Abstract
Study purposes were to determine the occurrence rate for preoperative breast pain; describe the characteristics of this pain; evaluate for differences in demographic and clinical characteristics; and evaluate for variations in pro- and anti-inflammatory cytokine genes between women who did and did not report pain. Patients (n=398) were recruited prior to surgery and completed self-report questionnaires on a number of pain characteristics. Genotyping was done using a custom genotyping array. Women (28.2%) who reported breast pain were significantly younger (p < 0.001); more likely to be non-white (p= 0.032); reported significantly lower Karnofsky Performance Status scores (p = 0.008); were less likely to be post menopausal (p = 0.012), and had undergone significantly more biopsies (p=0.006). Carriers of the minor allele for a single nucleotide polymorphism (SNP) in interleukin (IL)1-receptor 1 (IL1R1) (rs2110726) were less likely to report breast pain prior to surgery (p = 0.007). Carriers of the minor allele for a SNP in IL13 (rs1295686) were more likely to report breast pain prior to surgery (p= 0.019). Findings suggest that breast pain occurs in over a quarter of women who are about to undergo breast cancer surgery. Based on phenotypic and genotypic characteristics found, inflammatory mechanisms contribute to preoperative breast pain.
Keywords: breast pain, inflammation, cytokine genes, preoperative pain
INTRODUCTION
Acute postoperative pain and chronic pain associated with breast cancer and its treatment are common problems in women with breast cancer.1,3,18,23,27,30,44,63–65] However, only five papers have described breast pain prior to surgery.14,44,63–65 In a paper published in 1952,14 Corry noted that “the occurrence of pain in operable cases of carcinoma of the breast is well known to surgeons” and that its occurrence ranged from 14% to 45%. In one of the earliest studies of chronic pain after breast cancer surgery,65 30% of the 93 patients surveyed reported preoperative pain in their affected breast. Pain intensity scores ranged from 0.6 to 6.9 on a 0 to 10 centimeter visual analog scale. Ten percent of these women reported preoperative pain in both the affected breast and ipsilateral arm. Activities that aggravated the preoperative pain included reaching out, doing housework, driving a car, and sleeping on the affected side. In a second paper from the same cohort,63 patients with preoperative pain recalled higher levels of postoperative pain compared to patients without preoperative pain. In a recent study of risk factors for chronic pain following breast cancer surgery,44 28% of patients (n=93) reported preoperative breast pain. Of note, the presence of preoperative pain was not associated with the development of chronic pain following surgery. While these studies documented the occurrence of preoperative breast pain, detailed information on specific pain characteristics and risk factors for preoperative breast pain were not reported.
Potential causes for breast pain prior to surgery include the release of algogenic meditators from the tumor;24,26,41 perineural involvement by the cancer; and inflammation associated with tissue injury following a breast biopsy. This tissue injury is associated with the release of pro-inflammatory cytokines (e.g., interleukin (IL)1, tumor necrosis factor alpha (TNF-α)) that results in inflammatory pain. In addition, variations in a number of genes in inflammatory pathways (e.g., cyclooxygenase 2,52 TNFα,49,51,52 nuclear factor kappa beta (NFΚB1),52 IL1,4,50 IL8,50–51 IL1622) are associated with increases in acute4,77 and cancer49–52 pain. For example, a polymorphism in the promoter region of IL8 (rs4073) was associated with increased pain in patients with pancreatic cancer.50 However, no studies have evaluated for variation in pro-inflammatory cytokine genes in patients with breast pain prior to surgery for breast cancer.
Given the paucity of research on breast pain in women prior to breast cancer surgery and emerging evidence that cytokine gene polymorphisms may be associated with acute pain, the purposes of this study, in a sample of women who were to undergo surgery for breast cancer were to: determine the occurrence rate for preoperative breast pain; describe the characteristics of this pain; evaluate for differences in demographic and clinical characteristics between women who did and did not report pain prior to surgery; and evaluate for variations in pro- and anti-inflammatory cytokine genes between the two pain groups.
MATERIALS AND METHODS
Patients and Settings
This analysis is part of a larger study that evaluated neuropathic pain and lymphedema in women who underwent breast cancer surgery. Patients were recruited from breast care centers located in a comprehensive cancer center, two public hospitals, and four community practices.
Patients were eligible to participate if they: were an adult woman (≥18 years) who underwent breast cancer surgery on one breast; were able to read, write, and understand English; agreed to participate; and gave written informed consent. Patients were excluded if they were having breast cancer surgery on both breasts and/or had distant metastasis at the time of diagnosis. A total of 516 patients were approached to participate, 410 were enrolled in the study (response rate 79.4%), and 398 completed the baseline assessment. The most common reasons for refusal were: too busy, overwhelmed with the cancer diagnosis, or insufficient time available to do the baseline assessment prior to surgery.
Instruments
The demographic questionnaire obtained information on age, marital status, education, ethnicity, employment status, and living situation.
Karnofsky Performance Status (KPS) scale is widely used to evaluate functional status in patients with cancer and has well established validity and reliability.34–35 Patients rated their functional status using the KPS scale that ranged from 30 (I feel severely disabled and need to be hospitalized) to 100 (I feel normal; I have no complaints or symptoms).
Self-Administered Comorbidity Questionnaire (SCQ) is a short and easily understood instrument that was developed to measure comorbidity in clinical and health service research settings.56 The questionnaire consists of 13 common medical conditions that were simplified into language that could be understood without any prior medical knowledge. Patients were asked to indicate if they had the condition using a “yes/no” format. If they indicated that they had a condition, they were asked if they received treatment for it (yes/no; proxy for disease severity) and did it limit their activities (yes/no; indication of functional limitations). Patients were given the option to add two additional conditions not listed on the instrument. For each condition, a patient can receive a maximum of 3 points. Because the SCQ contains 13 defined medical conditions and 2 optional conditions, the maximum score totals 45 points if the open-ended items are used and 39 points if only the closed-ended items are used. The SCQ has well-established validity and reliability and has been used in studies of patients with a variety of chronic conditions.5,11
Breast Symptoms Questionnaire (BSQ), which consists of three parts, was used to obtain information on a number of pain characteristics. Part 1 obtained information on the prevalence, frequency, severity, and distress of symptoms in the breast (i.e., pain, swelling, numbness, strange sensations, hardness) prior to surgery. The symptoms that were assessed by Part 1 of the BSQ were identified in studies by Tasmuth and colleagues.64–65 The assessment of these symptoms is based on the format used in the Memorial Symptom Assessment Scale (MSAS).45–46 Frequency of occurrence of the symptom, if present, was rated using a 1 to 4 scale (1= rarely to 4 = constantly). Severity was rated on a 1 to 4 scale (1=slight to 4=very severe). Distress was rated on a 0 to 4 scale (0=not at all to 4=very much). Occurrence rates for each symptom were determined using the responses in the “did not have” portion of the symptom assessment scale. Adaptations of the MSAS were used in previous studies.36–37
If the patient had pain in the breast, they completed Part 2 of the BSQ. Patients were asked to rate the intensity of their pain (i.e., pain right now and average and worst pain) using a numeric rating scale (NRS) that ranged from 0 (no pain) to 10 (worst imaginable pain). NRSs are valid and reliable measures of pain intensity.31
Patients who completed Part 2 were asked to complete Part 3. With Part 3 of the BSQ, patients rated the level of interference caused by breast pain with sixteen activities using a 0 (does not interfere) to 10 (completely interferes) NRS. This interference scale is an adaptation of the interference scale from the Wisconsin Brief Pain Inventory (BPI).15 This interference scale is a valid and reliable measure that has been used to evaluate the extent to which a person’s pain interferes with their ability to function.12,57 In addition to the original eight items on the interference scale of the BPI (i.e., general activity, mood, walking ability, normal work, relations with other people, sleep, enjoyment of life, sexual activity), the eight additional activities that were evaluated were those that were evaluated in the studies by Tasmuth and colleagues64–65 (i.e., ability to sleep on the operated side, touch, ability to reach out, ability to carry things, ability to get up from bed, ability to do handicrafts, ability to drive a car, ability to write).
Pain Qualities Assessment Scale (PQAS)32,72 is an adaptation of the Neuropathic Pain Scale developed by Galer and Jensen21 that consists of 20 items. The first 18 items are measured with NRSs that evaluate the magnitude of the different pain qualities (e.g., sharp, hot, aching, cold). The last two questions ask for an estimate of the intensity of deep pain and surface pain. Scores for individual pain qualities are reported and a mean score across the 20 items was calculated. In addition, three subscale scores were calculated (i.e., surface pain, paroxysmal pain, deep pain).72 The PQAS has well-established validity and reliability.32,72
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Boards at each of the study sites. During the patient’s preoperative visit, a clinical staff member explained the study to the patient and determined her willingness to participate. For those women who were willing to participate, the staff member introduced the patient to the research nurse. The research nurse met with the women, determined eligibility, and obtained written informed consent prior to surgery. After obtaining consent, patients completed the enrollment questionnaires on average 4 days prior to surgery. Medical records were reviewed for disease and treatment information.
Genomic analyses
Gene selection
Cytokines and their receptors are classes of polypeptides that mediate inflammatory processes.71 Cytokine dysregulation is associated with increased inflammatory responses in acute pain71,73–76 and in a variety of chronic medical conditions.2,9,22,43,54, 60, 68 These polypeptides are divided into pro- and anti-inflammatory cytokines. Pro-inflammatory cytokines promote systemic inflammation and include: interferon (IFN) gamma, IFNG 1 receptor (IFNGR1), IL1R1, IL2, IL8, IL17A, nuclear factor kappa beta (NFKB1), NFKB2, and TNFα.58,71 Anti-inflammatory cytokines suppress the activity of pro-inflammatory cytokines and include: IL1R2, IL4, IL10, and IL13.58,71 Of note, IFNG1, IL1β, and IL6 possess pro- and anti-inflammatory functions.58
Blood collection and genotyping
Of the 398 patients who completed the baseline assessment, 302 provided a blood sample from which DNA could be isolated from peripheral blood mononuclear cells (PBMCs). No differences were found in any demographic and clinical characteristics between patients who did and did not choose to participate in the study or in those patients who did and did not provide a blood sample for genomic analyses.
Genomic DNA was extracted from PBMCs, that were maintained by the UCSF Genomic Markers of Symptoms Tissue Bank, using the PUREGene DNA Isolation System (Invitrogen, Carlsbad, CA). DNA samples were quantitated with a Nanodrop Spectrophotometer (ND-1000) and normalized to a concentration of 50 ng/μL (diluted in 10 mM Tris/1 mM EDTA). Genotyping was performed blinded to clinical status and positive and negative controls were included. Samples were genotyped using the Golden Gate genotyping platform (Illumina, San Diego, CA) and processed according to the standard protocol using GenomeStudio (Illumina, San Diego, CA). Two blinded reviewers visually inspected signal intensity profiles and resulting genotype calls for each SNP. Disagreements were adjudicated by a third reviewer. If consensus could not be reached, the SNP was excluded.
SNP selection
A combination of tagging SNPs and literature driven SNPs (i.e., reported as being associated with altered function and/or symptoms) were selected for analysis. Tagging SNPs were required to be common (defined as having a minor allele frequency ≥ 0.05) in public databases (e.g., HapMap). In order to ensure robust genetic association analyses, quality control filtering of SNPs was performed. SNPs with call rates <95%, or Hardy-Weinberg p<0.001 were excluded. As shown in Table 1, a total of 103 SNPs among the 15 candidate genes (IFNG1: 6 SNPs, IFNGR1: 1SNP; IL1B: 12 SNPs; IL1R1: 5 SNPs; IL1R2: 3 SNPs; IL2: 5 SNPs; IL4: 9 SNPs; IL6: 12 SNPs; IL8: 3 SNPs; IL10: 8 SNPs; IL13: 5 SNPs; IL17A: 6 SNPs; NFKB1: 14 SNPs; NFKB2: 4 SNPs; TNFA: 10 SNPs) passed all quality control filters and were included in the genetic association analyses. Potential functional roles of SNPs associated with pain were examined using PUPASuite 2.0,13 a comprehensive search engine that tests a series of functional effects (i.e., non-synonymous changes, altered transcription factor binding sites, exonic splicing enhancing or silencing, splice site alterations, microRNA target alterations).
Table 1.
Genes and Single Nucleotide Polymorphisms Analyzed for Pain versus No Pain in Women Prior to Breast Cancer Surgery
| Gene | SNP | Position | Chr | MAF | Alleles | Chi Square | p-value | Model |
|---|---|---|---|---|---|---|---|---|
| IFNG1 | rs2069728 | 66834051 | 12 | .079 | G>A | 2.12 | .347 | A |
| IFNG1 | rs2069727 | 66834490 | 12 | .411 | A>G | 1.72 | .422 | A |
| IFNG1 | rs2069718 | 66836429 | 12 | .442 | C>T | 2.83 | .242 | A |
| IFNG1 | rs1861493 | 66837463 | 12 | .264 | A>G | 2.39 | .303 | A |
| IFNG1 | rs1861494 | 66837676 | 12 | .279 | T>C | 2.50 | .287 | A |
| IFNG1 | rs2069709 | 66839970 | 12 | .008 | G>T | 0.39 | .534 | A |
| IFNGR1 | rs9376268 | 137574444 | 6 | .246 | G>A | 4.28 | .118 | A |
| IL1B | rs1071676 | 106042060 | 2 | .189 | G>C | 0.30 | .863 | A |
| IL1B | rs1143643 | 106042929 | 2 | .383 | G>A | 1.51 | .469 | A |
| IL1B | rs1143642 | 106043180 | 2 | .082 | C>T | 2.87 | .238 | A |
| IL1B | rs1143634 | 106045017 | 2 | .187 | C>T | 0.51 | .774 | A |
| IL1B | rs1143633 | 106045094 | 2 | .392 | G>A | 2.85 | .241 | A |
| IL1B | rs1143630 | 106046282 | 2 | .115 | C>A | 0.64 | .728 | A |
| IL1B | rs3917356 | 106046990 | 2 | .450 | A>G | 0.29 | .864 | A |
| IL1B | rs1143629 | 106048145 | 2 | .389 | T>C | 1.03 | .599 | A |
| IL1B | rs1143627 | 106049014 | 2 | .397 | T>C | 1.15 | .562 | A |
| IL1B | rs16944 | 106049494 | 2 | .386 | G>A | 0.64 | .726 | A |
| IL1B | rs1143623 | 106050452 | 2 | .277 | G>C | 2.10 | .350 | A |
| IL1B | rs13032029 | 106055022 | 2 | .448 | C>T | 0.09 | .958 | A |
| IL1R1 | rs949963 | 96533648 | 2 | .223 | G>A | 1.94 | .379 | A |
| IL1R1 | rs2228139 | 96545511 | 2 | .053 | C>G | 1.66 | .436 | A |
| IL1R1 | rs3917320 | 96556738 | 2 | .047 | A>C | 0.90 | .637 | A |
| IL1R1 | rs2110726 | 96558145 | 2 | .317 | C>T | FE | .007 | D |
| IL1R1 | rs3917332 | 96560387 | 2 | .187 | T>A | 2.25 | .324 | A |
| IL1R2 | rs4141134 | 96370336 | 2 | .362 | T>C | 0.77 | .680 | A |
| IL1R2 | rs11674595 | 96374804 | 2 | .247 | T>C | 1.36 | .507 | A |
| IL1R2 | rs7570441 | 96380807 | 2 | .408 | G>A | 1.70 | .428 | A |
| IL2 | rs1479923 | 119096993 | 4 | .308 | C>T | 1.89 | .388 | A |
| IL2 | rs2069776 | 119098582 | 4 | .184 | T>C | n/a | n/a | n/a |
| IL2 | rs2069772 | 119099739 | 4 | .241 | A>G | 0.19 | .911 | A |
| IL2 | rs2069777 | 119103043 | 4 | .047 | C>T | 5.21 | .074 | A |
| IL2 | rs2069763 | 119104088 | 4 | .277 | T>G | 0.85 | .653 | A |
| IL4 | rs2243248 | 127200946 | 5 | .086 | T>G | 1.06 | .588 | A |
| IL4 | rs2243250 | 127201455 | 5 | .269 | C>T | n/a | n/a | n/a |
| IL4 | rs2070874 | 127202011 | 5 | .245 | C>T | n/a | n/a | n/a |
| IL4 | rs2227284 | 127205027 | 5 | .387 | C>A | n/a | n/a | n/a |
| IL4 | rs2227282 | 127205481 | 5 | .390 | C>G | n/a | n/a | n/a |
| IL4 | rs2243263 | 127205601 | 5 | .124 | G>C | 1.90 | .386 | A |
| IL4 | rs2243266 | 127206091 | 5 | .237 | G>A | n/a | n/a | n/a |
| IL4 | rs2243267 | 127206188 | 5 | .237 | G>C | n/a | n/a | n/a |
| IL4 | rs2243274 | 127207134 | 5 | .261 | G>A | n/a | n/a | n/a |
| IL6 | rs4719714 | 22643793 | 7 | .255 | A>T | 1.59 | .452 | A |
| IL6 | rs2069827 | 22648536 | 7 | .069 | G>T | 0.84 | .658 | A |
| IL6 | rs1800796 | 22649326 | 7 | .134 | G>C | n/a | n/a | n/a |
| IL6 | rs1800795 | 22649725 | 7 | .285 | C>G | 3.35 | .187 | A |
| IL6 | rs2069835 | 22650951 | 7 | .130 | T>C | n/a | n/a | n/a |
| IL6 | rs2066992 | 22651329 | 7 | .091 | G>T | 2.37 | .306 | A |
| IL6 | rs2069840 | 22651652 | 7 | .333 | C>G | 3.19 | .203 | A |
| IL6 | rs1554606 | 22651787 | 7 | .319 | T>G | 1.36 | .507 | A |
| IL6 | rs2069845 | 22653229 | 7 | .319 | G>A | 1.36 | .507 | A |
| IL6 | rs2069849 | 22654236 | 7 | .024 | C>T | 2.65 | .266 | A |
| IL6 | rs2069861 | 22654734 | 7 | .056 | C>T | 2.06 | .357 | A |
| IL6 | rs35610689 | 22656903 | 7 | .259 | A>G | 2.03 | .363 | A |
| IL8 | rs4073 | 70417508 | 4 | .455 | T>A | 0.35 | .838 | A |
| IL8 | rs2227306 | 70418539 | 4 | .366 | C>T | 1.06 | .588 | A |
| IL8 | rs2227543 | 70419394 | 4 | .368 | C>T | 0.61 | .738 | A |
| IL10 | rs3024505 | 177638230 | 1 | .129 | C>T | 2.85 | .241 | A |
| IL10 | rs3024498 | 177639855 | 1 | .204 | A>G | 0.86 | .650 | A |
| IL10 | rs3024496 | 177640190 | 1 | .421 | T>C | 0.79 | .674 | A |
| IL10 | rs1878672 | 177642039 | 1 | .416 | G>C | 0.08 | .960 | A |
| IL10 | rs3024492 | 177642438 | 1 | .161 | A>T | n/a | n/a | n/a |
| IL10 | rs1518111 | 177642971 | 1 | .303 | G>A | 2.04 | .361 | A |
| IL10 | rs1518110 | 177643187 | 1 | .301 | G>T | 1.82 | .402 | A |
| IL10 | rs3024491 | 177643372 | 1 | .408 | T>G | 0.08 | .961 | A |
| IL13 | rs1881457 | 127184713 | 5 | .210 | A>C | 2.20 | .332 | A |
| IL13 | rs1800925 | 127185113 | 5 | .233 | C>T | 5.14 | .077 | A |
| IL13 | rs2069743 | 127185579 | 5 | .019 | A>G | 2.62 | .270 | A |
| IL13 | rs1295686 | 127188147 | 5 | .265 | G>A | 7.89 | .019 | A |
| IL13 | rs20541 | 127188268 | 5 | .212 | C>T | 2.18 | .337 | A |
| IL17A | rs4711998 | 51881422 | 6 | .346 | G>A | 5.02 | .081 | A |
| IL17A | rs8193036 | 51881562 | 6 | .327 | T>C | 1.77 | .412 | A |
| IL17A | rs3819024 | 51881855 | 6 | .372 | A>G | 0.52 | .772 | A |
| IL17A | rs2275913 | 51882102 | 6 | .361 | G>A | 1.29 | .525 | A |
| IL17A | rs3804513 | 51884266 | 6 | .023 | A>T | FE | .544 | A |
| IL17A | rs7747909 | 51885318 | 6 | .217 | G>A | 2.70 | .259 | A |
| NFKB1 | rs3774933 | 103645369 | 4 | .409 | T>C | 1.139 | .566 | A |
| NFKB1 | rs170731 | 103667933 | 4 | .397 | T>A | 2.89 | .576 | A |
| NFKB1 | rs17032779 | 103685279 | 4 | .023 | T>C | 0.00 | .968 | A |
| NFKB1 | rs230510 | 103695201 | 4 | .366 | T>A | 1.37 | .504 | A |
| NFKB1 | rs230494 | 103706005 | 4 | .477 | A>G | 1.01 | .604 | A |
| NFKB1 | rs4648016 | 103708706 | 4 | .017 | C>T | 0.09 | .765 | A |
| NFKB1 | rs4648018 | 103709236 | 4 | .025 | G>C | 0.02 | .881 | A |
| NFKB1 | rs3774956 | 103727564 | 4 | .479 | C>T | 1.05 | .591 | A |
| NFKB1 | rs10489114 | 103730426 | 4 | .025 | A>G | 0.02 | .881 | A |
| NFKB1 | rs4648068 | 103737343 | 4 | .366 | A>G | 2.42 | .299 | A |
| NFKB1 | rs4648095 | 103746914 | 4 | .052 | T>C | 0.00 | .977 | A |
| NFKB1 | rs4648110 | 103752867 | 4 | .205 | T>A | 1.50 | .472 | A |
| NFKB1 | rs4648135 | 103755716 | 4 | .060 | A>G | 0.07 | .792 | A |
| NFKB1 | rs4648141 | 103755947 | 4 | .188 | G>A | 3.02 | .221 | A |
| NFKB1 | rs1609798 | 103756488 | 4 | .337 | C>T | 1.02 | .600 | A |
| NFKB2 | rs12772374 | 104146901 | 10 | .157 | A>G | 0.10 | .949 | A |
| NFKB2 | rs7897947 | 104147701 | 10 | .229 | T>G | 0.67 | .717 | A |
| NFKB2 | rs11574849 | 104149686 | 10 | .085 | G>A | 0.47 | .792 | A |
| NFKB2 | rs1056890 | 104152760 | 10 | .317 | C>T | 2.47 | .291 | A |
| TNFA | rs2857602 | 31533378 | 6 | .341 | T>C | 0.69 | .708 | A |
| TNFA | rs1800683 | 31540071 | 6 | .390 | G>A | 1.85 | .397 | A |
| TNFA | rs2239704 | 31540141 | 6 | .335 | G>T | 0.42 | .810 | A |
| TNFA | rs2229094 | 31540556 | 6 | .278 | T>C | 1.71 | .426 | A |
| TNFA | rs1041981 | 31540784 | 6 | .386 | C>A | 1.68 | .433 | A |
| TNFA | rs1799964 | 31542308 | 6 | .224 | T>C | 2.34 | .311 | A |
| TNFA | rs1800750 | 31542963 | 6 | .016 | G>A | FE | .712 | A |
| TNFA | rs1800629 | 31543031 | 6 | .149 | G>A | 3.29 | .193 | A |
| TNFA | rs1800610 | 31543827 | 6 | .100 | C>T | 0.71 | .702 | A |
| TNFA | rs3093662 | 31544189 | 6 | .074 | A>G | 0.68 | .712 | A |
A = additive model, Chr = chromosome, D = dominant model, IFNG = interferon gamma, IL = interleukin, MAF = minor allele frequency, n/a = not assayed because SNP violated Hardy-Weinberg expectations (p<0.001), NFKB = nuclear factor kappa beta, R = recessive model, SNP= single nucleotide polymorphism, TNFA = tumor necrosis factor alpha
Single nucleotide polymorphisms (SNPs) that violated Hardy-Weinberg expectations are denoted in italics in the MAF column.
Statistical Analyses for the Phenotypic Data
Data were analyzed using SPSS version 18 (SPSS, Chicago, IL) and STATA Version 9 (STATA Corp). Descriptive statistics and frequency distributions were generated for sample characteristics. Independent sample t-tests (for continuous variables), Mann-Whitney U test (for continuous variables not normally distributed), and Chi square analyses (for categorical variables) were used to evaluate for differences in demographic and clinical characteristics between patients who did and did not report breast pain prior to surgery. All calculations used actual values. Adjustments were not made for missing data. Therefore, the cohort for each analysis was dependent on the largest set of available data between groups.
Statistical Analyses for the Genetic Data
Allele and genotype frequencies were determined by gene counting. Hardy-Weinberg equilibrium was assessed by the Chi-square or Fisher Exact tests. Measures of linkage disequilibrium (i.e., D’ and r2) were computed from the participants’ genotypes with Haploview 4.2. Linkage disequilibrium (LD)-based haplotype block definition was based on D’ confidence interval.20
For SNPs that were members of the same haploblock, haplotype analyses were conducted in order to localize the association signal within each gene and to determine if haplotypes improved the strength of the association with the phenotype. Haplotypes were constructed using the program PHASE version 2.1.62 In order to improve the stability of haplotype inference, the haplotype construction procedure was repeated 5 times using different seed numbers with each cycle. Only haplotypes that were inferred with probability estimates of ≥ 0.85, across the five iterations, were retained for downstream analyses. Only inferred haplotypes that occurred with a frequency estimate of ≥15% were included in the association analyses, assuming a dosage model (i.e., analogous to the additive model).
For association tests, three genetic models were assessed for each SNP: additive, dominant, and recessive. Barring trivial improvements (i.e., delta <10%), the genetic model that best fit the data, by maximizing the significance of the p-value was selected for each SNP. Logistic regression analysis that controlled for significant covariates as well as race/ethnicity, was used to evaluate the association between genotype and pain group membership. Only those genetic associations identified as significant from the univariate analyses were evaluated in the multivariate analyses. A backwards stepwise approach was used to create the most parsimonious model. Except for race/ethnicity, only predictors with a p-value of <0.05 were retained in the final model. Genetic model fit and both unadjusted and covariate-adjusted odds ratios were estimated using the STATA software package, version 9.61 Based on the recommendations of Rothman,53 adjustments were not made for multiple testing.
Ancestry informative markers (AIMs) can be used as a tool to minimize confounding due to population stratification in case-control association studies.25,28,66 Homogeneity in ancestry among participants was verified by principal component analysis,47 using HelixTree (GoldenHelix, Bozeman, MT). Briefly, the number of principal components (PCs) was sought which distinguished the major racial/ethnic groups in the sample by visual inspection of scatter plots of orthogonal PCs (i.e., PC 1 versus PC2, PC2 versus PC3). This procedure was repeated until no discernable clustering of patients by their self-reported race/ethnicity was possible (data not shown). The first three PCs were selected to adjust for potential confounding due to population substructure (i.e., race/ethnicity) by including them in all logistic regression models (described in the preceding paragraph). One hundred and six ancestry informative markers were included in the analysis.
RESULTS
Differences in demographic and clinical characteristics between the pain groups
Of the 398 who completed the baseline assessment, 390 (98%) completed the BSQ at enrollment. One hundred and ten women (28.2%) reported pain in their breast prior to surgery. As shown in Table 2, no between group differences were found in education, marital status, or living arrangements. However, women who reported pain were significantly younger (p < 0.001) and a higher percentage of them were non-white (p= 0.018). In terms of clinical characteristics (Table 2), women in the pain group reported significantly lower KPS scores (p = 0.008); were less likely to be post menopausal (p = 0.012), and had undergone significantly more biopsies (Mann Whitney U = 12887.0; p=0.006).
Table 2.
Differences in Demographic and Clinical Characteristics Between Patients With (n= 110) and Without (n= 280) Breast Pain
| Characteristic | No pain | Pain | Statistic and p-value |
|---|---|---|---|
| mean (SD) | mean (SD) | ||
| Age (years) | 56.5 (11.8) | 50.9 (9.8) | t= 4.81; p< 0.001 |
| Education (years) | 15.8 (2.7) | 15.4 (2.6) | t= 1.42; p= 0.16 |
| Self-administered Comorbidity Questionnaire score | 4.3 (2.8) | 4.2 (3.1) | t= 0.40; p= 0.69 |
| Karnofsky Performance Status score | 94.0 (10.3) | 90.9 (10.1) | t= 2.66; p= 0.008 |
| Number biopsies in past year | 1.5 (0.8) | 1.6 (0.8) | U= 12887.0, p< 0.01 |
| % (N) | % (N) | ||
| Married | 41.9 (117) | 43.0 (46) | FE; p= 0.91 |
| Employed | 48.4 (134) | 50.0 (55) | FE; p= 0.82 |
| Lives alone | 24.1 (67) | 25.2 (27) | FE; p= 0.90 |
| Ethnicity White Black/African American Asian/Pacific Islander Hispanic and Mixed Ethnic Background |
68.1 (190) 7.2 (20) 11.8 (33) 12.9 (36) |
55.0 (60) 15.6 (17) 15.6 (17) 13.8 (15) |
χ2= 8.82; p=0.03 |
| Stage at diagnosis 0 I IIA, IIB IIIA, IIIB, IIIC, IV |
19.3 (54) 38.9 (109) 34.6 (97) 7.1(20) |
17.3 (19) 33.6 (37) 37.3 (41) 11.8 (13) |
KW; 0.40 |
| Estrogen receptor positive | 77.5 (213) | 76.1 (83) | FE; p= 0.79 |
| Progesterone receptor positive | 69.3 (194) | 72.5 (70) | FE; p= 0.62 |
| Her2 neu positive | 16.1 (40) | 16.7 (17) | FE; p=0.88 |
| Post menopausal* | 67.9 (186) | 53.8 (57) | FE; p= 0.012 |
| Received neoadjuvant chemotherapy | 21.1 (59) | 17.3 (19) | FE; p=0.48 |
| Mastitis* | 11.6 (32) | 14.0 (15) | FE; p= 0.49 |
| Fibrocystic or cystic breast disease* | 17.8 (48) | 22.9 (24) | FE; p= 0.31 |
| History of breast feeding* | 49.6 (138) | 39.1 (43) | FE; p= 0.07 |
| Injury to affected arm* | 26.1 (72) | 20.2 (22) | FE; p= 0.24 |
| Injury to affected hand* | 22.3 (62) | 27.4 (29) | FE; p= 0.35 |
| Non-cancer surgery on the affected breast* | 12.9 (36) | 16.5 (18) | FE; p= 0.41 |
| Non-cancer surgery on the affected arm* | 6.1 (17) | 6.5 (7) | FE; p= 1.00 |
| Non-cancer surgery on the affected hand* | 8.7 (24) | 8.3 (9) | FE; p= 1.00 |
Abbreviations: FE = Fisher Exact test, KW = Kruskal-Wallis, U = Mann Whitney U test
Percentage of patients (N) who self-reported this condition
Pain characteristics
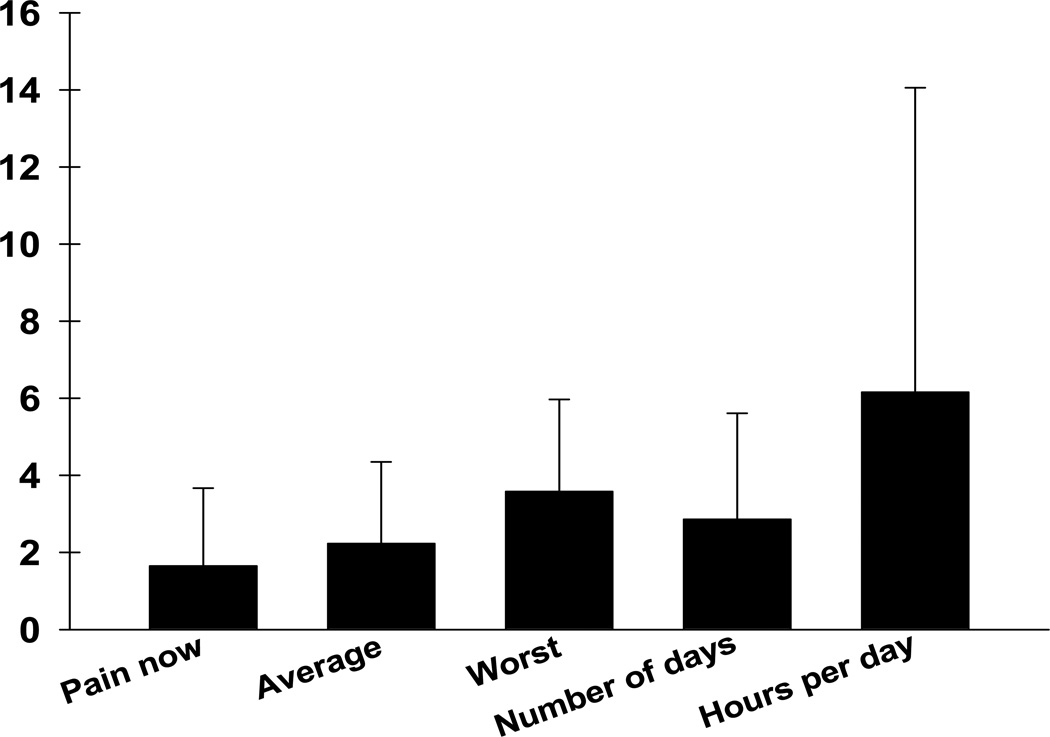
As illustrated in Figure 1, the women with pain (n=110) reported an average pain intensity score of 2.2 (standard deviation (SD) = 2.1) and a worst pain intensity score of 3.6 (SD=2.4). Women reported significant amounts of pain (i.e., pain that interfered with their mood or function) for an average of 6.2 (SD=7.9) hours per day, on an average of 2.9 (SD=2.8) days per week.
Figure 1.
Ratings of present, average, and worst pain intensity as well as number of hours per day and number of days per week that breast pain interferes with mood and/or activities.
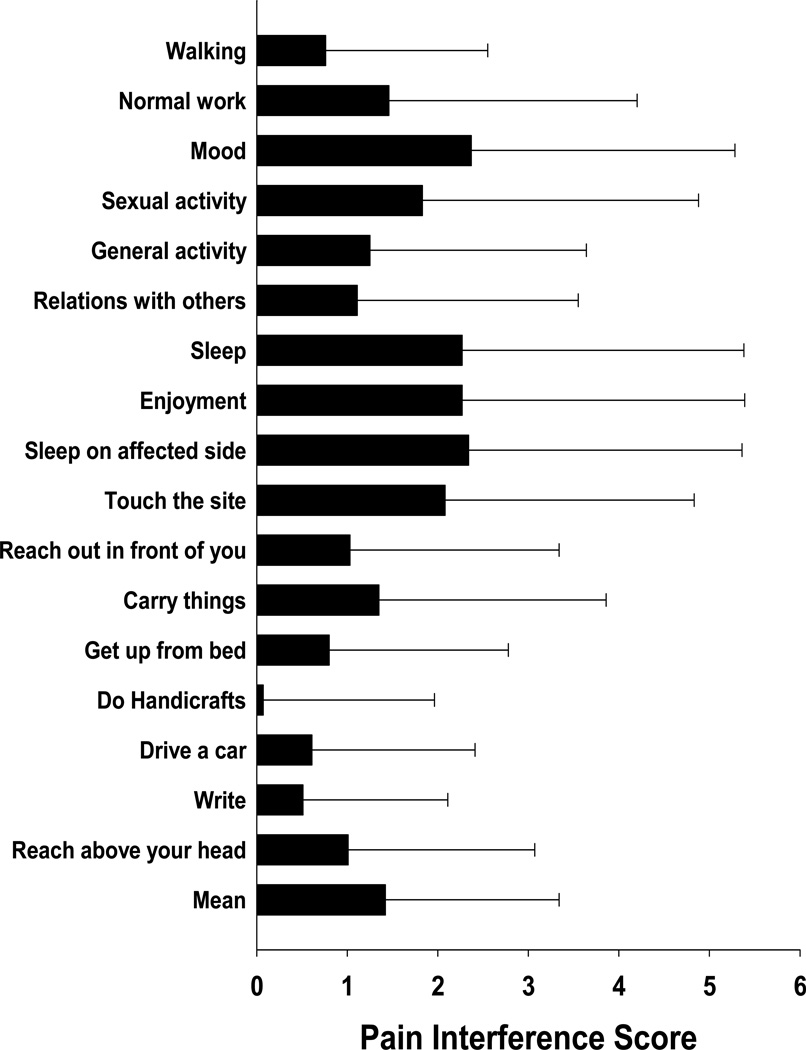
Patients’ ratings of pain interference with routine activities and specific upper extremity functions are illustrated in Figure 2. Interference ratings ranged from 2.4 (SD=2.9) (for mood) to 0.5 (SD=1.6) (for ability to write). The mean interference score was 1.7 (SD=2.2). Patients’ ratings on the PQAS are summarized in Table 3. The five descriptors with the highest ratings were tender, intense, dull, unpleasant, and aching.
Figure 2.
Ratings of pain interference items. All values are plotted as means ± standard deviations.
Table 3.
Individual Item Scores* and Subscale Scores for the Pain Qualities Assessment Scale (PQAS)
| Descriptor | Mean (SD) | Range |
|---|---|---|
| tender | 3.62 (3.20) | 0–10 |
| intense | 2.82 (2.49) | 0–10 |
| dull | 2.80 (2.61) | 0–10 |
| unpleasant | 2.72 (2.49) | 0–10 |
| aching | 2.64 (2.83) | 0–10 |
| shooting | 2.49 (2.91) | 0–10 |
| sharp | 2.35 (2.77) | 0–10 |
| sensitive | 1.86 (2.75) | 0–10 |
| radiating | 1.67 (2.42) | 0–10 |
| heavy | 1.66 (2.69) | 0–10 |
| electrical | 1.62 (2.60) | 0–10 |
| throbbing | 1.63 (2.63) | 0–10 |
| hot | 1.52 (2.55) | 0–10 |
| itchy | 1.38 (2.54) | 0–10 |
| tingling | 1.35 (2.49) | 0–10 |
| cramping | 1.16 (2.41) | 0–10 |
| numb | 0.99 (1.99) | 0–8 |
| cold | 0.36 (1.29) | 0–8 |
| Intense surface pain | 2.15 (2.58) | 0–10 |
| Intense deep pain | 2.92 (2.58) | 0–10 |
| PQAS subscale scores | ||
| Surface pain subscale | 1.19 (1.72) | |
| Paroxysmal pain subscale | 1.95 (2.22) | |
| Deep pain subscale | 1.99 (2.07) | |
Individual item scores are listed in descending order
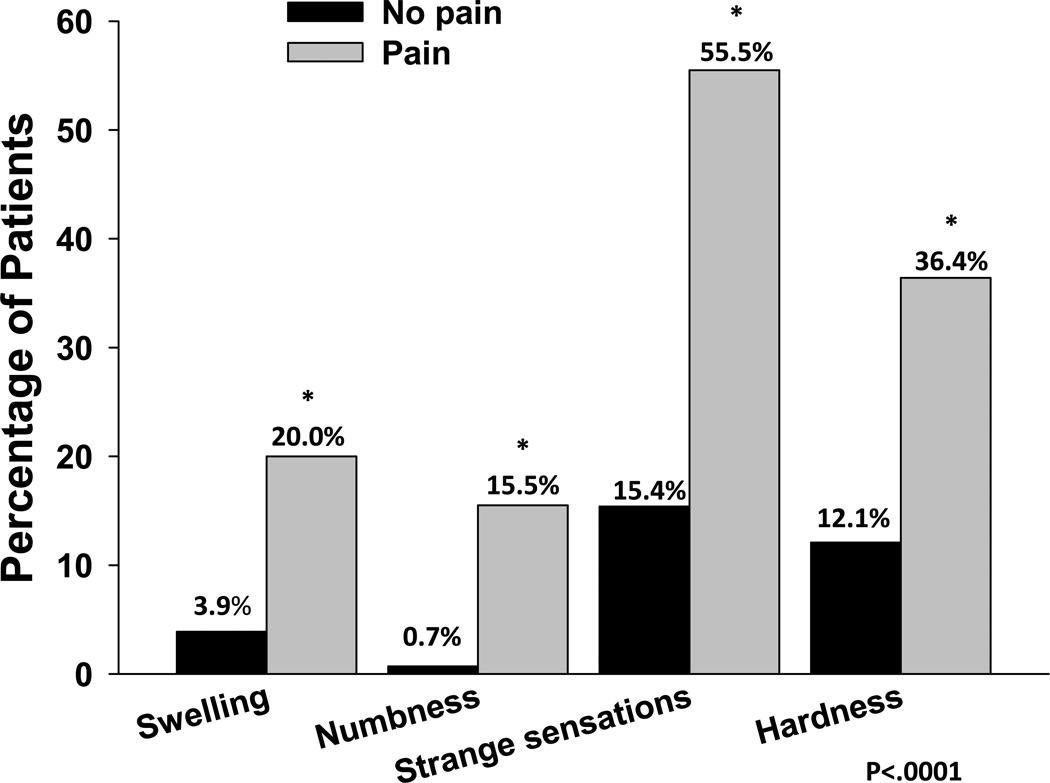
Patients with and without pain completed Part 1 of the BSQ. As shown in Figure 3, a significantly higher percentage of women with breast pain prior to surgery reported swelling (20.0% versus 3.9%), numbness (15.5% versus 0.7%), strange sensations (55.5% versus 15.4%), and hardness (36.4% versus 12.1%; all p<0.0001) in their affected breast.
Figure 3.
Differences in percentages of patients with and without pain who reported swelling, numbness, strange sensations and hardness in their affected breast (all p < 0.0001).
Candidate gene analysis for the occurrence of preoperative breast pain
Tag SNPs spanning IFNG1, IFNGR1, IL1B, IL1R1, IL1R2, IL2, IL4, IL6, IL8, IL10, IL13, IL17A, NFKB1, NFKB2, and TNF-A were chosen for analysis. Of those SNPs chosen, all had genotype distributions that met Hardy-Weinberg expectations with the exception of one each in IL2 and IL10, two in IL6, and seven in IL4. Because these SNPs did not meet this quality control criterion, they were not utilized in subsequent analyses. Statistically significant differences in minor allele distribution between the pain and no pain groups were found for rs2110726 (p = 0.007) in IL1R1 and rs1295686 (p= 0.019) in IL13. While the pre-specified level of significance was not reached, some SNPs had p-values that approached significance: rs2069777 (p= 0.07) in IL2, rs2069840 (p= 0.08) in IL6, rs1800925 (p=0.08) in IL13, and rs4711998 (p= 0.08) in IL17A.
Of note, the observation that 7 of the 9 tag SNPs selected to measure the common variability at the IL4 gene locus failed to meet Hardy-Weinberg expectations (i.e., rs2243250, rs2070874, rs2227284, rs2227282, rs2243266, rs2243267, rs2243274) suggested that the allele frequencies in these SNPs might vary among the major ethnic groups found in our sample. In fact, the minor allele frequencies of all 7 of these SNPs did vary among the ethnic groups (data not shown). However, no evidence of association was found between these IL4 SNPs and the occurrence of preoperative breast pain within or across the population subgroups.
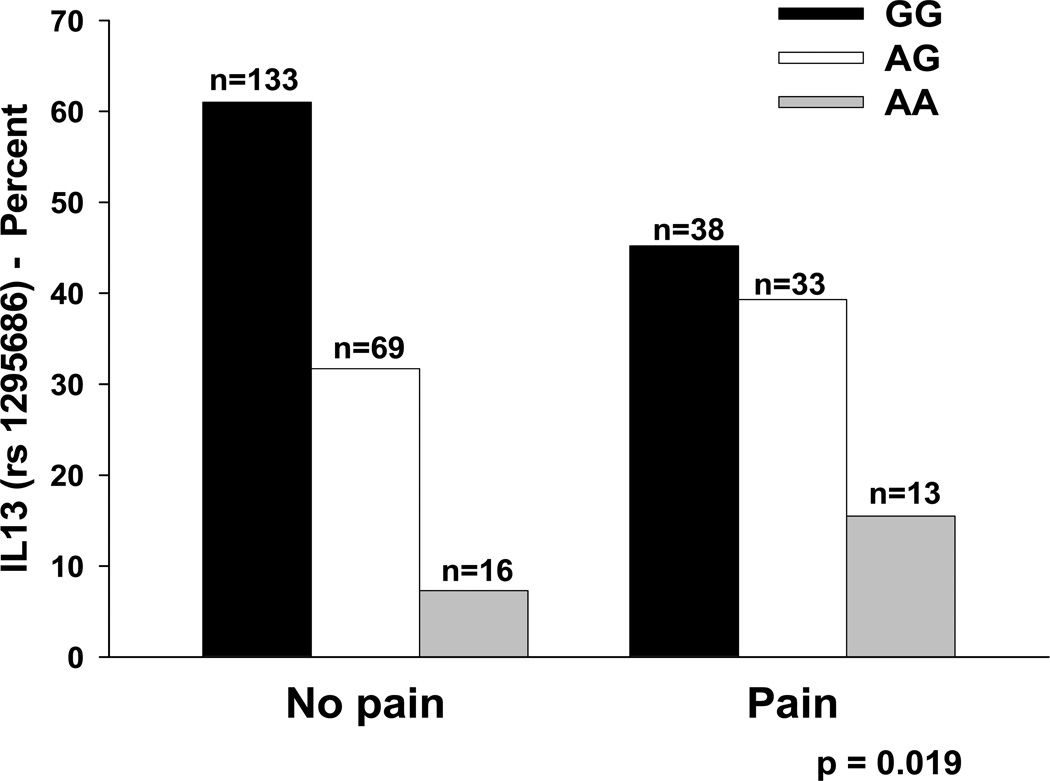
As summarized in Table 1, the minor allele frequency was significantly different between women with and without breast pain for two SNPs: IL1R1 rs2110726 and IL13 rs1295686. For IL1R1 rs2110726, a dominant model fit the data best (p=0.007, Figure 4). For IL13 rs1295686, an additive model fit the data best (p=0.019, Figure 5). In order to better estimate the magnitude (i.e., odds ratio, OR) and precision (95% confidence interval, CI) of the association of genotype on pain group membership, multivariate logistic regression models were fit that included genotype as well as age in years, ethnicity (i.e., White, Black, Asian/Pacific Islander, Hispanic/Mixed ethnic background/Other), functional status (i.e., KPS score), menopausal status, history of breastfeeding, and number of biopsies.
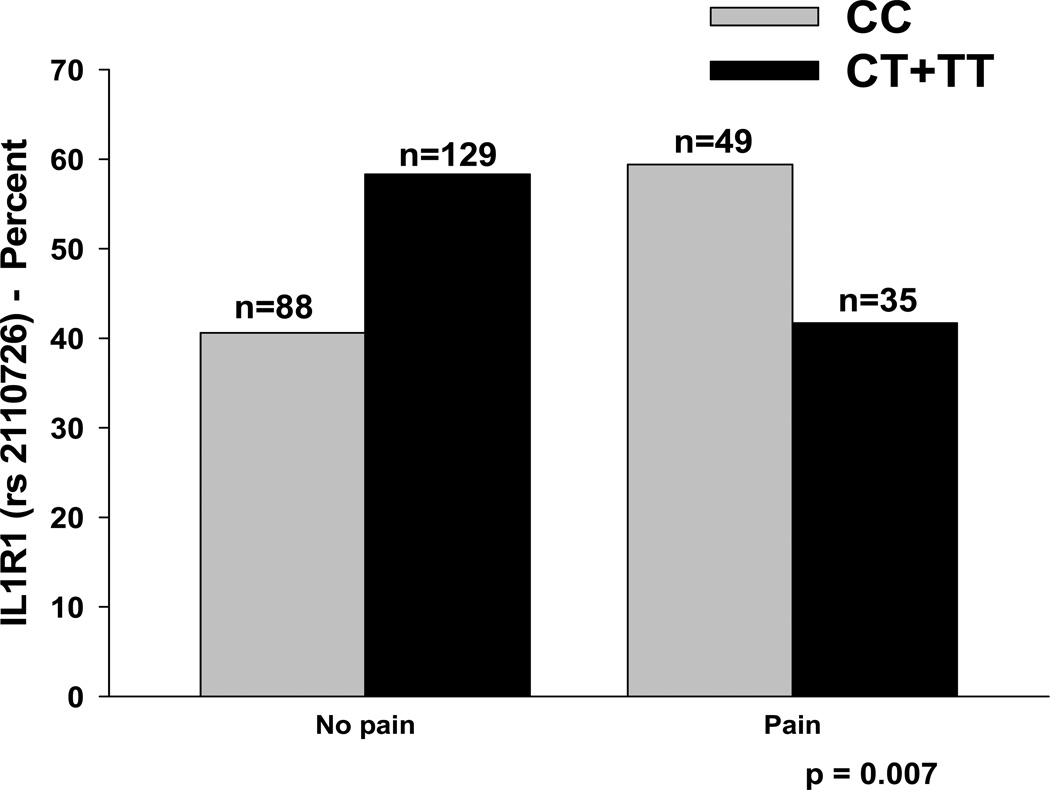
Figure 4.
Differences in the percentages of patients with and without pain who were homozygous for the common allele (CC) or heterozygous or homozygous for the rare allele (CT + TT) for rs2110726 in IL1RI.
Figure 5.
Differences in the percentages of patients with and without pain who were homozygous for the common allele (GG), heterozygous (AG), or homozygous for the rare allele (AA) for rs1295686 in IL13.
In the model fitted for breast pain prior to surgery for IL1R1 (rs2110726), genotype, ethnicity (i.e., white (reference group), Black, Asian/Pacific Islander, Hispanic/Mixed ethnic background/Other), and age were the only predictors retained in the final model (p<0.0001). After controlling for age and ethnicity, carriers of the minor allele (i.e., CT + TT) had a 53% decrease in the odds of reporting breast pain prior to surgery (95% CI: 18.5%, 73.2%, p=0.007). After controlling for IL1R1 genotype and ethnicity, for every 5-year increase in age, the odds of reporting breast pain prior to surgery decreased by 23% (95% CI: 12.1%, 32.0%, p<0.0001). After controlling for IL1R1 genotype and age, for individuals that self-identified as Asian or Pacific Islander, the odds of reporting breast pain prior to surgery decreased by 82% (95% CI: 2.1%, 96.5%, p=0.047). In the model for IL1R1, age and genotype accounted for 8.9% of the variance in the odds of reporting breast pain prior to surgery. Of note, the association between report of breast pain prior to surgery and the IL1R1 two-SNP haplotype (i.e., rs2110726, rs3917332) was explained by the association observed with rs2110726.
In the model fitted for breast pain prior to surgery for IL13 (rs1295686), genotype and age were the only predictors retained in the final model (p<0.0001). After controlling for age, each dose of the minor allele was associated with a 1.57 fold increase in the odds of reporting breast pain prior to surgery (95% CI: 1.037, 2.390, p=0.033). After controlling for IL13 genotype, for every 5-year increase in age, the odds of reporting breast pain prior to surgery decreased by 22.6% (95% CI: 12.1%, 31.9%, p<0.001). In the model for IL13, age and genotype accounted for 8.1% of the variance in odds of reporting breast pain prior to surgery. Of note, the association between report of breast pain prior to surgery and the IL13 two-SNP haplotype (i.e., rs1295686, rs20541) was collinear with the association observed with rs1295686, Therefore, this SNP could not be evaluated for its unique contribution to the odds of reporting breast pain prior to surgery when controlling for rs1295686.
DISCUSSION
This study is the first to describe the characteristics of preoperative breast pain in a sample of women prior to breast cancer surgery and to evaluate for genetic variations in pro-and anti-inflammatory genes in women who did and did not report pain. Consistent with previous studies,14,44,63–65 over one quarter of these patients experienced pain prior to surgery. This number is not insignificant given that in 2011 an estimated 230,480 new cases of breast cancer will be diagnosed in the United States.59 While the worst pain scores were in the mild to moderate range, a large amount of inter-individual variability was noted in this sample. In fact, 36.7% of the women reported a worst pain score of ≥ 4. In addition, these women reported that pain interfered with their activities or mood on approximately 3 days per week for about 6 hours per day. In terms of level of interference (Figure 2), this pain had the largest effect on patients’ mood, sleep, enjoyment of life, and ability to sleep on the affected side. Again, a large amount of inter-individual variability was noted in patients’ interference ratings. Taken together these findings suggest that preoperative breast pain is a significant problem for a subset of women.
Consistent with previous reports, women who reported pain were more likely to be younger1 and have poorer functional status10,39,40,69 than the no pain group. However, while the differences in KPS scores were statistically significant, both groups of women reported high levels of function.
Another interesting but not easily explained finding is that a higher percentage of non-white women reported breast pain prior to surgery. While findings from several studies suggest that members of minority groups report higher rates of chronic pain16–17 and increased sensitivity to painful stimuli,6–8,48 other studies have not demonstrated ethnic differences.17,38 One potential reason for the ethnic differences found in this study is that a higher percentage of non-white women were diagnosed with more advanced disease (61% versus 41%, p=0.035). However, stage of disease was not associated with the occurrence of breast pain in this study. The potential link between ethnicity, stage of disease, and pain warrants investigation in future studies. Finally, women in the pain group were less likely to be post-menopausal, which is consistent with the younger age of this group, and the potential effects of the menstrual cycle and estrogen upon nociception.19,42 These demographic and clinical characteristics suggest a profile of women who are at higher risk for pain prior to surgery.
Possible contributors to presurgical breast pain are tissue injury or nerve damage and inflammation associated with tumor growth, the number of biopsies performed prior to surgery, or both mechanisms. These mechanical injuries could result in the release of inflammatory mediators. This hypothesis is supported by several findings. First, women in the pain group reported a significantly higher number of biopsies. While the total number of biopsies was not normally distributed, 48% of the women in the pain group compared to only 29% in the no pain group had more than one biopsy. Unfortunately, data are not available on the type of biopsy performed, nor when the last biopsy was performed in relationship to completion of the enrollment questionnaire. A higher percentage of patients in the pain group reported swelling, numbness, strange sensations and hardness in their breasts (Figure 3). However, the exact causes for these differences are not readily apparent. Additional analyses were done within the pain group to evaluate whether women who had mastitis or fibrocystic disease reported higher occurrence rates for these four breast qualities. No differences in occurrence rates were found for any of these qualities between women with or without mastitis who reported pain in their breast prior to surgery. The same negative findings were found for fibrocystic disease (data not shown). Of note, the pain qualities reported by the patients with preoperative breast pain are suggestive of nociceptive pain rather than neuropathic pain.72 These phenotypic findings support the data from the genomic analyses that suggest that some innate differences in inflammatory responses may be influencing the development of pre-surgical pain in breast cancer patients.
The results of the SNP analyses suggest that variation in inflammatory pathways involving IL1R1 and IL13 are involved in preoperative pain. In this study, carriers of the minor allele for IL1R1 (rs2110726) had a 53% decrease in the odds of reporting preoperative breast pain. This finding is consistent with studies of IL1 function in mice, in which removal of IL1R function or blockade of IL1 led to a decrease in inflammation and pain behaviors.67 Additional functional studies are needed to determine if the minor allele of rs2110726 is associated with a decrease in IL1R1 function and therefore a decrease in the pro-inflammatory effects of IL1. The rs2110726 is in the 3’ untranslated region of the IL1R1 gene.29
This SNP analysis supports our hypothesis that genetic variation in anti-inflammatory cytokines may be involved in the development of breast pain prior to surgery. IL13, unlike IL1R1, is a cytokine with anti-inflammatory activity. Therefore, its role in pain may be as a moderator of the inflammatory response. In fact, patients with chronic widespread pain syndrome have reduced levels of a number of anti-inflammatory cytokines (i.e., IL2, IL4, IL8, IL10).68 Furthermore, IL4,70 IL10,70 and IL1333,70 are known to have antinociceptive effects in mice, independent of endogenous opioid release, possibly through inhibition of TNFα and IL1β release. The SNP rs1295686 is located in intron 3 of the IL13 gene.55 Given that neither tag SNP is in a coding region of the gene nor predicted to impact gene function (i.e., splicing, alteration of transcription factor binding sites), it is likely that each SNP is in linkage disequilibrium with a functional SNP(s).
Several study limitations need to be acknowledged. No direct measurements of systemic levels of inflammatory markers or physical examination for signs of inflammation at the site were performed to provide additional data on the underlying mechanisms for the preoperative breast pain. In addition, type of biopsy, needle size, and time since biopsy were not obtained which would have provided additional information on the pain phenotype. While proportions of African Americans, Asian/Pacific Islanders, and Caucasians were more representative of the United States population than previous studies on pretreatment breast cancer pain,44,63–65 the relatively small number of non-whites (36%) may have limited our ability to detect genotypic differences among the various ethnic groups. However, the rigorous approach used to control for population substructure (i.e., race/ethnicity) makes it unlikely that the genetic associations observed are due to this important source of confounding. Finally, future studies with a larger sample size, would increase the power to detect differences in the other cytokine genes. This hypothesis might be true for those SNPs in this study where genotypic differences approached statistical significance.
In conclusion, findings from this study and others44,63–65 suggest that preoperative breast pain affects a significant proportion of patients. In addition, the genomic data support the hypothesis that this pain problem involves inflammatory processes. This information may help to identify women who are at greater risk for preoperative breast pain. Subsequent studies will need to confirm these findings and evaluate the specific etiologies for this preoperative breast pain. For example, subsequent studies could evaluate whether the severity of pre-existing breast conditions (e.g., fibrocystic disease, mastitis), tumor characteristics (e.g., size, specific type of breast cancer), or preoperative biopsies contribute to the pain, numbness, hardness, and strange sensations reported by women in the pain group. In addition, future research needs to determine whether preoperative pain influences the severity of postoperative pain and/or the development of chronic pain following breast cancer surgery.
Perspective.
In women with breast cancer, preoperative pain may be associated with increases in inflammatory responses associated with an increased number of biopsies. In addition, differences in cytokine genes may contribute to this preoperative breast pain.
Acknowledgments
Disclosures: This study was funded by grants from the National Cancer Institute (CA107091 and CA118658). Dr. Bradley Aouizerat was funded through the National Institutes of Health (NIH) Roadmap for Medical Research Grant (KL2 RR624130). Dr. Dunn received funding from the Mount Zion Health Fund. Dr. Christine Miaskowski is an American Cancer Society Clinical Research Professor. Dr. Langford is supported by a Department of Defense Breast Cancer Research Program Postdoctoral Fellowship. This project is supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
REFERENCES
- 1.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Barkhordari E, Rezaei N, Ansaripour B, Larki P, Alighardashi M, Ahmadi-Ashtiani HR, Mahmoudi M, Keramati MR, Habibollahi P, Bashashati M, Ebrahimi-Daryani N, Amirzargar AA. Proinflammatory cytokine gene polymorphisms in irritable bowel syndrome. J Clin Immunol. 2010;30(1):74–79. doi: 10.1007/s10875-009-9342-4. [DOI] [PubMed] [Google Scholar]
- 3.Baron RH, Fey JV, Borgen PI, Van Zee KJ. Eighteen sensations after breast cancer surgery: a two-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Oncol Nurs Forum. 2004;31(4):691–698. doi: 10.1188/04.ONF.691-698. [DOI] [PubMed] [Google Scholar]
- 4.Bessler H, Shavit Y, Mayburd E, Smirnov G, Beilin B. Postoperative pain, morphine consumption, and genetic polymorphism of IL-1beta and IL-1 receptor antagonist. Neurosci Lett. 2006;404((1–2)):154–158. doi: 10.1016/j.neulet.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Brunner F, Bachmann LM, Weber U, Kessels AG, Perez RS, Marinus J, Kissling R. Complex regional pain syndrome 1--the Swiss cohort study. BMC Musculoskelet Disord. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113((1–2)):20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in diffuse noxious inhibitory controls. J Pain. 2008;9(8):759–766. doi: 10.1016/j.jpain.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in the nociceptive flexion reflex (NFR) Pain. 2008;134(1–2):91–96. doi: 10.1016/j.pain.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen RH, Chang CT, Chen WC, Tsai CH, Tsai FJ. Proinflammatory cytokine gene polymorphisms among Hashimoto's thyroiditis patients. J Clin Lab Anal. 2006;20(6):260–265. doi: 10.1002/jcla.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng KK, Lee DT. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol. 2011;78(2):127–137. doi: 10.1016/j.critrevonc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Cieza A, Geyh S, Chatterji S, Kostanjsek N, Ustun BT, Stucki G. Identification of candidate categories of the International Classification of Functioning Disability and Health (ICF) for a Generic ICF Core Set based on regression modelling. BMC Med Res Methodol. 2006;6:36. doi: 10.1186/1471-2288-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleeland CS, Gonin R, Baez L, Loehrer P, Pandya KJ. Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med. 1997;127(9):813–816. doi: 10.7326/0003-4819-127-9-199711010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Conde L, Vaquerizas JM, Dopazo H, Arbiza L, Reumers J, Rousseau F, Schymkowitz J, Dopazo J. PupaSuite: finding functional single nucleotide polymorphisms for large-scale genotyping purposes. Nucleic Acids Res. 2006;34:W621–W625. doi: 10.1093/nar/gkl071. Web Server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corry DC. Pain in carcinoma of the breast. Lancet. 1952;1(6702):274–276. doi: 10.1016/s0140-6736(52)90339-5. [DOI] [PubMed] [Google Scholar]
- 15.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17(2):197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 16.Day MA, Thorn BE. The relationship of demographic and psychosocial variables to pain-related outcomes in a rural chronic pain population. Pain. 2010;151(2):467–474. doi: 10.1016/j.pain.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic similarities and differences in the chronic pain experience: a comparison of African American, Hispanic, and white patients. Pain Med. 2005;6(1):88–98. doi: 10.1111/j.1526-4637.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg E, Pud D, Koltun L, Loven D. Effect of early administration of the N-methyl-d-aspartate receptor antagonist amantadine on the development of postmastectomy pain syndrome: a prospective pilot study. J Pain. 2007;8(3):223–229. doi: 10.1016/j.jpain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Fillingim RB, Edwards RR. The association of hormone replacement therapy with experimental pain responses in postmenopausal women. Pain. 2001;92(1–2):229–234. doi: 10.1016/s0304-3959(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 21.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48(2):332–338. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- 22.Gan XL, Lin YH, Zhang Y, Yu TH, Hu LN. Association of an interleukin-16 gene polymorphism with the risk and pain phenotype of endometriosis. DNA Cell Biol. 2010;29(11):663–667. doi: 10.1089/dna.2010.1049. [DOI] [PubMed] [Google Scholar]
- 23.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 24.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(Suppl 1):i104–i108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 25.Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Hum Mutat. 2008;29(5):648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Hickey OT, Burke SM, Hafeez P, Mudrakouski AL, Hayes ID, Shorten GD. Severity of acute pain after breast surgery is associated with the likelihood of subsequently developing persistent pain. Clin J Pain. 2010;26(7):556–560. doi: 10.1097/AJP.0b013e3181dee988. [DOI] [PubMed] [Google Scholar]
- 28.Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, McKeigue PM. Control of confounding of genetic associations in stratified populations. Am J Hum Genet. 2003;72(6):1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iida A, Saito S, Sekine A, Mishima C, Kitamura Y, Kondo K, Harigae S, Osawa S, Nakamura Y. Catalog of 668 SNPs detected among 31 genes encoding potential drug targets on the cell surface. J Hum Genet. 2003;48(1):23–46. doi: 10.1007/s100380300004. [DOI] [PubMed] [Google Scholar]
- 30.Janz NK, Mujahid M, Chung LK, Lantz PM, Hawley ST, Morrow M, Schwartz K, Katz SJ. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health. 2007;16(9):1348–1361. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4(1):2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MP, Gammaitoni AR, Olaleye DO, Oleka N, Nalamachu SR, Galer BS. The pain quality assessment scale: assessment of pain quality in carpal tunnel syndrome. J Pain. 2006;7(11):823–832. doi: 10.1016/j.jpain.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Karam MC, Al-Kouba JE, Bazzi SI, Smith CB, Leung L. Interleukin-13 reduces hyperalgesia and the level of interleukin-1beta in BALB/c mice infected with Leishmania major with an up-regulation of interleukin-6. J Neuroimmunol. 2011;234:49–54. doi: 10.1016/j.jneuroim.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Karnofsky D. Performance scale. New York: Plenum Press; 1977. [Google Scholar]
- 35.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 36.Kim E, Jahan T, Aouizerat BE, Dodd MJ, Cooper BA, Paul SM, West C, Lee K, Swift PS, Wara W, Miaskowski C. Changes in symptom clusters in patients undergoing radiation therapy. Support Care Cancer. 2009;17(11):1383–1391. doi: 10.1007/s00520-009-0595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim E, Jahan T, Aouizerat BE, Dodd MJ, Cooper BA, Paul SM, West C, Lee K, Swift PS, Wara W, Miaskowski C. Differences in symptom clusters identified using occurrence rates versus symptom severity ratings in patients at the end of radiation therapy. Cancer Nurs. 2009;32(6):429–436. doi: 10.1097/NCC.0b013e3181b046ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Neubert JK, Rowan JS, Brahim JS, Iadarola MJ, Dionne RA. Comparison of experimental and acute clinical pain responses in humans as pain phenotypes. J Pain. 2004;5(7):377–384. doi: 10.1016/j.jpain.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Knudsen AK, Brunelli C, Kaasa S, Apolone G, Corli O, Montanari M, Fainsinger R, Aass N, Fayers P, Caraceni A, Klepstad P. European Palliative Care Research Council, European Pharmacogenetic Study: Which variables are associated with pain intensity and treatment response in advanced cancer patients?--Implications for a future classification system for cancer pain. Eur J Pain. 2011;15(3):320–327. doi: 10.1016/j.ejpain.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Langford DJ, Tripathy D, Paul SM, West C, Dodd MJ, Schumacher K, Miaskowski C. Trajectories of pain and analgesics in oncology outpatients with metastatic bone pain. J Pain. 2011;12:495–507. doi: 10.1016/j.jpain.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Grivennikov SI, Karin M. The Unholy Trinity: Inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19(4):429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin VT. Ovarian hormones and pain response: a review of clinical and basic science studies. Gend Med. 2009;6(Suppl 2):168–192. doi: 10.1016/j.genm.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Nakki A, Kouhia ST, Saarela J, Harilainen A, Tallroth K, Videman T, Battie MC, Kaprio J, Peltonen L, Kujala UM. Allelic variants of IL1R1 gene associate with severe hand osteoarthritis. BMC Med Genet. 2010;11:50. doi: 10.1186/1471-2350-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, Dworkin RH. Risk factors for chronic pain following breast cancer surgery: A prospective study. J Pain. 2006;7(9):626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Coyle N, Smart-Curley T, Kemeny N, Norton L, Hoskins W. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3(3):183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 46.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L. The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 47.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 48.Rahim-Williams FB, Riley JL, 3rd, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 2007;129(1–2):177–184. doi: 10.1016/j.pain.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes-Gibby CC, El Osta B, Spitz MR, Parsons H, Kurzrock R, Wu X, Shete S, Bruera E. The influence of tumor necrosis factor-alpha-308 G/A and IL-6-174 G/C on pain and analgesia response in lung cancer patients receiving supportive care. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3262–3267. doi: 10.1158/1055-9965.EPI-08-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes-Gibby CC, Shete S, Yennurajalingam S, Frazier M, Bruera E, Kurzrock R, Crane CH, Abbruzzese J, Evans D, Spitz MR. Genetic and nongenetic covariates of pain severity in patients with adenocarcinoma of the pancreas: Assessing the influence of cytokine genes. J Pain Symptom Manage. 2009;38(6):894–902. doi: 10.1016/j.jpainsymman.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reyes-Gibby CC, Spitz M, Wu X, Merriman K, Etzel C, Bruera E, Kurzrock R, Shete S. Cytokine genes and pain severity in lung cancer: Exploring the influence of TNF-{alpha}-308 G/A IL6-174G/C and IL8-251T/A. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2745–2751. doi: 10.1158/1055-9965.EPI-07-0651. [DOI] [PubMed] [Google Scholar]
- 52.Reyes-Gibby CC, Spitz MR, Yennurajalingam S, Swartz M, Gu J, Wu X, Bruera E, Shete S. Role of inflammation gene polymorphisms on pain severity in lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2636–2642. doi: 10.1158/1055-9965.EPI-09-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 54.Sadeghnejad A, Karmaus W, Arshad SH, Kurukulaaratchy R, Huebner M, Ewart S. IL13 gene polymorphisms modify the effect of exposure to tobacco smoke on persistent wheeze and asthma in childhood, a longitudinal study. Respir Res. 2008;9:2. doi: 10.1186/1465-9921-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sadeghnejad A, Karmaus W, Hasan Arshad S, Ewart S. IL13 gene polymorphism association with cord serum immunoglobulin. E. Pediatr Allergy Immunol. 2007;18(4):288–292. doi: 10.1111/j.1399-3038.2006.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity1 for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 57.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 58.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 59.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 60.Smith AJ, Keen LJ, Billingham MJ, Perry MJ, Elson CJ, Kirwan JR, Sims JE, Doherty M, Spector TD, Bidwell JL. Extended haplotypes and linkage disequilibrium in the IL1R1- IL1A-IL1B-IL1RN gene cluster: Association with knee osteoarthritis. Genes Immun. 2004;5(6):451–460. doi: 10.1038/sj.gene.6364107. [DOI] [PubMed] [Google Scholar]
- 61.StataCorp. Stata Statistical Software: Release 9. College Station, Texas: Stata Corporation; 2005. [Google Scholar]
- 62.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tasmuth T, Estlanderb AM, Kalso E. Effect of present pain and mood on the memory of past postoperative pain in women treated surgically for breast cancer. Pain. 1996;68(2–3):343–347. doi: 10.1016/s0304-3959(96)03219-8. [DOI] [PubMed] [Google Scholar]
- 64.Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453–459. doi: 10.1093/oxfordjournals.annonc.a059215. [DOI] [PubMed] [Google Scholar]
- 65.Tasmuth T, von Smitten K, Kalso E. Pain and other symptoms during the first year after radical and conservative surgery for breast cancer. Br J Cancer. 1996;74(12):2024–2031. doi: 10.1038/bjc.1996.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Genet. 2008;17(R2):R143–R150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres R, Macdonald L, Croll SD, Reinhardt J, Dore A, Stevens S, Hylton DM, Rudge JS, Liu-Bryan R, Terkeltaub RA, Yancopoulos GD, Murphy AJ. Hyperalgesia, synovitis and multiple biomarkers of inflammation are suppressed by interleukin 1 inhibition in a novel animal model of gouty arthritis. Ann Rheum Dis. 2009;68(10):1602–1608. doi: 10.1136/ard.2009.109355. [DOI] [PubMed] [Google Scholar]
- 68.Uceyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum. 2006;54(8):2656–2664. doi: 10.1002/art.22026. [DOI] [PubMed] [Google Scholar]
- 69.Utne I, Miaskowski C, Bjordal K, Paul SM, Jakobsen G, Rustoen T. Differences in the use of pain coping strategies between oncology inpatients with mild vs. moderate to severe pain. J Pain Symptom Manage. 2009;38(5):717–726. doi: 10.1016/j.jpainsymman.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Vale ML, Marques JB, Moreira CA, Rocha FA, Ferreira SH, Poole S, Cunha FQ, Ribeiro RA. Antinociceptive effects of interleukin-4-10, and-13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther. 2003;304(1):102–108. doi: 10.1124/jpet.102.038703. [DOI] [PubMed] [Google Scholar]
- 71.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112(1):116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Victor TW, Jensen MP, Gammaitoni AR, Gould EM, White RE, Galer BS. The dimensions of pain quality: Factor analysis of the Pain Quality Assessment Scale. Clin J Pain. 2008;24(6):550–555. doi: 10.1097/AJP.0b013e31816b1058. [DOI] [PubMed] [Google Scholar]
- 73.Wang XM, Hamza M, Wu TX, Dionne RA. Upregulation of IL-6, IL-8 and CCL2 gene expression after acute inflammation: Correlation to clinical pain. Pain. 2009;142(3):275–283. doi: 10.1016/j.pain.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang XM, Wu TX, Hamza M, Ramsay ES, Wahl SM, Dionne RA. Rofecoxib modulates multiple gene expression pathways in a clinical model of acute inflammatory pain. Pain. 2007;128(1–2):136–147. doi: 10.1016/j.pain.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24(6):968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. 2005;14(4):166–174. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- 77.Wolf G, Livshits D, Beilin B, Yirmiya R, Shavit Y. Interleukin-1 signaling is required for induction and maintenance of postoperative incisional pain: genetic and pharmacological studies in mice. Brain Behav Immun. 2008;22(7):1072–1077. doi: 10.1016/j.bbi.2008.03.005. [DOI] [PubMed] [Google Scholar]