Abstract
The 31P magnetization transfer (MT) effects among NMR resonances of PCr, γ-ATP and Pi have been attributed to the chemical exchange reactions among PCr, ATP and Pi catalyzed by creatine kinase (CK) and ATPase enzymes; therefore, are commonly applied in situ to measure chemical exchange fluxes involving two chemically coupled CK and ATPase reactions (i.e., PCr↔ATP↔Pi) by selectively saturating γ-ATP resonance. Beside the expected reductions in the Pi and PCr NMR signals upon saturating γ-ATP resonance, one particularly interesting phenomenon showing decreases in α-ATP and β-ATP signals was also observed. The underlying mechanism was investigated and identified via saturating NMR resonance of β-ATP in the present study. The unique relayed magnetization transfer effects through spin diffusion were observed in the rat brain using in vivo 31P MRS.
Keywords: In vivo31P MRS, spin diffusion, magnetization transfer, chemical exchange, Nuclear Overhauser effect (NOE)
Introduction
The magnetization transfer (MT) effects among three NMR-detectable resonances of phosphocreatine (PCr), γ-adenosine-5′-triphosphate (γ-ATP) and inorganic phosphate (Pi) have been commonly observed by in vivo 31P MRS in the rat and human brains (1–5). These MT effects have been attributed to the chemical exchange reactions catalyzed by the creatine kinase (CK) and ATPase enzymes as illustrated the following, respectively.
| (1) |
| (2) |
These two chemical reactions linking PCr, ATP and Pi play a key role in regulating brain energy metabolism (6). Consequently, a variety of MT methods combined with in vivo 31P MRS (defined as in vivo 31P MT approach in this article) have been widely used to measure chemical exchange rate constants and fluxes of the CK and ATPase reactions, for instance, using intact cells (7), perfused animal hearts (8–10) and human hearts (11), skeletal muscles (12) and brains (1–5,11,13). Among these approaches, the saturation transfer (ST) method (14) is popularly used in most pathologic or physiologic studies because of its high efficiency and simplicity (4). By using the ST approach, the pseudo unidirectional forward chemical exchange rate constants and fluxes of CK and ATPase reactions can be deduced from the magnetization reductions of PCr and Pi, respectively, in the presence of steady-state saturation of the γ-ATP resonance (4). Beside the expected magnetization reductions in the Pi and PCr signals upon saturating γ-ATP resonance, one particularly interesting phenomenon, i.e., decreases of signal intensity in α-ATP and β-ATP resonances, was also commonly observed in vivo (1–4,15). Nevertheless, the reason leading to these magnetization reductions is still not fully understood. One alternative explanation is that the observed MT effect on α-ATP could possibly be attributed to the negative 31P NOE among the γ-ATP, β-ATP and α-ATP spins in situ. Another possible cause of the decrease in signal intensity in α-ATP and β-an ATP resonance is chemical exchange between ADP and ATP catalyzed by the adenylate kinase (AK) enzyme (i.e. ADP+ADP ↔ ATP+AMP) because β-ADP is saturated together with γ-ATP due to the overlapped chemical shifts. To date, no strong evidences could verify these assumptions. The previous reports are ambiguous. The purpose of this study is to identify the possible sources which contribute to the magnetization reductions of α-ATP and β-ATP when γ-ATP is selectively saturated and to study their possible impact on the measurements of chemical exchange fluxes involved in the three-site exchange system of PCr↔ATP↔Pi. To clarify these possibilities, the β-ATP resonance was saturated in our current study and the magnetization reductions in the resonances of α-ATP, γ-ATP and PCr were observed. Since the β-ATP resonance is not overlapped with any other resonances, the only possible reason leading to α-ATP signal intensity reduction comes from the negative 31P NOE upon β-ATP resonance saturation. Therefore our current experiment clearly indicated that a relayed magnetization transfer network via chemical exchange and NOE exists among NMR spins of PCr, ATP and Pi in vivo. The unique relayed magnetization transfer effects through spin diffusion were observed in the rat brain using in vivo 31P MRS.
Materials and Method
Animal and phantom preparation
Five male Sprague-Dawley rats (250–400 g) anesthetized with 2% isoflurane were used for this study. The femoral arteries were catheterized for blood sampling and physiology monitoring to maintain animals at the normal physiological condition during the whole NMR measurements. All surgical procedures and experimental protocols were performed according to the guidelines of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
In order to compare in vitro MT effects with that observed in in vivo measurements, four phantoms including [PCr] =15.0 mM, [ATP] = 9.0 mM, [Pi] = 12.0 mM, [Mg2+] = 1.5 mM and [NaN3] = 0.25 mM (pH=7.1) with varied viscosity (0%, 2%, 4% and 6% w/v agarose, respectively) were prepared and measured using the same MRS measurement procedures and parameters as that of animal studies. To test off-resonance saturation effect of the applied saturation pulse trains, a phantom of [Pi]=200 mM was prepared. All the chemicals were bought from Sigma-Aldrich Co (St. Louis, MO). Both animal rectal temperature and phantom solution temperature were maintained at 37±1 °C inside the magnet during NMR measurements.
NMR Experiments
All NMR experiments were carried out at a 9.4-T/31-cm horizontal magnet (Magnex Scientific, UK) interfaced with a Varian INOVA console (Varian Inc., Palo Alto, CA). A dual RF surface-coil probe consisting of a butterfly-shape 1H surface coil and an elliptical-shape 31P surface coil with axes of 12 mm and 10 mm was used. The 1H surface coil was used for acquiring anatomy images and for shimming magnetic field homogeneity. The 31P surface coil was directly placed on the rat head for acquiring in vivo 31P MT spectra. The majority of rat brain (the cortical and sub-cortical region) was measured considering the intrinsic low sensitivity of in vivo 31P MRS. The RF pulse sequence used for the in vivo 31P MT experiments in this study has been described previously (3–5). Briefly, the BISTRO saturation pulse train scheme (16) was applied to selectively saturate the targeted resonance; Steady-state saturation transfer experiments were performed using a long saturation time (6.6 s) to achieve the steady-state magnetizations of unsaturated spins. The 31P spectra were acquired under approximately fully relaxed condition (repetition time = 9 s) with other acquisition parameters: 512 data points, 5000 Hz spectral width and 256 signal averages. The relative MT effect was quantified by the magnetization reduction ratio (R), which is defined as:
where, M* and M0 are the steady-state magnetization upon saturating the targeted NMR resonance such as γ-ATP and the magnetization at thermal equilibrium, respectively. They were quantified by the AMARES spectral fitting method provided by the MRUI software package (17). The chemical shift of the PCr resonance was assigned to zero ppm.
The results are presented by mean±standard deviation (SD).
Results
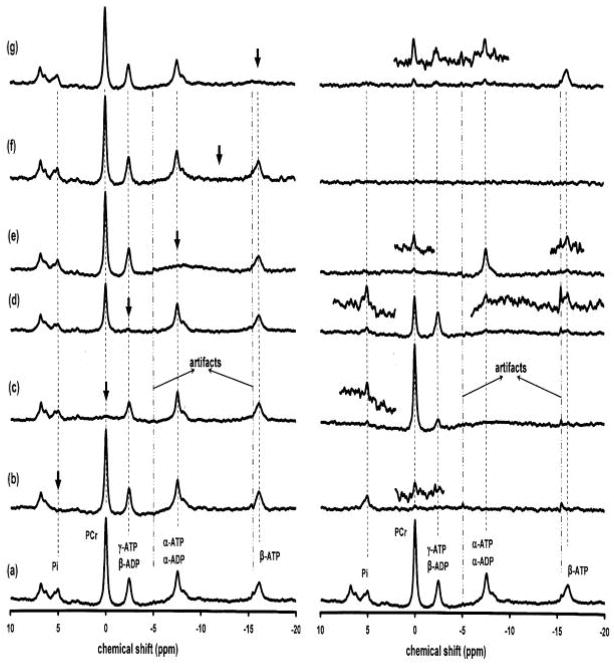
The 31P MRS of a phantom ([pi] =200 mM) was presented in Fig 1, which illustrated the saturation profile of the applied BISTRO saturation pulse trains. The in vivo 31P MRS in the absence of the BISTRO saturation pulse trains from a rat brain was illustrated in Fig. 2a. The MT effects from the same rat brain were demonstrated when the NMR resonance of Pi (Fig. 2b), PCr (Fig. 2c), γ-ATP (Fig. 2d), α-ATP (Fig. 2e) and β-ATP (Fig. 2g) was saturated, respectively. Fig. 2f illustrates the comparison spectrum in which the carry frequency of the BISTRO saturation pulse train was applied to the chemical shift at −12.5 ppm between the α-ATP and β-ATP resonances. The panels in the right column of Fig. 2 show the difference spectra between the saturated spectra and the control spectrum in the absence of the BISTRO saturation pulse train (Fig. 2a) beside the low panel which is the same as Fig. 2a in the left column.
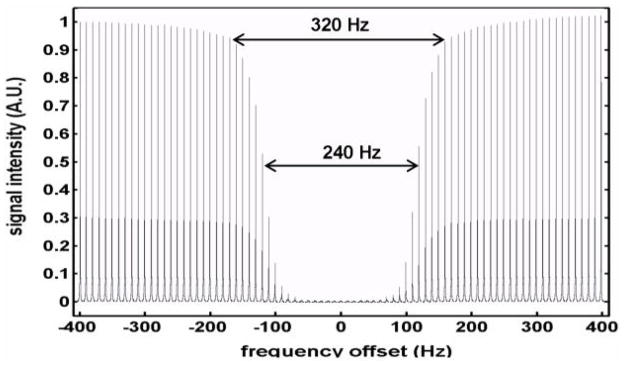
Fig. 1.
The frequency profile (±400 Hz with 10 Hz step) of the applied BISTRO saturation pulse trains was achieved through varying the carry frequency of the BISTRO saturation pulse trains. The measurements were conducted from a phantom ([Pi] = 200 mM). The frequency offset at 0 Hz means that carry frequency was on the resonance of Pi. The signal intensity in the plot was normalized to the NNR signal which was acquired with the same NMR pulse sequence and parameters except without BISTRO saturation pulse trains. The frequency width of 5% and 50% signal deduction was 320 and 240 Hz, respectively.
Fig. 2.
In vivo 31P spectra acquired from a representative rat brain in the absence (a) and presence of saturation (b) to (g) shown in the left column. The panels in the right column show the subtracted difference spectra between the control spectrum (a) and the spectrum with the steady-state saturation of the resonance of Pi (b), PCr (c), γ-ATP/β-ADP (d), α-ATP/α-ADP (e), and β-ATP (g), respectively; and at −12.5 ppm (f). The arrows indicate the carrier frequency of BISTRO saturation pulse train. The glitches around −5 and −16 ppm were artifacts. The partially expanded spectra were enlarged by increasing the vertical display scale (×4).
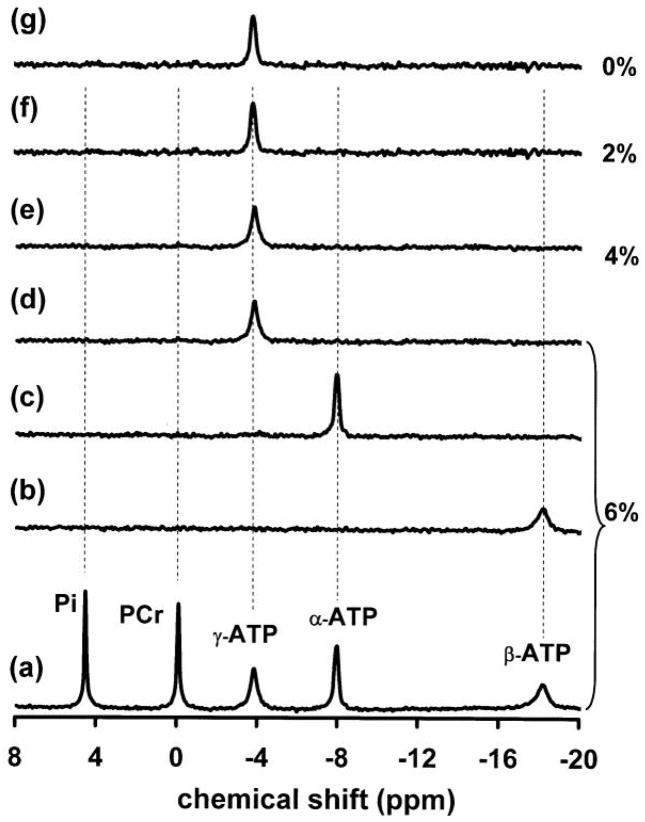
The steady-state saturation of the γ-ATP resonance resulted in not only significant magnetization reductions in the Pi and PCr resonances, but also in the α-ATP and β-ATP resonances (Fig. 2d), indicating a complex MT network in the rat brain. The results of relative magnetization reductions (i.e., percent reductions) measured in the rat brain in situ are summarized in Table 1. In contrast to the in vivo measurements, no MT effects were observed in all the prepared phantom solutions with various viscosities (see Fig. 3). For instance, the steady-state saturation of γ-ATP did not lead to any signal deductions in the β-ATP, α-ATP, PCr and Pi resonances.
Table 1.
Magnetization saturation transfer results measured in the rat brain (n=5, mean±SD)
| Relative magnetization reduction by MT effects | |||||
|---|---|---|---|---|---|
| Saturating position | Pi | PCr | γ-ATP/β-ADP | α-ATP/α-ADP | β-ATP |
| Pi | – | 0.02±0.01 | x | x | x |
| PCr | 0.16±0.06 | – | 0.25±0.04 | x | x |
| γ-ATP/β-ADP | 0.43±0.07 | 0.40±0.04 | – | 0.07±0.05 | 0.08±0.05 |
| α-ATP/α-ADP | x | 0.02±0.01 | x | – | 0.06±0.04 |
| β-ATP | x | 0.08±0.03 | 0.17±0.06 | 0.16±0.04 | – |
In the subtracted spectra (see Fig. 2), when magnitude of NMR peaks are within the spectral noise level, it was not quantified and was noted as “x”, which indicates an insignificant reduction in the measured magnetization.
Fig. 3.
31P spectra acquired from phantoms with varied concentration of agarose. Spectra (a) to (d) were acquired from the phantom solution containing 6% agarose (maximum viscosity). Spectrum (a) was the control (in the absence of saturation) and spectra (b) to (d) were the differenced spectra between the control and spectra with the steady-state saturation of β-ATP (b), α-ATP (c) and γ-ATP (d), respectively. The different spectra (e) to (g) were obtained from the phantom solutions with 4% (e), 2% (f) and 0% (g) agarose, respectively, when γ-ATP was saturated.
Discussion
The direct off-resonance RF saturation effects can be ignored in our experiments because of ideal performance (characterized by an excellent saturation frequency profile with negligible RF off-resonance saturation effect of the applied BISTRO saturation pulse train (3,4), which was demonstrated in Fig. 1). This is also clearly evident in Fig. 2b, which showed that when Pi was saturated the two partially overlapped resonances (assigned to phosphoethanolamine and phosphocholine) at the down field within 7 ppm (the chemical shift difference from Pi is about 300 Hz at 9.4T) had no detectable changes. Thus, the control spectra in order to correct direct off-resonance RF saturation effects are not necessary(3–5). In addition, any magnetization reductions observed in the present study will be attributed to either chemical exchange effect or nuclear Overhauser effect (NOE) (18). It is known that water molecules in a biological system exist in sub-compartmental pools, deemed “free-water” and “bound water”, respectively. “Bound water” is normally not NMR visible due its binding to macromolecules with a very short T2 relaxation time. The exchange between “free water” and “bound water” leads to the MT effect and provide a unique MRI contrast, which can be detected by the MRI-MT measurement, in which the 1H NMR signal of “free water” at 4.7 ppm decreases when the magnetization of “bound water” is saturated, even though the carrier frequency of saturation pulse is far away from the resonance of “free water” (19). Nevertheless, such an MT effect commonly detected by 1H MRI was not observed in the in vivo 31P MRS measured in the brain. This is evident in Fig. 2f, showing no MT effects (or magnetization reductions) in all detected resonances when the BISTRO saturation pulse train was applied to the chemical shift at the middle of the α-ATP and β-ATP resonances (−12.5 ppm). These observations have several interesting implications. First, the MT effect caused by the exchange between “free” and “bound” phosphate metabolites can be excluded from the possible contribution to the magnetization reductions as observed in the in vivo 31P MT measurements (see Fig. 2). This is presumably owing to the lack of the exchange pathway between “free” and “bound” phosphate metabolites or to an exchange rate constant that is too slow to be detected by 31P MT approach. Second, the observations also suggest that the NMR invisible pool of bounded phosphates in the brain might be very small and negligible in vivo, thus, the phosphate compounds measured by in vivo 31P MRS are likely fully detectable in the brain. This conclusion is crucial for the absolute quantification of cerebral phosphate metabolites as well as the cerebral metabolic fluxes of ATP, which rely on the absolute concentration of [Pi] or [PCr] (1–5).
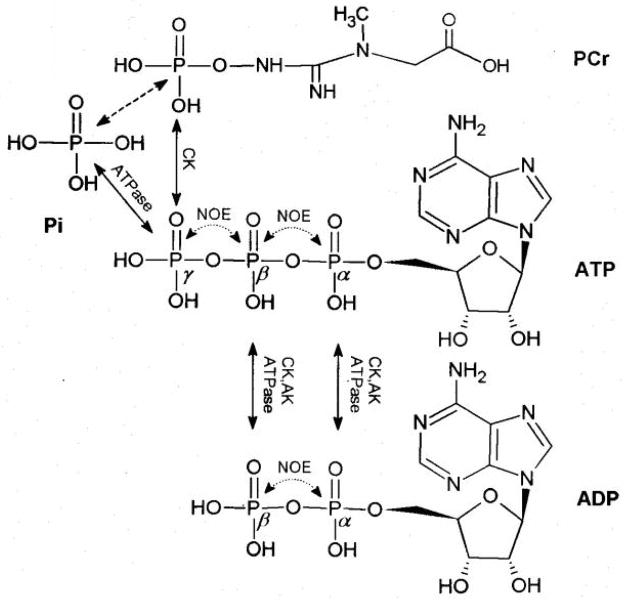
In the brain, PCr, γ-ATP and Pi constitute a chemically coupled three-spin system through the chemical exchanges of PCr↔ATP↔Pi as illustrated in Fig. 4, which demonstrates the molecular structures of PCr, ATP, ADP and Pi as well as two possible MT mechanisms through chemical exchange and NOE. In principle, saturating any resonance of PCr, γ-ATP and Pi can result in magnetization reductions of other two chemically coupled resonances. This phenomenon is demonstrated in Fig. 2b (by saturating Pi), Fig. 2c (by saturating PCr) and Fig. 2d (by saturating γ-ATP). Due to the low inherent sensitivity of 31P MRS and the relatively small brain size in the rat, some magnetization deductions (e.g., the magnetization reduction of γ-ATP when Pi was saturated; see Fig. 2b) were not significant in the rat brain, though it has been reliably detected in the human brain at 7T (1,3,4). Saturating the NMR resonance of γ-ATP resulted in significant magnetization reductions of PCr and Pi which can be attributed to the chemical exchanges of PCr→ATP←Pi. Therefore, these magnetization reductions have been commonly applied to deduce the unidirectional forward chemical exchange fluxes of CK and ATPase reactions under physiologic or pathologic conditions.
Fig. 4.
Sketched molecular structures of PCr, ATP, ADP and Pi and the possible MT pathways via NOEs (illustrated by doted lines) and chemical exchanges (illustrated by the solid lines) catalyzed by CK, ATPase and AK, respectively. The dashed line indicates an indirect chemical exchange.
The MT effect to the β-ATP resonance by saturating the γ-ATP resonance (Fig. 2d) may result from β-ADP through the adenylate kinase (AK) reaction (i.e. ADP+ADP ↔ ATP+AMP), CK or ATPase reactions because β-ADP is saturated together with γ-ATP due to the overlapped chemical shifts between them (see Figs. 2 and 4). However, it is more difficult to explain the MT effect to α-ATP based on chemical exchanges when γ-ATP is saturated because of the lack of direct chemical exchange pathways. One alternative explanation is that the observed MT effect on α-ATP could possibly be attributed to the negative 31P NOE among the γ-ATP, β-ATP and α-ATP spins in situ. To identify this possibility, the β-ATP resonance was saturated and the magnetization reductions in the resonances of α-ATP, γ-ATP and PCr were clearly visible in our current study (see Fig. 2g). Since the β-ATP resonance is not overlapped with any other resonances, the only possible reason leading to α-ATP signal intensity reduction comes from the negative 31P NOE upon β-ATP resonance saturation.
In addition, there are two arguments for further supporting the notion that there exists a negative 31P NOE between γ-ATP and β-ATP. First, the magnetization reduction of PCr upon saturating β-ATP can be achieved only by the relayed MT effect from γ-ATP via the chemical exchange of PCr↔γ-ATP. Second, it is possible that the signal reduction observed near the γ-ATP chemical shift can be attributed to the β-ADP reduction rather than the γ-ATP reduction because of the complete chemical shift overlapping between these two resonances; and the possible MT effect on the β-ADP resonance via the chemical exchange between ATP and ADP catalyzed by the enzymes of CK, AK, and ATPase when β-ATP is saturated (see Fig. 4). However, [ADP] in the rat brain is approximately 10 times lower than that of [ATP] in the brain (6); β-ADP is usually undetectable within a limited NMR acquisition time. Therefore, the observed signal reduction is likely attributed to the γ-ATP resonance and the negative NOE between γ-ATP and β-ATP spins when the β-ATP resonance is saturated. This conclusion is further supported by a recent study reporting that the saturation of γ-ATP resulted in a similar β-ATP reduction in the skeletal muscle of the mice lack of both cytosolic CK and AK enzymes (20). Our current results clearly indicate a significant NOE among the three ATP spins.
The triphosphate chain with folded conformation in the ATP molecule has been determined by x-ray in the crystal structure of ATP (21). The folded conformation shortens the distance among the α-, β-, and γ-phosphates and thereby enhances the dipolar-dipolar interactions and negative NOEs among them. This could provide an explanation for the NOEs measured in vivo presumably due to the unique molecular conformation in the brain tissue. In contrast, the similar NOEs were not detected in the phantom solutions. Therefore, the in vivo 31P results from the present study reveal a complex MT network linking the brain phosphate metabolites through the relayed chemical exchanges and NOEs, which has been widely observed in the in vitro protein structure studies by using 1H MRS (22). The relative magnetization deductions of α-ATP and β-ATP are very small compared to the magnetization deductions of PCr and Pi upon saturating the γ-ATP resonance (see Fig. 2d and Table 1). What’s more, the concentration of ADP (~0.3 mM) (6) is relatively low compared to [ATP] = 3.0 mM, [PCr] = 5.0 mM and [Pi] = 1.2 mM.” Thus, the magnetization reductions in PCr and Pi during the steady-state saturation of the γ-ATP resonance are mainly dominated by the MT effects related to the chemical exchanges involving in the three-spin system of PCr ↔ATP↔ Pi, and they can be used to deduce the cerebral metabolic fluxes for both the CK and ATPase reactions with a good approximation by ignoring NOEs. In principle, a quantitative examination of the impact of spin diffusion effects on the chemical exchange rates measurements in the three-site exchange system (PCr, r-ATP and Pi) can be simulated using a complete relaxation matrix. However, it is very complicated because of too many unknown parameters. Fig. 4 illustrates the whole relayed magnetization transfer network via chemical exchanges and NOEs involving a seven-site spin system. Generally, in order to separate NOEs and chemical exchanges, intrinsic spin-lattice relaxation times (T1) in the absence of chemical exchange and tumbling rates (ιc) of seven-coupled spins are need, just to mention a few. However, few parameters are available. It is difficult to measure T1s of β, α-ADP and β, α-ATP considering the small size of rat brain and the low sensitivity of in vivo 31P MRS. Another problem, tumbling rates of 31P spins are unknown. Therefore, a quantitative simulation was not conducted in the current study.
Conclusion
The in vivo magnetization reductions of α-ATP and β-ATP upon saturating γ-ATP possibly result mainly from the relayed MT effects from NOEs, although the chemical exchange contribution cannot be completely excluded for the magnetization reduction of β-ATP resonance based on the present study. The relayed MT effects were observed by 31P MRS only in the brain but not in the phantom solution. These effects should not significantly affect the measured chemical exchange fluxes when spin diffusion effect is ignored. Finally, the results also suggest that the most brain phosphate metabolites are fully visible in an in vivo 31P spectrum.
Acknowledgments
The authors gratefully acknowledge Ms. Alissa Cooper for insightful discussion and manuscript editing. This work was partially supported by NIH grants NS41262, EB00329, EB00513, P41 RR08079, 1R21MH092704 and P30NS057091; the W.M. Keck Foundation and MIND institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shoubridge EA, Briggs RW, Radda GK. 31P NMR saturation transfer measurements of the steady state rates of creatine kinase and ATP synthetase in the rat brain. FEBS Lett. 1982;140(2):289–292. doi: 10.1016/0014-5793(82)80916-2. [DOI] [PubMed] [Google Scholar]
- 2.Balaban RS, Kantor HL, Ferretti JA. In vivo flux between phosphocreatine and adenosine triphosphate determined by two-dimensional phosphorous NMR. J Biol Chem. 1983;258(21):12787–12789. [PubMed] [Google Scholar]
- 3.Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2003;100(24):14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57(1):103–114. doi: 10.1002/mrm.21107. [DOI] [PubMed] [Google Scholar]
- 5.Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105(17):6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siesjo BK. Brain energy metabolism. New York: Wiley; 1978. pp. 101–110. [Google Scholar]
- 7.Brown TR, Ugurbil K, Shulman RG. 31P nuclear magnetic resonance measurements of ATPase kinetics in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977;74(12):5551–5553. doi: 10.1073/pnas.74.12.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugurbil K. Magnetization transfer measurements of creatine kinase and ATPase rates in intact hearts. Circulation. 1985;72(5 Pt 2):IV94–96. [PubMed] [Google Scholar]
- 9.Ugurbil K, Petein M, Maidan R, Michurski S, From AH. Measurement of an individual rate constant in the presence of multiple exchanges: application to myocardial creatine kinase reaction. Biochemistry. 1986;25(1):100–107. doi: 10.1021/bi00349a015. [DOI] [PubMed] [Google Scholar]
- 10.Spencer RG, Balschi JA, Leigh JS, Jr, Ingwall JS. ATP synthesis and degradation rates in the perfused rat heart. 31P-nuclear magnetic resonance double saturation transfer measurements. Biophys J. 1988;54(5):921–929. doi: 10.1016/S0006-3495(88)83028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottomley PA, Hardy CJ. Mapping creatine kinase rates in human brain and heart with 4 tesla saturation transfer 31P NMR. J Magn Reson. 1992;99:443–448. [Google Scholar]
- 12.Brindle KM, Blackledge MJ, Challiss RA, Radda GK. 31P NMR magnetization-transfer measurements of ATP turnover during steady-state isometric muscle contraction in the rat hind limb in vivo. Biochemistry. 1989;28(11):4887–4893. doi: 10.1021/bi00437a054. [DOI] [PubMed] [Google Scholar]
- 13.Lei H, Zhu XH, Zhang XL, Ugurbil K, Chen W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn Reson Med. 2003;49(2):199–205. doi: 10.1002/mrm.10379. [DOI] [PubMed] [Google Scholar]
- 14.Frosen S, Hoffman RA. Study of moderately rapid chemical exchange by means of nuclear magnetic double resonance. J Chem Phys. 1963;39:2892–2901. [Google Scholar]
- 15.Matthews PM, Bland JL, Gadian DG, Radda GK. The steady-state rate of ATP synthesis in the perfused rat heart measured by 31P NMR saturation transfer. Biochem Biophys Res Commun. 1981;103(3):1052–1059. doi: 10.1016/0006-291x(81)90915-3. [DOI] [PubMed] [Google Scholar]
- 16.de Graaf RA, Luo Y, Garwood M, Nicolay K. B1-insensitive, single-shot localization and water suppression. J Magn Reson B. 1996;113(1):35–45. doi: 10.1006/jmrb.1996.0152. [DOI] [PubMed] [Google Scholar]
- 17.van den Boogaart A, Van Hecke A, Van Huffel P, Graveron-Demilly S, van Ormondt D, de Beer R. MRUI: a graphical user interface for accurate routine MRS data analysis. Prague. 1996:318. [Google Scholar]
- 18.Ernst RR, Bodenhausen B, Wokaun A. Principles of Nuclear Magnetic Resonances in One or Two Dimensions. Oxford University Press; 1992. [Google Scholar]
- 19.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10(1):135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 20.Nabuurs C, Kan H, Wiering A, Heerschap A. Do adenylate and creatine kinase contribute to saturation transfer effects among ADP and ATP resonances in 31P MRS of skeletal muscle? Proc Intl Soc Magn Reson Med. 2007;15:3794. [Google Scholar]
- 21.Kennard O, Isaacs NW, Motherwell WDS, Coppola JC, Wampler DL, Larson AC, Watson DG. The crystal and molecular structure of adenosine triphosphate. Proc R Soc Lond A. 1971;325:401–436. [Google Scholar]
- 22.Johnston PD, Redfield AG. Nuclear magnetic resonance and nuclear Overhauser effect study of yeast phenylalanine transfer ribonucleic acid imino protons. Biochemistry. 1981;20(5):1147–1156. doi: 10.1021/bi00508a016. [DOI] [PubMed] [Google Scholar]