocardial infarction, and stroke in 25 year follow up of 49 618 young Swedish men. BMJ. 1999;319:821–822. doi: 10.1136/bmj.319.7213.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hippe M, Vestbo J, Hein HO, Borch-Johnsen K, Jensen G, Sorensen TI. Familial predisposition and susceptibility to the effect of other risk factors for myocardial infarction. J Epidemiol Community Health. 1999;53:269–276. doi: 10.1136/jech.53.5.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grønbæk M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, et al. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med. 2000;133:411–419. doi: 10.7326/0003-4819-133-6-200009190-00008. [DOI] [PubMed] [Google Scholar]
- 85.Liao YL, McGee DL, Cao GC, Cooper RS. Alcohol intake and mortality: Findings from the National Health Interview Surveys (1988 and 1990) Am J Epidemiol. 2000;151:651–659. doi: 10.1093/oxfordjournals.aje.a010259. [DOI] [PubMed] [Google Scholar]
- 86.Genchev GD, Georgieva LM, Weijenberg MP, Powles JW. Does alcohol protect against ischaemic heart disease in Bulgaria? A case-control study of non-fatal myocardial infarction in Sofia. Cent Eur J Public Health. 2001;9:83–86. [PubMed] [Google Scholar]
- 87.Tavani A, Bertuzzi M, Negri E, Sorbara L, La VC. Alcohol, smoking, coffee and risk of non-fatal acute myocardial infarction in Italy. Eur J Epidemiol. 2001;17:1131–1137. doi: 10.1023/a:1021276932160. [DOI] [PubMed] [Google Scholar]
- 88.Romelsjö A, Branting M, Hallqvist J, Alfredsson L, Hammar N, Leifman A, et al. Abstention, alcohol use and risk of myocardial infarction in men and women taking account of social support and working conditions: the SHEEP case-control study. Addiction. 2003;98:1453–1462. doi: 10.1046/j.1360-0443.2003.00488.x. [DOI] [PubMed] [Google Scholar]
- 89.Marques-Vidal P, Montaye M, Arveiler D, Evans A, Bingham A, Ruidavets JB, et al. Alcohol consumption and cardiovascular disease: differential effects in France and Northern Ireland. The PRIME study. Eur J Cardiovascr Prev Rehabil. 2004;11:336–343. doi: 10.1097/01.hjr.0000136416.24769.42. [DOI] [PubMed] [Google Scholar]
- 90.Wells S, Broad J, Jackson R. Alcohol consumption and its contribution to the burden of coronary heart disease in middle-aged and older New Zealanders: A population-based case-control study. N Z J Med. 2004;117:U793. [PubMed] [Google Scholar]
- 91.Tavani A, Bertuzzi M, Gallus S, et al. Risk factors for non-fatal acute myocardial infarction in Italian women. Prev Med. 2004;39:128–134. doi: 10.1016/j.ypmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 92.Mäkelä P, Paljarvi T, Poikolainen K. Heavy and nonheavy drinking occasions, all-cause and cardiovascular mortality and hospitalizations: A follow-up study in a population with a low consumption level. J Stud Alcohol. 2005;66:722–728. doi: 10.15288/jsa.2005.66.722. [DOI] [PubMed] [Google Scholar]
- 93.Kabagambe EK, Baylin A, Ruiz-Narvaez E, Rimm EB, Campos H. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. Am J Clin Nutr. 2005;82:1336–1345. doi: 10.1093/ajcn/82.6.1336. [DOI] [PubMed] [Google Scholar]
- 94.Mukamal KJ, Chung HJ, Jenny NS, Kuller LH, Longstreth WT, Mittleman MA, et al. Alcohol consumption and risk of coronary heart disease in older adults: The cardiovascular health study. J Am Geriatr Soc. 2006;54:30–37. doi: 10.1111/j.1532-5415.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 95.Tolstrup J, Jensen MK, Tjonneland A, Overvad K, Mukamal KJ, Grønbæk M. Prospective study of alcohol drinking coronary heart disease in women and patterns and men. BMJ. 2006;332:1244–1247. doi: 10.1136/bmj.38831.503113.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gun RT, Pratt N, Ryan P, Gordon I, Roder D. Tobacco and alcohol-related mortality in men: estimates from the Australian cohort of petroleum industry workers. Aust N Z J Public Health. 2006;30:318–324. doi: 10.1111/j.1467-842x.2006.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 97.Harriss LR, English DR, Hopper JL, Powles J, Simpson JA, O’Dea K, et al. Alcohol consumption and cardiovascular mortality accounting for possible misclassification of intake: 11-year follow-up of the Melbourne Collaborative Cohort Study. Addiction. 2007;102:1574–1585. doi: 10.1111/j.1360-0443.2007.01947.x. [DOI] [PubMed] [Google Scholar]
- 98.Dorn JM, Hovey K, Williams BA, Freudenheim JL, Russell M, Nochajski TH, et al. Alcohol drinking pattern and non-fatal myocardial infarction in women. Addiction. 2007;102:730–739. doi: 10.1111/j.1360-0443.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- 99.Henderson SO, Haiman CA, Wilkens LR, Kolonel LN, Wan P, Pike MC. Established risk factors account for most of the racial differences in cardiovascular disease mortality. PLoS ONE. 2007;2(4) doi: 10.1371/journal.pone.0000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hart CL, Smith GD. Alcohol consumption and mortality and hospital admissions in men from the Midspan Collaborative cohort study. Addiction. 2008;103:1979–1986. doi: 10.1111/j.1360-0443.2008.02373.x. [DOI] [PubMed] [Google Scholar]
- 101.Ikehara S, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, et al. Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women: The Japan Collaborative Cohort Study. Stroke. 2008;39:2936–2942. doi: 10.1161/STROKEAHA.108.520288. [DOI] [PubMed] [Google Scholar]
- 102.Ikehara S, Iso H, Yamagishi K, Yamamoto S, Inoue M, Tsugane S, et al. Alcohol consumption, social support, and risk of stroke and coronary heart disease among Japanese men: the JPHC Study. Alcohol Clin Exp Res. 2009;33:1025–1032. doi: 10.1111/j.1530-0277.2009.00923.x. [DOI] [PubMed] [Google Scholar]
- 103.Bazzano LA, Gu D, Reynolds K, Chen J, Wu X, Chen CS, et al. Alcohol consumption and risk of coronary heart disease among Chinese men. Int J Cardiol. 2009;135:78–85. doi: 10.1016/j.ijcard.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford) Am J Clin Nutr. 2009;89:1613S–1619. doi: 10.3945/ajcn.2009.26736L. [DOI] [PubMed] [Google Scholar]
- 105.Mukamal KJ, Chen CM, Rao SR, Breslow RA. Alcohol consumption and cardiovascular mortality among US adults, 1987 to 2002. J Am Coll Cardiol. 2010;55:1328–1335. doi: 10.1016/j.jacc.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arriola L, Martinez-Camblor P, Larranaga N, Basterretxea M, Amiano P, Moreno-Iribas C, et al. Alcohol intake and the risk of coronary heart disease in the Spanish EPIC cohort study. Heart. 2010;96:124–130. doi: 10.1136/hrt.2009.173419. [DOI] [PubMed] [Google Scholar]