Abstract
The sarcin-ricin loop (SRL) is one of the longest conserved sequences in the 23S rRNA. The SRL has been accepted as crucial for the activity of the ribosome because it is targeted by cytotoxins such as α-sarcin and ricin that completely abolish translation. Nevertheless, the precise functional role of the SRL in translation is not known. Recent biochemical and structural studies indicate that the SRL is critical for triggering GTP hydrolysis on elongation factors Tu and G (EF-Tu and EF-G). To determine the functional role of the SRL in the elongation stage of protein synthesis, we analyzed mutations in the SRL that are known to abolish protein synthesis and are lethal to cells. Here, we show that the SRL is not critical for GTP hydrolysis on EF-Tu and EF-G. The SRL also is not essential for peptide bond formation. Our results, instead, suggest that the SRL is crucial for anchoring EF-G on the ribosome during mRNA-tRNA translocation.
Keywords: ribosome, tRNA selection, peptide bond, translocation, GTP hydrolysis
Introduction
Ribosomes perform the complex task of protein synthesis in all cells. Several translation factors interact with the ribosome at different steps to accelerate protein synthesis. Chief among them are guanosine triphosphatase (GTPase) factors that orchestrate critical steps in proteins synthesis. These GTPase factors interact with conserved elements of the ribosome. One of the highly conserved elements in the ribosomal large subunit that GTPase factors interact with is the sarcin-ricin loop (SRL) in helix 95 of 23S rRNA (nucleotides 2646-2674 in E. coli) 1 (Figure 1). The SRL is considered to play an essential role in protein synthesis because it is targeted by deadly cytotoxins such as α-sarcin and ricin that completely inactivate the ribosome 2; 3. α-Sarcin cleaves the phosphodiester bond between G2661 and A2662 and ricin depurinates A2660 in the SRL 4; 5. These covalent modifications to the SRL inhibit the binding of GTPase factors resulting in the shutdown of protein synthesis 6; 7; 8,9.
Figure 1. Interaction of the SRL with elongation factor Tu.
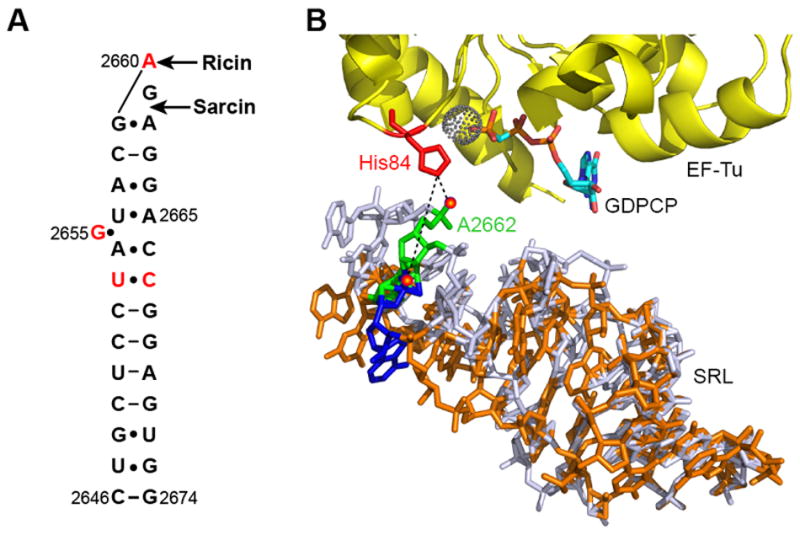
(A) Secondary structure of the SRL. The bases analyzed in this study are shown in red and the sites targeted by ricin and α-sarcin are also indicated. (B) Interaction of the SRL with the EF-Tu ternary complex (PDB: 2XQE). The catalytic His84 (red) in EF-Tu (yellow) and GDPCP (cyan) are indicated. The dotted sphere indicates a water molecule close to the γ-phosphate of GDPCP. Wild type SRL (light blue) showing the position of the phosphate oxygen (red sphere) at A2662 (green), which forms a hydrogen bond with His84 in EF-Tu. Superimposed on the wild type SRL is a model showing how deletion of bases U2653 and C2667 in the SRL (orange) will change the interaction of the phosphate oxygen (red sphere) at A2662 (blue) with EF-Tu.
In the elongation stage of protein synthesis, the SRL iteratively interacts with the GTPase factors elongation factor Tu (EF-Tu) and elongation factor G (EF-G). EF-Tu delivers aminoacyl tRNA as an EF-Tu•GTP•tRNA ternary complex to the ribosomal A site. EF-G•GTP catalyzes the translocation of the peptidyl-tRNA from the A site to the P site and the deacylated tRNA from the P site to the E site in the ribosome. The associated mRNA also moves by one codon to the 3’ end. Both, EF-Tu and EF-G protect bases G2655, A2660 and G2661 in the SRL from chemical modifications 10. EF-Tu additionally protects base A2665 in the SRL 10. Short oligoribonucleotides representing the SRL sequence have been shown to bind EF-G 11. Cryo-EM reconstructions 12; 13; 14; 15; 16; 17; 18 and X-ray crystal structures 19; 20; 21 showed that the SRL interacts with the GTP-binding domains in EF-Tu and EF-G. However, the precise functional role of the SRL is far from clear. Studies suggest that conformational changes in the SRL may regulate the cyclic binding of EF-Tu ternary complex and EF-G•GTP to the ribosome 22. A more recent study, however, indicates that the SRL is not important for binding EF-G to the ribosome but that the exocyclic N6 amino group at A2660 in the SRL is important for triggering GTP hydrolysis on EF-G 23. In contrast, a model proposed from structural data suggests that it is the phosphate oxygen at A2662 in the SRL that is critical for triggering GTP hydrolysis on EF-Tu and EF-G 21 (Figure 1B). Thus, it is not clear whether the SRL serves as a mere GTPase factor-binding site or whether it is actively involved in promoting GTP hydrolysis and other key steps of translation.
To understand the precise function of the SRL, we made mutations in the SRL that are known to inactivate the ribosome. Mutant ribosomes were purified to homogeneity and analyzed using kinetic assays. Our results show that the SRL is important for the binding of EF-Tu•GTP•tRNA ternary complex and EF-G to the ribosome. Surprisingly, however, the SRL is not essential for GTP hydrolysis on EF-Tu and for peptide bond formation suggesting that it is not crucial for tRNA selection. Furthermore, GTP hydrolysis on EF-G was not inhibited. Instead, mutations that changed the orientation of the SRL severely impaired the ability of the ribosome to translocate the mRNA-tRNA complex. These results indicate that the interaction of EF-G with the SRL is critical for mRNA-tRNA translocation.
Results
Mutations in the SRL inhibit subunit association
Previous studies have shown that mutations G2655C, A2660U, and the deletion of bases U2653 and C2667 that form a non-canonical base pair in the SRL abolish protein synthesis and are lethal to cells 7; 24; 25; 26; 27 (Figure 1A). To dissect the role of the SRL in the elongation cycle of protein synthesis, we choose to study these mutations using highly purified mutant ribosomes and pre-steady state kinetic assays. The three mutations were constructed in plasmid pLK35•50S•MS2, which contains a MS2 binding hairpin inserted in helix 98 of 23S rRNA 28. The mutant ribosomes were purified using the MS2 affinity tag method 29. Primer extension analysis showed that the mutant 50S subunits were >95% pure.
To test whether the mutations in the SRL inhibit the association of the 50S subunit to the 30S subunit, we performed in vitro subunit association experiments. The mutant 50S subunits were mixed with a 1.5-fold molar excess of the wild type 30S subunits, and the formation of the 70S ribosome was analyzed by sucrose density-gradient sedimentation. Peaks representing the 30S subunit, 50S subunit, and the 70S ribosome were observed with the wild type 50S subunit (Figure 2A). Similarly, G2655C and A2660U mutant 50S subunits associated with the 30S subunits to form 70S ribosomes indicating that these bases are not critical for subunit association. In contrast, the U2653Δ-C2667Δ deletion mutant showed two peaks instead of a single peak corresponding to the 50S subunit indicating defects in assembly. Furthermore, the 70S ribosome formed was significantly reduced indicative of subunit association defect. Previous studies have shown that the binding mRNA and tRNA to the ribosome can sometimes rescue defects in subunit association 28; 30. Therefore, we repeated the subunit association experiments in the presence of mRNA and tRNA. In the presence of mRNA and tRNA, sucrose gradient analysis showed a larger 70S peak with the U2653Δ-C2667Δ mutant than without mRNA and tRNA (Figure 2B). Thus, the addition of mRNA and tRNA significantly improved the association of the U2653Δ-C2667Δ mutant 50S subunit with the 30S subunit. We, however, note that the in vitro subunit association assay cannot reveal subtle differences in subunit affinity or in the rate of subunit association for the mutant 50S subunits.
Figure 2. Mutations in the SRL affect 50S subunit assembly and function.
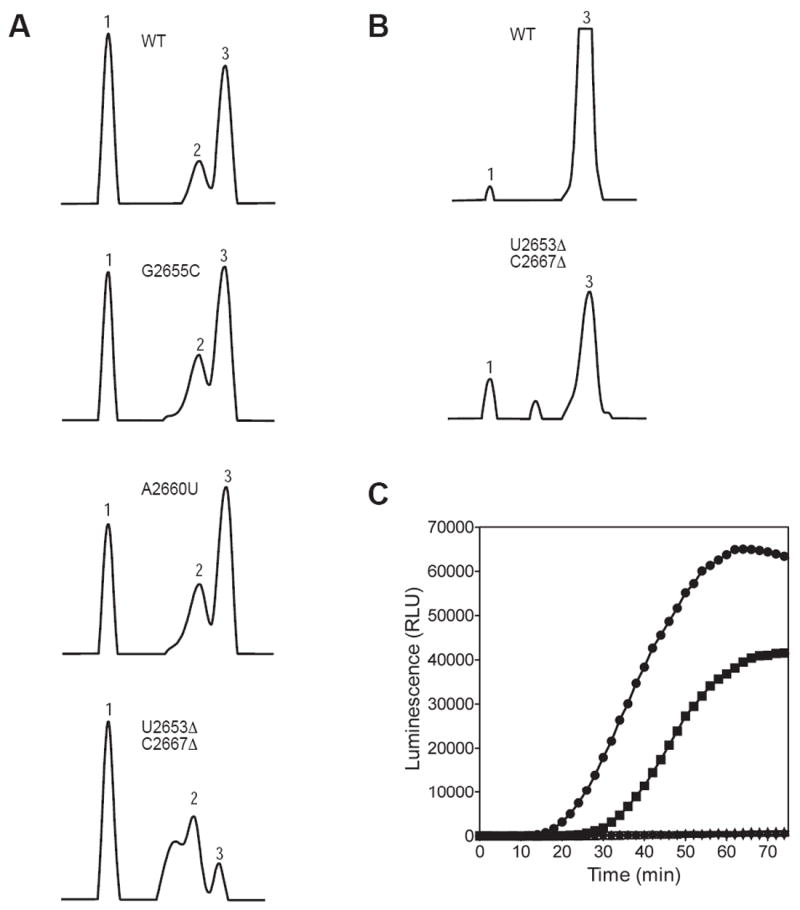
(A) Sucrose gradient profiles showing the 30S subunit (peak 1), 50S subunit (peak 2) and 70S ribosome (peak 3) for the wild type and the indicated mutants. (B) The addition of mRNA and tRNA improves the association of the U2653Δ-C2667Δ mutant 50S subunit with the 30S subunit. (C) In vitro translation. Synthesis of the reporter enzyme Renilla luciferase by the wild type (ℓ), G2655C (
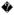 ), A2660U (←), and U2653Δ-C2667Δ (−) mutant ribosomes. RLU, relative luminescence units.
), A2660U (←), and U2653Δ-C2667Δ (−) mutant ribosomes. RLU, relative luminescence units.
We next analyzed the activity of the mutant ribosomes using an in vitro protein synthesis assay. In this assay, the ability of ribosomes to synthesize the Renilla luciferase reporter enzyme was monitored over time 31. Renilla luciferase catalyzes the oxidation of coelenterazine to produce light, which was monitored in real time with a plate reader. The G2655C mutant ribosomes showed a slight defect in luciferase synthesis compared to the wild type ribosomes (Figure 2C). In contrast, the A2660U and U2653Δ-C2667Δ mutant ribosomes were completely inactive in luciferase synthesis.
The SRL is not critical for tRNA selection
The SRL is proposed to play a key role during tRNA selection by the ribosome. Structural data suggest that the phosphate oxygen at A2662 in the SRL triggers GTP hydrolysis on EF-Tu by correctly positioning the catalytic histidine 84 of EF-Tu 21 (Figure 1B). Following GTP hydrolysis, EF-Tu•GDP dissociates from the ribosome. The cognate tRNA is then accommodated into the 50S subunit A site and participates in peptide bond formation. We used a nitrocellulose filter-binding assay to determine the role of the SRL in binding EF-Tu•GTP•tRNA ternary complex to the ribosome 32. The EF-Tu mutant His84Ala, which is defective in GTP hydrolysis, was used to form ternary complex with GTP and radioactively labeled Phe-tRNAPhe. Therefore, the reversible binding of the GTPase activated state of the ternary complex to the ribosome was measured in these experiments 33. Equilibrium binding experiments were performed with a fixed concentration of ternary complex and varying concentrations of ribosome having tRNAfMet in the P site and the cognate phenylalanine codon (UUU) in the A site (Figure 3A). The equilibrium dissociation constant (KD) for the wild type, the G2655C, the A2660U, and the U2653Δ-C2667Δ ribosomes were 1.8 ± 0.3 nM, 3.1 ± 0.6 nM, 3.0 ± 0.4 nM, and 29.4 ± 4.4 nM, respectively. These results show that the binding of the ternary complex to the ribosome is inhibited by less than 2-fold with the transversion mutations in the SRL and by 16-fold with the U2653Δ-C2667Δ deletion mutation.
Figure 3. Effect of the SRL mutations on the binding of EF-Tu ternary complex to the ribosome and GTP hydrolysis.
(A) Binding of EF-Tu ternary complex to the ribosomal A site determined by filter binding. Graph showing the equilibrium binding of EF-Tu•GTP•Phe-tRNAPhe ternary complex to the wild type (ℓ), G2655C (
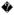 ), A2660U (←), and U2653Δ-C2667Δ (−) mutant ribosomes. (B) Plot of the observed rates of GTP hydrolysis on EF-Tu ternary complex at several ribosome concentrations. The curves represent the best fit to a single Michaelis Menten expression to calculate the K1/2 and the kGTP. Symbols: wild type (ℓ) and U2653Δ-C2667Δ (−) mutant ribosomes.
), A2660U (←), and U2653Δ-C2667Δ (−) mutant ribosomes. (B) Plot of the observed rates of GTP hydrolysis on EF-Tu ternary complex at several ribosome concentrations. The curves represent the best fit to a single Michaelis Menten expression to calculate the K1/2 and the kGTP. Symbols: wild type (ℓ) and U2653Δ-C2667Δ (−) mutant ribosomes.
To determine the role of the SRL in the tRNA selection process, we determined the rate of GTP hydrolysis on EF-Tu by the wild type and the U2653Δ-C2667Δ mutant ribosomes. We focused only on the U2653Δ-C2667Δ mutant ribosome because the interaction of the phosphate oxygen at A2662 with the catalytic histidine 84 in EF-Tu is expected to be disrupted in this mutant (Figure 1B). Ribosome with tRNAfMet in the P site and the codon UUU in the A site was rapidly mixed with EF-Tu•[32P]GTP•Phe-tRNAPhe ternary complex to obtain the time course of GTP hydrolysis. The rate of GTP hydrolysis was determined at several ribosome concentrations, and the data were analyzed by fitting to a Michaelis Menten expression to obtain the apparent affinity of the ternary complex for the ribosome (K1/2) and the maximum rate of GTP hydrolysis (kGTP) (Figure 3B). The K1/2 was 3 μM and 2.8 μM for the wild type and the U2653Δ-C2667Δ ribosomes, respectively. The similar K1/2 values for the wild type and mutant ribosomes are because of the rapid rate of GTP hydrolysis compared to the rate of dissociation of the ternary complex from the ribosome 33; 34. The kGTP was 56 s-1 and 16 s-1 for the wild type and the U2653Δ-C2667Δ ribosomes, respectively. The K1/2 and kGTP values determined here for the wild type ribosome are similar to the values reported previously 33; 34; 35; 36. Interestingly, our results show that the rate of GTP-hydrolysis is reduced by only 3.5-fold with the U2653Δ-C2667Δ ribosome compared to the wild type ribosome. Considering that 50% of the U2653Δ-C2667Δ ribosomes are inactive (see below), the rate of GTP hydrolysis on EF-Tu is reduced by less than 2-fold.
After GTP hydrolysis on EF-Tu, the acceptor end of the aminoacyl tRNA is accommodated into the peptidyl transferase center in the large ribosomal subunit. The rate of accommodation of the tRNA is slow and is the rate limiting step for peptide bond formation by the ribosome 33. We analyzed the extent and the rate of peptide bond formation by the wild type and mutant ribosomes. Ribosome with f[35S]-Met-tRNAMet bound to the P site was mixed with a limiting concentration of the cognate EF-Tu•GTP•Phe-tRNAPhe ternary complex and the extent of f[35S]-Met-Phe dipeptide formed in 10 seconds was determined 8; 37. Under these conditions, the extent of peptide bond formed reveals whether the tRNAs are preferentially accommodated or rejected by the ribosome after GTP hydrolysis on EF-Tu. The extent of dipeptide formed was similar with the wild type and the mutant ribosomes indicating that Phe-tRNAPhe is not rejected to a greater extent by the mutant ribosomes (Figure 4A and 4B).
Figure 4. Peptide bond formation and the error rate of translation.
(A) Peptide bonds formed by the wild type and mutant ribosomes. The % f[35S]Met-Phe dipeptide formed in 10 seconds after adding EF-Tu•GTP•Phe-tRNAPhe ternary complex to the ribosome is indicated below the lanes. (B) Bar graph showing the % of peptide bonds formed in 10 seconds. The standard deviation from 3 experiments is shown. (C) The time course of peptide bond formation. The lines are the best fit to a single exponential equation. The standard deviation from 3 experiments is shown. (D) The SRL mutants show increased fidelity of tRNA selection. A control reaction with the CUC codon in the A site was carried out to serve as a marker for f[35S]Met-Leu dipeptide. Wild type and mutant ribosomes showed only two spots corresponding to the cognate f[35S]Met-Phe dipeptide and the near-cognate f[35S]Met-Leu dipeptide as indicated. (E) Bar graph showing the fraction of incorrect f[35S]Met-Leu dipeptide formed by the wild type and mutant ribosomes. The standard deviation from 3 experiments is shown.
We next determined the rate of peptide bond formation (kpep) under conditions where the concentration of the cognate EF-Tu•GTP•Phe-tRNAPhe ternary complex is in large excess over the ribosome and nearly saturating 37. The kpep for the wild type ribosome was 10.7 + 1.2 s-1 and is similar to values reported previously 33; 34; 35; 36. The kpep for the G2655C (kpep = 9.9 + 2.0 s-1) and the A2660U (kpep = 8.2 + 2.6 s-1) ribosomes were similar to the wild type ribosome. The U2653Δ-C2667Δ ribosome showed a ≈2-fold reduced rate of peptide bond formation (kpep = 5.4 + 0.6 s-1) (Figure 4C). Since 50% of U2653Δ-C2667Δ ribosomes are inactive (see below), the extent of peptide bond formed is also reduced by 50%. We also analyzed the overall error rate of the mutant ribosomes by measuring the amount of near-cognate Leu-tRNALeu incorporated with the codon UUU in the A site 37. All three mutant ribosomes showed ≈2-fold reduced error rate compared to the wild type ribosome (Figure 4D and 4E). Our results indicate that although the mutations in the SRL inhibit the binding of EF-Tu ternary complex to the ribosome, they do not drastically affect the overall process of tRNA selection.
Mutations in the SRL inhibit the binding of EF-G
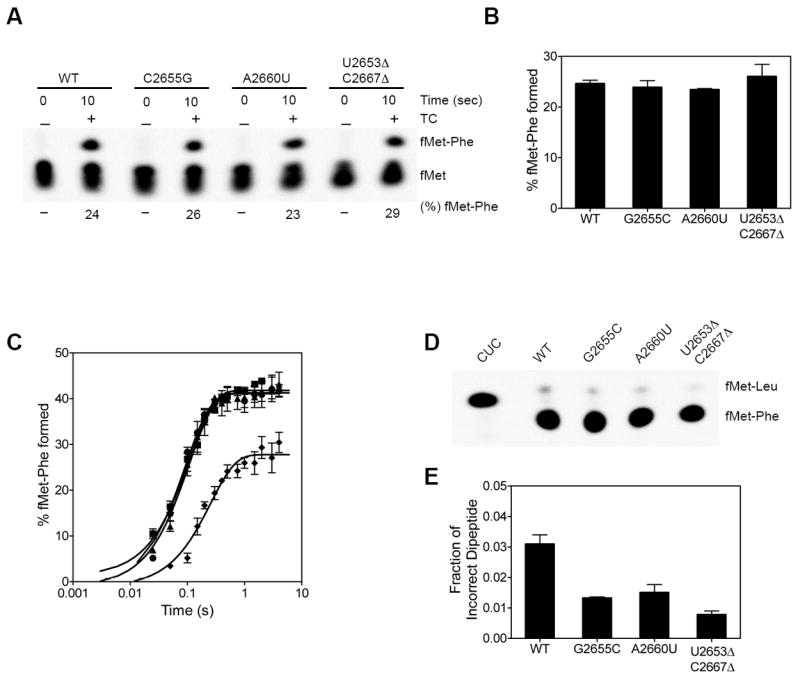
In the elongation cycle of protein synthesis, following tRNA selection and peptide bond formation, the mRNA-tRNA complex is translocated by EF-G. The SRL interacts with the GTP-binding domain of EF-G. Initially, we used a qualitative assay to analyze whether mutations in the SRL affects the binding of EF-G to the ribosome. Ribosomes were incubated with EF-G•GTP, EF-G•GDPNP, or EF-G•GDP•fusidic acid and the free EF-G were separated from the EF-G bound to the ribosome by microsedimentation in an ultracentrifuge 38. The pellet containing EF-G bound to the ribosome was analyzed by SDS-PAGE (Figure 5A). EF-G•GDPNP and EF-G•GDP•fusidic acid bound to a similar extent to the wild type and the G2655C ribosomes (Figure 5B). In contrast, EF-G•GDPNP bound to the A2660U and the U2653Δ-C2667Δ ribosomes was reduced by 4-fold. However, in the presence of fusidic acid, EF-G bound to the A2660U and the U2653Δ-C2667Δ ribosomes was reduced only by ≈2-fold compared to the wild type ribosome (Figure 5B). It should be noted that high concentrations of ribosome and EF-G were used in this assay resulting in significant amount of EF-G binding to the mutant ribosomes. Nevertheless, these results indicate that EF-G has a reduced binding affinity to the A2660U and the U2653Δ-C2667Δ ribosomes.
Figure 5. Effect of the SRL mutations on the binding of EF-G to the ribosome.
(A) Binding of EF-G to the ribosome in the presence of GTP, GDPNP or, GTP and fusidic acid (FA) determined by a microsedimentation assay. SDS-PAGE showing bands corresponding to EF-G, ribosomal protein S1 and the 75 KD protein from the molecular weight ladder are indicated. (B) Bar graph showing EF-G bound to the wild type and mutant ribosomes in the presence of GDPNP and FA. The amount of EF-G bound was normalized relative to ribosomal protein S2. (C to E) Equilibrium binding of EF-G to the wild type and the indicated mutant ribosomes determined using a fluorescence-based assay. The binding was performed in the presence of GTP and fusidic acid. The starting fluorescence intensity was normalized to 1 and the change in fluorescence intensity is shown in arbitrary units (a.u.).
To quantitatively measure the binding affinity of EF-G for the mutant ribosomes, we used a fluorescence-based assay. EF-G with fluorescein attached at position 591 shows a decrease in fluorescence intensity when bound to the ribosome in the presence of fusidic acid 39. Equilibrium binding experiments were performed with a fixed concentration of EF-G and varying concentrations of the ribosome (Figure 5C, 5D and 5E). The equilibrium dissociation constant (KD) of EF-G binding to the wild type, the G2655C, and the A2660U ribosomes were 2.8 ± 0.3 nM, 8.6 ± 1.3 nM, and 42 ± 7 nM, respectively. The binding of EF-G to the U2653Δ-C2667Δ ribosome was very weak and could not be determined using this assay because of the increased light scattering at high ribosome concentrations. These results show that mutations in the SRL appreciably reduce the affinity of EF-G for the ribosome.
The SRL is not essential for EF-G-dependent GTP hydrolysis
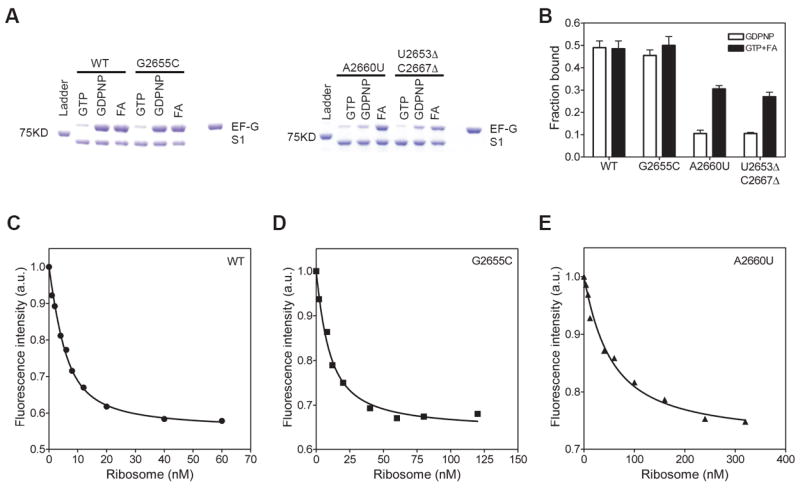
Structural data showed that the SRL interacts intimately with the GTP-binding domain of EF-G suggesting that it may be important for triggering GTP hydrolysis 20. We measured the rate of GTP hydrolysis by EF-G under multiple turnover conditions 8. In these experiments the concentration of the ribosome was fixed and limiting. The concentration of EF-G was in excess and varied. The initial velocity of GTP hydrolysis at increasing concentration of EF-G was plotted to calculate the KM and kcat (Figure 6A). The KM for GTP hydrolysis for the wild type, the G2655C, the A2660U and the U2653Δ-C2667Δ ribosomes were 1.8 μM, 1.9 μM, 1.5 μM, and 12 μM, respectively. Thus, the productive binding affinity of EF-G•GTP to the U2653Δ-C2667Δ ribosome is reduced by at least 5-fold, which agrees with the data described above showing that this mutant ribosome binds EF-G weakly compared to the other two mutants. The kcat of GTP hydrolysis were 9 s-1, 9 s-1, 7 s-1, and 4 s-1 for the wild type, the G2655C, the A2660U and the U2653Δ-C2667Δ ribosomes, respectively. Considering that 50% of the U2653Δ-C2667Δ ribosomes are inactive, the kcat of GTP hydrolysis is about the same as the wild type ribosomes. These results suggest that the SRL is not essential for GTP hydrolysis on EF-G.
Figure 6. Rate of GTP hydrolysis on EF-G and the kinetics of translocation.
(A) Graph showing the steady-state rate of GTP hydrolysis on EF-G with the wild type and mutant ribosomes. (B) Translocation monitored by the toeprinting assay. The toeprints for the pre-translocation (Pre) and post-translocation (Post) complexes are indicated. (-) and (+) indicate the absence and presence of EF-G, respectively. The extent of translocation in % is indicated below the lanes. (C) Translocation with increasing concentrations of tRNAPhe. The concentration of A site tRNAPhe was increased from 1 to 4 μM. The extent of translocation in % is indicated below the lanes. (D to G) Graphs showing the rate of translocation at increasing concentrations of EF-G. The kinetics of translocation are biphasic with a fast (filled symbol) and a slow (empty symbols) apparent rates. Ribosome with the U2653Δ-C2667Δ mutation showed only a single slow rate of translocation. The lines are the best fit to a single Michaelis Menten expression to calculate the K1/2 and the ktrans. Symbols: wild type (ℓ), G2655C (
 ), A2660U (←), and U2653Δ-C2667Δ (−) ribosomes.
), A2660U (←), and U2653Δ-C2667Δ (−) ribosomes.
The SRL is important for mRNA-tRNA translocation
Following GTP hydrolysis on EF-G, the mRNA-tRNA complex is translocated by the ribosome. We used a toeprinting assay to monitor EF-G-dependent translocation of mRNA-tRNA complex 40. The extent of translocation by the wild type, the G2655C, the A2660U and the U2653Δ-C2667Δ ribosomes were 83%, 78%, 66% and 44%, respectively (Figure 6B). Increasing the time of translocation did not increase the extent of translocation by the U2653Δ-C2667Δ ribosomes indicating that about 50% of the ribosomes are inactive (data not shown). The toeprinting assay showed that the U2653Δ-C2667Δ ribosomes could bind tRNAfMet in the P site to the same extent as the wild type ribosomes. This is caused by the wild type 30S subunits added to form 70S ribosomes, which will bind tRNAfMet in the P site to give a similar toeprint. The toeprinting assay cannot distinguish between the 30S complex and the 70S complex. To test the possibility that the U2653Δ-C2667Δ ribosomes are defective in binding tRNAPhe to the A site, we performed the translocation experiment with varying concentrations of tRNAPhe (Figure 6C). No significant improvement was observed even after adding 4 μM of tRNAPhe suggesting that about ≈50% of the U2653Δ-C2667Δ ribosomes are inactive and cannot be rescued by adding higher concentrations of tRNAPhe to saturate the A site. These results are consistent with the subunit association experiments, which showed a smaller 70S peak with the U2653Δ-C2667Δ 50S subunits compared to wild type 50S subunits even in the presence of mRNA and tRNA (Figure 2B). Furthermore, peptide bond formed was also reduced by ≈50% with the U2653Δ-C2667Δ ribosomes compared to the wild type ribosomes (Figure 4C). Thus, multiple lines of evidence indicate that only ≈50% of the U2653Δ-C2667Δ 50S subunits can form functional 70S ribosomes that can translocate the mRNA-tRNA complex.
To determine the effect of the SRL mutations on the rate of translocation we carried out a rapid kinetic assay 41. The kinetics of mRNA translocation showed a fast phase and a slow phase with apparent rate constants k1 and k2, respectively. The reason for the biphasic kinetics of mRNA translocation is not clear but has been reported previously 42; 43; 44. Translocation rates were measured at different concentrations of EF-G to determine the apparent affinity of EF-G (K1/2) for the mutant ribosomes and the maximum rate of translocation (ktrans) (Figure 6D to 6G). The K1/2 and ktrans were calculated by fitting k1 obtained at increasing EF-G concentrations to a Michaelis Menten expression. The K1/2 for the wild type, the G2655C, the A2660U and the U2653Δ-C2667Δ ribosomes were 0.7 μM, 0.6 μM, 1 μM and 3 μM, respectively. Thus, consistent with results described above, the U2653Δ-C2667Δ ribosomes have a lower binding affinity for EF-G. The ktrans for the wild type, the G2655C, the A2660U and the U2653Δ-C2667Δ ribosomes were 28 s-1, 11 s-1, 15 s-1, and 0.1 s-1, respectively. The 300-fold slower rate of translocation by the U2653Δ-C2667Δ ribosome compared to the wild type ribosome show that the SRL is essential for the movement of the mRNA-tRNA complex catalyzed by EF-G.
Discussion
The SRL is a universally conserved structural element that projects from the large ribosomal subunit 1; 45. The SRL is composed of a 12 base pair stem formed by several non-canonical base pairs and is capped by a GNRA tetraloop 45; 46; 47 (Figure 1). The SRL has long been recognized as critical for ribosome function because of its interaction with the elongation factors EF-Tu and EF-G. However, it is not clear whether the SRL serves only as a docking site for the elongation factors or whether it participates in additional functions such as inducing GTP hydrolysis on EF-Tu and EF-G and in triggering conformational changes that are important for tRNA selection and translocation. To dissect the precise function of the SRL we made mutations G2655C, A2660U, and deleted the non-canonical base pair U2653Δ-C2667Δ in the SRL. These mutations in the SRL are known to be lethal and inhibit protein synthesis 7; 24; 25; 26; 27. Most previous studies have examined the activity of the mutant ribosomes in the presence of the wild type ribosomes. However, the recent advent of affinity tag methods to purify mutant ribosomes to homogeneity makes it possible to characterize these mutant ribosomes in the absence of the wild type ribosomes using kinetic methods 29.
Base G2655 is bulged out from the SRL stem and forms a base-triple with U2656 and A2665 45; 46; 47. G2655 is protected from chemical probes by EF-Tu and EF-G 10. Previous studies, carried out in cells expressing both the wild type and the mutant ribosomes, showed that deletion of G2655 or transversions to U or C was lethal to cells and the mutant ribosomes were inactive in protein synthesis 24. In contrast, cells expressing the G2655A mutation in the 23S rRNA had a growth rate similar to wild type cells and the mutant ribosomes were active in protein synthesis 24. Earlier, Dontsova and co-workers used an affinity tag approach to purify the G2655C mutant ribosomes 26. Using qualitative experiments, they showed that the G2655C mutant ribosomes are not defective in GTP hydrolysis by EF-G, slightly defective in binding EF-G but more defective in translocation. Our results are consistent with this study. We show that the G2655C ribosomes are not defective in GTP hydrolysis on EF-G but have a 3-fold lowered affinity for binding EF-G and a 3-fold reduced rate of translocation compared to wild type ribosomes. In addition, we show that the G2655C ribosomes have a 2-fold defect in binding EF-Tu ternary complex; however, the rate of peptide bond formation is not affected. The G2655C ribosomes also showed a 2-fold lower error rate possibly resulting from the reduced affinity for EF-Tu ternary complex. We propose that the G2655C ribosomes are not very active in protein synthesis mainly because of defects in EF-G binding and in translocation.
Bases A2660 and G2661 are the only two nucleotides in the GNRA tetraloop of the SRL that are unpaired 45; 46; 47. Ricin targets the SRL of both bacterial and eukaryotic ribosomes and is one of most deadly cytotoxins on earth. Ricin is an N-glycosidase that catalyzes the depurination of A2660 and A4324 in bacterial and eukaryotic ribosomes, respectively 4. Depurination of A2660 inhibits the binding of elongation factors. This is consistent with footprinting studies, which showed that EF-Tu and EF-G protect A2660 from chemical modification 10. Mutation A2660U causes a growth defect and inhibits protein synthesis 7. We show that the A2660U 50S subunits have no major assembly defects and associate with the 30S subunits to form 70S ribosomes but are inactive in synthesizing Renilla luciferase in vitro. The binding affinity of EF-Tu ternary complex to the A2660U ribosomes is reduced by less than 2-fold compared to the wild type ribosomes. In addition, the rates of peptide bond formation are similar for the A2660U and the wild type ribosomes. Therefore, it is unlikely that the A2660U ribosome is inactive in protein synthesis because of defects in tRNA selection.
A previous study using an oligoribonucleotide that mimics the SRL showed that the A2660U mutation does not affect the binding of EF-G 11. In contrast, we show that the A2660U ribosome has a 10-fold defect in binding EF-G. In the earlier study, the binding of EF-G to even the wild type SRL oligoribonucleotide mimic was weak (KD ≈ 7 μM) compared to the high affinity interaction of EF-G with the ribosome reported here (KD ≈ 3 nM). Therefore, it is possible that our binding assay is more sensitive to subtle changes in the SRL structure. Interestingly, at saturating concentrations of EF-G, the A2660U ribosome showed no defect in GTP hydrolysis on EF-G. A recent study showed that the N6 amino group of adenine at position 2660 in the SRL is critical for promoting GTP hydrolysis on EF-G 23. Based on this result, it was proposed that either a steric clash between the N6 amino group of A2660 and EF-G residues or a favorable stacking interaction between A2660 and the G-domain of EF-G might induce GTP hydrolysis on EF-G 23. Our studies show that the A2660U mutant ribosome is not defective in GTP hydrolysis on EF-G suggesting that the steric clash between the larger purine base (A2660) and EF-G residues is not responsible for inducing GTP hydrolysis on EF-G. Finally, the A2660U ribosome has a 2-fold defect in translocation. Taken together, our data indicate that protein synthesis is abolished by the A2660U mutation mainly because of the 10-fold reduced binding affinity for EF-G.
A model for inducing GTP hydrolysis on EF-Tu was proposed based on a recent crystal structure of the EF-Tu ternary complex bound to the ribosome 21. According to this model the phosphate oxygen at A2662 in the SRL plays a critical role in inducing GTP hydrolysis by properly positioning the catalytic histidine 84 in EF-Tu (Figure 1B). Histidine 84 in EF-Tu coordinates a water molecule responsible for the nucleophilic attack on the γ-phosphate of GTP. Furthermore, it was proposed that the interaction of the phosphate oxygen at A2662 in the SRL with the catalytic histidine in translational GTPase factors might be a universal mechanism used by the ribosome to activate GTP hydrolysis 21. Although the precise mechanism used by the ribosome to activate GTP hydrolysis on translational GTPase factors is not clear, and has been debated recently 48; 49, the crystal structure nevertheless suggests an important role for the phosphate oxygen at A2662 in organizing the GTPase center. The U2653Δ-C2667Δ mutant ribosome is interesting in this context because the deletion of the non-canonical U2653Δ-C2667Δ base pair will shorten and rotate the SRL stem, moving the position of the phosphate oxygen at A2662 by more than 8 Å from the catalytic histidine residue in GTPase factors (Figure 1B). Deletion of the U2653Δ-C2667Δ base pair will also decrease the flexibility of the SRL stem 25. These changes to the SRL are, therefore, expected to inhibit GTP hydrolysis on EF-Tu and EF-G.
Consistent with a previous report 25, we show that the deletion of bases U2653 and C2667 in the 23S rRNA are lethal to cells and the mutant ribosomes are inactive in protein synthesis. In addition, the U2653Δ-C2667Δ mutation causes defects in 50S subunit assembly and in subunit association. The defects in 50S subunit assembly and in subunit association may be resulting from the disruption of the tertiary interactions that the SRL makes with helix 91 in the 23S rRNA 45. Indeed, a previous study showed that replacing the entire SRL sequence with a GAAA tetraloop causes major assembly defects 39. The U2653Δ-C2667Δ mutation is subtler and the defect in 50S assembly and subunit association can be partly rescued by adding mRNA and tRNA. We show that the binding affinity of the EF-Tu ternary complex for the U2653Δ-C2667Δ ribosome is reduced by 16-fold. Taking into account that about 50% of the U2653Δ-C2667Δ ribosomes is inactive, the defect in binding the EF-Tu ternary complex maybe closer to 8-fold. Interestingly, the rates of GTP hydrolysis on EF-Tu and peptide bond formation by the active population of U2653Δ-C2667Δ ribosomes is less than 2-fold slower than the wild type ribosomes. These results suggest that the interaction of the phosphate oxygen at A2662 with the catalytic histidine in EF-Tu is not critical for the overall process of tRNA selection. Alternatively, in the U2653Δ-C2667Δ ribosome, it is possible that some other functional group in the SRL may fulfill the critical role played by A2662 in positioning the catalytic histidine 84 in EF-Tu to induce GTP hydrolysis.
Our studies show that the rate of GTP hydrolysis on EF-G is reduced by 2-fold with the U2653Δ-C2667Δ ribosome, which again argues that the interaction of the phosphate oxygen at A2662 with the catalytic histidine in EF-G is not crucial for GTP hydrolysis. More important, our studies reveal that the U2653Δ-C2667Δ ribosomes are primarily defective in EF-G-dependent translocation. The U2653Δ-C2667Δ ribosomes showed a 300-fold reduced rate of translocation compared to the wild type ribosomes. In contrast, the K1/2 for translocation is increased by only 5-fold for the U2653Δ-C2667Δ ribosomes compared to the wild type ribosomes. These results reveal that EF-G-dependent translocation of the mRNA-tRNA complex is the major defect in the U2653Δ-C2667Δ ribosome. Structural studies have shown that both the ribosome and EF-G undergo large-scale conformational changes during translocation 17; 50; 51. The 30S subunit undergoes a ratchet-like rotation relative to the 50S subunit and the head domain of the 30S subunit pivots relative to the body of the 30S subunit 51; 52. Interestingly, the GTP-binding domain of EF-G maintains its interaction with the SRL in the pre- and post-translocation states, while domain IV of EF-G moves from the shoulder region to the A site in the 30S subunit 17. The SRL remains immobile during these structural changes in the ribosome and may serve as a crucial anchor for EF-G. Since the U2653Δ-C2667Δ mutation considerably weakens the interaction of EF-G with the SRL, after GTP hydrolysis, EF-G may prematurely dissociate from the mutant ribosome without promoting mRNA-tRNA movement explaining the drastic inhibition in translocation. Based on our analysis of the three mutants, we propose that the main functional role of the highly conserved SRL is to stabilize the binding of EF-G on the ribosome during the extensive conformational changes that accompany mRNA-tRNA movement.
Materials and Methods
Site-directed mutagenesis of 16S rRNA
Site-directed mutagenesis was performed with a QuickChange PCR mutagenesis kit (Stratagene). Plasmid pLK35•50S•MS2 28 was used as the template for introducing mutations G2655C, A2660U and C2653Δ/U2667Δ into 23S rRNA. All clones were verified by automated DNA sequencing of the entire 23S rRNA operon.
Plasmid replacement strategy
Plasmid replacement was performed as described previously 53. Briefly, E. coli strain SQZ10 (Δ7rrn) containing plasmid pHK-rrnC+sacB (kanamycin resistant) was transformed with pLK35-23S-MS2 containing the desired mutations. The transformants were grown overnight in LB medium (10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl per liter of medium) with 100 μg/mL ampicillin at 37 °C with shaking. The cultures were diluted and plated on 2YT agar plates with 8% sucrose and 100 μg/mL ampicillin. The colonies on the plates were screened for sensitivity to kanamycin by replica plating. Plasmid replacement was confirmed by isolating plasmids and automated DNA sequencing.
Purification of MS2-tagged 50S subunits
Mutations in the SRL are lethal, and the mutant 50S subunits were purified from pop 2136 cells using the MS2 affinity tag. Expression and purification were performed as described previously 28; 29. The purity of the MS2-tagged 50S subunits were assayed by primer extension after total RNA extraction. Reverse transcription using the primer 5′-TCA ACGTTCCTTCAGGACCCT -3′ and NTP(-dCTP) with ddCTP gave different products for tagged and untagged 23S rRNA.
Subunit association
We conducted subunit association by incubating 30S subunits and 50S subunits separately at 42 °C for 10 min in buffer A [50 mM Tris-HCl (pH 7.6), 100 mM NH4Cl, and 6 mM β-mercaptoethanol, 20 mM MgCl2], slowly cooling the subunit to 37 °C, holding it for an additional 10 min at 37 °C. Subunit association was initiated by combining 20 pmol 50S subunits and 30 pmol 30S subunits and incubating at 37°C for 10 min. In some experiments, 1.5-fold excess mRNA and tRNAfMet were added to the reaction to stabilize the 70S ribosome. The samples were separated in 10-35% sucrose gradients prepared in the same buffer and analyzed as described previously 53. For functional assays, subunit association was performed as described above in the appropriate buffer containing 20 mM MgCl2. Then the mixture was diluted with buffer having no MgCl2 to lower it to the desired concentration.
In vitro translation of reporter protein
The activity of the purified ribosomes was analyzed by in vitro translation of the reporter protein Renilla luciferase as described previously 31. Briefly, activated ribosomes were added to the S-100 in vitro translation mix and transferred to a 96-well plate. The 96-well plate was incubated at 37 °C in a plate reader (Genios, Tecan), and the synthesis of the luciferase enzyme was monitored in real-time by measuring the luminescence every 2 min. Duplicates of the samples were used for each experiment, and the assays were repeated at least two times.
KD measurement of ternary complex binding to ribosome
E. coli tRNAPhe was labeled with [α-32P]ATP as described previously 54. The KD of ternary complex binding to ribosomes was determined using a 96 well dot blot apparatus (Shleicher and Schuell), with an upper nitrocellulose membrane (BA-85, Whatman) and a lower nylon membrane (Hybond-N+, Amersham) 55. Ternary complex was prepared with a 10-fold excess of EF-Tu (H84A) over Phe-[32P]-tRNAPhe in buffer B [50 mM Tris-HCl (pH 7.5), 15 mM MgCl2, 70 mM NH4Cl, 30 mM KCl, 8 mM putrescine, 2 mM DTT, and 0.5 mM spermidine]. Ternary complex (0.2 nM) was incubated with P site blocked ribosomes (0-200 nM) for one minute at room temperature in the same buffer. Reactions were performed simultaneously in rows of 12 in a 96 well microtiter plate (NUNC conical bottom). 25 μl were taken using a multichannel pipette and immediately passed through the double filter system and washed with 100 μl buffer B three times. After a series of filtrations were completed, the membranes were removed from the filter apparatus, dried, and exposed to a phosphorimager screen (Bio-Rad). Data were fitted to a hyperbolic equation.
GTP Hydrolysis by EF-Tu ternary complex
GTP hydrolysis experiments were performed in buffer C [50 mM Tris-HCl (pH 7.5), 3.5 mM MgCl2, 70 mM NH4Cl, 30 mM KCl, 8 mM putrescine, 2 mM DTT, and 0.5 mM spermine], essentially as described previously 37. Ternary complexes were incubated at 37 °C for 5 min using nucleotide free EF-Tu. Concentration of the ternary complex was 100 nM and the concentration of the ribosome was 0.4 μM, 1.25 μM or 1.6 μM. We could not test higher concentrations of ribosome because of the low yield of affinity-purified mutant ribosomes. Time courses were performed at 20 °C with a quench-flow instrument (μQFM-400, BioLogic). Free phosphate was analyzed by PEI-cellulose TLC developed in 0.5 M potassium phosphate (pH 3.5). The extent of GTP hydrolysis was quantified with a phosphorimager (Bio-Rad). The time course of GTP hydrolysis was fitted to a single exponential equation to determine the apparent rate of GTP hydrolysis. To calculate the K1/2 and the rate of GTP hydrolysis at saturation (kGTP), the apparent rates of GTP hydrolysis at increasing ribosome concentrations were fitted to a Michaelis Menten curve as described previously 33; 34; 37.
Peptidyl transferase assay
Peptidyl transferase experiments were performed at 20 °C in buffer C with a quench-flow instrument (μQFM-400, BioLogic) as described previously 37. Concentration of ribosome and ternary complexe were 1.25 μM and 0.25 μM, respectively. Dipeptide was resolved by electrophoresis on cellulose TLC plates and quantified using a phosphorimager (Bio-Rad). The time course of peptide bond formation was fit to a single exponential equation to determine the apparent rate of peptide bond formation. To determine the extent of dipeptide formation for mutant ribosomes, the reaction was incubated for 10 seconds and quenched manually with 1M KOH.
Fidelity of tRNA selection
Fidelity experiments were done in buffer C as described earlier 37. Dipeptides f[35S]Met-Phe and f[35S]Met-Leu were resolved by electrophoresis on cellulose TLC plates and quantified using a phosphorimager (Bio-Rad). The extent of misincorporation was determined from the ratio of f[35S]Met-Leu to f[35S]Met-Phe plus f[35S]Met-Leu.
EF-G binding by microsedimentation
Binding of EF-G to the ribosome was analyzed by a microsedimentation assay 38. Activated ribosomes (0.12 μM) pre-associated with 1.5-fold mRNA+9 and tRNAfMet were mixed with the same amount of EF-G in buffer D [20 mM Hepes-KOH (pH 7.6), 6 mM MgCl2, 150 mM NH4Cl, 4 mM β-mercaptoethanol, 0.05 mM spermine, and 2 mM spermidine]. 0.5mM GTP or GDPNP and 1 mM fusidic acid were added and the final volume was adjusted to 100 μl. Mixtures were incubated at 37 °C for 10 min and loaded on a micro-sediment tube (0.3 ml, Sarstedt 702) inserted into the cap of a Ti-80 centrifuge bottle (Beckman). The top of the micro-sediment tube was covered with parafilm and the tubes were centrifuged at 36,000 rpm in a Ti-80 rotor for 1.5 hour. The supernatant was carefully removed, and the pellet was resuspended by vortexing overnight in 10 μl protein loading buffer. Samples were analyzed by 10% SDS-PAGE and the bands were quantified by Quantity One software (Bio-Rad).
EF-G-591-IAF binding to ribosome
His-tagged EF-G containing a single cysteine at position 591 was labeled with 5-iodoacetamidofluorescein (IAF) as described previously 56. EF-G binding was carried out as described by Noller and co-workers 39 with following modification. Ribosome complex containing mRNA and tRNAfMet were titrated in reactions that contained 1 nM EF-G-591-IAF, 0.5 mM GTP and 1 mM fusidic acid, 0.015% Nikkol, 1.6 mM DTT, 10 mM MgCl2, 100 mM NH4Cl, 50 mM Tris-HCl (pH 7.0) in a total volume of 160 μl and incubated for 10 min at 37 °C. Fluorescence intensity of EF-G-591-IAF were acquired using a Fluoromax-P spectrofluorometer at 25°C with excitation at 492 nm and emission at 518 nm in a 160 μl cuvette (Starna Cells).
GTP hydrolysis by EF-G
GTP hydrolysis was measured by combining activated ribosomes (0.25 μM final concentration, pre-associated with 1.5-fold excess mRNA+9 and tRNAfMet) with EF-G (0.5 to 14 μM final concentration) and GTP (1 mM final concentration with trace amount of [γ-32P]-GTP) in buffer D at room temperature. 3 μl aliquots were withdrawn at different time point and quenched with 5% SDS. 1 μl samples were spotted on cellulose TLC plates and developed in 0.5 KH2PO4 (pH 3.5). The amount of 32Pi formed was quantified using a phosphorimager. The initial velocities were plotted versus each EF-G concentration and fitted to a Michaelis-Menten equation. Experiments were repeated at least two times.
Toeprinting Assay
Pretranslocation complexes was formed and translocation was monitored by the toeprinting assay as described previously 40.
Translocation Kinetics
Rapid kinetic experiments were performed essentially as described previously 41; 53. The experiments were conducted at 25 °C in buffer D. Briefly, 80 μL of pretranslocation complex (0.25 μM, after mixing) containing and fMet-Phe-tRNAPhe in ribosomal P and A sites, respectively, and pyrene-labeled mRNA+9 was rapidly mixed with 80 μL of EF-G·GTP (1.25 μM, after mixing) using a stopped-flow instrument (μSFM-20, BioLogic). The samples were excited at 343 nm and the change in fluorescence emission intensity at 376 nm was measured after the emission had passed through a long-pass filter (361 AELP, Omega Optical). Approximately five traces were averaged for each experiment and the experiments were repeated four times. The decreases in fluorescence intensity were analyzed by nonlinear least-squares fitting to the double-exponential equation Y = ax + b + A1 exp(-k1x) + A2 exp(-k2x) using Bio-Kine (BioLogic)
Modeling the Structure of the SRL with Bases U2653 and C2667 Deleted
The SRL deletion was modeled using the X-ray crystallography structure of Ramakrishnan and co-workers (PDB accession code 2XQE) 21. The base pair was first deleted from the structure. Next, the helix was shifted by simultaneously aligning the phosphates, C3’ and C4’ atoms of nucleotides 2651 and 2669 with the same atoms of the original nucleotides 2552 and 2668.
Highlights.
The sarcin-ricin loop in 23S rRNA interacts with elongation factors Tu and G.
We studied the role of the sarcin-ricin loop in tRNA selection and translocation.
We find that the sarcin-ricin is important for anchoring EF-G during translocation.
Acknowledgments
We thank Norbert Polacek and Ulrich Muller for comments on the manuscript. This work was supported by NIH Grant GM065265 to S.J.
Abbreviations
- SRL
sarcin-ricin loop
- rRNA
ribosomal RNA
- EF-Tu
elongation factor Tu
- EF-G
elongation factor G
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cannone JJ, Subramanian S, Schnare MN, Collett JR, D’Souza LM, Du Y, Feng B, Lin N, Madabusi LV, Müller KM, Pande N, Shang Z, Yu N, Gutell RR. The Comparative RNA Web (CRW) Site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics. 2002;3:2. doi: 10.1186/1471-2105-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endo Y, Huber PW, Wool IG. The ribonuclease activity of the cytotoxin alpha-sarcin. The characteristics of the enzymatic activity of alpha-sarcin with ribosomes and ribonucleic acids as substrates. J Biol Chem. 1983;258:2662–7. [PubMed] [Google Scholar]
- 3.Fernandez-Puentes C, Vazquez D. Effects of some proteins that inactivate the eukaryotic ribosome. FEBS Lett. 1977;78:143–6. doi: 10.1016/0014-5793(77)80292-5. [DOI] [PubMed] [Google Scholar]
- 4.Endo Y, Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987;262:8128–30. [PubMed] [Google Scholar]
- 5.Endo Y, Wool IG. The site of action of alpha-sarcin on eukaryotic ribosomes. The sequence at the alpha-sarcin cleavage site in 28 S ribosomal ribonucleic acid. J Biol Chem. 1982;257:9054–60. [PubMed] [Google Scholar]
- 6.Hausner TP, Atmadja J, Nierhaus KH. Evidence that the G2661 region of 23S rRNA is located at the ribosomal binding sites of both elongation factors. Biochimie. 1987;69:911–23. doi: 10.1016/0300-9084(87)90225-2. [DOI] [PubMed] [Google Scholar]
- 7.Chan YL, Wool IG. The integrity of the sarcin/ricin domain of 23 S ribosomal RNA is not required for elongation factor-independent peptide synthesis. J Mol Biol. 2008;378:12–9. doi: 10.1016/j.jmb.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Ortega L, Alvarez-Garcia E, Gavilanes JG, Martinez-del-Pozo A, Joseph S. Cleavage of the sarcin-ricin loop of 23S rRNA differentially affects EF-G and EF-Tu binding. Nucleic Acids Res. 2010;38:4108–19. doi: 10.1093/nar/gkq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanchard SC, Gonzalez RL, Kim HD, Chu S, Puglisi JD. tRNA selection and kinetic proofreading in translation. Nat Struct Mol Biol. 2004;11:1008–14. doi: 10.1038/nsmb831. [DOI] [PubMed] [Google Scholar]
- 10.Moazed D, Robertson JM, Noller HF. Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature. 1988;334:362–364. doi: 10.1038/334362a0. [DOI] [PubMed] [Google Scholar]
- 11.Munishkin A, Wool IG. The ribosome-in-pieces: binding of elongation factor EF-G to oligoribonucleotides that mimic the sarcin/ricin and thiostrepton domains of 23S ribosomal RNA. Proc Natl Acad Sci U S A. 1997;94:12280–4. doi: 10.1073/pnas.94.23.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuette JC, Murphy FVt, Kelley AC, Weir JR, Giesebrecht J, Connell SR, Loerke J, Mielke T, Zhang W, Penczek PA, Ramakrishnan V, Spahn CM. GTPase activation of elongation factor EF-Tu by the ribosome during decoding. Embo J. 2009;28:755–65. doi: 10.1038/emboj.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villa E, Sengupta J, Trabuco LG, LeBarron J, Baxter WT, Shaikh TR, Grassucci RA, Nissen P, Ehrenberg M, Schulten K, Frank J. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc Natl Acad Sci U S A. 2009;106:1063–8. doi: 10.1073/pnas.0811370106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connell SR, Takemoto C, Wilson DN, Wang H, Murayama K, Terada T, Shirouzu M, Rost M, Schuler M, Giesebrecht J, Dabrowski M, Mielke T, Fucini P, Yokoyama S, Spahn CM. Structural basis for interaction of the ribosome with the switch regions of GTP-bound elongation factors. Mol Cell. 2007;25:751–64. doi: 10.1016/j.molcel.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J. Locking and unlocking of ribosomal motions. Cell. 2003;114:123–34. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DJ, Nilsson J, Merrill AR, Andersen GR, Nissen P, Frank J. Structures of modified eEF2 80S ribosome complexes reveal the role of GTP hydrolysis in translocation. EMBO J. 2007;26:2421–31. doi: 10.1038/sj.emboj.7601677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark H, Rodnina MV, Wieden HJ, van Heel M, Wintermeyer W. Large-scale movement of elongation factor G and extensive conformational change of the ribosome during translocation. Cell. 2000;100:301–309. doi: 10.1016/s0092-8674(00)80666-2. [DOI] [PubMed] [Google Scholar]
- 18.Stark H, Rodnina MV, Wieden HJ, Zemlin F, Wintermeyer W, van Heel M. Ribosome interactions of aminoacyl-tRNA and elongation factor Tu in the codon-recognition complex. Nat Struct Biol. 2002;9:849–54. doi: 10.1038/nsb859. [DOI] [PubMed] [Google Scholar]
- 19.Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FVt, Weir JR, Ramakrishnan V. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science. 2009;326:688–94. doi: 10.1126/science.1179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao YG, Selmer M, Dunham CM, Weixlbaumer A, Kelley AC, Ramakrishnan V. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science. 2009;326:694–9. doi: 10.1126/science.1179709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voorhees RM, Schmeing TM, Kelley AC, Ramakrishnan V. The mechanism for activation of GTP hydrolysis on the ribosome. Science. 2010;330:835–8. doi: 10.1126/science.1194460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, Chan YL, Wool IG. The identification of the determinants of the cyclic, sequential binding of elongation factors Tu and G to the ribosome. J Mol Biol. 2009;386:802–13. doi: 10.1016/j.jmb.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 23.Clementi N, Chirkova A, Puffer B, Micura R, Polacek N. Atomic mutagenesis reveals A2660 of 23S ribosomal RNA as key to EF-G GTPase activation. Nat Chem Biol. 2010;6:344–51. doi: 10.1038/nchembio.341. [DOI] [PubMed] [Google Scholar]
- 24.Macbeth MR, Wool IG. The phenotype of mutations of G2655 in the sarcin/ricin domain of 23 S ribosomal RNA. J Mol Biol. 1999;285:965–75. doi: 10.1006/jmbi.1998.2388. [DOI] [PubMed] [Google Scholar]
- 25.Macbeth MR, Wool IG. Characterization of in vitro and in vivo mutations in non-conserved nucleotides in the ribosomal RNA recognition domain for the ribotoxins ricin and sarcin and the translation elongation factors. J Mol Biol. 1999;285:567–80. doi: 10.1006/jmbi.1998.2337. [DOI] [PubMed] [Google Scholar]
- 26.Leonov AA, Sergiev PV, Bogdanov AA, Brimacombe R, Dontsova OA. Affinity purification of ribosomes with a lethal G2655C mutation in 23 S rRNA that affects the translocation. J Biol Chem. 2003;278:25664–70. doi: 10.1074/jbc.M302873200. [DOI] [PubMed] [Google Scholar]
- 27.Marchant A, Hartley MR. Mutational studies on the alpha-sarcin loop of Escherichia coli 23S ribosomal RNA. Eur J Biochem. 1994;226:141–7. doi: 10.1111/j.1432-1033.1994.tb20035.x. [DOI] [PubMed] [Google Scholar]
- 28.Ali IK, Lancaster L, Feinberg J, Joseph S, Noller HF. Deletion of a conserved, central ribosomal intersubunit RNA bridge. Mol Cell. 2006;23:865–74. doi: 10.1016/j.molcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Youngman EM, Green R. Affinity purification of in vivo-assembled ribosomes for in vitro biochemical analysis. Methods. 2005;36:305–12. doi: 10.1016/j.ymeth.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Belitsina NV, Spirin AS. Studies on the structure of ribosomes. IV. Participation of aminoacyl-transfer RNA and peptidyl-transfer RNA in the association of ribosomal subparticles. J Mol Biol. 1970;52:45–55. doi: 10.1016/0022-2836(70)90176-2. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Ortega L, Stephen J, Joseph S. Precise alignment of peptidyl tRNA by the decoding center is essential for EF-G-dependent translocation. Mol Cell. 2008;32:292–9. doi: 10.1016/j.molcel.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daviter T, Wieden HJ, Rodnina MV. Essential role of histidine 84 in elongation factor Tu for the chemical step of GTP hydrolysis on the ribosome. J Mol Biol. 2003;332:689–99. doi: 10.1016/s0022-2836(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 33.Pape T, Wintermeyer W, Rodnina MV. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J. 1998;17:7490–7. doi: 10.1093/emboj/17.24.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ledoux S, Uhlenbeck OC. Different aa-tRNAs are selected uniformly on the ribosome. Mol Cell. 2008;31:114–23. doi: 10.1016/j.molcel.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cochella L, Green R. An active role for tRNA in decoding beyond codon:anticodon pairing. Science. 2005;308:1178–80. doi: 10.1126/science.1111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kothe U, Rodnina MV. Codon reading by tRNAAla with modified uridine in the wobble position. Mol Cell. 2007;25:167–74. doi: 10.1016/j.molcel.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Hetrick B, Khade PK, Lee K, Stephen J, Thomas A, Joseph S. Polyamines accelerate codon recognition by transfer RNAs on the ribosome. Biochemistry. 2010;49:7179–89. doi: 10.1021/bi1009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dix DB, Wittenberg WL, Uhlenbeck OC, Thompson RC. Effect of replacing uridine 33 in yeast tRNAPhe on the reaction with ribosomes. J Biol Chem. 1986;261:10112–8. [PubMed] [Google Scholar]
- 39.Lancaster L, Lambert NJ, Maklan EJ, Horan LH, Noller HF. The sarcin-ricin loop of 23S rRNA is essential for assembly of the functional core of the 50S ribosomal subunit. RNA. 2008;14:1999–2012. doi: 10.1261/rna.1202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph S, Noller HF. EF-G-catalyzed translocation of anticodon stem-loop analogs of transfer RNA in the ribosome. EMBO J. 1998;17:3478–3483. doi: 10.1093/emboj/17.12.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Studer SM, Feinberg JS, Joseph S. Rapid Kinetic Analysis of EF-G-dependent mRNA Translocation in the Ribosome. J Mol Biol. 2003;327:369–381. doi: 10.1016/s0022-2836(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 42.Walker SE, Shoji S, Pan D, Cooperman BS, Fredrick K. Role of hybrid tRNA-binding states in ribosomal translocation. Proc Natl Acad Sci U S A. 2008;105:9192–7. doi: 10.1073/pnas.0710146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ermolenko DN, Noller HF. mRNA translocation occurs during the second step of ribosomal intersubunit rotation. Nat Struct Mol Biol. 2011;18:457–62. doi: 10.1038/nsmb.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khade PK, Joseph S. Messenger RNA interactions in the decoding center control the rate of translocation. Nat Struct Mol Biol. 2011;18:1300–2. doi: 10.1038/nsmb.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science. 2000;289:905–20. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 46.Correll CC, Munishkin A, Chan YL, Ren Z, Wool IG, Steitz TA. Crystal structure of the ribosomal RNA domain essential for binding elongation factors. Proc Natl Acad Sci U S A. 1998;95:13436–41. doi: 10.1073/pnas.95.23.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szewczak AA, Moore PB, Chang YL, Wool IG. The conformation of the sarcin/ricin loop from 28S ribosomal RNA. Proc Natl Acad Sci U S A. 1993;90:9581–5. doi: 10.1073/pnas.90.20.9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voorhees RM, Schmeing TM, Kelley AC, Ramakrishnan V. Response to Comment on “The Mechanism for Activation of GTP Hydrolysis on the Ribosome”. Science. 2011;333:37. doi: 10.1126/science.1194460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liljas A, Ehrenberg M, Aqvist J. Comment on “The Mechanism for Activation of GTP Hydrolysis on the Ribosome”. Science. 2011;333:37. doi: 10.1126/science.1202532. [DOI] [PubMed] [Google Scholar]
- 50.Agrawal RK, Heagle AB, Penczek P, Grassucci RA, Frank J. EF-G-dependent GTP hydrolysis induces translocation accompanied by large conformational changes in the 70S ribosome. Nat Struct Biol. 1999;6:643–647. doi: 10.1038/10695. [DOI] [PubMed] [Google Scholar]
- 51.Frank J, Agrawal RK. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature. 2000;406:318–22. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- 52.Ratje AH, Loerke J, Mikolajka A, Brunner M, Hildebrand PW, Starosta AL, Donhofer A, Connell SR, Fucini P, Mielke T, Whitford PC, Onuchic JN, Yu Y, Sanbonmatsu KY, Hartmann RK, Penczek PA, Wilson DN, Spahn CM. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature. 2010;468:713–6. doi: 10.1038/nature09547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi X, Chiu K, Ghosh S, Joseph S. Bases in 16S rRNA important for subunit association, tRNA binding, and translocation. Biochemistry. 2009;48:6772–82. doi: 10.1021/bi900472a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ledoux S, Uhlenbeck OC. [3’-32P]-labeling tRNA with nucleotidyltransferase for assaying aminoacylation and peptide bond formation. Methods. 2008;44:74–80. doi: 10.1016/j.ymeth.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fahlman RP, Uhlenbeck OC. Contribution of the esterified amino acid to the binding of aminoacylated tRNAs to the ribosomal P- and A-sites. Biochemistry. 2004;43:7575–83. doi: 10.1021/bi0495836. [DOI] [PubMed] [Google Scholar]
- 56.Ticu C, Nechifor R, Nguyen B, Desrosiers M, Wilson KS. Conformational changes in switch I of EF-G drive its directional cycling on and off the ribosome. Embo J. 2009;28:2053–65. doi: 10.1038/emboj.2009.169. [DOI] [PMC free article] [PubMed] [Google Scholar]


