Abstract
Although public health campaigns advise pregnant women to abstain from ethanol, drinking during pregnancy is pervasive. Here, we highlight recent studies that have clearly demonstrated long-lasting neurobehavioral deficits in offspring of laboratory animals exposed to moderate levels of ethanol during development. Alterations in learning, memory, motor coordination, social behavior, and stress responses were identified in these animals. Increased vulnerability to substance abuse was also demonstrated. These behavioral alterations have been associated with impairments in neurotransmitter systems, neuromodulators, and/or synaptic plasticity in several brain regions. With this review, we hope to contribute to a better appreciation of the potential effects of developmental exposure to moderate ethanol levels, leading to better interventions aimed at relieving fetal alcohol spectrum disorders.
Keywords: alcohol, ethanol, neurotransmission, fetal alcohol syndrome, animal model, pregnancy
Introduction
Exposure to ethanol during pregnancy produces fetal alcohol spectrum disorders (FASDs), a major public health problem with an estimated prevalence of 1-5% in the United States and Western Europe, and an even higher prevalence in other regions, including South Africa [1]. The high prevalence of FASDs is not surprising given that a significant percentage of pregnant women consume ethanol (e.g., 10-20% in the U.S.A., 40% in Uruguay; 50% in some parts of Italy) [2-4]. The most severe form of FASDs is fetal alcohol syndrome (FAS), characterized by growth retardation, facial abnormalities, and neurobehavioral alterations. FAS is often associated with heavy ethanol consumption throughout pregnancy, including binge drinking. Most patients with FASDs exhibit only a subset of the characteristics of FAS, such as cognitive and behavioral deficits, in the absence of facial alterations.
Although it is widely accepted that exposure to high doses of ethanol has long-lasting detrimental effects on brain development, the case for moderate exposure (Box 1) remains controversial [5-7]. On one side are a number of studies suggesting that moderate prenatal alcohol exposure (MPAE) is associated with a higher incidence of behavioral and cognitive problems during childhood and/or adolescence, including mood disorders, deficits in working memory and attention, increases in aggression, and alterations in peer relationships [8-12]. Conversely, other studies have shown that MPAE is not associated with an increased incidence of social, motor, or emotional problems [13-17]. Reasons for the discrepancies between these studies are likely to include differences in methodology, sample demographic characteristics, confounding variables (e.g., stress, genetic factors, socioeconomic status, nutrition, or co-exposure to other harmful substances), and accurate determination of drinking patterns [7] (Box 1).
Box 1. What is moderate drinking?
The definition of both the content of ethanol in a standard drink and moderate drinking varies by country (http://www.icap.org/table/Internationaldrinkingguidelines). In general, government guidelines recommend that women consume no more than 10-40 g of ethanol/day. In the U.S.A., 1 standard drink (14 g of ethanol)/day is considered moderate consumption for non-pregnant women [77]. BALs depend on many factors, including gender, weight, and presence of food in the stomach. Consumption of 28 g of ethanol in 1 hr by a 60 kg non-pregnant woman is estimated to yield a BAL ≈ 0.06 g/dl (http://www.dot.wisconsin.gov/safety/motorist/drunkdriving/calculator.htm). However, the effect of pregnancy on ethanol metabolism in not fully understood, with studies showing increased metabolism in rats [78] or decreased metabolism and accumulation in the amniotic fluid in guinea pigs [79, 80]. A study with women revealed faster elimination kinetics of ethanol from maternal blood during the early 2nd trimester in comparison to non-pregnant women; however, this study also found a slower rate of ethanol clearance from the amniotic fluid suggesting that this compartment acts as an ethanol reservoir [81]. In humans, the dose-response relationship between prenatal ethanol exposure and fetal effects needs to be better established. To this end, developing better methods to accurately quantify prenatal ethanol exposure is required. A method that accounts not only for dose but also for pattern and timing of exposure was recently shown to be more sensitive at detecting neurobehavioral effects of MPAE [10]. As a reference, moderate consumption was defined in that study as ≤70 g ethanol/week and 3-4 standard drinks/occasion (standard drink = 10 g of ethanol in Australia).
Although human epidemiological studies are inconclusive regarding the impact of MPAE, primate and rodent models of FASDs have convincingly demonstrated significant effects in several brain regions. In this review, we focus on studies completed in the last decade where animals were exposed in vivo to blood alcohol levels (BALs) ≤ 0.17 g/dl, which have been assumed to model human MPAE (Box 1; for reference, the U.S. legal intoxication limit is 0.08 g/dl = 17.4 mM). We elected to concentrate on studies with BALs up to approximately twice the legal intoxication limit in the U.S. based on a recent computational modeling study indicating that significantly higher BALs are required to produce neurodevelopmental effects in rodents than in primates [18]. Studies discussed here used a variety of exposure paradigms (Fig 1) to assess ethanol’s effects across different brain regions. The reader is referred to more comprehensive reviews for additional information on FASD, including studies that have used other modes of exposure and higher ethanol doses [19-24].
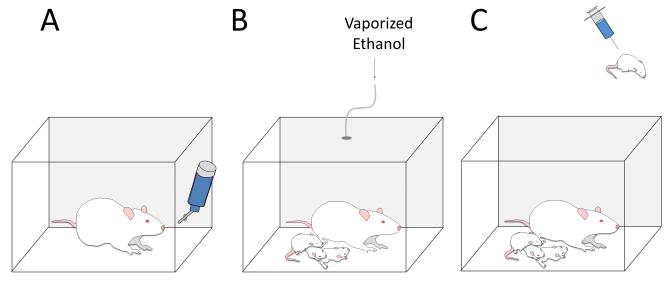
Fig 1. Schematic representation of ethanol exposure paradigms used in the studies reviewed here.
To model 1st and 2nd trimester ethanol exposure, pregnant rodents were exposed to moderate doses of ethanol using: A) forced (i.e., ethanol containing solutions were the only source of water and/or food [25, 46, 58]) and continuous or limited voluntary drinking paradigms [26, 29, 30]. Voluntary drinking has also been used to expose monkeys to ethanol at different stages of pregnancy [62]. B) To model human exposure during the 3rd trimester, rat pups and dams were exposed via ethanol vapor inhalation chambers [35, 55] or C) pups via intraperitoneal or subcutaneous ethanol injections [48, 64].
Hippocampal Formation
Glutamatergic and Histaminergic Transmission
Studies with rodents have provided strong evidence indicating that MPAE impairs hippocampal-dependent memory. MPAE adult rat offspring (liquid diet during pregnancy; BALs of 0.03 and 0.08 g/dl) displayed impaired performance on a moving platform version of the Morris Water Task [25]. Furthermore, hippocampal slice experiments from prenatally exposed rats revealed a reduction in activity-dependent potentiation of evoked [3H]-D-aspartate release, suggesting that abnormalities in glutamatergic plasticity may underlie the behavioral alterations [25].
A more recent study confirmed that voluntary drinking in pregnant rats (BAL = 0.08 g/dl) induced long-lasting learning and memory deficits in their offspring [26]. MPAE adult rat offspring exhibited alterations in hippocampal-dependent memory assessed using both the Morris Water Task and 1-trial contextual fear conditioning [26] (Glossary) (Fig 2A-B). In vivo electrophysiological experiments under urethane anesthesia demonstrated a MPAE-induced deficit in glutamatergic long-term potentiation (LTP) in the dentate gyrus (DG), providing a possible underlying mechanism for observed alterations in spatial memory [27] (Fig 2C). As discussed below, histamine neurotransmission may also be involved.
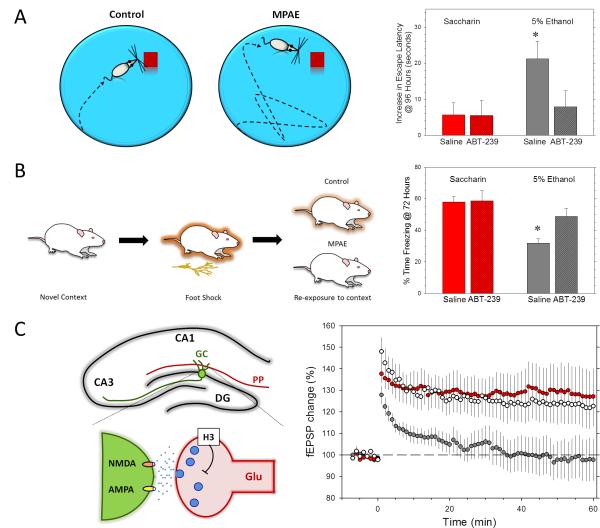
Fig 2. Prenatal exposure to moderate ethanol levels impairs hippocampal-dependent memory and plasticity.
A) Left panel: schematic representation of the Morris Water Task, which measures training-induced changes in the time to find a hidden platform (escape latency) in a tub full of opaque water. Animals typically use environmental cues to locate the platform. MPAE animals typically need longer time to find the escape platform. Right panel: escape latency was significantly increased in MPAE adult rat offspring (voluntary drinking paradigm during pregnancy; 5% ethanol (v/v) plus 0.066% saccharin (v/v) in water) and this effect was reversed by the H3 receptor antagonist, ABT-239 [26]. B) Left panel: schematic representation of the contextual fear conditioning test, which measures immobility (i.e., freezing) time in rodents re-exposed to the environmental context where they received a foot shock. MPAE animals typically freeze less (i.e., fail to link the context with the shock received previously) than control animals. Right panel: freezing in a contextual fear conditioning test was reduced in MPAE adult offspring and this effect was reversed by ABT-239 [26]. C) Left panel: shown in the top panel is a schematic representation of a coronal section of the hippocampal formation showing the CA1 and CA3 hippocampal subfields, as well as the dentate gyrus (DG). A granule cell (GC) in the DG is shown in green. GCs receive glutamatergic input from the enthorhinal cortex via the perforant path (PP). The lower panel illustrates a PP-GC synapse, including presynaptic H3 receptors and postsynaptic NMDA and AMPA receptors. Prenatal ethanol exposure may result in a long-lasting increase in the activity of H3 receptors, decreasing glutamate release and synaptic plasticity. Right panel: LTP recorded in the DG from urethane anesthetized rats was impaired in MPAE adult offspring (gray circles) with respect to saccharin control group offspring (red circles) and this effect was reversed by the H3 receptor blocker ABT-239 (white circles). The graph shows the change in field excitatory postsynaptic potential (fEPSP) amplitude over a 1hr period. Reprinted with permission from [26] (Panels A, B; © Wiley-Blackwell) and [27] (Panel C; © American Society for Pharmacology and Experimental Therapeutics).
Histamine is a monoamine neurotransmitter synthesized by the hypothalamic tuberomammillary nuclei neurons that project to several brain regions, including the DG. Histamine has been demonstrated to regulate the sleep-wake cycle, feeding behavior, and memory formation [28]. The neuronal actions of histamine are mediated by four subtypes of receptors (H1-4). H3 receptors have generated significant interest, as these could be potential drug targets for the treatment of several neuropsychiatric disorders [28]. The investigational H3 antagonist, ABT-239, ameliorated the MPAE-induced deficits in the Morris Water Task, contextual fear conditioning, and DG LTP (Fig 2), suggesting that this agent may be potentially useful in the treatment of FASDs [26, 27]. Although the mechanism of ABT-239 action is complex, it possibly involves facilitation of glutamate, acetylcholine and/or histamine release via inhibition of presynaptic H3 receptors. These findings are especially exciting as there are limited existing pharmacological treatments against FASDs.
Studies have also observed deficits in hippocampal-dependent behavioral tests (contextual fear conditioning, trace fear conditioning, and radial-arm maze tasks) in MPAE adult mouse offspring (voluntary drinking paradigm; BAL = 0.09-0.12 g/dl) [29, 30]. Immunohistochemical experiments showed that MPAE (BAL = 0.08 g/dl) decreases NMDA receptor (NMDAR)-dependent activation of extracellular signal-regulated kinase 1/2 (ERK1/2) in the DG, but not the CA1 and CA3 hippocampal regions, indicating that alterations in the DG may underlie the behavioral deficits [31]. Importantly, ERK1/2 activation is involved in some forms of NMDAR-dependent LTP in the DG [32].
Moderate ethanol exposure of rats during the 3rd trimester-equivalent [postnatal days 1-8 (P1-8); vapor chambers; BAL = 0.17 g/dl] was also shown to persistently alter hippocampal-dependent learning [33]. These behavioral deficits were associated with increases in levels of vesicular glutamate transporter 1, excitatory amino acid transporters 1 and 3, and GluN2A-D NMDAR subunits in the hippocampal formation and/or neocortex [33].
GABAergic Plasticity
During the 3rd trimester-equivalent, GABAergic synapses are actively refined by synaptic plasticity-dependent mechanisms. In the hippocampus, activity-dependent retrograde release of brain-derived neurotrophic factor (BDNF) from CA3 pyramidal neuron dendrites causes persistent potentiation of GABAergic interneuron-CA3 pyramidal neuron synapses, a phenomenon known as GABAA-LTP [34]. It was found that moderate exposure of rats during the 3rd trimester-equivalent (vapor chambers; P2-6; lowest BAL tested = 0.02 g/dl) inhibited GABAA-LTP [35]. The mechanism of action of ethanol is hypothesized to involve persistent inhibition of L-type voltage-gated Ca2+ channels, leading to a reduction in dendritic release of BDNF [35]. These findings indicate that ethanol exposure can potently affect GABAergic transmission and plasticity during development, which could partially be responsible for the long-lasting alterations in neuronal circuits associated with FASDs. It should be investigated whether ethanol exposure during the 3rd trimester-equivalent persistently decreases GABAergic transmission altering the balance of excitatory and inhibitory inputs.
Adult Neurogenesis
A number of studies suggest that alterations in adult neurogenesis could contribute to the pathophysiology of FASDs [23]. The effects of MPAE on adult neurogenesis were first characterized in mice (voluntary drinking paradigm; BAL = 0.12 g/dl) [36]. This study found no significant difference in the survival or differentiation of progenitor cells in the DG of adult offspring under standard housing conditions, but did report that MPAE blocked an environmental enrichment-induced increase in survival of progenitor cells. Conversely, a recent study found reduced generation of new neurons and glial cells in MPAE rat female adult offspring (liquid diet paradigm; BAL = 0.14 g/dl) [37]. These findings indicate that the effects of MPAE on adult neurogenesis in the DG depend on the method of ethanol exposure and the characteristics of the animal model (i.e., age, species, and gender).
MPAE also has profound consequences on neurogenesis in other areas of the brain. MPAE adult mice (voluntary drinking; BAL = 0.12 g/dl) were shown to be significantly less capable of discriminating between two odors versus controls [38]. Further experiments revealed that ethanol-exposed mice have a reduced number of olfactory bulb precursor neurons and fewer new neurons in the olfactory bulb granule cell layer during the early postnatal period.
The mechanisms responsible for the long-lasting effects of MPAE on neurogenesis are presently unknown but could involve intrinsic alterations in the progenitor cells themselves and/or deficits in the stem cell niche. A recent study found evidence of reduced intrinsic neurogenic capacity of neural progenitor cells derived from the adult subventricular zone of MPAE mice [39]. Alternatively, MPAE-induced alterations in neurogenesis may be secondary to chronic stress [40]. It has been shown that MPAE programs the hypothalamic-pituitary-adrenal (HPA) axis, increasing its activity, leading to sensitization to stressors throughout life. This effect increases vulnerability to stress-related disorders, such as anxiety and/or depression, which could be mediated by alterations in adult neurogenesis [41]. MPAE (liquid diet; BAL 0.14 g/dl) alone was found to inhibit adult neurogenesis in the DG, and restraint stress could not further reduce neurogenesis unlike in control rats [42]. One possibility is that the MPAE-induced increase of HPA axis activity reduces neurogenesis to a degree that cannot be dampened further by additional stress. Clearly, future studies are needed to understand the role of HPA axis alterations in the neurogenesis deficits associated with MPAE.
Thyroid Hormone and Retinoic Acid
The thyroid hormone, triiodothyronine (T3), is degraded by iodothyronine deiodinase-III, which is encoded by an imprinted gene, leading to differential expression of maternal vs. paternal genes. A recent study demonstrated that MPAE (liquid diet; BAL = 0.12) reduced the paternal and total expression of iodothyronine deiodinase-III in the hippocampus of fetal and adult rat males only, leading to an increase in T3 levels [43]. These alterations were linked to deficits in the Morris Water Task in adult male rats. In the frontal cortex, MPAE increased total expression of iodothyronine deiodinase-III and reduced T3 levels in both genders. The effects of MPAE in the hippocampus and frontal cortex were dependent on the genetic background, as offspring of Sprague-Dawley mothers crossed with Brown-Norway fathers exhibited MPAE-induced alterations but not offspring of reciprocal crosses. This study indicates that gender-, brain region-, and genotype-specific imprinting processes could interact with MPAE to produce long-lasting deficits in hippocampal-dependent spatial memory.
MPAE (liquid diet; BAL = 0.01-0.12 g/dl) has also been shown to dramatically increase all-trans retinoic acid (RA) levels both in the hippocampus and neocortex of embryonic day 19 (E19) fetuses [44]. Given that RA is an important regulator of adult neurogenesis and neurotransmission [45], it should be investigated whether alterations in the levels of this biologically active form of vitamin A can be an additional factor underlying the neurobehavioral alterations induced by MPAE.
Cerebellum
The cerebellum—involved in motor coordination and learning, as well as cognitive and emotional functions—has been shown to be affected by MPAE. Exposure of pregnant macaque monkeys to moderate levels of ethanol (voluntary drinking; BAL = 0.02-0.05 g/dl) caused alterations in motor coordination and response speed in infant offspring (reviewed in [21]). Similarly, MPAE mouse offspring (forced drinking; BAL = 0.07 g/dl) exhibited significant motor impairments determined by rotarod and runway assays (at P 24) in addition to deficits in motor learning as measured by eyeblink conditioning [46]. In vivo electrophysiological recordings showed that spontaneous firing of Purkinje neurons is significantly increased and accompanied by fast local field potential oscillations, which may be associated with disruptions in information flow within the cerebellar cortex. In vitro cerebellar slice recordings showed reduced glutamate release at parallel fiber (i.e., axons from cerebellar granule cells) to Purkinje cell synapses [46]. Long-term depression (LTD) was shifted toward LTP at these synapses, an effect associated with a reduction in both protein kinase C-γ expression and voltage-gated Ca2+ channel function [46].
MPAE has also been shown to produce abnormalities in other cerebellar neuronal populations. Exposure of rats through gestation and weaning (voluntary drinking; BAL = 0.07 g/dl) increased soma size and dendritic length in cerebellar granule cells, and delayed maturation of Bergmann glia [47]. Moreover, a recent study reported alterations in cerebellar granule cell migration in a mouse model of ethanol exposure during the 3rd trimester-equivalent (1 g/kg intraperitoneal injection at P10; BAL = 0.07 g/dl) [48]. In addition, 3rd trimester-equivalent exposure (1-2 g/kg/day P2-11; intragastric intubation; BAL = 0.05-0.15 g/dl) impaired eyeblink conditioning in adult rats, which could be related to alterations in deep cerebellar nuclear neurons [49]. Collectively, these studies clearly indicate that the developing cerebellum is a sensitive target of MPAE.
Neocortex
Several brain regions have been linked to social behavior in rats, including the frontal cortex. Long-lasting alterations in social behavior were recently demonstrated in MPAE adult rats (voluntary drinking; BAL = 0.08 g/dl) [50]. Impairments in social behavior were more consistently observed in adult male rats in response to a new cage mate. Ethanol exposed rats exhibited increases in anogenital and body sniffing (social investigation), and wrestling behavior (either aggressive or playful behavior). Exposure to a new cage mate increased expression of activity-related immediate early genes [c-fos and/or activity-regulated cytoskeletal (Arc) gene] in the agranular insular cortex (equivalent to the orbital prefrontal cortex in primates), the prelimbic cortex, and lateral orbital area in control offspring but this effect was not observed in MPAE offspring [50, 51]. Furthermore, when control rats were exposed to various cage-mates, they exhibited an increase in dendritic length and spine density in the agranular insular cortex. Conversely, MPAE offspring exhibited an increase in dendritic length with no change in spine density. It should be noted that another study with rats demonstrated that MPAE (forced drinking; BAL = 0.015-0.04 g/dl) results in deficits in social recognition memory, which were correlated with neocortical and hippocampal increases in levels of an endogenous neurosteroid (3α-hydroxy, 5α-pregnan-20-one) that potently modulates GABAA receptor (GABAAR) function [52]. These studies suggest that MPAE causes long-lasting alterations in social behaviors that could possibly be a consequence of synaptic plasticity deficits in the cerebral cortex.
MPAE has also been shown to affect neocortical GABAergic neuron development. During early development in mice (E0.5-14.5), it was found that MPAE (liquid diet; BAL = 0.03 g/dL) increases the density of GABAergic interneurons in the neocortex [53]. Ethanol exposure caused premature migration of medial ganglionic eminence-derived GABAergic neurons into the neocortex and also promoted differentiation of these neurons. Studies with slice co-cultures showed that ethanol increases ambient GABA levels, an effect that was also demonstrated in acute slices by monitoring tonic GABAergic currents in migrating cells [53]. In addition, it was demonstrated that MPAE increases sensitivity of GABAARs to exogenous GABA, suggesting a change in the subunit composition of these receptors. Related to this study, MPAE (liquid diet during preconception, pregnancy and gestation; BAL=0.09 g/dl) was recently shown to decrease cortical thickness in rat fetuses and/or neonates, an effect that could be a consequence of alterations in radial glia [resulting from reduced expression of the paired box 6 (Pax6) transcription factor], delayed neuronal migration, and decreased numbers of neuroblasts [54].
Exposure to ethanol during the 3rd trimester equivalent was recently demonstrated to produce long-lasting impairments in the excitability of layer 5 pyramidal neurons of the rat neocortex [55]. Rats were exposed to ethanol during P2-6 via vapor chambers (lowest range of BAL = 0.15 g/dl) and slice electrophysiological recordings were performed at P30-60. Depolarizing current injection resulted in dendritic spikes with significantly lower frequency and duration in neurons from ethanol exposed animals. The somatic response to dendritic current injection was also reduced in neurons from the ethanol group. Imaging studies revealed that dendritic Ca2+ transients mediated by voltage-gated Ca2+ channels were reduced in neurons from ethanol treated animals. Taken together with the results of other studies (see hippocampal and cerebellar sections) [35, 46], these findings further support the possibility that voltage-gated Ca2+ channels are important targets of ethanol exposure during development.
Dopaminergic and Serotonergic Systems
Dopaminergic System
The dopaminergic system has been tightly linked to drug abuse and a number of studies have demonstrated that MPAE increases vulnerability to ethanol and other abused substances. Behavioral studies have shown that MPAE increases: 1) orienting responses towards ethanol odor, 2) palatability and consumption of ethanol, and 3) reinforcing effects of ethanol [56, 57]. Consonant with these results, ethanol exposure during pregnancy and lactation (forced drinking; maternal BAL= 0.009-0.12 g/dl; suckling offspring BAL = 0.019 g/dl), increased preference and consumption of ethanol-containing solutions in adult rat offspring [58, 59]. Adult rats from the ethanol group also exhibited increased cocaine and amphetamine consumption after cocaine exposure, as well as enhanced conditioned place preference for ethanol and cocaine. Interestingly, these animals had an increased sensitivity to the anxiolytic effects of acute ethanol exposure and stimulant effects of ethanol, cocaine, and amphetamine (but decreased sensitivity to the sedative effects of ethanol). These animals exhibited complex age-dependent changes in mRNA expression for BDNF, the GluN1 NMDAR subunit, type-1 cannabinoid receptor, and dopamine receptors (D1 and D2) in the neocortex, thalamus, striatum, hippocampus and/or cerebellum [58, 59]. Furthermore, reductions in radioligand binding to D1 and D2 receptors, and/or dopamine transporters were also evident in the striatum of ethanol-exposed rats (at 1-2 months of age) [58].
Other studies have examined the effect of MPAE on ethanol consumption and preference. It was recently reported that MPAE guinea pigs (continuous-access voluntary drinking; expected BAL = 0.05 g/dl) showed increased ethanol preference [60]. However, another study reached a different conclusion: prenatal co-exposure of rats to ethanol (liquid diet; BAL = 0.1 g/dl) and nicotine (osmotic minipump; 3-6 mg/kg/day) decreased ethanol preference in adolescent males, and reduced both consumption and preference in adult female rats [61]. This raises the possibility that prenatal co-exposure to ethanol and other drugs of abuse has a different effect on drug-seeking behavior compared to ethanol exposure alone.
Studies with primates have also revealed MPAE-induced dopaminergic alterations. Pregnant rhesus monkeys were exposed to ethanol (voluntary drinking; BAL = 0.02-0.05 g/dl) during early pregnancy (gestational days 0-50), middle-late pregnancy (gestational days 50-135) or throughout pregnancy (gestational days 0-135) [62]. Positron emission tomography studies revealed that exposure during early pregnancy and throughout pregnancy reduces striatal dopaminergic function in young adult offspring (as measured by the ratio of striatal D2 receptor binding over dopamine synthesis). Interestingly, mid-late gestation exposure had the opposite effect indicating that the timing of prenatal exposure dictates the outcome. A subsequent study by the same group of investigators showed a reduction in aversive responses to repetitive tactile stimulation in young adult monkeys exposed to ethanol throughout gestation that could be related to changes in dopaminergic function in the striatum [63]. Given the regulatory role of the striatum on the function of other brain regions, this alteration in striatal dopaminergic signaling could result in impaired control of sensory input [21].
The above-described studies with primates indicate that MPAE during late pregnancy could significantly affect striatal function. In general agreement with these results, it was reported that exposure to moderate ethanol levels (0.63 g/kg subcutaneously at P 7; BAL=0.05 g/dl) increases caspase-3 activation in the mouse caudate nucleus (but not the thalamus) 4 hr after injection, suggesting activation of apoptotic pathways in this brain region [64].
Serotonergic System
Human and animal studies have implicated serotonin neurotransmitter system alterations in the pathophysiology of FASDs [65, 66]. MPAE (Liquid diet; BAL=0.017-0.14 g/dl) was shown to impair neural tube midline development in fetal mice, which negatively impacts the development of serotoninergic neurons in the raphe nucleus of the brainstem [67]. A recent study with primates demonstrated a gene-environment interaction between the serotonin transporter gene-linked polymorphic region (5-HTTLPR) and MPAE. A variation in this region has been identified in humans resulting in long and short forms of the serotonin transporter (SERT), the former being associated with higher expression of SERT [68]. Long and short forms of this region have also been identified in rhesus monkeys and the effect of MPAE (voluntary drinking; BAL = 0.02-0.05 g/dl) has been studied [69]. In neonatal MPAE offspring, monkeys with the short form were more irritable, and had significant adrenocorticotropic hormone and cortisol increases in response to mother-infant separation. These findings suggest that having the short variant of 5-HTTLPR may result in altered responsiveness of the serotonin system to modulation by stress hormones, an effect that is exacerbated by MPAE. Alternatively, altered serotoninergic modulation of the HPA axis could be the cause of the increased responsiveness to stress in these animals, a conclusion that is generally supported by studies with rodents [70]. Importantly, this study further highlights the role that genetics can play in modulating susceptibility to the effects of MPAE [43].
Another effect of MPAE on the serotonin system was identified in respiratory centers in the brain stem [71]. In brain stem slices from control rats, the amplitude of hypoglossal nerve rootlet burst activity can be persistently increased in response to brief episodes of anoxia, a phenomenon that is known as respiratory LTP. In slices from rats exposed to ethanol during pregnancy and lactation (forced drinking; maternal BAL = 0.08 g/dl; nursing pup BAL = 0.019 g/dl), respiratory LTP was converted into LTD. Serotonin accumulates during hypoxia and plays a critical role in the induction of respiratory long-term facilitation. Therefore, the authors measured expression of serotonin (5-HT) 2A/2C receptors, which have been implicated in this process. It was shown that mRNA levels for these receptors were decreased in the ethanol group and higher concentrations of serotonin were required to induce facilitation of respiration [70]. These findings may be therapeutically significant, as alterations in brain stem serotonergic signaling could explain the higher incidence of sudden infant death syndrome in patients with FASDs [72].
Concluding Remarks
The collective evidence from the animal studies reviewed above strongly suggests that MPAE can persistently alter multiple neurotransmitter and neuromodulatory systems throughout the brain, leading to significant neurobehavioral alterations in offspring (Fig 3). Some studies showed significant effects at BALs as low as 0.02 g/dl, which could be achieved after consumption of just 1 drink/hr (Box 1). Several mechanisms that could account for the behavioral effects of ethanol have been demonstrated, including alterations in neuronal migration, adult neurogenesis, neurotransmitter receptor function, synaptic plasticity, and intracellular signaling pathways. Importantly, modulation of histamine neurotransmission has been identified as having potentially beneficial effects to reverse some of the deficits induced by MPAE.
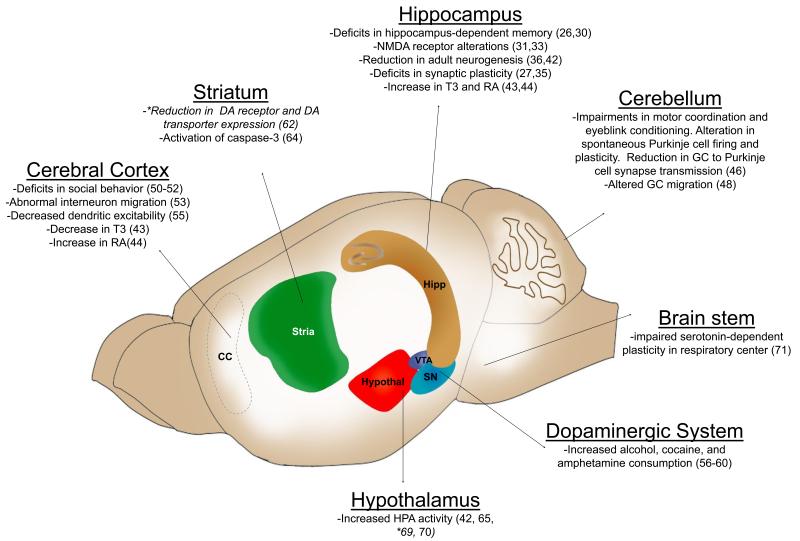
Fig 3. Schematic representation of the rodent brain indicating examples of the effect of moderate ethanol exposure in different brain regions.
*For comparison, the results of studies with primates are also mentioned in italics. Abbreviations: DA, dopamine; GC, granule cells; HPA, hypothalamic-pituitary-adrenal axis; RA, all-trans retinoic acid; SN, substantia nigra; T3, triiodothyronine; VTA, ventral tegmental area.
The findings of the above-reviewed preclinical studies could be translated into the clinic in several ways. First, public educational campaigns on the potential effects of MPAE should be widely implemented and training on this important issue should be provided to medical students, residents, and other health care professionals involved in the care of pregnant women, as these professionals may be unaware of the potential dangers of MPAE [73]. In addition, public health campaigns against FASDs should include information on studies of the effects of MPAE. Second, health care professionals should be made aware of the current guidelines/methods for ethanol consumption screening during antenatal and prenatal care visits, and interventions that could aid in the prevention of drinking during pregnancy [74]. Third, development of laboratory tests that can objectively assess MPAE should continue to be actively pursued [75]. Fourth, offspring of mothers who consumed ethanol at moderate levels should be assessed for cognitive alterations at multiple levels and at different stages of development, as deficits could be significantly ameliorated by early behavioral or pharmacological interventions [76]. Although further research is required to fully understand the potential consequences of MPAE (Box 2), studies with animal models clearly indicate that MPAE can significantly affect brain development. Clinicians should take the findings of these studies into account when advising women about ethanol consumption during pregnancy and should also consider MPAE as a potential cause of neurobehavioral disorders identified during adulthood.
Box 2. Outstanding Issues.
Although studies have demonstrated potent effects of acute ethanol exposure on developing neurons in vitro [35, 82, 83], animal studies that have specifically characterized the long-lasting neurobehavioral effects of sporadic light drinking during pregnancy remain to be performed (for instance, the equivalent of 1-4 drinks/month in humans [84]).
Pregnant women often consume ethanol at moderate doses concomitantly with other substances (e.g., nicotine and cannabis). The possibility that co-exposure to these substances modifies the effect of ethanol should be further studied [61].
The role of genetic, epigenetic, and environmental factors in determining sensitivity of the developing brain to MPAE should be further explored.
Studies of the effect of MPAE should be expanded to other brain regions. Investigators have begun to address this issue; e.g., reduced soma size of M-type neurons in the lateral geniculate nucleus (a thalamic nucleus that relays visual information) was recently demonstrated in non-human primate adult offspring (voluntary drinking during pregnancy; BAL=0.06-0.13 g/dl) [85].
The majority of animal studies have demonstrated correlations between behavioral deficits and alterations in neuronal function. Future studies should assess whether restoration of neuronal function in specific brain regions reverses or ameliorates behavioral impairments. Studies of this nature are essential for developing an integrated view of the neurobehavioral impact of MPAE-induced alterations in multiple neurotransmitter systems across different brain regions.
In order to better understand the mechanism of action of ethanol, its effect on the developmental trajectory of affected cell populations should be assessed. This type of study will increase understanding of how a relatively transient developmental insult (i.e., ethanol exposure) results in alterations that persist into adulthood.
Postmortem brain tissue from patients with FASDs is not widely available to the research community. Brain banks across the world should make a concerted effort to increase availability of these samples, allowing confirmation of key findings of animal studies with human tissue.
In light of the results of animal experiments, human epidemiological studies should assess the effect of MPAE on a wide range of cognitive domains across different stages of development.
Acknowledgements
Supported by National Institutes of Health grants RO1-AA015614, RO1-AA014973, T32-AA014127, K12-GM088021 and P20-AA17068. We are grateful to Dan Savage, Kevin Caldwell, and Rafael Varaschin for critically reading the manuscript.
Glossary
- Brain reward system
a neuronal circuit that includes dopaminergic neurons in the ventral tegmental area projecting to several target regions including the nucleus accumbens, prefrontal cortex, and amygdala.
- Contextual fear conditioning
a behavioral test in which animals learn to associate fear with spatial environmental cues.
- Eyeblink conditioning
a behavioral test in which animals learn to associate auditory or visual stimuli with a stimulus that induces an eyeblink response (e.g., airpuff to the cornea).
- Positron emission tomography
an imaging technique that is capable of detecting radioactive chemicals in brain and other organs.
- Stem cell niche
microenvironment that regulates self-renewal, differentiation, and maturation of stem cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riley EP, et al. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutson JR, et al. The incidence of prenatal alcohol exposure in Montevideo Uruguay as determined by meconium analysis. Ther Drug Monit. 2010;32:311–317. doi: 10.1097/FTD.0b013e3181dda52a. [DOI] [PubMed] [Google Scholar]
- 3.Center for Disease Contron and Prevention Alcohol use among pregnant and nonpregnant women of childbearing age—United States, 1991-2005. MMWR Morb Mortal Wkly Rep. 2009;58:529–532. [PubMed] [Google Scholar]
- 4.Ceccanti M, et al. Clinical delineation of fetal alcohol spectrum disorders (FASD) in Italian children: comparison and contrast with other racial/ethnic groups and implications for diagnosis and prevention. Neurosci Biobehav Rev. 2007;31:270–277. doi: 10.1016/j.neubiorev.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Henderson J, et al. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. Bjog. 2007;114:243–252. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Leary CM, Bower C. Guidelines for pregnancy: What’s an acceptable risk, and how is the evidence (finally) shaping up? Drug Alcohol Rev. 2011 doi: 10.1111/j.1465-3362.2011.00331.x. doi: 10.1111/j.1465-3362.2011.00331.x. [DOI] [PubMed] [Google Scholar]
- 7.Todorow M, et al. Investigating the effects of low to moderate levels of prenatal alcohol exposure on child behaviour: a critical review. J Popul Ther Clin Pharmacol. 2010;17:e323–330. [PubMed] [Google Scholar]
- 8.Burden MJ, et al. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29:443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- 9.Coles CD, et al. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol Teratol. 1991;13:357–367. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- 10.O’Leary CM, et al. A new method of prenatal alcohol classification accounting for dose, pattern and timing of exposure: improving our ability to examine fetal effects from low to moderate alcohol. J Epidemiol Community Health. 2010;64:956–962. doi: 10.1136/jech.2009.091785. [DOI] [PubMed] [Google Scholar]
- 11.Sayal K, et al. Prenatal alcohol exposure and gender differences in childhood mental health problems: a longitudinal population-based study. Pediatrics. 2007;119:e426–434. doi: 10.1542/peds.2006-1840. [DOI] [PubMed] [Google Scholar]
- 12.Sood B, et al. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose-response effect. Pediatrics. 2001;108:E34. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- 13.Bay B, Kesmodel US. Prenatal alcohol exposure - a systematic review of the effects on child motor function. Acta Obstet Gynecol Scand. 2010 doi: 10.1111/j.1600-0412.2010.01039.x. doi: 10.1111/j.1600-0412.2010.01039.x. [DOI] [PubMed] [Google Scholar]
- 14.Bay B, et al. Low to Moderate Alcohol Intake During Pregnancy and Risk of Psychomotor Deficits. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01657.x. doi: 10.1111/j.1530-0277.2011.01657.x. [DOI] [PubMed] [Google Scholar]
- 15.Kelly Y, et al. Light drinking in pregnancy, a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38:129–140. doi: 10.1093/ije/dyn230. [DOI] [PubMed] [Google Scholar]
- 16.Kelly YJ, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health. 2010;66:41–48. doi: 10.1136/jech.2009.103002. [DOI] [PubMed] [Google Scholar]
- 17.Robinson M, et al. Low-moderate prenatal alcohol exposure and risk to child behavioural development: a prospective cohort study. Bjog. 2010;117:1139–1150. doi: 10.1111/j.1471-0528.2010.02596.x. [DOI] [PubMed] [Google Scholar]
- 18.Gohlke JM, et al. Computational models of ethanol-induced neurodevelopmental toxicity across species: Implications for risk assessment. Birth Defects Res B Dev Reprod Toxicol. 2008;83:1–11. doi: 10.1002/bdrb.20137. [DOI] [PubMed] [Google Scholar]
- 19.Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- 20.Medina AE. Fetal alcohol spectrum disorders and abnormal neuronal plasticity. Neuroscientist. 2011;17:274–287. doi: 10.1177/1073858410383336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider ML, et al. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valenzuela CF, et al. Focus on: Neurotransmitter Systems. Alcohol Res Health. 2011;34:106–120. [PMC free article] [PubMed] [Google Scholar]
- 23.Gil-Mohapel J, et al. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev. 2010;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- 25.Savage DD, et al. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcohol Clin Exp Res. 2002;26:1752–1758. doi: 10.1097/01.ALC.0000038265.52107.20. [DOI] [PubMed] [Google Scholar]
- 26.Savage DD, et al. Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcohol Clin Exp Res. 2010;34:1793–1802. doi: 10.1111/j.1530-0277.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varaschin RK, et al. Effects of the cognition-enhancing agent ABT-239 on fetal ethanol-induced deficits in dentate gyrus synaptic plasticity. J Pharmacol Exp Ther. 2010;334:191–198. doi: 10.1124/jpet.109.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passani MB, Blandina P. Histamine receptors in the CNS as targets for therapeutic intervention. Trends Pharmacol Sci. 2011;32:242–249. doi: 10.1016/j.tips.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Allan AM, et al. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcohol Clin Exp Res. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- 30.Brady ML, et al. A Limited Access Mouse Model of Prenatal Alcohol Exposure that Produces Long-Lasting Deficits in Hippocampal-Dependent Learning and Memory. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01644.x. doi: 10.1111/j.1530-0277.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samudio-Ruiz SL, et al. Prenatal ethanol exposure persistently impairs NMDA receptor-dependent activation of extracellular signal-regulated kinase in the mouse dentate gyrus. J Neurochem. 2009;109:1311–1323. doi: 10.1111/j.1471-4159.2009.06049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis S, et al. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zink M, et al. Perinatal exposure to alcohol disturbs spatial learning and glutamate transmission-related gene expression in the adult hippocampus. Eur J Neurosci. 2011;34:457–468. doi: 10.1111/j.1460-9568.2011.07776.x. [DOI] [PubMed] [Google Scholar]
- 34.Gaiarsa JL. Plasticity of GABAergic synapses in the neonatal rat hippocampus. J Cell Mol Med. 2004;8:31–37. doi: 10.1111/j.1582-4934.2004.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zucca S, Valenzuela CF. Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. J Neurosci. 2010;30:6776–6781. doi: 10.1523/JNEUROSCI.5405-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi IY, et al. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcohol Clin Exp Res. 2005;29:2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- 37.Uban KA, et al. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Horm Behav. 2010;58:835–843. doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akers KG, et al. Fetal alcohol exposure leads to abnormal olfactory bulb development and impaired odor discrimination in adult mice. Mol Brain. 2011;4:29. doi: 10.1186/1756-6606-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roitbak T, et al. Moderate fetal alcohol exposure impairs neurogenic capacity of murine neural stem cells isolated from the adult subventricular zone. Exp Neurol. 2011;229:522–525. doi: 10.1016/j.expneurol.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joels M, et al. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Hellemans KG, et al. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sliwowska JH, et al. Stress-induced suppression of hippocampal neurogenesis in adult male rats is altered by prenatal ethanol exposure. Stress. 2010;13:301–313. doi: 10.3109/10253890903531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sittig LJ, et al. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. Faseb J. 2011;25:2313–2324. doi: 10.1096/fj.10-179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kane MA, et al. Ethanol elevates physiological all-trans-retinoic acid levels in select loci through altering retinoid metabolism in multiple loci: a potential mechanism of ethanol toxicity. Faseb J. 2010;24:823–832. doi: 10.1096/fj.09-141572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson CR, Mello CV. Significance of vitamin A to brain function, behavior and learning. Mol Nutr Food Res. 2010;54:489–495. doi: 10.1002/mnfr.200900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Servais L, et al. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc Natl Acad Sci U S A. 2007;104:9858–9863. doi: 10.1073/pnas.0607037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez-Burgos I, Alejandre-Gomez M. Cerebellar granule cell and Bergmann glial cell maturation in the rat is disrupted by pre- and post-natal exposure to moderate levels of ethanol. Int J Dev Neurosci. 2005;23:383–388. doi: 10.1016/j.ijdevneu.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Kumada T, et al. Inhibition of cerebellar granule cell turning by alcohol. Neuroscience. 2010;170:1328–1344. doi: 10.1016/j.neuroscience.2010.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green JT, et al. The effects of moderate neonatal ethanol exposure on eyeblink conditioning and deep cerebellar nuclei neuron numbers in the rat. Alcohol. 2006;39:135–150. doi: 10.1016/j.alcohol.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton DA, et al. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamilton DA, et al. Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behav Brain Res. 2010;214:66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbaccia ML, et al. Cognitive impairment and increased brain neurosteroids in adult rats perinatally exposed to low millimolar blood alcohol concentrations. Psychoneuroendocrinology. 2007;32:931–942. doi: 10.1016/j.psyneuen.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Cuzon VC, et al. Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci. 2008;28:1854–1864. doi: 10.1523/JNEUROSCI.5110-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aronne MP, et al. Effects of prenatal ethanol exposure on rat brain radial glia and neuroblast migration. Exp Neurol. 2011;229:364–371. doi: 10.1016/j.expneurol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Granato A, et al. Early exposure to alcohol leads to permanent impairments of dendritic excitability in neocortical pyramidal neurons. J Neurosci. 2012;32:1377–1382. doi: 10.1523/JNEUROSCI.5520-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abate P, et al. Fetal learning about ethanol and later ethanol responsiveness: evidence against “safe” amounts of prenatal exposure. Exp Biol Med (Maywood) 2008;233:139–154. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chotro MG, Arias C. Exposure to low and moderate doses of alcohol on late gestation modifies infantile response to and preference for alcohol in rats. Ann Ist Super Sanita. 2006;42:22–30. [PubMed] [Google Scholar]
- 58.Barbier E, et al. Effects of prenatal and postnatal maternal ethanol on offspring response to alcohol and psychostimulants in long evans rats. Neuroscience. 2009;161:427–440. doi: 10.1016/j.neuroscience.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 59.Barbier E, et al. Long-term alterations in vulnerability to addiction to drugs of abuse and in brain gene expression after early life ethanol exposure. Neuropharmacology. 2008;55:1199–1211. doi: 10.1016/j.neuropharm.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 60.Shea KM, et al. Maternal ethanol consumption by the pregnany guinea pig causes neurobehavioral deficits and increases ethanol preference in offspring. Behavioral Pharmacology. 2012;23:105–112. doi: 10.1097/FBP.0b013e32834ed866. [DOI] [PubMed] [Google Scholar]
- 61.Williams SK, et al. Simultaneous prenatal ethanol and nicotine exposure affect ethanol consumption, ethanol preference and oxytocin receptor binding in adolescent and adult rats. Neurotoxicol Teratol. 2009;31:291–302. doi: 10.1016/j.ntt.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider ML, et al. Moderate-level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcohol Clin Exp Res. 2005;29:1685–1697. doi: 10.1097/01.alc.0000179409.80370.25. [DOI] [PubMed] [Google Scholar]
- 63.Schneider ML, et al. Sensory processing disorder in a primate model: evidence from a longitudinal study of prenatal alcohol and prenatal stress effects. Child Dev. 2008;79:100–113. doi: 10.1111/j.1467-8624.2007.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young C, Olney JW. Neuroapoptosis in the infant mouse brain triggered by a transient small increase in blood alcohol concentration. Neurobiol Dis. 2006;22:548–554. doi: 10.1016/j.nbd.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 65.Macri S, et al. Early adversity and alcohol availability persistently modify serotonin and hypothalamic-pituitary-adrenal-axis metabolism and related behavior: what experimental research on rodents and primates can tell us. Neurosci Biobehav Rev. 2007;31:172–180. doi: 10.1016/j.neubiorev.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 66.Zhou FC, et al. Fetal alcohol exposure reduces serotonin innervation and compromises development of the forebrain along the serotonergic pathway. Alcohol Clin Exp Res. 2005;29:141–149. doi: 10.1097/01.alc.0000150636.19677.6f. [DOI] [PubMed] [Google Scholar]
- 67.Zhou FC, et al. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Brain Res Dev Brain Res. 2003;144:43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]
- 68.Heils A, et al. The human serotonin transporter gene polymorphism--basic research and clinical implications. J Neural Transm. 1997;104:1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- 69.Kraemer GW, et al. Moderate level fetal alcohol exposure and serotonin transporter gene promoter polymorphism affect neonatal temperament and limbic-hypothalamic-pituitary-adrenal axis regulation in monkeys. Biol Psychiatry. 2008;63:317–324. doi: 10.1016/j.biopsych.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hofmann CE, et al. Hypothalamic-pituitary-adrenal responses to 5-HT1A and 5-HT2A/C agonists are differentially altered in female and male rats prenatally exposed to ethanol. Alcohol Clin Exp Res. 2007;31:345–355. doi: 10.1111/j.1530-0277.2006.00316.x. [DOI] [PubMed] [Google Scholar]
- 71.Kervern M, et al. Perinatal alcohol exposure in rat induces long-term depression of respiration after episodic hypoxia. Am J Respir Crit Care Med. 2009;179:608–614. doi: 10.1164/rccm.200703-434OC. [DOI] [PubMed] [Google Scholar]
- 72.Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol. 2009;51:223–233. doi: 10.1002/dev.20367. [DOI] [PubMed] [Google Scholar]
- 73.Vagnarelli F, et al. A survey of Italian and Spanish neonatologists and paediatricians regarding awareness of the diagnosis of FAS and FASD and maternal ethanol use during pregnancy. BMC Pediatr. 2011;11:51. doi: 10.1186/1471-2431-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Potter B, Fleming MF. Obstetrics and gynecology resident education in tobacco, alcohol, and drug use disorders. Obstet Gynecol Clin North Am. 2003;30:583–599. viii. doi: 10.1016/s0889-8545(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 75.Bakhireva LN, Savage DD. Focus On: Biomarkers of Fetal Alcohol Exposure and Fetal Alcohol Effects. Alcohol Res Health. 2011;34:56–63. [PMC free article] [PubMed] [Google Scholar]
- 76.Kodituwakku PW. A neurodevelopmental framework for the development of interventions for children with fetal alcohol spectrum disorders. Alcohol. 2010;44:717–728. doi: 10.1016/j.alcohol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.U.S. Department of Human Services and U.S. Department of Agriculture . Dietary guidelines for americans. 7th Edition edn. U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Badger TM, et al. The effects of pregnancy on ethanol clearance. Life Sci. 2005;77:2111–2126. doi: 10.1016/j.lfs.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 79.Litvin J, Switzer BR. Pharmacokinetics of ethanol in the guinea pig. Alcohol Clin Exp Res. 1988;12:71–76. doi: 10.1111/j.1530-0277.1988.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 80.Clarke DW, et al. Disposition of ethanol and activity of hepatic and placental alcohol dehydrogenase and aldehyde dehydrogenases in the third-trimester pregnant guinea pig for single and short-term oral ethanol administration. Alcohol Clin Exp Res. 1986;10:330–336. doi: 10.1111/j.1530-0277.1986.tb05099.x. [DOI] [PubMed] [Google Scholar]
- 81.Nava-Ocampo AA, et al. Elimination kinetics of ethanol in pregnant women. Reprod Toxicol. 2004;18:613–617. doi: 10.1016/j.reprotox.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 82.Galindo R, et al. Alcohol is a potent stimulant of immature neuronal networks: implications for fetal alcohol spectrum disorder. J Neurochem. 2005;94:1500–1511. doi: 10.1111/j.1471-4159.2005.03294.x. [DOI] [PubMed] [Google Scholar]
- 83.Mameli M, et al. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci. 2005;25:8027–8036. doi: 10.1523/JNEUROSCI.2434-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dufour MC. What is moderate drinking? Alcohol Res Health. 1999;23:5–14. [PMC free article] [PubMed] [Google Scholar]
- 85.Papia MF, et al. Reduced soma size of the M-neurons in the lateral geniculate nucleus following foetal alcohol exposure in non-human primates. Exp Brain Res. 2010;205:263–271. doi: 10.1007/s00221-010-2364-6. [DOI] [PubMed] [Google Scholar]