Abstract
The selective attention to particular aspects of incoming sensory information is enabled by a network of neural areas that includes frontal cortex, posterior parietal cortex, and, in the visual domain, visual sensory regions. Although progress has been made in understanding the relative contribution of these different regions to the process of visual attentional selection, primarily through studies using neuroimaging, rather little is known about the temporal relationships between these disparate regions. To examine this, participants viewed two rapid serial visual presentation (RSVP) streams of letters positioned to the left and right of fixation point. Before each run, attention was directed to either the left or the right stream. Occasionally, a digit appeared within the attended stream indicating whether attention was to be maintained within the same stream (‘hold’ condition) or to be shifted to the previously ignored stream (‘shift’ condition). By titrating the temporal parameters of the time taken to shift attention for each participant using a fine-grained psychophysics paradigm, we measured event-related potentials time-locked to the initiation of spatial shifts of attention. The results revealed shifts of attention were reflected earlier in the response recorded over frontal than over parietal electrodes and, importantly, that the early activity over frontal electrodes was associated with a successful shift of attention. We conclude that frontal areas are engaged early for the purpose of executing an attentional shift, likely triggering a cascade through the fronto-parietal network and, ultimately, resulting in attentional modulation of sensory events in posterior cortices.
INTRODUCTION
The human visual system sorts through massive amounts of sensory input, which is sampled almost continuously, to arrive at a coherent perception of the scene. This process of searching through the environment for behaviorally-relevant information is a ubiquitous component of sensory processing, and it reflects the remarkable ability of the perceptual system to select dynamically information that is compatible with the current goal of the organism. Such perceptual selectivity, referred to as attention, is considered central to cognition, with selected or attended information subsequently receiving preferential or enhanced processing. One of the key elements to understanding attentional selection is to determine what representations are engaged by this process such that they serve as potential candidates for selection. Several possible representations have been identified including those that are space- (Eriksen & Hoffman, 1972; Posner, Snyder, & Davidson, 1980; Yantis, et al., 2002), feature- (Corbetta, Miezin, Dobmeyer, Shulman, & Petersen, 1991; Liu, Slotnick, Serences, & Yantis, 2003), object- (Corbetta, Tansy, et al., 2005; Duncan, 1984; Kanwisher & Driver, 1992; Shomstein & Behrmann, 2006), and/or modality-based (Bushara, et al., 1999; Shomstein & Yantis, 2004), and much recent psychophysical and imaging work has explored the similarities and distinctions between these forms of attentional selection and underlying representations.
Of all of these different potential candidate representations from which selection can occur, selection from space-based representations is perhaps the most pervasive and fundamental. Not only do space-based representations reflect topographical organization and layout of early visual cortex, but these representations describe the sensory environment with a unique set of 3D identifiers (i.e., each stimulus in the sensory environment occupies a unique set of spatial coordinates), thereby facilitating location-based selection in a direct and isomorphic manner. This space-based selection is reflected in multiple visual cortical areas as increased activity of neurons representing the attended location (Bisley & Goldberg, 2003; Moran & Desimone, 1985; Saalmann, Pigarev, & Vidyasagar, 2007; Somers, Dale, Seiffert, & Tootell, 1999; Treue & Maunsell, 1996). The behavioral benefit of this enhanced neural selectivity is that stimuli that appear in attended spatial locations are processed more efficiently and more accurately than stimuli that appear elsewhere (Chawla, Rees, & Friston, 1999; Posner, 1980; Treue & Martinez Trujillo, 1999; Yantis, et al., 2002).
Despite the growing understanding of attentional selection gleaned from numerous studies, we do not yet have a full understanding of the mechanism that serves as the source to initiate the attentional orienting signal, which, ultimately, results in the neural modulation and behavioral benefit for attended locations. Investigations of this issue have uncovered a network of regions spanning frontal and parietal cortices that triggers a control signal for shifting from one representation to another, be it one that is space-based (Corbetta & Shulman, 2002; Hopfinger, Buonocore, & Mangun, 2000; Serences & Yantis, 2007), feature-based (Greenberg, Esterman, Wilson, Serences, & Yantis, 2010; Liu, et al., 2003), or object-based (Shomstein & Behrmann, 2006). Although there is general consensus concerning regions that are engaged in this attentional shifting process, the relative contributions of the identified frontal and parietal regions have been difficult to characterize. Moreover, some studies have yielded conflicting findings, with several investigations suggesting that the initial spatial re-orienting signal is elicited by the frontal cortex, while others suggest that it is the parietal cortex that initiates the re-orienting signal with frontal cortex following suit (Brignani, Lepsien, Rushworth, & Nobre, 2009; Buschman & Miller, 2007; Green & McDonald, 2008; Simpson, et al., 2011). It should be noted that while most investigations of bottom-up attentional capture have convincingly demonstrated that the shifting signal originates over the parietal cortex (Fu, Greenwood, & Parasuraman, 2005; Green, Doesburg, Ward, & McDonald, 2011; Hopfinger & Ries, 2005; Leblanc, Prime, & Jolicoeur, 2008; Ptak, Camen, Morand, & Schnider, 2011), most of the controversy regarding the temporal relationship between the source signals over frontal or parietal cortex has been exclusive to the investigations of top-down attentional control.
Part of the difficulty in determining the relative contribution of frontal and parietal regions to the attentional control signal lies in the fact that the neural profiles of these areas observed in response to the initiation of a spatial shift are similar, and, consequently, it is difficult to untangle and disambiguate their independent contributions. For example, both frontal and parietal regions contain topographically mapped priority maps. Single-unit physiology experiments with awake behaving monkeys have found evidence that both the frontal eye fields (FEFs) and the lateral intraparietal area (LIP) contain representations compatible with priority maps (Balan & Gottlieb, 2006; Bisley & Goldberg, 2010; Thompson & Bichot, 2005; Thompson, Bichot, & Sato, 2005), usually assumed to be the first step in triggering the shift signal. Concordantly, functional imaging studies in humans have found that corresponding frontal and parietal areas contain topographic representations related to saccade planning and attention (Chiu, Esterman, Gmeindl, & Yantis, 2011; Esterman, Chiu, Tamber-Rosenau, & Yantis, 2009; Greenberg, et al., 2010; Greenberg, et al., in press; Silver & Kastner, 2009), suggesting that these areas in humans may also contain priority maps utilized for the upcoming shift of attention. Moreover, the shift-related signal elicited over frontal and parietal regions is similar with the result that both regions are best described as initiating a transient signal, as measured by both fMRI and ERP. This identified transient signal is interpreted as being responsible for issuing, or initiating, an attention control signal to switch the current spatial focus of attention but a more detailed account of the dynamics of these disparate regions remains elusive (Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000; Hopfinger, et al., 2000; Rushworth, Paus, & Sipila, 2001; Yantis, et al., 2002).
One possible clue that might assist in uncovering the relative contribution of frontal and parietal areas to the control of spatial attention lies in the ability to identify the relative timing of the corresponding activations in the different regions. Measuring event-related potentials (ERP) provides an ideal opportunity to exploit high temporal-resolution data and to examine the temporal relationship between the initiation of the spatial attentional control signal observed over the frontal and the parietal cortex. The goal of the present investigation was, thus, to elucidate the relative functional roles of two major nodes of the human attentional network, the frontal and parietal cortices, by focusing on the temporal relationships between these important subregions.
In order to assess the relative timing of the contribution of frontal and parietal cortices to spatial shifts of attention, we adopted a two-pronged approach. First, we conducted detailed psychophysical investigations to determine the timing thresholds required, on an individual-by-individual basis, to initiate a spatial shift of attention so as to delineate the particular switch signature for each participant. At the same time, we determined a threshold at which each participant was able to detect a target after the switch of attention so that the signal for trials in which the shift was successful could be separated from trials in which it was not. Second, in a separate session, each participant's neural activity was recorded by ERP, while the individual completed the behavioral attentional shifting task with the unique parameters for stimulus presentation adopted from the individual thresholding session. Critically, these attentional switch thresholds ensured that we were indexing the ERP components that occurred before the attentional shift initiation (i.e., source of the attentional shifting signal) as opposed to those components that occur after the execution of the shift. In this way, we can isolate the components that are related to the initiation of a spatial shift of attention, rather than a host of perceptual/post-perceptual processes that are involved in target detection, more generally.
Elucidating the neural mechanism of top-down spatial shifts of attention can also prove useful for understanding the behavioral deficits following damage to the parietal lobe. Clinical symptoms of hemispatial neglect have been strongly associated with damage to the parietal lobe including the temporo-parietal junction (TPJ) as well as inferior parietal lobule (IPL) as well as connections between frontal and parietal cortices, all regions associated with shifts of spatial attention (Bartolomeo, Thiebaut de Schotten, & Doricchi, 2007; Corbetta, Kincade, Lewis, Snyder, & Sapir, 2005; Friedrich, Egly, Rafal, & Beck, 1998; Ptak & Schnider, 2010; Shomstein, Lee, & Behrmann, 2010; Thiebaut de Schotten, et al., 2005; Vallar & Perani, 1986).
METHOD
Participants
Twelve neurologically healthy right-handed adults (ages 21-33, 5 female) with normal or corrected-to-normal visual acuity participated in two experimental sessions (psychophysical and ERP recording). Participants provided written consent to participate in the protocol that was approved by the Institutional Review board of Carnegie Mellon University and were paid for their participation.
Paradigm
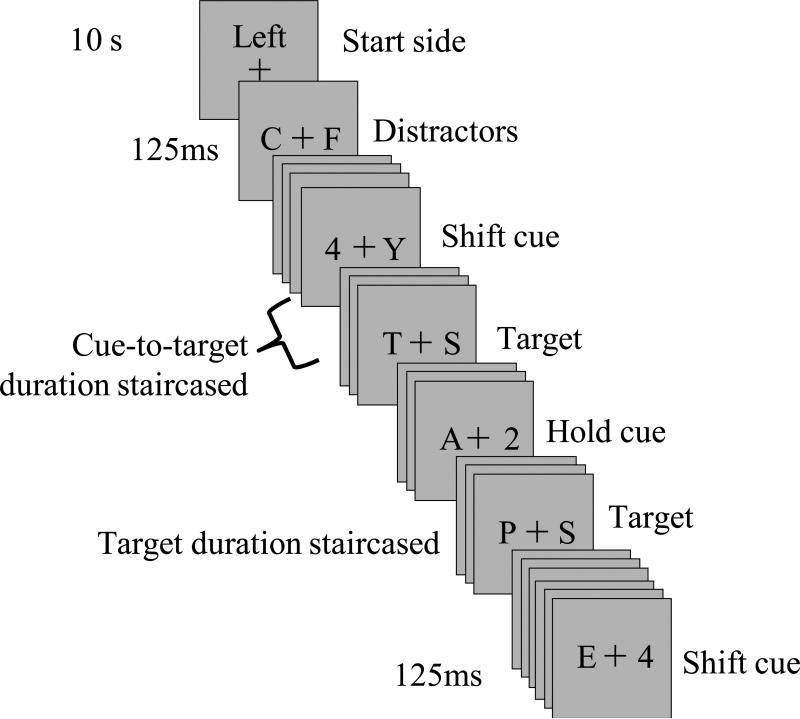
The behavioral task, depicted in Figure 1, is a variant of a previously described rapid serial visual presentation (RSVP) task (Sperling & Reeves, 1980). In this task, two streams of letters appear on a computer screen, one to the right and one to the left of a central fixation cross. Stimuli were rendered in black on a gray background (RGB: 128, 128, 128) and presented at a rate of 8Hz (125ms, unless otherwise noted). Subjects were instructed to maintain fixation on a central cross, presented on a 19” CRT monitor with a refresh rate of 60Hz and subtending 0.4° of visual angle from a viewing distance of 60cm. At the beginning of each run, an attentional cue with the words “left” or “right” (presented for 10s) instructed subjects which stream of letters was to be attended first. After the cue disappeared, the two streams of letters appeared 2.5° to the left and right of the fixation cross. Each letter in the stream changed identity synchronously every 125ms. Letters were chosen at random from a predetermined set (‘A’, ‘C’, ‘F’, ‘G’, ‘H’, ‘J’, ‘K’, ‘M’, ‘N’, ‘P’, ‘R’, ‘T’, ‘U’, ‘V’, ‘X’, ‘Y’) and subtended approximately 0.5° horizontally and 0.6° vertically. Occasionally a digit (“4” or “2”) appeared within the attended stream only.
Figure 1. Stimuli and Task.
The visual display contained two streams of letters, each of which could contain a cue and a target. Only one stream was attended at a time, with cues and targets appearing within the attended stream only. Cues indicated whether a spatial shift of attention was required (shift cue, 4) or whether attention was to be maintained in the currently attended stream. Participants pressed a button in response to the target (letter S). Over the course of a behavioral testing session, two parameters were staircaised – target duration and cue-to-target interval.
The participant's task was twofold. Firstly, participants were to detect digits embedded among the stream of letters, and all letters, aside from ‘S’ (see below), served as ‘distractors’ and were to be ignored. Digits served as attentional cues indicating whether attention was to be maintained within the same, already attended, stream (hold condition, digit “2”) or to be shifted to the previously ignored stream (shift condition, digit “4”). Cues did not require a response. Secondly, participants were asked to detect the appearance of the target letter “S” (by depressing a space bar) that appeared shortly after the digit cue. Target letters appeared only within the attended stream, and followed 66% of the cues (the remaining cues had no subsequent target and, thus, served as catch trials). No targets appeared without the prior appearance of a cue. Following the target (or the time at which it would have occurred in a catch trial), the next cue occurred randomly between 2 and 4 seconds later.
Psychophysics
The first experimental session was used to derive a psychophysical estimate of the time required to execute an attentional shift for each individual subject. Two variables were manipulated in order to arrive at an accurate estimation – target letter presentation time and cue-to-target duration time. First, using the staircasing method (adaptive method based on estimating the most informative intervals for deriving each participant's distribution thresholds based on an assumed distribution psychometric method described elsewhere (Watson & Pelli, 1983)), we manipulated the exposure duration of the target “S” with accuracy to identify the “S” fixed at 90%, while the cue-to-target interval was set at 800ms to allow for sufficient time between the cue and target to move spatial selection from one stream to another without any time constraints. The thresholding procedure lasted approximately 20 minutes and the final estimated threshold at the end of that time was taken as that participant's target duration. Once the target duration was established, an additional staircase procedure was run to determine the amount of time following the cue that was required for participants to detect 66% of the targets, in other words to determine the amount of time needed in order to initiate and execute a spatial shift of attention. This latter threshold was estimated by first applying the derived individual target detection thresholds while staircasing the duration of the cue-to-target interval. Participants performed 30 blocks consisting of 64 (32 shifts and 32 holds) trials each and individual target thresholds were computed – 15 blocks for target thresholding and 15 blocks for cue-to-target interval thresholding procedures (approximately 25 minutes). During the ERP recording, the final temporal thresholds for target duration and cue-to-target interval established during the psychophysical session were used and 66% of cues were followed by targets (the remainder served as ‘catch’ trials). In this session, participants completed a total of twenty blocks of 64 trials each.
Electrophysiological recording and analysis
EEG was recorded using Ag/AgCl electrodes embedded in a fabric cap (Neuroscan, El Paso, TX), from 64 scalp locations. Electrodes were also placed on the right mastoid, above and below the left eye, and on the outer canthi of both eyes. The ground was placed at location AFz. All electrode recording was referenced to the left mastoid, and electrode impedances were kept below 10 kΩ. EEG data were collected using SynAmps2 amplifiers (Neuroscan) from 0.1 to 200 Hz, sampled at 1000Hz with a resolution of 29.8 ηV, and amplified with a gain of 2,816.
Following data acquisition, the continuous EEG data were corrected for ocular movement artifacts, and separate cue- and target-locked 1000ms epochs were extracted (epochs with changes exceeding 100 μV were discarded) from each electrode. Epochs were baseline corrected relative to the period of -100 to 0 msec before the cue or target onset, depending on the analysis. Signals obtained on the electrodes were then averaged to form four regions-of-interest (ROI), reflecting a side x cortical region 2×2 design: frontal right (F4, F6, F8), frontal left (F3, F5, F7), parietal right (P4, P6, P8) and left (P3, P5, P7). Inspection of responses showed a large impact of whether stimuli were in the ipsilateral or contralateral field. To increase power and better capture these effects, data were further averaged across hemispheres (e.g. left/right frontal) by whether the stimuli were in the ipsilateral or contralateral field and hence the statistical analysis is done by ROIs (Frontal, Parietal) × side (ipsi/contralateral). Note that because our interest was primarily in the more anterior sites (frontal, parietal), we focused our analytic explorations in this region. Unfortunately, perhaps because we only had a single electrode in each occipital hemisphere, we were unable to reliably separate occipital signals, which may have provided a marker for the final deployment of spatial attention from signals arising in parietal cortex. However, previous studies have demonstrated that there is robust signal in these early cortices that reflect the consequences of the attentional switch accompanied by enhancement of topographic regions associated with the selected spatial locations (Shomstein & Behrmann, 2006).
ERP grand averages
Artifact-free data from four ROIs (frontal (ipsi/contralateral) and parietal (ipsi/contralateral electrodes)) were then used to create ERP waveforms separately for each event. The waveforms were referenced to the average of the left mastoid and low-pass filtered at 30Hz. Trials were then averaged together to create grand average waveforms for each combination of condition (Shift Hit, Hold Hit, or Random Letter) and field (Contralateral, Ipsilateral) at each ROI to allow us to examine the signal associated with the shift of attention versus the hold of attention. In addition to comparing waveforms from shift versus hold trials, we also compared shift versus the Random Letter (a distractor) trials as this serves as a neutral condition or baseline in which there is a sudden stimulus onset at the locus of attention but without any cue or attentional consequence.
We performed two analyses on the waveforms associated with the event types to establish the differences in the signal between the events of interest (e.g., shift vs. random letter, hold vs. random letter, and shift vs. hold). For the first analysis, for each comparison and ROI, the earliest separation in the waveform for the two events under consideration was computed by performing a series of paired t-tests between the two waveforms across participants. The earliest separation was defined as the first timepoint of the first run of 16 consecutive timepoints at which the difference between the two waveforms was significantly different at p < 0.01. This analytic approach, recently used by Pitts et al. (2010), computes a series of paired t-tests at each timepoint, across all the subject's data. The procedure uses the between-subjects error to establish the variance around any particular point and each test is (at least theoretically) independent. By using a strenuous threshold p <.01 and requiring sixteen consecutive timepoints to be significant, this analysis ensures that the resulting separation between the waveforms is statistically robust.
For the second analysis, the time at peak and the amplitude of the peak response of the early components (before the average behavioral threshold) was extracted in each participant and subjected to ANOVAs to test for difference between successful shifts and holds.
RESULTS
Our goal was to investigate the temporal relationships among the key areas of the fronto-parietal network during a task that required the shifting of attention from one spatial location to another (Figure 1 and Methods).
Psychophysics of attentional shifting
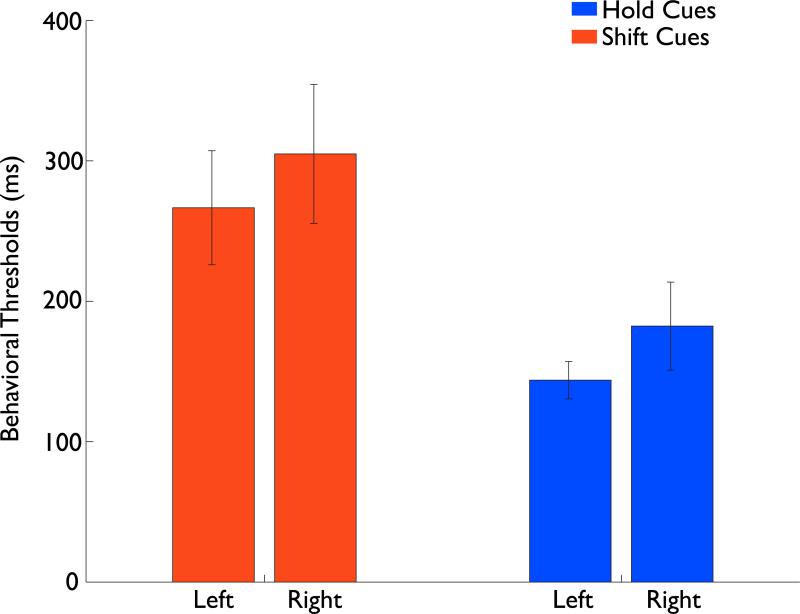
To examine whether the derived thresholds for target detection (with fixed 800ms target-to-cue interval) varied as a function of condition or of side, the thresholds obtained for each participant were submitted to an omnibus ANOVA with target type (successful target identification after a shift or after a hold cue) and side (ipsilateral and contralateral to the target) as within-subjects factors. There was no significant difference in target duration as a function of whether the condition was a shift or hold nor was duration affected as a function of side of space on which the target appeared (F<1). There was also no interaction between target type and side of space (F<1). The average threshold across participants for target duration was 134ms with a subsequent average RT for target letter detection of 295ms. Following this, we performed a similar analysis with the derived cue-to-target thresholds (with target duration fixed at individual thresholds); these were submitted to an omnibus ANOVA with cue-type (“4”, “2” i.e. shift, hold) and field (left, right) as within-subject factors (Figure 2). The ANOVA revealed a significant main effect of cue-type [F(1,11)=9.86, p<0.05], with significantly longer thresholds required for shifts of attention (M=286ms) than for maintenance of attention (M=163ms). There was also a main effect of the field in which the cue appears [F(1,11)=4.94, p<0.05], with thresholds to shift attention from left to right being slightly shorter (M=215ms) than from right to left (M=245ms). This left/right difference potentially indexes the general view that the right hemisphere plays a greater role in attentional selection than does the left hemisphere (Corbetta & Shulman, 2002; Mesulam, 1999). The interaction between cue-type (shift/hold) and side (left/right) did not reach significance (all F<1). Given these results, it is clear that while there is a small effect of the field in which the cues were presented, the primary difference in thresholds is due to the distinction between shift and hold cues. These results establish that it takes approximately 286ms on average for participants to complete a successful shift of attention between hemifields, and this threshold provides a firm limit on the portion of the ERP response that should be considered as critical to accomplishing that shift rather than processing occurring after the shift has been made.
Figure 2. Behavioral Thresholds.
The average behavioral thresholds derived for the gap between a cue and target. The two red bars show the thresholds for left and right shift cues while the two blue bars show the same for hold cues. Note the slightly longer thresholds for right than left cues for both shift and hold cues. Note also the larger thresholds for the shift cues. The error bars in this and all plots indicate the between-subject standard error.
ERP differences between successful shifts of attention and random letters
In order to assess the relative timing of the contribution of frontal and parietal cortex to successful shifts of attention, we recorded participant's brain activity in a separate session while they performed the behavioral attentional shifting task with ERP, with the parameters for stimulus presentation adopted from the thresholding session. Critically, the individually established attentional switch thresholds allowed us to ensure that any effects we observe occurred prior to the completion of an attentional shift.
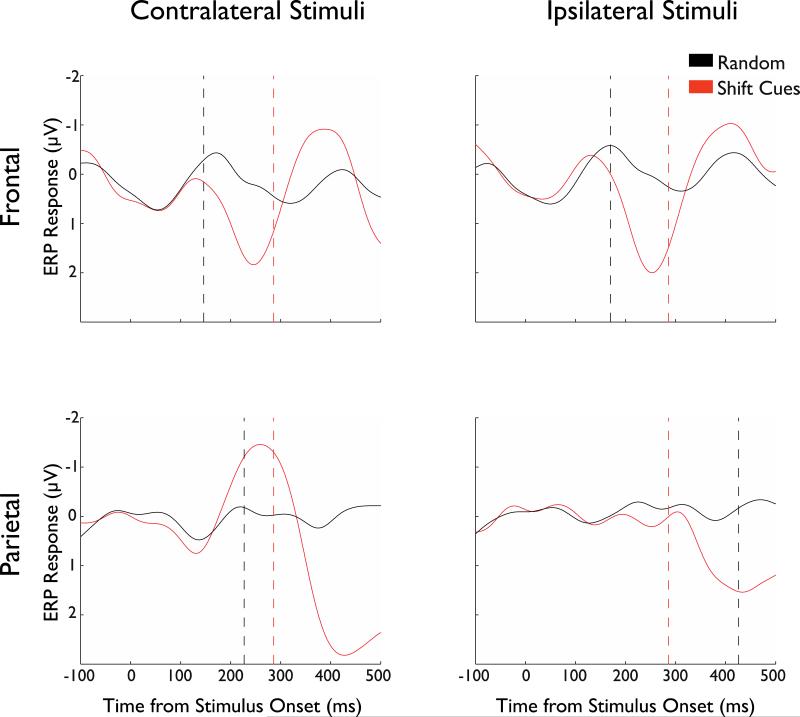
First, following standard preprocessing (see Methods) we extracted the grand averaged waveforms for Shift Hits (targets following a shift cue) and Random Letters (a random letter chosen from a no-target trial, within the temporal window of where the target would appear if it were a target-present trial) (Figure 3). The comparison of these two conditions provides information about where and when the processing of the shift cue begins. The comparison of shift versus the baseline affords a clean determination of the shift (rather than any processes engaged in inhibiting the shift as might be true in the hold condition; see below for further analyses of the hold condition). We calculated the earliest separation times (see Methods) between the shift and random letter conditions in the frontal and parietal ROIs. We first consider the response to contralateral stimuli – these are trials in which the shift cue (i.e., digit ‘4’) appeared on one side of space and the target appeared on the opposite side in the subsequent display (see Figure 1 for examples). The waveforms showing the responses to contralateral stimuli are depicted in Figure 3 (left panel), and suggest that the first significant divergence occurred in the frontal electrodes at 146ms, well before the average behavioral threshold of 286ms. The parietal electrodes showed the divergence only at 227ms, a full 81ms after the frontal electrodes but still in advance of the behavioral threshold. Qualitatively, note that the difference between frontal and parietal latencies of separation is substantial and greater than 25% of the available time range pre-shift (286ms). These results suggest that the frontal cortex likely initiates the processing of the shift cue and then triggers the response of the parietal cortex. There was also a divergence between the conditions in the ipsilateral field in frontal electrodes prior to the behavioral threshold (170ms). However, the divergence in the ipsilateral parietal electrodes (426ms) occurred well after the behavioral threshold, implicating its involvement in post-perceptual and post-attentional shift processes.
Figure 3. Differences between Shift Hits and Random.
Raw ERP timecourses for Shift Hits and Random Letter. The first row shows these timecourses derived from the frontal electrodes, while the second row shows the same derived from the parietal electrodes. The first column shows responses to contralateral stimuli, while the second column shows the responses to ipsilateral stimuli. The red dotted lines show the average behavioral threshold for shift cues (see Figure 2). The black dotted line indicates the first timepoint at which the Shift Hits and Random responses reliably separated in each plot. Note the later separation in parietal than frontal electrodes. Note also that the parietal produces very weak responses to ipsilateral cues and no separation in the responses until well past the behavioral threshold.
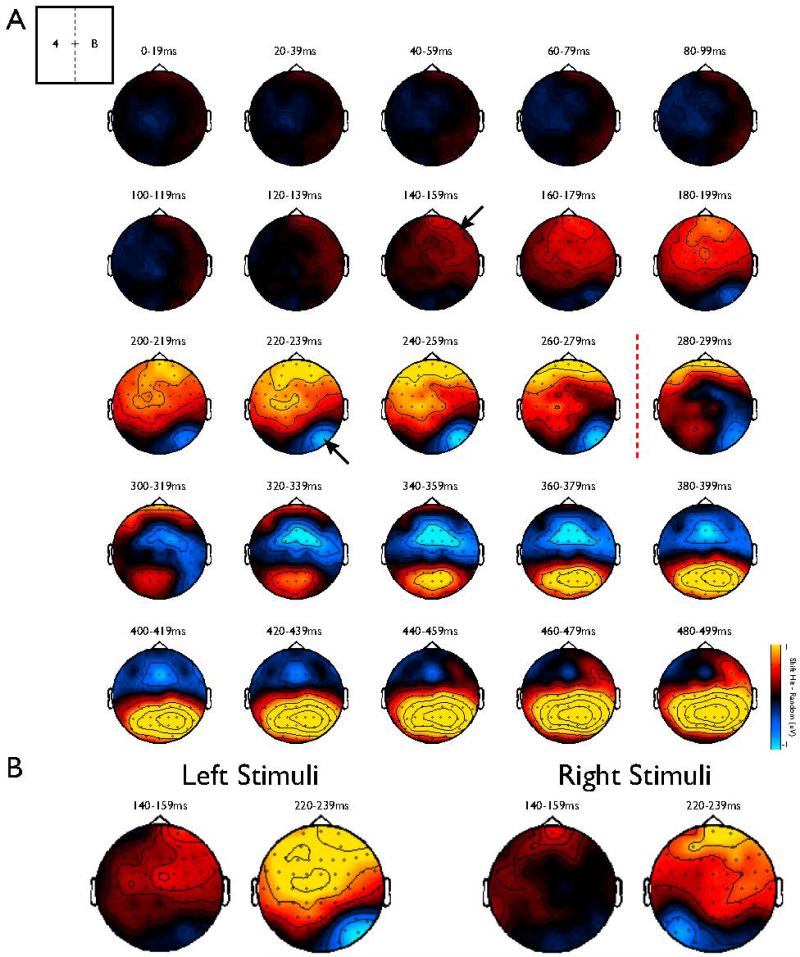
In order to qualitatively visualize the spatial and temporal distribution of the difference between Shift Hits and Random Letters, we created a topographic plot of the difference between these waveforms across electrodes and time (Figure 4A). As is evident from the plot, the earliest difference is a positive deflection across the contralateral frontal electrodes that then spreads to the ipsilateral frontal electrodes, followed by a quite spatially punctate negative deflection in the contralateral parietal electrodes. Note that the hemisphere engaged by the cues is always contralateral, with a strong flipping of the lateralization observed between left and right cues (Figure 4B).
Figure 4. Topography and timecourse of differences between Shift Hits and Random.
(A) The pattern of results was similar for left and right cues but flipped across hemisphere (see Figure 4B). Therefor, the data have been collapsed over left and right presentations of cues by flipping the identity of electrodes across hemispheres in to increase power and reduce complexity. The Shift cue or Random Letter occurred in the left hemifield (top inset) at time 0 (first topographic plot). The first significant difference between the two conditions occurred in the contralateral frontal electrodes (black arrow) at 146ms (Figure 3), spreading shortly thereafter to the ipsilateral frontal electrodes. The next significant difference emerged in the contralateral parietal electrodes (black arrow) at 246ms (Figure 3), which was before the mean behavioral threshold of 286ms (dashed red line). (B) Plot of the difference between left and right cues in the critical time bins.
Taken together, these results suggest that whereas contralateral frontal cortex participates in successful shifts of attention, both contralaterally and ipsilaterally and roughly at the same temporal point, the parietal engagement in shifts of attention appears to only be evident for contralateral but not ipsilateral shifts within the time range established for it to be functionally relevant.
Differences between shifts and maintenance of attention
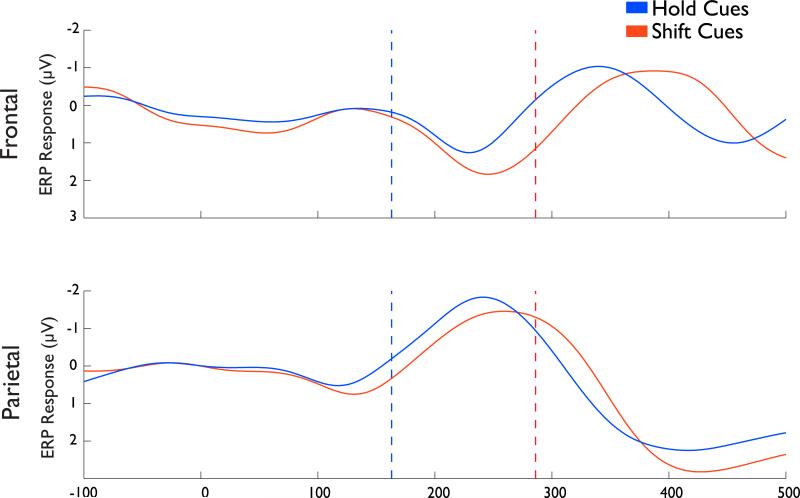
In order to investigate the difference in the ERP signal between processes engaged in shifting attention to a particular location versus maintaining attention on the same location, we next examined the responses to contralateral cues resulting in shift hits and hold hits (Figure 5). Note that we focus only on the contralateral trial as the ipsilateral trials (see above) have a very delayed divergence. Interestingly, in both the frontal and parietal electrodes, these two conditions were not statistically different, in that the waveforms had the same basic components and appear qualitatively equivalent. Interestingly, this equivalence is apparent even though, in the case of the hold cues, most of the response occurs well after the behavioral threshold (168ms), suggesting some variability in the signal.
Figure 5. Differences between Shift Hits and Hold Hits.
Raw ERP timecourses for Shift Hits and Hold Hits. The top plot is derived from frontal electrodes and the bottom from parietal electrodes. The red and blue dotted lines indicate the behavioral thresholds for shift and hold cues, respectively. Note the larger and earlier second component for Hold Hits compared to Shift Hits.
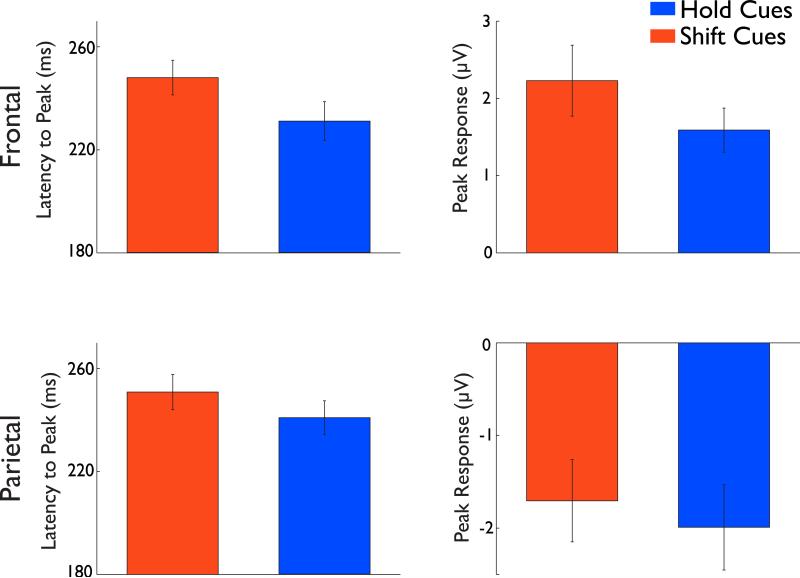
Closer scrutiny of the waveforms, however, reveals that the shift and hold waveforms are not formally equivalent: relative to the hold cues, the peak response to shift cues was delayed in both ROIs and this is especially evident in the second major component of the response, which occurred just before the behavioral threshold for successful shifts of attention (286ms). Peak times for this component, which occurred in the standard range for P2 (180-270ms) were extracted for each individual participant and entered into a two-way ANOVA with ROI (frontal, parietal) and cue-type (shift, hold) as factors. There was a main effect of cue-type [F(1,11)=9.80, p=0.01)], with longer latencies to peak for shift (frontal = 248ms, parietal = 251ms) than hold (frontal = 231ms, parietal = 240ms) cues (Figure 6). No other effects reach significance (all p>0.1), though there was a weak trend for a main effect of ROI [F(1,11)=2.56, p = 0.14]. These results suggest that shift cues trigger additional processing in frontal and parietal relative to hold cues.
Figure 6. Analysis of the second component of Shift and Hold Hits.
Latency and peak response of the second component for Shift and Hold Hits in frontal (top row) and parietal (bottom row) electrodes. The first column shows the latency from stimulus onset to the peak response. The second column depicts the peak response. Note the longer latency in both frontal and parietal electrodes for shift compared to hold hits. Note also the stronger response in frontal electrodes for the shift compared to hold cues.
In addition to the apparent temporal disparity between the peak of the shift and hold cues, there was also a difference in the strength of the ERP components. To compare the signal magnitude in the ROIs, the absolute peak value for the component for each individual was entered into a two-way ANOVA with ROI (frontal, parietal) and cue-type (shift, hold) as factors, revealing an ROIxCue-type interaction [F(1,11)=4.81, p=0.05)]. Subsequent pairwise comparisons revealed that this interaction arose from the component being stronger in frontal electrodes for shift (2.28μv) than hold (1.6mv) cues [t(11)=2.04, p<0.05]. This effect was absent in parietal electrodes [t(11)=0.55] but showed a trend in the opposite direction with a slightly weaker response to shift (-1.85μv) than hold (1.99μv) cues (Figure 6). These results indicate that shift cues cause additional and delayed processing in frontal cortex relative to hold cues but that the signal is of greater magnitude when it emerges. Parietal cortex evidences a delay in the onset of the component, perhaps reflecting the delay in the signal from frontal cortex.
DISCUSSION
The goal of the current study was to investigate the relative contribution of frontal and parietal cortices to processes involved in the control of spatial attentional allocation. Specifically, given that both regions issue a similar, transient spatial re-orienting signal (Bisley & Goldberg, 2003; Corbetta & Shulman, 2002; Ipata, Gee, Goldberg, & Bisley, 2006; Yantis & Serences, 2003), we focused on the temporal profile of the shifting signal by examining the time course of the relationship between the control signal initiated over frontal as compared to parietal cortical regions. A novel psychophysical approach was adopted in which we were able to quantify the time required for an initiation of a successful spatial shift of attention (shift events) for each participant as well as the time required to re-engage attention on an already attended spatial location (hold events). With this level of parametric specificity, we then examined the event-related potentials generated over frontal and parietal regions for each participant and, across the group of participants. In so doing, we were able to identify the earliest temporal separation between the ERPs in response to shifting attention as compared to holding attention, as well as in comparison to a neutral baseline. We interpret these early temporal separations as an index of the first meaningful signal that drives shifts of spatial attention.
Using the standard grand-averaging procedure, we compared the earliest divergence in signals between the attentional shift condition versus the random letter condition, in which no attentional cues (shift or hold) are presented. This comparison revealed that for both contralateral and ipsilateral shifts of attention, the divergence between shift/random letter conditions was apparent in electrodes positioned over frontal cortex earlier than was true for the condition divergence in parietal cortex (see Figure 4). This pattern of results suggests that the attentional shift signal is initiated in frontal cortex and then subsequently propagated to the parietal cortex, ultimately passed to the early sensory regions (from V4 through to V1 (Buffalo, Fries, Landman, Liang, & Desimone, 2010)).
The particular, perhaps disproportionate, engagement of frontal cortex is also evident in the direct comparison of the waveform separation in the shift versus hold conditions. While there was no difference in the separation point of these conditions in early frontal or parietal cortex, suggesting that perhaps the latency is not differentiable (although earlier than the Random Letter baseline comparison, see above), there was significantly higher amplitude in the signal in frontal cortex for the shift over hold comparison. This was not apparent in parietal cortex. Of note is that all these differences in the ERP waveforms reported here occur early in the ERP waveform and we can anchor them to the timing of the behavioral response. Taken together, these results provide evidence consistent with current models of top-down attentional-control which suggest that the signal to shift spatial attention originates within the frontal cortex (Corbetta & Shulman, 2002; Grent-'t-Jong & Woldorff, 2007; Herrington & Assad, 2010; Serences & Yantis, 2006).
Previous studies with a similar goal of elucidating the temporal relationship between control signals elicited over frontal and parietal cortices (Fu, et al., 2005; Green, et al., 2011; Hopfinger & Ries, 2005; Leblanc, et al., 2008; Ptak, et al., 2011) reported effects that appear to emerge much earlier in the ERP response (e.g., 105-145ms after the cue onset). While at a first glance it might appear that these results are inconsistent with ours, a closer look reveals that previous research has almost exclusively focused on a much more rapid bottom-up, or reflexive, shifting of attention. It is thus not surprising that shifts resulting from a pure top-down selection, as those investigated in the current experiment, operate on a different and somewhat slower time scale (for review see Egeth & Yantis, 1997). For example, Leblanc et al. (2008) in Experiment 1 examined the time course of N2pc component in response to salient stimuli eliciting bottom-up capture of attention and observed the earliest effects in the 105-145ms time range. It should be noted that in follow-up experiments, which involved a contingent capture paradigm and in which capture was constrained by a top-down task contingent (Folk, Leber, & Egeth, 2002), the time course of the N2pc component increased to 170-300ms, a time frame closely aligned with findings observed in this investigation.
While some investigations, as discussed above, observed effects much earlier than the 170ms reported here, other studies have reported effects that emerge far later in the ERP response, namely 400ms or 350ms after the cue (Brignani, et al., 2009; Green & McDonald, 2008; Grent-'t-Jong & Woldorff, 2007; Simpson, et al., 2011). For example, Grent-‘t-Jong and Woldorff (2007) recorded ERPs in response to cues that required spatial shifts (followed by a target) and those that signaled a no-target trial. Under these conditions, at approximately 400msec post-cue, ERPs were observed in the frontal areas and these signals preceded those observed over the parietal areas. In light of behavioral spatial effects reported in the literature (Gibson & Bryant, 2005; Posner, 1980) and more precisely, the thresholded estimate of about 300ms that it takes to shift spatial attention in this type of an RSVP task, these ERP responses seem rather delayed and most likely reflect the actual shift, rather than the initiation of the shift per se, or even post-shift related perceptual responses. Therefore, although the findings of the Grent-`t-Jong and Woldorff study are consistent with our observation of an advance frontal response, the temporal profiles themselves are rather different. A more recent study by Brignani et al. (2009) adopted a RSVP paradigm similar to the one we have exploited here, and reported somewhat earlier ERP signals observed over frontal and parietal cortices in the range of 330ms and 370ms, respectively, compared to those documented by the Grent-‘t-Jong and Woldorff (2007) investigation. Compared with our findings, however, they still occur relatively late; again, though, we note the consistency in delineating the temporal advantage for the frontal over parietal signals in this study too. As suggested above, these later effects are more likely to reflect processes involved not only in initiating and executing an attentional shift, but also in processes such as target identification, response selection, error monitoring, etc.
A major departure from the previous studies, reported in the present paper, then, is the earliest separations observed over frontal and parietal regions. Indeed, the waveform signatures associated with the attentional shift all occurred within the interval established by the psychophysical measurements for the initiation and execution of the attentional shift. As such, these waveforms, using the indices of temporal divergence, provide an uncontaminated response profile of an attentional shift. It is of course understood that a shifting threshold will be entirely dependent on the specific paradigm that is adopted for the purposes of eliciting spatial shifts of attention. Indeed, given that neither Grent-‘t-Jong and Woldorff (2007) nor Brignani et al. (2009) documented the time of the psychophysical attentional shift in their participants, we cannot know definitively whether the ERP changes they report are consistent with the behavioral responses of their participants or not. As the current investigation both derived the threshold measure and used this value in the ERP study, we were able to make direct links between signals elicited over frontal and parietal areas and their relative contribution to initiation and execution of a spatial shift.
Several other important and novel findings emerged as a result of this investigation. Firstly, a probe into the contribution of ipsilateral control regions to spatial shifts of attention revealed that the signal elicited over ipsilateral frontal cortex occurred well before the behavioral threshold (170ms), thus suggesting direct involvement of ipsilateral frontal areas in planning and execution of a spatial shift of attention. Interestingly, the signal associated with a shift of attention elicited by the ipsilateral parietal cortex emerged well after the derived behavioral threshold for shifts of attention (426ms). This later involvement possibly reflects processes that are engaged after planning and executing a spatial shift of attention (e.g., target processing, response selection, etc.). Secondly, when comparing directly signals elicited in response to contralateral shift vs. holds of attention, we observed a remarkable similarity in the fundamental components of signals originating in the frontal and parietal cortices, suggesting that shifts and hold of attention are perhaps mechanistically more similar than not. What is different, however, is the magnitude and rise time of the components in response to shifts and holds of attention, with shift related responses having a greater magnitude and a later peak. These results indicate that shift cues cause additional and delayed processing in frontal cortex relative to hold cues but that the signal is of greater magnitude when it emerges. Parietal cortex evidences a delay in the onset of the component, perhaps reflecting the delay in the signal from frontal cortex.
While the evidence provided here is strong, and the use of the ROI-based analysis is superior to focusing on any single electrode and/or component, there are several limitations to the current study. Firstly, while we argue that frontal electrodes reflect activity of frontal cortex and parietal electrodes reflect activity of parietal cortex, we do so cautiously. Anatomical data were not acquired as a part of this study, thus prohibiting definitive source localization analysis. However, our analysis examining the distribution of effects across the entire set of electrodes (Figure 4), clearly shows that the observed effects are well localized both spatially and temporally to the electrodes of interest, and that the effects in the parietal electrodes are circumscribed, with little evidence of the observed effects even in very nearby occipital electrodes. Secondly, we do not directly determine whether the difference between the latencies of the parietal and frontal cortex differ statistically. The reason for this is that there is no variance left in the data with which to establish the difference, as the between-trial variance is removed by the averaging necessary to establish the waveforms within each subject and the between-subject variance is consumed by derived temporal difference within each set. We attempted a number of randomization tests but simply lacked enough power to firmly establish the difference of the differences across 500 potential timepoints. However, the difference between the latencies of separation is 81ms, which is quite large numerically (see also new Figure 4). Nonetheless, we are careful to phrase this section as a qualitative rather than quantitative comparison of the two sets of electrodes.
By using a careful psychophysical method for determining the exact amount of time necessitated for the initiation of a successful shift of spatial attention and by recording neural responses over the fronto-parietal attentional network, we were able to investigate the temporal relationship of neural processes underlying spatial shifts of attention. Our findings support several conclusions. Consistent with previous studies, we show that parietal and frontal cortices are involved in initiating the attentional shift (Brignani, et al., 2009; Grent-'t-Jong & Woldorff, 2007; Moran & Desimone, 1985; O'Craven, Downing, & Kanwisher, 1999; Shomstein & Yantis, 2004; Simpson, et al., 2011; Yantis, et al., 2002). Moreover, we observed a highly structured temporal sequence of responses elicited following an intent to spatially re-orient attentional locus, such that attentional control signal was first elicited by the frontal lobe then followed by the parietal lobe. Needless to say, much remains to be done including further research to uncover the process by which the shift trigger is instantiated in frontal cortex, and to elucidate the mechanism by which this top-down cascade of shift signals is implemented. Electrophysiological techniques, extending beyond ERP to magneto-encephalography, offer great promise in this regard and future explorations of long-range synchrony and frequency oscillations may help uncover the cortical dynamics, which ultimately underlie these processes.
Highlights.
Here we investigate the relative contribution of frontal and parietal cortex to the initiation of a spatial shift of attention.
By titrating the temporal parameters of the time taken to shift attention for each participant using a fine-grained psychophysics paradigm, we determined the upper limit on latency of ERP effects.
By using ERPs, we show that the response associated with a shift of attention of frontal electrodes precedes that of the response observed in parietal cortex.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Balan PF, Gottlieb J. Integration of exogenous input into a dynamic salience map revealed by perturbing attention. The Journal of Neuroscience. 2006;26:9239–9249. doi: 10.1523/JNEUROSCI.1898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cerebral cortex. 2007;17:2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annual review of neuroscience. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignani D, Lepsien J, Rushworth MF, Nobre AC. The timing of neural activity during shifts of spatial attention. Journal of cognitive neuroscience. 2009;21:2369–2383. doi: 10.1162/jocn.2008.21176. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Liang H, Desimone R. A backward progression of attentional effects in the ventral stream. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:361–365. doi: 10.1073/pnas.0907658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Weeks RA, Ishii K, Catalan MJ, Tian B, Rauschecker JP, Hallett M. Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nature neuroscience. 1999;2:759–766. doi: 10.1038/11239. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nature neuroscience. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Esterman MS, Gmeindl L, Yantis S. Tracking cognitive fluctuations with multivoxel pattern time course (MVPTC) analysis. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nature neuroscience. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. Journal of neuroscience. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews: Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Tansy AP, Stanley CM, Astafiev SV, Snyder AZ, Shulman GL. A functional MRI study of preparatory signals for spatial location and objects. Neuropsychologia. 2005;43:2041–2056. doi: 10.1016/j.neuropsychologia.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Duncan J. Selective attention and the organization of visual information. Journal of Experimental Psychology: General. 1984;113:501–517. doi: 10.1037//0096-3445.113.4.501. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual Attention: Control, Representation, and time course. Ann. Rev. Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Hoffman JE. Temporal and spatial characteristics of selective encoding from visual displays. Perception & psychophysics. 1972;12:201–204. [Google Scholar]
- Esterman M, Chiu YC, Tamber-Rosenau BJ, Yantis S. Decoding cognitive control in human parietal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17974–17979. doi: 10.1073/pnas.0903593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk CL, Leber AB, Egeth HE. Made you blink! Contingent attentional capture produces a spatial blink. Perception & psychophysics. 2002;64:741–753. doi: 10.3758/bf03194741. [DOI] [PubMed] [Google Scholar]
- Friedrich FJ, Egly R, Rafal RD, Beck D. Spatial attention deficits in humans: a comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology. 1998;12:193–207. doi: 10.1037//0894-4105.12.2.193. [DOI] [PubMed] [Google Scholar]
- Fu S, Greenwood PM, Parasuraman R. Brain mechanisms of involuntary visuospatial attention: an event-related potential study. Human brain mapping. 2005;25:378–390. doi: 10.1002/hbm.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BS, Bryant TA. Variation in cue duration reveals top-down modulation of involuntary orienting to uninformative symbolic cues. Perception & psychophysics. 2005;67:749–758. doi: 10.3758/bf03193530. [DOI] [PubMed] [Google Scholar]
- Green JJ, Doesburg SM, Ward LM, McDonald JJ. Electrical neuroimaging of voluntary audiospatial attention: evidence for a supramodal attention control network. Journal of neuroscience. 2011;31:3560–3564. doi: 10.1523/JNEUROSCI.5758-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JJ, McDonald J. Electrical neuroimaging reveals timing of attentional control activity in human brain. PLOS biology. 2008;6:0730–0738. [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. Journal of neuroscience. 2010;30:14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Verstynen T, Chiu YC, Yantis S, Schneider W, Behrmann M. Visuotopic cortical connectivity underlying attention revealed with high definition fiber tracking. Journal of neuroscience. doi: 10.1523/JNEUROSCI.5419-11.2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Woldorff MG. Timing and sequence of brain activity in top-down control of visual-spatial attention. PLOS biology. 2007;5:e12. doi: 10.1371/journal.pbio.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington TM, Assad JA. Temporal sequence of attentional modulation in the lateral intraparietal area and middle temporal area during rapid covert shifts of attention. Journal of neuroscience. 2010;30:3287–3296. doi: 10.1523/JNEUROSCI.6025-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Ries AJ. Automatic versus contingent mechanisms of sensory-driven neural biasing and reflexive attention. Journal of cognitive neuroscience. 2005;17:1341–1352. doi: 10.1162/0898929055002445. [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. Journal of neuroscience. 2006;26:3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Driver J. Objects, attributes, and visual attention: Which, what, and where? Currrent Directions in Psychological Science. 1992;1:26–31. [Google Scholar]
- Leblanc E, Prime DJ, Jolicoeur P. Tracking the location of visuospatial attention in a contingent capture paradigm. Journal of cognitive neuroscience. 2008;20:657–671. doi: 10.1162/jocn.2008.20051. [DOI] [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Serences JT, Yantis S. Cortical mechanisms of feature-based attentional control. Cerebral cortex. 2003;13:1334–1343. doi: 10.1093/cercor/bhg080. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philosophical Transaction of the Royal Society of London B. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Downing PE, Kanwisher N. fMRI evidence for objects as the units of attentional selection. Nature. 1999;401:584–587. doi: 10.1038/44134. [DOI] [PubMed] [Google Scholar]
- Pitts MA, Martinez A, Hillyard SA. When and where is binocular rivalry resolved in the visual cortex? Journal of vision. 2010;10 doi: 10.1167/10.14.25. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. Journal of experimental psychology. 1980;109:160–174. [PubMed] [Google Scholar]
- Ptak R, Camen C, Morand S, Schnider A. Early event-related cortical activity originating in the frontal eye fields and inferior parietal lobe predicts the occurrence of correct and error saccades. Human brain mapping. 2011;32:358–369. doi: 10.1002/hbm.21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R, Schnider A. The dorsal attention network mediates orienting toward behaviorally relevant stimuli in spatial neglect. Journal of neuroscience. 2010;30:12557–12565. doi: 10.1523/JNEUROSCI.2722-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Sipila PK. Attention systems and the organization of the human parietal cortex. Journal of neuroscience. 2001;21:5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends in cognitive sciences. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cerebral cortex. 2007;17:284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Behrmann M. Cortical systems mediating visual attention to both objects and spatial locations. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11387–11392. doi: 10.1073/pnas.0601813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Lee J, Behrmann M. Top-down and bottom-up attentional guidance: Investigating the role of the dorsal and ventral parietal cortices. Experimental Brain Research. Special issue on Visuo-Spatial Neglect. 2010;206:197–208. doi: 10.1007/s00221-010-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Control of attention shifts between vision and audition in human cortex. Journal of neuroscience. 2004;24:10702–10706. doi: 10.1523/JNEUROSCI.2939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends in cognitive sciences. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GV, Weber DL, Dale CL, Pantazis D, Bressler SL, Leahy RM, Luks TL. Dynamic activation of frontal, parietal, and sensory regions underlying anticipatory visual spatial attention. Journal of neuroscience. 2011;31:13880–13889. doi: 10.1523/JNEUROSCI.1519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling G, Reeves A. In: Attention and Performance VIII. Nickerson RS, editor. Erlbaum; Hillsdale, NJ: 1980. pp. 347–360. [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, Dubois B, Bartolomeo P. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Progress in brain research. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. Journal of neurophysiology. 2005;93:337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia. 1986;24:609–622. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Perception & psychophysics. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nature neuroscience. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Current opinion in neurobiology. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]