Abstract
The blood-brain barrier (BBB) is increasingly being recognized as a site of special scientific importance. Numerous models of the BBB have been constructed over the past years with increasingly mechanistic studies of fundamental questions of cell biology and neuroimmunology. However, there has been a limiting factor of not being able to perform real-time studies of BBB function utilizing 3D models. Equally, real-time models have been limited mainly to 2D models comprised solely of endothelial cells (ECs). To measure changes in the electrical resistance across a BBB model, when adding inflammatory or stem cells which will interact with co-cultured glial cells has, to date, been beyond the capabilities of models.
We have cultured an inverted BBB model with ECs on electrodes which are on the lower surface of xCELLigence Cell Invasion Migration plates. Glial cells were cultured in the basal well with foot processes extending through the filters to make contact with the ECs. SIV-infected macrophages decreased electrical resistance of the EC monolayer when added to the “parenchymal” face of the model.
We present a novel inverted blood-brain barrier model that allow real time analyses of endothelial cell adhesion during modeled neuroinflammation.
Keywords: blood-brain barrier, HIV, model, electrical resistance
1. Introduction
1.1. The importance of the blood-brain barrier
The blood-brain barrier (BBB) plays a critical role in normal physiology of the central nervous system by regulating what reaches the brain from the periphery. The BBB also plays a major role in neurologic disease including encephalitides associated with human immunodeficiency virus (HIV) infection and Lyme neuroborreliosis.
The BBB is composed of closely packed nonfenestrated brain microvascular endothelial cells (BMEC) situated between the bloodstream and the basement membrane. Surrounding the BMEC, and in contact with the basement membrane, are perivascular macrophages and the foot processes of astrocytes and microglia (Renner, 2011b). Astrocytes and microglia can play an immunoregulatory role in addition to supporting the BBB biochemically as a diffusion barrier. BMEC, astrocytes, perivascular macrophages, and parenchymal microglia are all likely to encounter agents entering the CNS via the circulation. Neuroinflammation can result from bacteria or viruses entering the parenchyma (Renner, 2011c, Ramesh, 2009). Several of these conditions have been modeled using in vitro models (MacLean, 2004, MacLean, 2002, Eugenin, 2006).
1.2. Neurological disease and the BBB
Inflammation in the brain, as in other tissues, involves a complex interaction between endothelial cells and leukocytes, mediated through a variety of adhesion molecules, cytokines, chemokines, and their receptors. BMEC are structurally and functionally distinct from peripheral endothelial cells (MacLean, 2001, Craig, 1998). Therefore, it is important to use endothelial cells that are derived from brain, rather than from other organs.
1.3. Models of the BBB
In vitro models of the BBB have been constructed to understand the basic physiology of BBB function, or to tease out mechanisms of neuropathology. A number of these models utilize endothelial cells of various origins including umbilical vein (Wang, 2011) and brain, either in 2D (MacLean, 2001, Hartmann, 2007) or 3D (MacLean, 2004, MacLean, 2002, Chaudhuri, 2008) conformations. 2D models have been used to determine how the endothelial cells of the BBB are activated without interference from the other cell types that comprise the BBB. However, 2D models have two unfortunate drawbacks: no ability to have cocultures with glial cells, and the inflammatory or other stimulus can only be added to the apical face of the ECs.
1.4. 3D coculture models of the BBB
When ECs are cultured on the opposite face of a filter from astrocytes (Persidsky, 1999a, Hatherell, 2011, Eugenin, 2006), or on top of a gel containing astrocytes (Biegel and Pachter, 1994), there is increased tight junction protein expression and electrical resistance across the monolayer. The orientation of these models are ideal for studies of how invasive cells cross the BBB from the vasculature (Persidsky, 1997) or pharmacological studies examining how agents cross the BBB.
1.5. Proposed inverted 3D model
Our novel model is cultured in an inverse configuration from conventional models such that endothelial cells are grown on the lower surface of the filter and astrocytes in the well. This allows for cellular stimuli to be in direct contact with the astrocytes. For consistency, we will refer to the orientation of cells within wells, rather than how they would be oriented in vivo. Thus, we will use “apical” to refer to the endothelial side of the filter and “basal” to be the astrocyte side of the filter.
2. Materials and Methods
2.1. Isolation and culture of astrocytes and BMEC
Frontal cortices were obtained from healthy control macaques at necropsy. Contaminating meninges were removed and microvessels and astrocytes cultured as previously described (MacLean, 2002). In brief, astrocytes were prepared from frontal cortices of normal rhesus macaques as mixed glia using 0.25% trypsin (Invitrogen, Carlsbad, CA) and 20U/ml DNAse (Sigma, St. Louis, MO) digestion for 60 minutes at 37°C. Cells were filtered through 110μm pore filters and plated. 10 days later, microglia were removed using 5mM l-leucine methyl ester (Sigma) for one hour.
Microvessels were isolated by mechanical means. Frontal cortices were minced before filtration through 350μm pore filters and retention by 120μm filters. The retained vessels were digested using 1mg/ml collagenase/dispase (Roche, Indianapolis, IN) and 20U/ml DNAse (Sigma). Endothelial cells were then plated on 1% gelatin (Sigma) coated flasks.
2.2. Setup of inverted BBB model
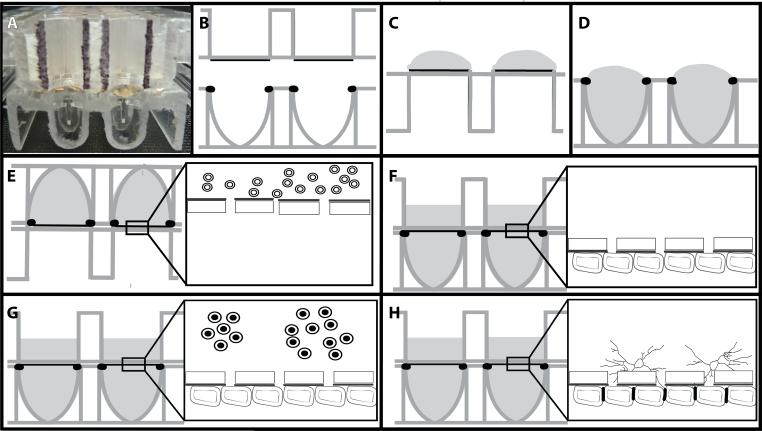
These studies used the xCELLigence cell invasion and migration (CIM) plates (Roche, Indianapolis, IN, see Figure 1A). For clarity, a cartoon version is used (Fig. 1B). Electrodes were coated with fibronectin (50μg/ml) in 1% gelatin for 2 hours (Fig. 1C). The fibronectin/gelatin was removed and monodispersed BMEC (at P3 or below) were seeded at 22,500 cells/ filter in the lower well (Fig. 1D). The 2 halves of the plates were assembled according to the manufacturer's instructions and connected to the xCELLigence DP system to produce a time zero control for each plate and well. The plates were then removed and inverted for 30 minutes at room temperature to prevent edge effects (Lundholt, 2003). Following this, the CIM plates were incubated (still inverted) at 37°C overnight (Fig. 1E). The next morning the plates were returned to the upright orientation and media added to the upper well (Fig. 1F). At this time the plates were returned to the xCELLigence DP system and monitoring resumed using the installed software.
Figure 1. Schematic of assembly of inverted BBB model.
The xCELLigence Cell Invasion Migration (CIM) plate consists of 16 wells in a standard 96-well plate format (A). Schematically, the upper well has an 8μm pore filter as the base (B). The lower surface of this filter has gold electrodes covering approximately 60% of the surface which are coated with fibronectin/gelatin (C). BMEC suspension is added at 22,500 cells to the lower well with a positive meniscus (D). The upper well is snapped in place and the assembled model inverted overnight (E). The plates were then reverted and media added to the upper well of the CIM plate (F) and the CIM plate returned to the xCELLigence system. Astrocytes are added when the traces plateau (G) resulting in tight junctions between the ECs and astrocyte foot processes (H).
Data were analyzed by Friedman Test (non-parametric repeated measures ANOVA) with Dunn's post test using InStat version 3.0a for Macintosh (GraphPad Software, San Diego California USA). For paired comparisons, Wilcoxon matched-pairs signed-ranks test was performed.
2.3. Induction of tight junctions
When the traces reached a plateau, commonly between 36 and 48 hours after EC plating, autologous astrocytes were seeded into the upper wells at 20,000 cells per well (Fig. 1G). Traces were followed automatically using the installed software to monitor the effects of astrocyte processes on electrical resistance of the BMEC monolayer (Fig. 1H).
2.4. Culture and infection of bone marrow-derived macrophages
Bone marrow-derived macrophages (BMDM) were cultured as previously described (Ivey, 2009). Bone marrow was obtained from juvenile normal macaques aseptically at necropsy, vortexed, and filtered through 70μm pore filters. Macrophages were grown in IMDM media (Mediatech) supplemented with 10% FCS and antibiotics (Invitrogen). BMDM were infected by incubating with SIVmac251 (a neuropathogenic strain) using 100 TCID50 per T25 flask for four hours before washing and culturing for a further 48 hours. At that point, media were harvested, pooled, and macrophages trypsinized. The macrophages were resuspended in fresh macrophage media. 20,000 SIV-infected or control BMDM were added to the upper well of the BBB model. For controls, TNF-α (100U/ml) was added to either the apical or the basal surface and altered adhesion monitored.
2.4. Confocal imaging of inverted BBB model
CIM plates were fixed with 1% paraformaldehyde. Filters were excised from CIM plates using scalpels, and blocked in PBS containing 1% bovine serum albumin and 0.1% Triton-X100 (both from Sigma) for one hour at room temperature, before incubating with primary antibodies to Von Willebrand factor (Dako, Carpinteria, CA), and/or GFAP-CY3 (Sigma) overnight at 4°C, washed three times with PBS with 0.2% fish skin gelatin (PBS/FSG), and then incubated in the dark for 60 min at room temperature with secondary antibodies directly conjugated with Alexa 488 (green) (Molecular Probes/Invitrogen, Carlsbad, CA). Filters were washed three times in PBS/FSG, coverslipped with Prolong Gold antiquenching reagent (Molecular Probes), and imaged on a Leica TCS SP II confocal microscope equipped with three lasers. Individual optical slices represent 0.2 μm, and 25 optical slices were collected at 2048 × 2048 pixel resolution for each section.
3. Results
3.1. Establishment of inverted BBB model
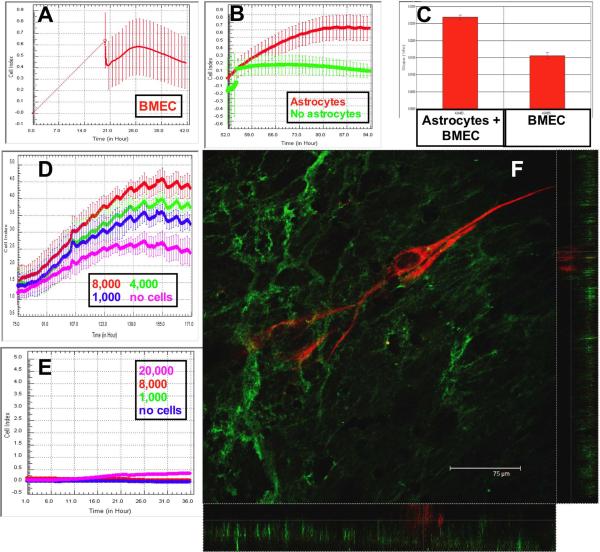
CIM plates were inverted for the first 16 hours. Therefore, it was not practical to measure impedance during this initial time. Once CIM plates were connected to the xCELLigence device, loosely adherent BMEC fell from the filters before a gradual increase in electrical resistance was observed (Figure 2A). Monitoring continued over the next 36–48 hours, until a plateau was reached (Figure 2A). During this growth / confluence phase, there was an increase in cell index (CI), calculated using the installed software.
Figure 2. BMEC adhesion is increased following astrocyte coincubation.
The Cell Index (CI) increases during the adhesion of BMEC to the inverted filter assembly. When the plate is reverted and connected to the xCELLigence system, it is apparent that BMEC have adhered and begun to spread (A). When astrocytes are added to the upper well, the CI is increased further compared to BMEC monocultures (B, p<0.0001). The change in CI is calculated using the installed software when astrocytes (C, left trace) are added to the wells. Astrocytes increased the CI in a dose-dependent manner (D). Astrocyte foot processes did not cross the filter and increase the CI in the absence of BMEC (E). Endothelial cells were immuno-labeled with antibodies to Von Willebrand Factor (F, green). Astrocytes were labeled with antibodies to GFAP-CY3 (red). Monolayers of endothelial cells were apparent below the GFAP-positive astrocyte processes. Confocal stacks were then examined in x–z and y–z format to definitively show that the astrocytes were physically separated from the endothelial cells (see also the supplementary video).
The addition of astrocytes to the upper wells resulted in a further increase in the CI over the next 24 hours compared with wells not receiving astrocytes (Figure 2B). The software was “zeroed” before either media (green trace) or astrocytes (red trace) were seeded in the basal well. Therefore, the relative change in CI is plotted in Figure 2B. The rate of increase in CI following addition of astrocytes was calculated using the manufacturer's software (Figure 2C). The addition of astrocytes induced a significant increase in cell index compared to control media (p<0.0001, n=16 for each group). This increase was dose-dependent, with lower numbers of astrocytes inducing a diminished increase in CI (Figure 2D, magenta trace- no astrocytes, blue trace-1,000 astrocytes, green- 4,000 astrocytes, red- 8,000). Astrocytes added to the upper well of plates with no BMEC did not migrate through the filters as evidenced by negligible cell index over 36 hours (Figure 2E).
3.3. Confocal microscopy of inverted BBB model
48 hours after the addition of astrocytes, the CIM plates were fixed and imaged by confocal microscopy. 3D confocal stacks were viewed in x–z and y–z orientations to confirm astrocyte-EC interaction (Figure 2F, and video in supplemental material). GFAP-positive astrocyte processes (red) were observed to extend above the endothelial cell monolayer (green).
3.4. SIV infected macrophages diminish BBB integrity
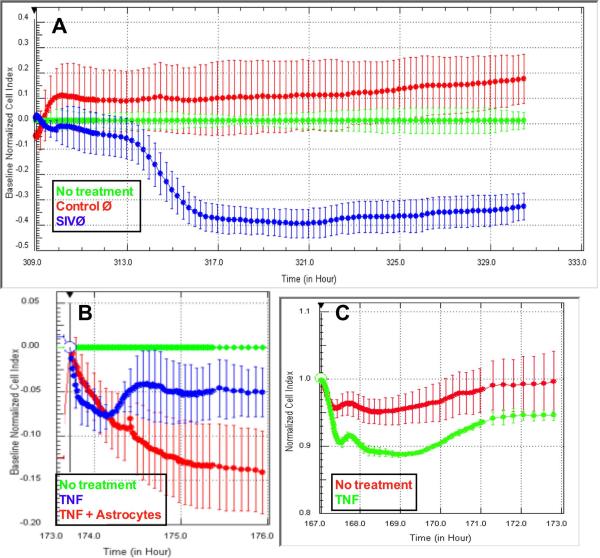
SIV-infected or control macrophages were added to the upper well of CIM plates. Control macrophages (Figure 3A, red trace) did not induce any significant alteration in electrical resistance compared with addition of culture media (green trace, p>0.05). Addition of SIV-infected macrophages to the upper well, and hence in contact with astrocytes, induced a significant drop in resistance (Figure 3A, blue trace, p<0.001 compared with either no treatment or control macrophages). This effect took four hours to initiate, and was complete within eight hours. The macrophages did not appear to transmigrate the filters as evidenced by no increase in CI for the next 20 hours. It is possible this lack of migration is due to chemokines being secreted by the astrocytes preventing migration (Renner, 2011a).
Figure 3.
SIV-infected macrophages decrease resistance of the BBB model. Addition of SIV-infected macrophages to the upper well (blue) induced a decrease in CI compared with control macrophages (red) or media alone (green, p<0.001). The decrease was mirrored by the addition of TNF-α to the astrocyte face of the CIM plate (B, p<0.001) or the luminal face of the E plates (C, p<0.0001).
To demonstrate that this model behaved similarly to previous models, we added TNF-α to the upper well (de Vries, 1996). TNF-α is known to alter the phenotype of astrocytes in culture (Renner, 2011a) with many downstream effects on BBB (Renner et al, under review). 100U/ml TNF-α decreased electrical resistance within 30 minutes (Figure 3B, red or blue traces) compared with control (green trace, p<0.001). Three hours after addition of TNF-α, the decreased resistance was not significantly different between wells with astrocytes (blue) or without astrocytes (red, p>0.05). Finally, TNF-α was added to the luminal surface of BMEC cultured on E-plates (Figure 3C, p<0.0001). Electrical resistance was decreased to a similar degree as that observed with the inverted model.
4. Discussion
Previous studies, including those from our lab, have shown that cocultures of astrocytes and endothelial cells have increased tight junctions and electrical resistance compared to EC cultures alone (MacLean, 2002, Persidsky, 1999a, Persidsky, 1997). We confirmed statistically significantly increased electrical resistance measured in real time in our inverted model following coculture with astrocytes.
These studies developed a new model for examining the BBB in health and disease. By inverting the model relative to our previous studies, we will be able to examine the downstream effects that are directly associated with contact between glia and inflammatory agents, be they bacterial, as in the causative agent in Lyme disease, Borrelia burgdorferi, or host inflammatory macrophages in lentivirus-mediated encephalitis following infection with HIV.
Traditional models, including the one we have previously used (MacLean, 2002, MacLean, 2004), are limited to either having the cellular stimulus being added to the apical face of the endothelial cells (and therefore not in the “parenchyma”) or not in direct contact with the astrocytes due to falling to the bottom of the lower well. These models do however allow for manipulation of the glial cells, such as infecting microglia with HIV to induce increased migration of monocytes (Persidsky, 1999b). For studies of how the BBB is affected by interactions between bacterial or viral infected macrophages and glia, we are introducing a model that allows addition of cellular stimuli to the “parenchymal” side of the BBB model and to be in direct contact with the glial cells. We believe this model will be useful for studies of encephalitis, including HIV neuropathogenesis and Lyme neuroborreliosis.
Models using real time analyses have either measured 2D cultures using electrodes patterned on the substrate upon which cells are grown, or require manipulation of the filters on which the cells are grown. ECIS (Applied BioPhysics, Troy, NY) and xCELLigence (Roche, Indianapolis, IN) are two leading technologies utilizing this first methodology. CIM plates for xCELLigence have electrodes covering 60% of the surface of an 8μm pore membrane suspended between two wells (a modified Boyden chamber). We have utilized this system to make an inverted BBB model suitable for real time analyses of neuroinflammation. It should be noted here that while our inverted model allows interactions of inflammatory cells with the glia, it does not provide contact with the luminal surface of the endothelial cells. E-plates (Roche) can be used for these studies (Figure 3C).
Electrical resistance is routinely used for real time analyses of barrier functions. Traditionally, electrical resistance has been measured using a “chopstick” approach. This technique measures the resistance across a filter. Due to the nature of having to move the filters to have the resistance measured, and of the importance of maintaining a constant gap between the electrodes “chopstick” based models have inherent problems with reproducibility. Additionally, these systems report on changes to populations of cells as a whole, requiring complete coverage of the filters. Examples of this technology are CellZscope (nanoAnalytics, Munster, Germany) and Evom (WPI, Inc., Sarasota, FL).
5. Conclusions
We have presented a model of the BBB that will allow direct contact between glial cells of the neurovascular unit and stem cells / inflammatory cells / tumor cells while simultaneously measuring real time changes in EC resistance. This will enable studies that measure differences in how soluble mediators and contact mediators influence disruption of the BBB. We propose using this model to examine interactions of tumor cells or stem cells with glia or inflammation mediated by cellular stimuli, be they bacterial (Lyme Neuroborreliosis) or viral (HIV encephalitis).
Supplementary Material
Highlights
Inverted model allows contact of inflammatory cells with glia
Lentiviral-infected macrophages induce BBB disruption
Presence of macrophages alone is not sufficient for BBB disruption
BBB disruption is measured in real time
Acknowledgements
This work was supported by PHS grants RR00164, MH077544, RR20159, OD11104. Nicole Renner was supported by a Louisiana Board of Regents Fellowship (LEQSF(2007–2012)-GF15). We thank David Core, TNPRC for assistance with CIM plates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Biegel D, Pachter JS. Growth of brain microvessel endothelial cells on collagen gels: applications to the study of blood-brain barrier physiology and CNS inflammation. In Vitro Cell Dev Biol Anim. 1994;30A(9):581–8. doi: 10.1007/BF02631256. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Yang B, Gendelman HE, Persidsky Y, Kanmogne GD. STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood-brain barrier. Blood. 2008;111(4) doi: 10.1182/blood-2007-05-091207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig LE, Spelman JP, Strandberg JD, Zink MC. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvasc Res. 1998;55(1) doi: 10.1006/mvre.1997.2045. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64(1) doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4) doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C, Zozulya A, Wegener J, Galla HJ. The impact of glia-derived extracellular matrices on the barrier function of cerebral endothelial cells: an in vitro study. Exp Cell Res. 2007;313(7) doi: 10.1016/j.yexcr.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods. 2011;199(2) doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Ivey NS, Renner NA, Moroney-Rasmussen T, Mohan M, Redmann RK, Didier PJ, Alvarez X, Lackner AA, Maclean AG. Association of FAK activation with lentivirus-induced disruption of blood-brain barrier tight junction-associated ZO-1 protein organization. J Neurovirol. 2009:1–12. doi: 10.1080/13550280902998413. PMCID: PMC2896435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholt BK, Scudder KM, Pagliaro L. A simple technique for reducing edge effect in cell-based assays. J Biomol Screen. 2003;8(5) doi: 10.1177/1087057103256465. [DOI] [PubMed] [Google Scholar]
- MacLean AG, Orandle MS, Alvarez X, Williams KC, Lackner AA. Rhesus macaque brain microvessel endothelial cells behave in a manner phenotypically distinct from umbilical vein endothelial cells. J Neuroimmunol. 2001;118(2) doi: 10.1016/s0165-5728(01)00348-4. [DOI] [PubMed] [Google Scholar]
- MacLean AG, Orandle MS, MacKey J, Williams KC, Alvarez X, Lackner AA. Characterization of an in vitro rhesus macaque blood-brain barrier. J Neuroimmunol. 2002;131(1–2):98–103. doi: 10.1016/s0165-5728(02)00256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean AG, Rasmussen TA, Bieniemy DN, Alvarez X, Lackner AA. SIV-induced activation of the blood-brain barrier requires cell-associated virus and is not restricted to endothelial cell activation. J Med Primatol. 2004;33(5–6):236–42. doi: 10.1111/j.1600-0684.2004.00077.x. [DOI] [PubMed] [Google Scholar]
- Persidsky Y. Model systems for studies of leukocyte migration across the blood - brain barrier. J Neurovirol. 1999a;5(6) doi: 10.3109/13550289909021287. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999b;155(5) doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158(7) [PubMed] [Google Scholar]
- Ramesh G, Borda JT, Gill A, Ribka EP, Morici LA, Mottram P, Martin DS, Jacobs MB, Didier PJ, Philipp MT. Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. J Neuroinflammation. 2009;6:23. doi: 10.1186/1742-2094-6-23. PMCID: 2748066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner NA, Ivey NS, Redmann RK, Lackner AA, MacLean AG. MCP-3/CCL7 production by astrocytes: implications for SIV neuroinvasion and AIDS encephalitis. Journal of neurovirology. 2011a;17(2) doi: 10.1007/s13365-010-0017-y. PMCID: PMC3086688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner NA, Lackner AA, Maclean AG. Blood-Brain Barrier Disruption and Encephalitis in Animal Models of AIDS. Encephalitis. 2011b;(2) in press. [Google Scholar]
- Renner NA, Redmann RK, Moroney-Rasmussen T, Sansing HA, Aye PP, Didier PJ, Lackner AA, Maclean AG. S100beta as a novel and accessible indicator for the presence of monocyte-driven encephalitis in AIDS. Neuropathology and applied neurobiology. 2011c doi: 10.1111/j.1365-2990.2011.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhen H, Zhang J, Zhang W, Zhang R, Cheng X, Guo G, Mao X, Wang J, Zhang X. Survivin promotes glioma angiogenesis through vascular endothelial growth factor and basic fibroblast growth factor in vitro and in vivo. Mol Carcinog. 2011 doi: 10.1002/mc.20829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.