Abstract
Clostridium perfringens type C is an important cause of enteritis and/or enterocolitis in several animal species, including pigs, sheep, goats, horses and humans. The disease is a classic enterotoxemia and the enteric lesions and associated systemic effects are thought to be caused primarily by beta toxin (CPB), one of two typing toxins produced by C. perfringens type C. This has been demonstrated recently by fulfilling molecular Koch’s postulates in rabbits and mice. We present here an experimental study to fulfill these postulates in goats, a natural host of C. perfringens type C disease. Nine healthy male or female Anglo Nubian goat kids were inoculated with the virulent C. perfringens type C wild-type strain CN3685, an isogenic CPB null mutant or a strain where the cpb null mutation had been reversed. Three goats inoculated with the wild-type strain presented abdominal pain, hemorrhagic diarrhea, necrotizing enterocolitis, pulmonary edema, hydropericardium and death within 24 h of inoculation. Two goats inoculated with the CPB null mutant and two goats inoculated with sterile culture media (negative controls) remained clinically healthy during 24 h after inoculation and no gross or histological abnormalities were observed in the tissues of any of them. Reversal of the null mutation to partially restore CPB production also increased virulence; 2 goats inoculated with this reversed mutant presented clinical and pathological changes similar to those observed in goats inoculated with the wild-type strain, except that spontaneous death was not observed. These results indicate that CPB is required for C. perfringens type C to induce disease in goats, supporting a key role for this toxin in natural C. perfringens type C disease pathogenesis.
Keywords: Beta toxin, Clostridium perfringens type C, enterotoxemia, goats
1. Introduction
Clostridium perfringens is an anaerobic, spore-forming, Gram-positive rod that is a pathogen of humans, domestic and wild animals (Miclard et al., 2009). C. perfringens produces a vast array of toxins, including four toxins that are used to classify C. perfringens isolates into five types (A-E); depending upon production of these four typing toxins, namely (alpha [CPA], beta [CPB], epsilon [ETX] and iota [ITX]) (Uzal and McClane, 2011). All types produce CPA; in addition, C. perfringens type B and C isolates produce CPB, type B and D isolates produce ETX and type E isolates produce ITX.
C. perfringens type C causes highly lethal diseases in many mammalian species that are characterized mainly by necrotizing enteritis or enterocolitis. Most clinical signs and lesions associated with C. perfringens type C disease are thought to be produced by CPB, a 35-kDa protein that forms pores in the membrane of susceptible cell lines, leading to cell swelling and lysis (Uzal and McClane, 2011). CPB is destroyed by proteolytic enzymes such as trypsin, explaining why the neonate is especially predisposed to CPB action; i.e., the trypsin inhibitor action of colostrum, which prevents proteolytic degradation of immunoglobulins during the first days of life, also protects CPB (Diab et al., 2011; Smith and Sherman, 1996).
Death is likely to result primarily from toxemia following absorption of CPB from the intestine into circulation, allowing targeting of internal organs (Ma et al., 2011; Uzal et al., 2009). It is also possible that other toxins present in the intestinal lumen gain access to the blood circulation following massive intestinal mucosal barrier damage, and that they contribute to the systemic effects observed in C. perfringens type C disease
The lethal and necrotizing effects of CPB play key roles in the tissue damage that is characteristic of C. perfringens type C infections (McClane et al., 2004). This was demonstrated when we recently constructed a series of C. perfringens type C toxin null mutants in strain CN3685, which demonstrated that CPB, but not CPA or perfringolysin O (PFO), is necessary for type C isolates to cause intestinal damage in a rabbit intestinal loop model and lethality in mice (Sayeed et al., 2008; Uzal et al., 2009).
In goats, information about the pathogenesis of C. perfringens type C infection is scant. To gain knowledge on the mechanism of type C infection in this species, we developed a model for type C infection in goats, and then tested the CPB null mutant and a reversed mutant strain in this model, fulfilling molecular Koch’s postulates in a natural host of C. perfringens type C disease.
2. Material and Methods
2.1 Animals
Conventionally-reared, healthy male and female Anglo Nubian goat kids were used. They were never vaccinated against clostridial or other disease and had been weaned at the age of 10 to 14 weeks (an age at which most maternal antibodies have disappeared), placed in a warm, dry room, and fed alfalfa hay and water ad-libitum for 1 or 2 weeks before the experiments. All animals were between 14 and 20 kg body weight. Fecal samples were collected 4 and 2 days before the beginning of the experiments. A fecal float analysis to detect intestinal parasite eggs and parasites was performed with each of these samples. All procedures involving animals were reviewed and approved by the University of California, Davis Committee for Animal Care and Use (permit 16383).
2.2 Bacterial Strains
C. perfringens type C isolate CN3685 was provided by Russell Wilkinson (University of Melbourne, Australia). This isolate, originally from the Burroughs-Welcome C. perfringens collection, had been isolated in 1954 from peritoneal fluid of a sheep with struck. It has been previously characterized (Fisher et al., 2006) for its genotype and toxin production, which showed that (under the growth conditions used in our current study) this isolate produces ~13 µg/ml of CPB. This toxin production level places CN3685 among the strongest 20% of CPB producers of the 45 C. perfringens type C disease isolates surveyed in that previous study (Fisher et al., 2006). Isogenic CN3685 CPB toxin null mutant BMC 104 had been constructed previously by insertion of a targeted group II intron into the cpb gene; characterization studies confirmed that this insertional event had inactivated CPB production (Sayeed et al., 2008). The reversed CPB mutant BMC 105 had also been constructed previously by introduction of an Ltr-encoding plasmid into BMC104. This allows removal of the intron from some disrupted cpb message, which is apparently transcribed as a monocistronic mRNA in BMC104, partially restoring CPB production by the reversed mutant to about one third of wild-type levels (Sayeed et al., 2008).
2.3 Production of type C cultures for goat inoculation
Wild-type CN3685, BMC 104 and BMC 105 were each grown for 8 h in TGY (tryptone, yeast extract, glucose, cysteine hydrochloride and distilled water) at 37°C. The reversed CPB mutant BMC105 was grown in the presence of 15 µg/ml of chloramphenicol to retain the LtrA-encoding plasmid needed to remove the intron insertion from the cpb message and partially restore CPB production. A 10% inoculum (50 ml) of each starter culture was then transferred into 500 ml of fresh TGY and grown at 30°C overnight (log phase culture); in BMC105, this incubation induced the expression of the LtrA protein required for trans-splicing removal of the sense oriented intron from the cpb mRNA (results not shown). Those culture supernatants were assayed by Western blotting (data not shown) as previously described (Sayeed et al., 2008), to confirm CPB production by CN3685 and BMC105, but not BMC104.
2.4 Intraduodenal inoculation of C. perfringens type C strain CN3685 and its derivatives in goats
The animals were divided into 4 treatment groups as follows. Three animals were inoculated with the wild-type strain CN3685. Two animals each were inoculated with the CPB null mutant BMC 104, the reversed CPB mutant BMC 105 or sterile, non-toxic TGY (negative controls). The goat kids were fasted for 24 hours before inoculation but were allowed access to water until 1 hr before surgery. The pre-anesthetic induction was carried out by intravenous injection of a mixture of ketamine and diazepam, and anesthesia was maintained with a mixture of isofluorane and oxygen administered by endotracheal tube in an open circuit. A laparotomy was performed via the right flank, and the pyloric area of the abomasum and the first portion of duodenum were exposed. Two hundred ml of a 20% solution of wheaten corn flour in 0.85 % saline were injected into the abomasum of all animals in each group. Then, 250 ml of a culture of the wild-type, CPB null mutant or reversed mutant were inoculated into the duodenum through a drip, over a period of approximately 10 minutes. Control animals received 250 ml of sterile, non-toxic, sterile TGY instead of bacterial culture. All animals received 0.25 g of trypsin inhibitor (Sigma, St. Louis, Missouri) mixed with the inoculum. The abdominal incision was closed by separate muscle and skin sutures.
The animals were allowed to recover from anesthesia and then clinically examined periodically after inoculation. Those animals that remained clinically healthy during that period, and those that showed moderate or mild clinical signs, were euthanized 24 hours after inoculation. Euthanasia was performed by an intravenous overdose of barbiturate.
2.5 Clinical signs
A scoring system from 0 to 3 was used to characterize overall clinical abnormalities. A score of 0 was assigned to animals that did not show clinical abnormalities during the whole experimental period. A score of 1 was assigned to animals that showed only mild, transient diarrhea, and that reminded alive during this time. A score of 2 was assigned to animals that showed moderate, hemorrhagic diarrhea accompanied by abdominal discomfort and depression during most of the observation period, and that reminded alive during this time. A score of 3 was assigned to animals that showed severe hemorrhagic diarrhea and abdominal pain and depression, dying spontaneously within 24 hs after inoculation.
2.6 Post-mortem examination, histopathology, microbiology testing and ELISA for C. perfringens toxins
Post-mortem examinations were performed immediately after death on all goats. Samples of the gastrointestinal tract, brain, kidneys, lungs, heart, spleen and liver were collected and fixed by immersion in 10% formalin pH 7.2 for a minimum of 24 h. After this, the brains were sliced into sections 0.5 cm thick, and fixed in fresh formalin for 7 more days before blocks were prepared from corpus striatum, parietal cortex, midbrain at the level of superior colliculi, pons, cerebellar peduncles, cerebellum and medulla at the level of the obex. All tissues were processed routinely to obtain 4 µm thick sections which were stained with hematoxylin and eosin. Selected sections were also stained with Gram or Phospho Tungstic Acid Hematoxylin (PTAH).
Small and large intestinal contents of each goat and samples of liver, spleen and lung were aseptically collected and inoculated onto reduced Brucella Blood Agar (Anaerobic Systems, Morgan Hill, California) and incubated anaerobically or aerobically at 37°C for 48 hs. Anaerobic isolates were identified by conventional biochemical techniques. C. perfringens isolates were typed by a multiplex PCR technique to amplify segments specific for the genes encoding CPA, CPB, ETX, ITX, CPE and CPB2, as previously described (Bueschel et al., 2003). Briefly, bacteria were grown on brain-heart infusion agar plates (Hardy Diagnostics, Santa Maria, California ), incubated anaerobically overnight at 37C and then processed for multiplex PCR analysis using colony lysates as templates. The multiplex PCR products were then separated in 2% agarose gels, stained with ethidium bromide and examined by ultraviolet light transillumination.
Samples of small and large intestinal contents from all goats were also tested for CPA, CPB and ETX via a commercial capture ELISA kit (BIO-X, Brussels, Belgium), following the manufacturer’s instructions. Briefly, the test used 96-flat-bottom-well plates sensitized by specific monoclonal antibodies to CPA, CPB or ETX. Samples were added to wells and plates were incubated for 60 min at room temperature, followed by washing and incubation for 60 min with peroxidase-labeled anti-CPA,- CPB or -ETX polyclonal antibodies. Plates were washed again, and a mixture of chromogen-substrate (hydrogen peroxide and tetramethyl bendizine) was added. The enzymatic reaction was stopped by acidification with phosphoric acid. Optical densities were read using an ELISA reader with a 450 nm filter. Purified CPA, CPB or ETX were used in positive control wells; toxins were replaced by buffer in negative control wells. Results were calculated according to the manufacturer’s instructions.
2.7 Immunohistochemistry
All sections of small intestine and colon were processed by an indirect immunoperoxidase technique for C. perfringens, as previously described (Diab et al, 2011) using the Dako EnVision kit (Dako, Carpenteria, CA) according to the instructions of the manufacturer. A rabbit polyclonal anti-C. perfringens antibody (GenWay Bio. San Diego, CA) was used as primary antibody. Small intestine from a goat that was culture - negative for C. perfringens was used as negative control. Also, sections of small intestine and colon from all goats were processed by an indirect immunoperoxidase technique for CPB as previously described (Miclard et al., 2009) using the LSAB kit (DakoCytomation; Dako, San Diego, California) according to the instructions of the manufacturer. An anti-CPB monoclonal antibody (Center for Veterinary Biologics, Ames, Iowa) was used as primary antibody.
3. Results
A summary of the overall severity of the clinical signs, gross and microscopic lesions is presented in Table 1. All animals inoculated with the wild-type isolate or with the reversed mutant presented hemorrhagic diarrhea, abdominal pain and depression, but these clinical signs were more severe in the 3 animals inoculated with the wild-type strain, which were the only animals that died spontaneously. The animals inoculated with the CPB null mutant and the negative controls did not show clinical abnormalities, except for one of the goats inoculated with the CPB null mutant strain that presented mild, transient diarrhea that resolved spontaneously 4 hours after onset. No other clinical abnormalities were observed in any other animal.
Table 1.
Summary of overall severity of clinical signs, gross and microscopic lesions observed in 9 goats inoculated with C. perfringens type C wild-type, its mutants, or with sterile non-toxic culture medium.
| Inoculum | Case № | Interval inoculation to death |
Death | Overall severity of clinics signs* |
Overall severity of gross lesions** |
Overall severity of microscopic lesions*** |
|---|---|---|---|---|---|---|
| C. perfringens type C wild type (CN3685) | 1 | 22.5 hs | Spontaneous | 3 | 2.5 | 3 |
| 2 | 6 hs | Spontaneous | 3 | 3 | 3 | |
| 3 | 3.20 hs | Spontaneous | 3 | 3 | 3 | |
| C. perfringens type C CPB null mutant(BMC 104) | 4 | 24 hs | Euthanasia | 1 | 0.5 | 0 |
| 5 | 24 hs | Euthanasia | 0 | 0 | 0 | |
| C. perfringens type C CPB reversed mutant (BMC 105) | 6 | 24 hs | Euthanasia | 2 | 1 | 2 |
| 7 | 24 hs | Euthanasia | 2 | 1 | 2 | |
| Sterile, non-toxic TGY | 8 | 24 hs | Euthanasia | 0 | 0 | 0 |
| 9 | 24 hs | Euthanasia | 0 | 0 | 0 | |
0: no clinical abnormalities; 1: mild, transient diarrhea; 2: moderate hemorrhagic diarrhea accompanied by abdominal discomfort and depression during most of the observation period; 3: similar signs to those with score 2, but of greater severity; animals died spontaneously within 24 hs of inoculation.
0: no gross lesions; 1 – 3: mild to severe gross lesions (See table 2 for details).
0: no microscopic lesions; 1 – 3: mild to severe microscopic lesions (See table 3 for details).
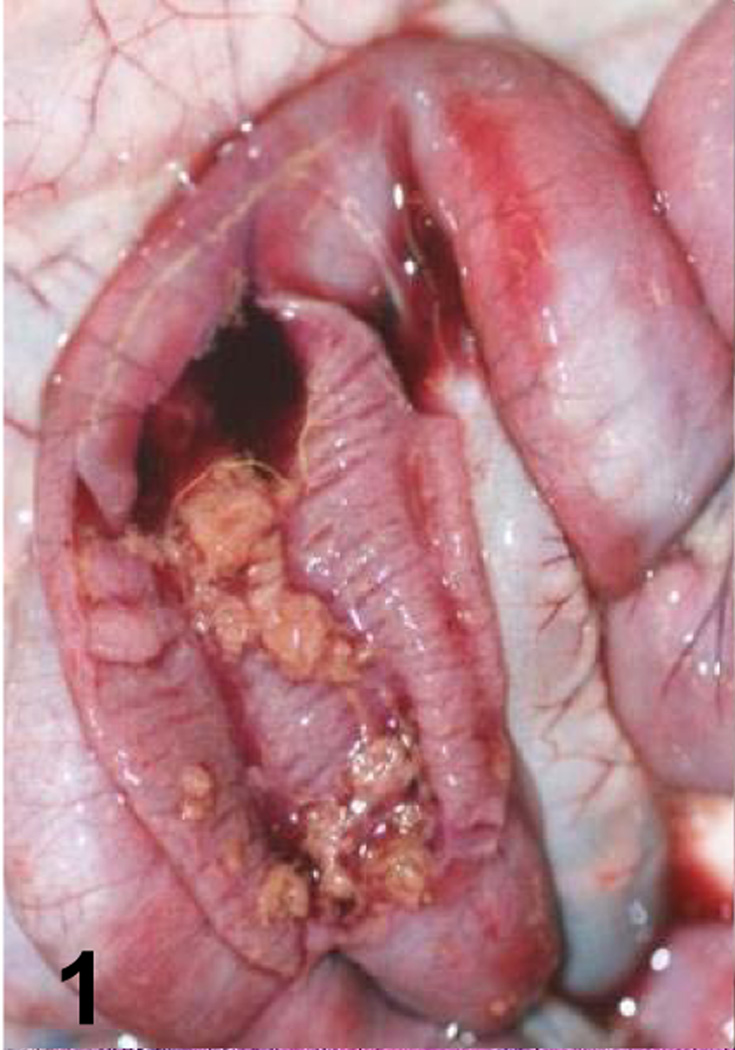
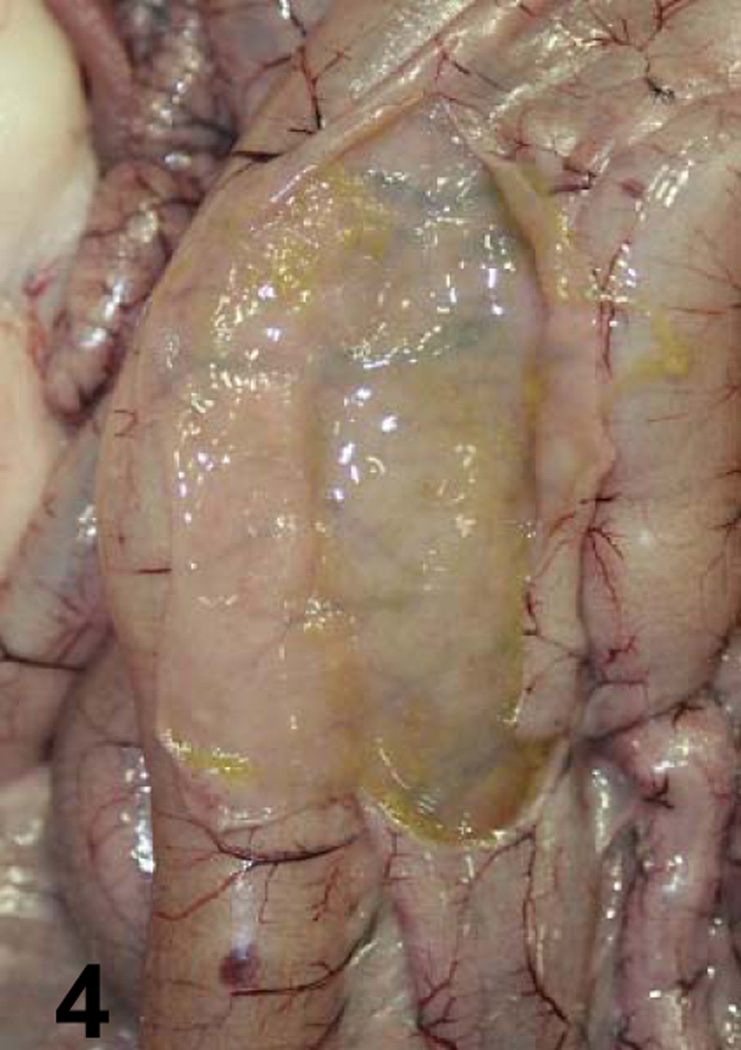
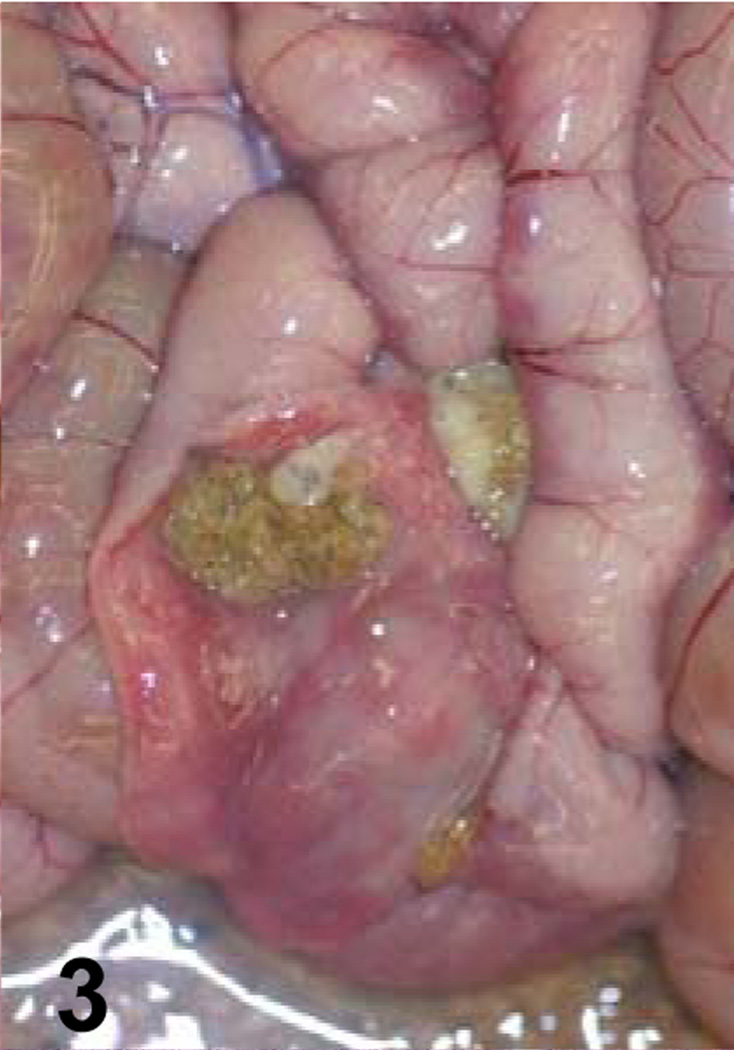
A summary of the main gross changes is presented in Table 2 and in Figures 1–4. Gross lesions characterized mostly by hemorrhagic fluid content with strands of fibrin, incipient pseudomembrane formation, mucosal hemorrhage and congestion involving jejunum, ileum and spiral colon were observed only in animals inoculated with the wild-type strain (Fig. 1). Two animals inoculated with the wild-type also presented transmural edema of small intestine and colon, and hemorrhagic mesenteric lymph nodes. Excess of hemorrhagic peritoneal and pleural fluid and fibrin strands on the intestinal serosa were also observed in one of the animals inoculated with the wild-type strain. The small intestine and colon of the 2 animals inoculated with the CPB reversed mutant strain presented hemorrhagic mucosa, fluid hemorrhagic content and fibrin in the lumen (Fig. 3). The colon content of one animal inoculated with the CPB null mutant was fluid. No other significant gross abnormalities were observed in any of the animals of the four groups.
Table 2.
Main gross lesions observed in 9 goats inoculated with C. perfringens type C wild-type, its mutants, or with sterile non-toxic culture medium.
| Inoculum | Case № | Small intestine* |
Colon* |
||||
|---|---|---|---|---|---|---|---|
| Mucosal congestion and hemorrhage |
Fluid content | Pseudomembrane | Mucosal congestion and hemorrhage |
Fluid content |
Pseudomembrane | ||
| C. perfringens type C wild type (CN 3685) | 1 | 3 | 3 | 2 | 0 | 3 | 2 |
| 2 | 3 | 2 | 3 | 0 | 3 | 3 | |
| 3 | 3 | 2 | 3 | 0 | 3 | 3 | |
| C. perfringens type C CPB null mutant (BMC 104) | 4 | 0 | 0 | 0 | 0 | 2 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C. perfringens type C CPB reversed mutant (BMC 105) | 6 | 1 | 1 | 1 | 1 | 1 | 0 |
| 7 | 1 | 1 | 1 | 1 | 1 | 0 | |
| Sterile, non-toxic TGY | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | |
0: no gross lesions; 1–3: mild to severe gross lesions
Fig. 1.
Jejunum of a goat inoculated with C. perfringens type C wild-type. Observe the presence of a pseudomembrane and blood in the intestinal lumen.
Fig. 4.
Jejunum of a goat inoculated with sterile, non-toxic TGY. No gross lesions are observed.
Fig. 3.
Jejunum of a goat inoculated with C. perfringens type C CPB reverse mutant. The mucosa is hemorrhagic and there is fibrin present in the intestinal lumen.
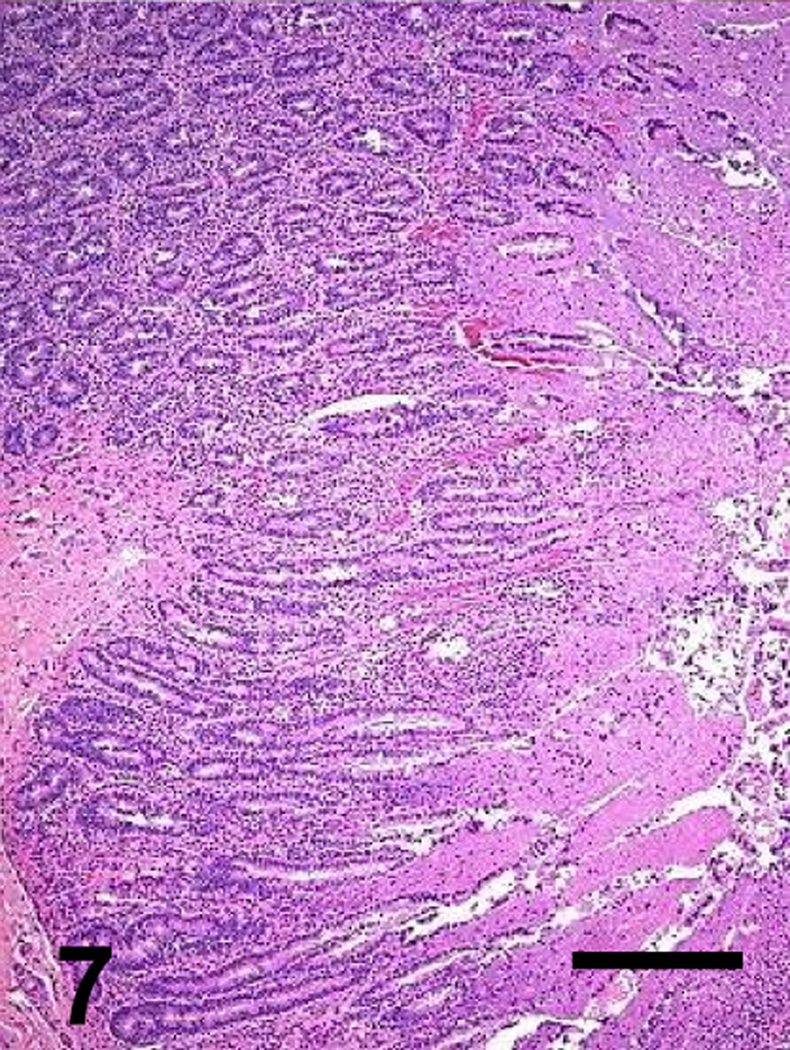
A summary of the main microscopic findings in the intestinal tract is presented in Table 3 and Figures 5–12. Microscopic lesions were observed only in animals inoculated with the wild-type or CPB reversed mutant, but the severity of the lesions was greater in the animals inoculated with the wild-type strain than in those inoculated with the CPB reversed mutant. When present, the lesions were similar in small intestine (affecting mostly jejunum and ileum) and colon, but they were generally more severe in the small intestine.
Table 3.
Microscopic abnormalities observed in the intestine of 9 goats inoculated with C. perfringens type C wild-type strain, its mutants or sterile non-toxic culture medium.
| Case № | Small intestine* |
Colon* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculum | Mucosal necrosis |
Villus blunting |
Pseudomembrane | Transmural edema |
Inflammatory cell infiltration |
Mucosal necrosis |
Pseudomembrane | Transmural edema |
Inflammatory cell infiltration |
|
| C.perfringes type C wild type (CN3685) | 1 | 3 | 2 | 2 | 2 | 1 | 3 | 2 | 2 | 1 |
| 2 | 3 | 2 | 0 | 2 | 1 | 3 | 0 | 1 | 1 | |
| 3 | 3 | 0 | 1 | 3 | 2 | 3 | 2 | 1 | 1 | |
| C. perfringens type C CPB null mutant (BMC 104) | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C.perfringens type C CPB reversed mutant (BMC 105) | 6 | 0 | 0 | 0.5 | 0 | 0 | 2 | 0 | 0 | 0 |
| 7 | 1 | 2 | 0.5 | 0 | 0 | 1 | 0 | 1 | 1 | |
| Sterile non-toxic TGY | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
0: no microscopic lesions; 1 – 3: mild to severe microscopic lesions.
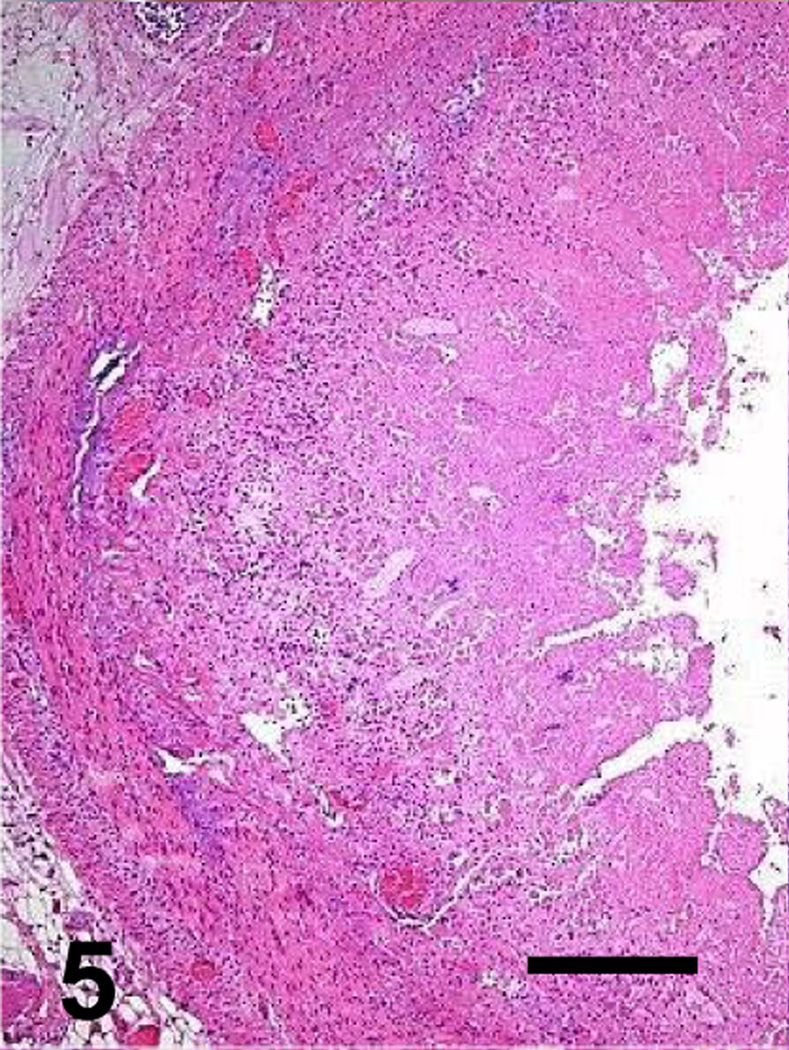
Fig. 5.
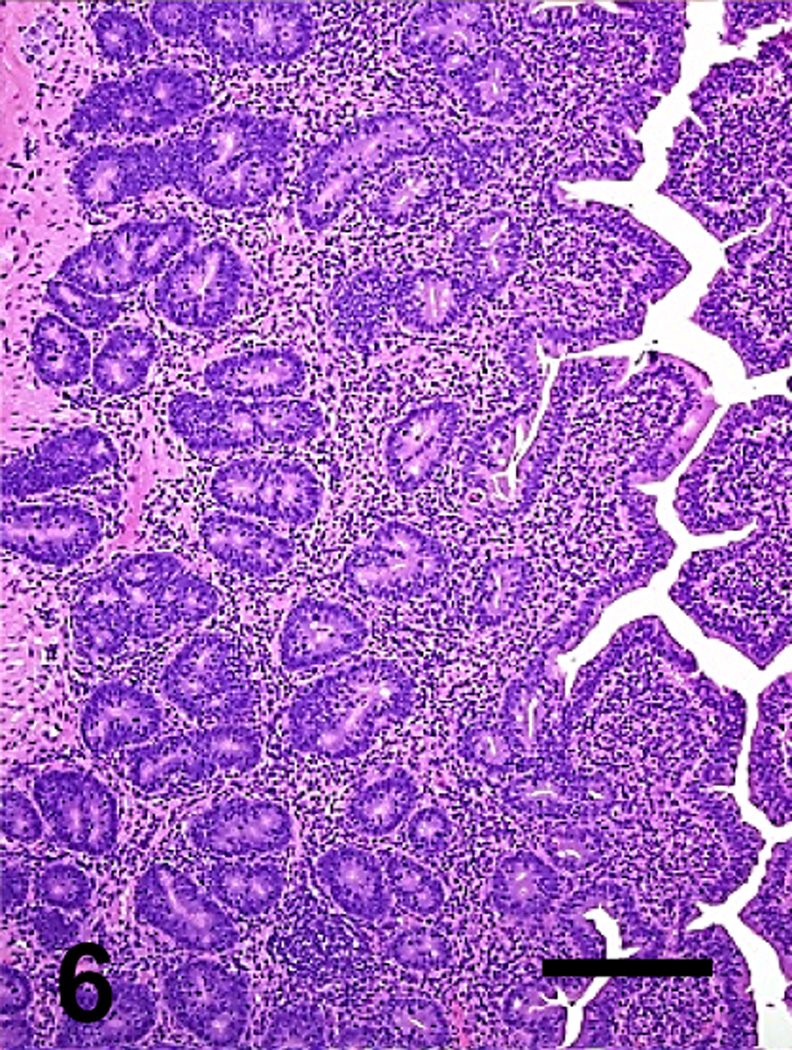
Jejunum of a goat inoculated with C. perfringens type C wild type. The mucosa and submucosa are diffusely necrotic. Bar: 100µm
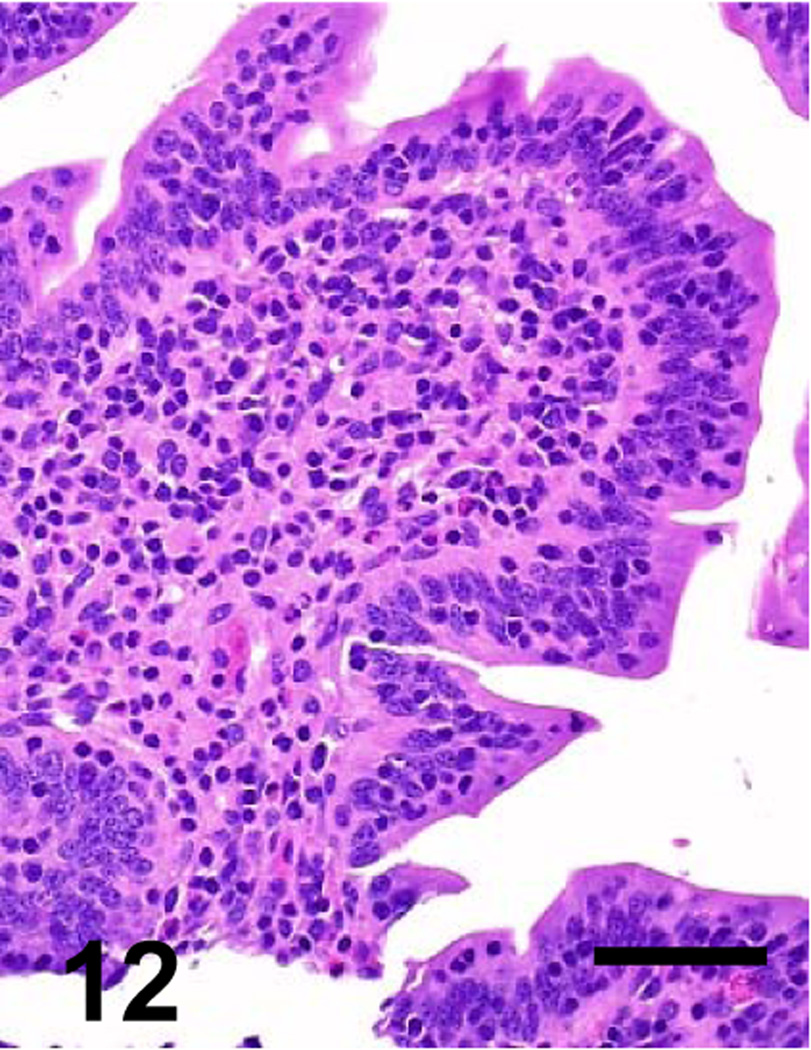
Fig. 12.
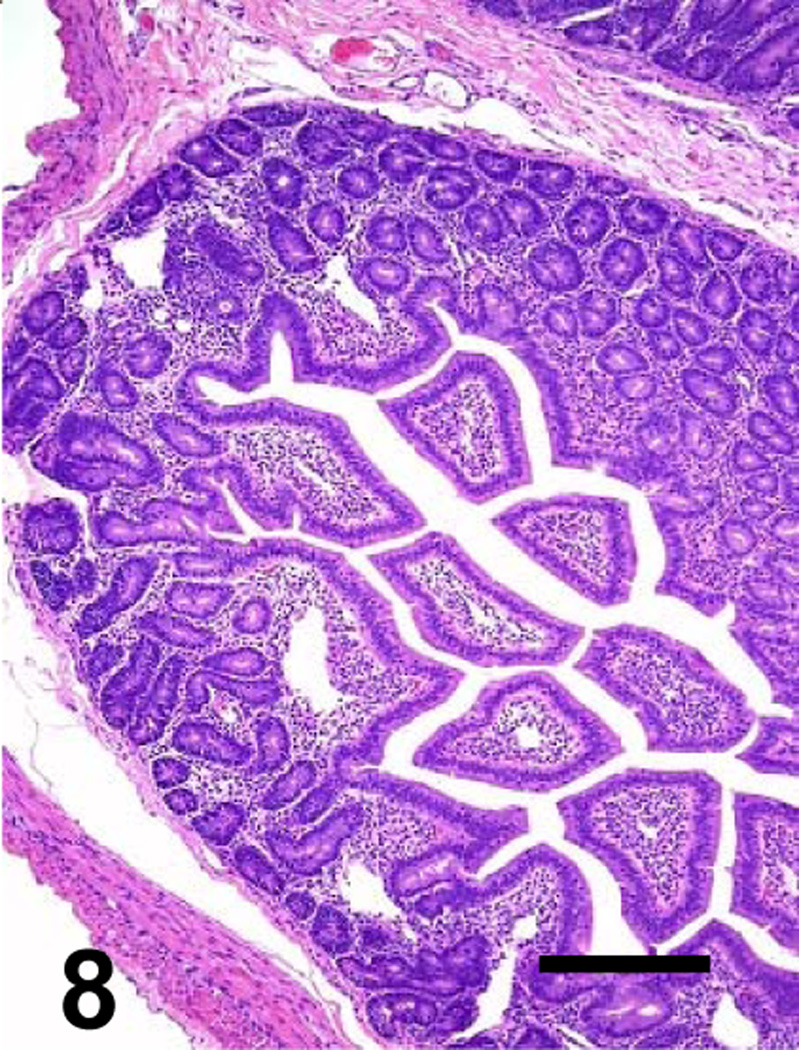
Higher magnification of the goat’s jejunum showed in Figure 8. No significant histological abnormalities are observed. Bar: 40 µm
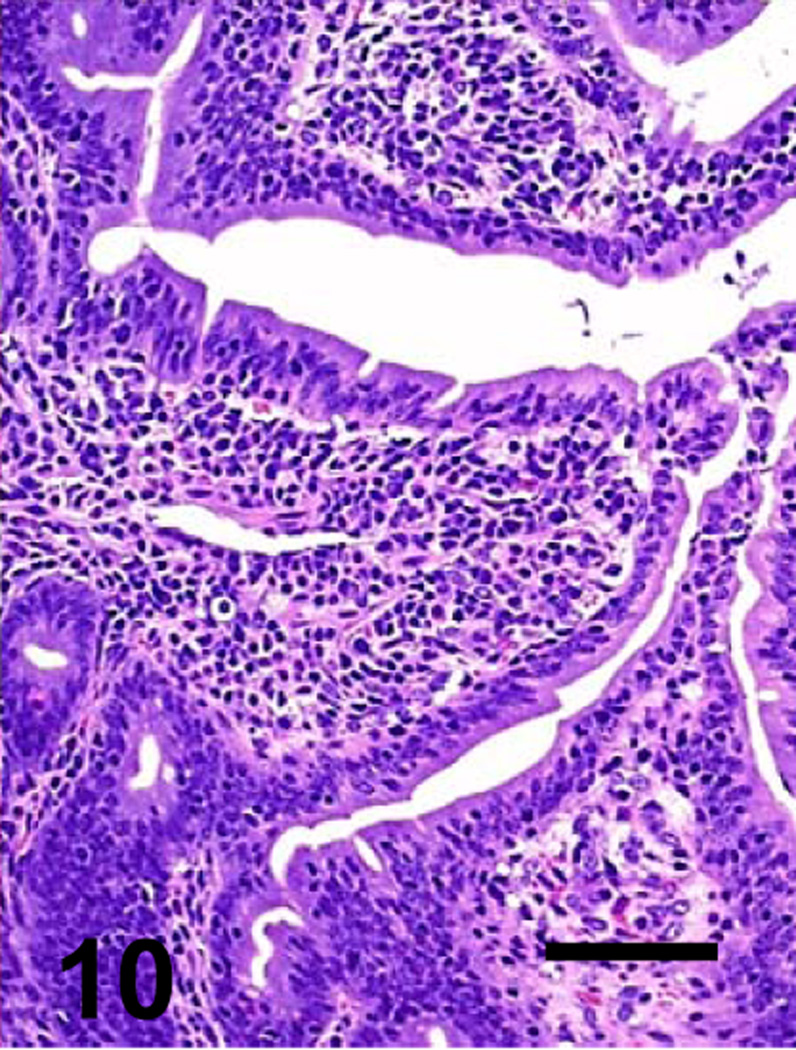
Most microscopic lesions were multifocal; however, the submucosa, muscularis and/or serosa, showed diffuse proteinaceous edema and mild inflammatory cell infiltration in all the animals inoculated with the wild-type and in one of the goats inoculated with the CPB reversed mutant (Table 3). The small intestine showed severe villous blunting, coagulative mucosal necrosis and an inflammatory cell infiltrate composed of neutrophils and fewer macrophages, lymphocytes and plasma cells (Figs. 5, 7, 9 and 11). Fibrin thrombi occluding superficial capillaries, arterioles or venules of the lamina propria were rarely observed. Numerous crypts were filled with neutrophils and a moderate number of neutrophils were seen migrating through the crypt epithelium. The luminal surface of the intestine of two goats inoculated with the wild-type was covered by a pseudomembrane composed of degenerated and necrotic desquamated epithelial cells, cell debris, neutrophils, lymphocytes, plasma cells, macrophages, fibrin and large thick bacilli with square ends and occasional subterminal spores. Similar bacilli were observed, singly or in clusters, free in the intestinal lumen, or lining the margin of the denuded mucosal surface. Although the bacterial population was usually greater in the intestinal lumen, a few bacilli were also seen in crypts and glands and invading the necrotic lamina propria. More than 95% of the bacilli observed in the lumen of the small intestine and colon of the goats inoculated with any of the C. perfringens strains were Gram-positive and stained strongly for C. perfringens by immunohistochemistry. The spiral colon crypts presented hypertrophy of the goblet cells and were full with abundant mucus.
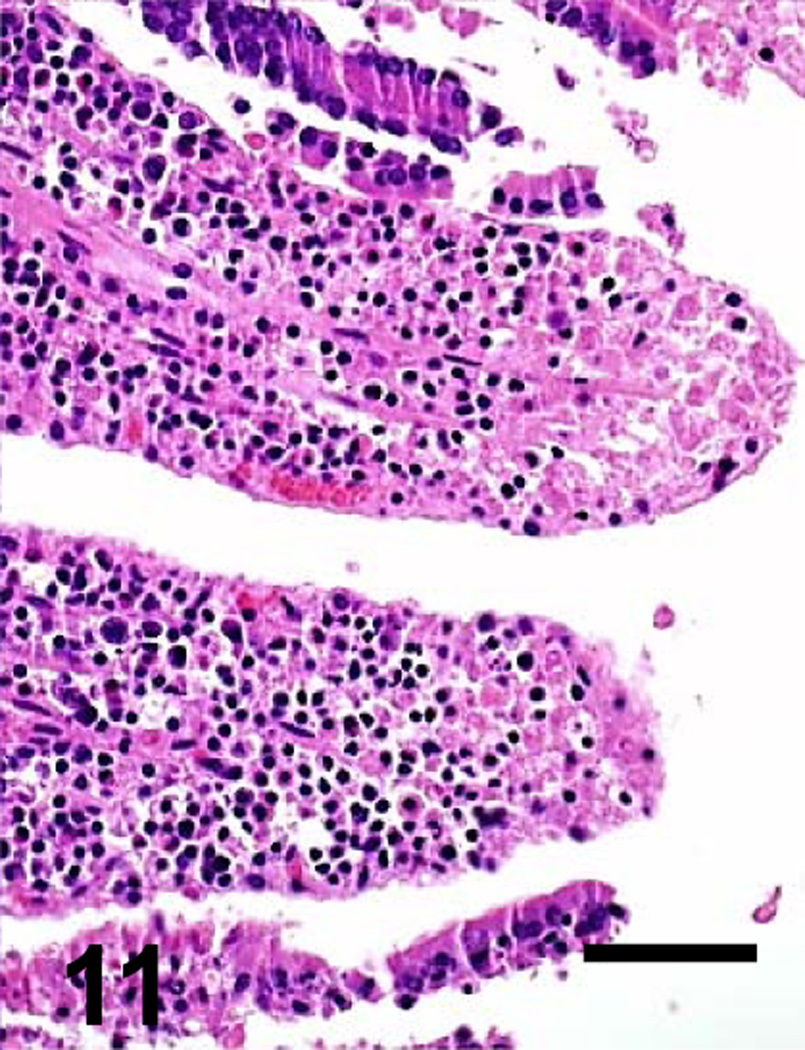
Fig. 7.
Jejunum of a goat inoculated with C. perfringens type C CPB reverse mutant. There is diffuse mucosal necrosis. Bar: 100µm
Fig. 9.
Higher magnification of the goat’s jejunum showed in Figure 5. There is complete loss of villus and crypt epithelium and necrosis of the lamina propria. Bar: 40 µm
Fig. 11.
Higher magnification of the goat’s jejunum showed in Figure 7. There is partial loss of villus epithelium. Bar: 40 µm
Histological changes were not usually observed outside the gastrointestinal tract of any of these animals. However, moderate to severe kidney and liver congestion, and mild myocardial hemorrhage developed in one animal inoculated with the reversed mutant. No significant histological abnormalities were observed in any tissues of the animals inoculated with the CPB null mutant or TGY (Figs. 6, 8, 10 and 12). Only a few thick Gram-positive bacilli with square ends were observed in the lumen of the small intestine and colon of the animals inoculated with TGY; these bacilli stained strongly positive by C. perfringens immunohistochemistry.
Fig. 6.
Jejunum of a goat inoculated with C. perfringens type C CPB null mutant. No significant histological lesions are observed. Bar: 100µm
Fig. 8.
Jejunum of a goat inoculated with TGY. No significant histological lesions are observed. Bar: 100µm
Fig. 10.
Higher magnification of the goat’s jejunum showed in Figure 6. No significant histological abnormalities are observed. Bar: 40 µm
Only one animal inoculated with the wild-type strain showed positive results after CPB immunohistochemistry. In this animal, positive staining of endothelial cells of medium size veins in the lamina propria and submucosa was observed. No CPB staining was observed in any of the intestinal sections of any other animal.
A small amount of mixed aerobic flora was isolated from the small and large intestine of all goats. C. perfringens was cultured from the small and large intestinal contents of all the goats inoculated with the wild-type, CPB null mutant and the reversed CPB mutant. The isolates from wild-type and complemented strain-inoculated animals were identified as type C by the toxin genotyping multiplex PCR assay. Those isolated from the animals inoculated with the CPB null mutant were identified as type A by this multiplex toxin genotyping PCR assay, which does not efficiently amplify a product from the intron-disrupted cpb gene (data not shown). No other aerobic or anaerobic microorganisms were isolated from any of the other samples from any of the goats cultured.
CPA and CPB were detected by ELISA in small and large intestinal contents of all goats inoculated with the wild-type and the reversed mutant. CPA, but not CPB, was detected in the small and large intestinal content of the two animals inoculated with the CPB null mutant and the two animal inoculated with TGY.
4. Discussion and conclusions
The objective of this study was to comprehensively document the lesions produced by C. perfringens type C in goats and also to test the hypothesis that CPB is necessary and sufficient to produce clinical signs and gross and microscopic lesions of C. perfringens type C disease in a natural host. In this study, the virulence of a wild-type C. perfringens type C was completely eliminated when the gene encoding CPB was inactivated in a virulent wild-type C strain. This virulence was partly restored when the CPB mutation was reversed in this isolate, thus indicating that the observed loss of pathogenicity for the CPB null mutant was not due to an unintended secondary mutation and fulfilling molecular Koch postulates. Molecular Koch’s postulates for C. perfringens type C infection have been fulfilled before in rabbits and mice, where virulence was eliminated when the gene that encodes CPB was inactivated in the virulent type C isolates and virulence was restored by reversing this mutation (Sayeed et al., 2008).
It was notable that the animals inoculated with the wild-type strain developed more severe clinical signs, gross and histopathological lesions than those inoculated with the reversed mutant. This phenomenon was observed before in rabbit intestinal loops and in mice inoculated intraduodenally with this mutant and it is believed to be a consequence of the reversed mutant producing, both in vitro and in vivo, substantially less CPB than the wild-type because of inefficient splicing to remove the intron insertion from the cpb mRNA (Sayeed et al., 2008).
The most consistent gross and histological finding in goats inoculated with the wild-type strain in our study was necrotizing enterocolitis. This is similar to the lesions described for spontaneous C. perfringens type C infections in other animal species, including horses, pigs and sheep (Diab et al., 2011; Songer and Uzal, 2005). Histologic evidence of mucosal or submucosal thrombosis was described before as a hallmark of type C infections in piglets, horses and humans (Diab et al., 2011; Miclard et al., 2009; Lawrence and Cocke, 1980). Miclard et al (2009) suggested that mucosal thrombosis was an early event in type C infections in piglets, leading to mucosal necrosis. However, the study referred to (Miclard et al, 2009) investigated end stage lesions of necrotizing enteritis and no conclusions on initial stages of the disease could be drawn. Our results lend support to the idea that, at least in goats, CPB has a direct effect on the intestinal mucosa and that the mucosal necrosis observed in our goats was due to the primary action of CPB on the intestinal epithelium rather than ischemia secondary to vascular thrombosis. It is therefore possible that the pathogenesis of C. perfringens type C disease is different in goats and in pigs. Nevertheless, extensive hemorrhage and tissue edema were present in all animals inoculated with CPB-producing strains, demonstrating that vascular leakage is an important component of type C infection. CPB was demonstrated to rapidly damage cultured endothelial cells (Gurtner et al, 2010; Popescu et al, 2011) and was detected at vascular endothelial cells in one of the affected goats in our study. Therefore it is possible that endothelial damage mediated by CPB also contributes to the pathogenesis of type C enteritis in goats.
A presumptive diagnosis of C. perfringens type C disease in most animal species can be achieved by observing necrotizing enteritis or enterocolitis, followed by isolation of C. perfringens type C, since this type of C. perfringens is rarely found in the intestine of healthy animals (Diab et al., 2011). However, final confirmation of a type C infection diagnosis should be based on the detection of CPB in intestinal contents (Diab et al., 2011). In this case, C. perfringens type C was isolated from small and large intestinal content of all the animals inoculated with the wild-type and the reversed mutant, and CPB was detected in small and large intestinal content of these animals, thus confirming that the clinical signs, gross and histological lesions observed were produced by C. perfringens type C.
No CPB was detected in the intestinal samples of the animals inoculated with the CPB null mutant strain, confirming that this strain did not produce CPB. The C. perfringens isolates from the intestine of the animals inoculated with the CPB null mutant were typed by PCR as type A. This is due to the presence of an intron insertion into the cpb gene in this strain, which (using cpb primers) would produce a PCR product that is poorly amplified under standard multiplex toxin genotyping PCR conditions.
A relatively small number of animals was used for these experiments due to humane reasons and also to reduce costs. It had initially been decided that the number of animals per group would be increased if needed after analyzing the results of the first set of experiments. However, given the high degree of consistency of the results obtained, it was decided not to increase the number of animals.
None of the animals inoculated with the CPB null mutant or TGY developed significant clinical signs, gross or histological lesions. This confirms that the alterations observed in the animals inoculated with the wild-type or the complemented strains were indeed due to CPB. This study therefore confirms that CPB is required to produce the clinical signs, gross and histological signs of C. perfringens type C infection in goats.
Fig. 2.
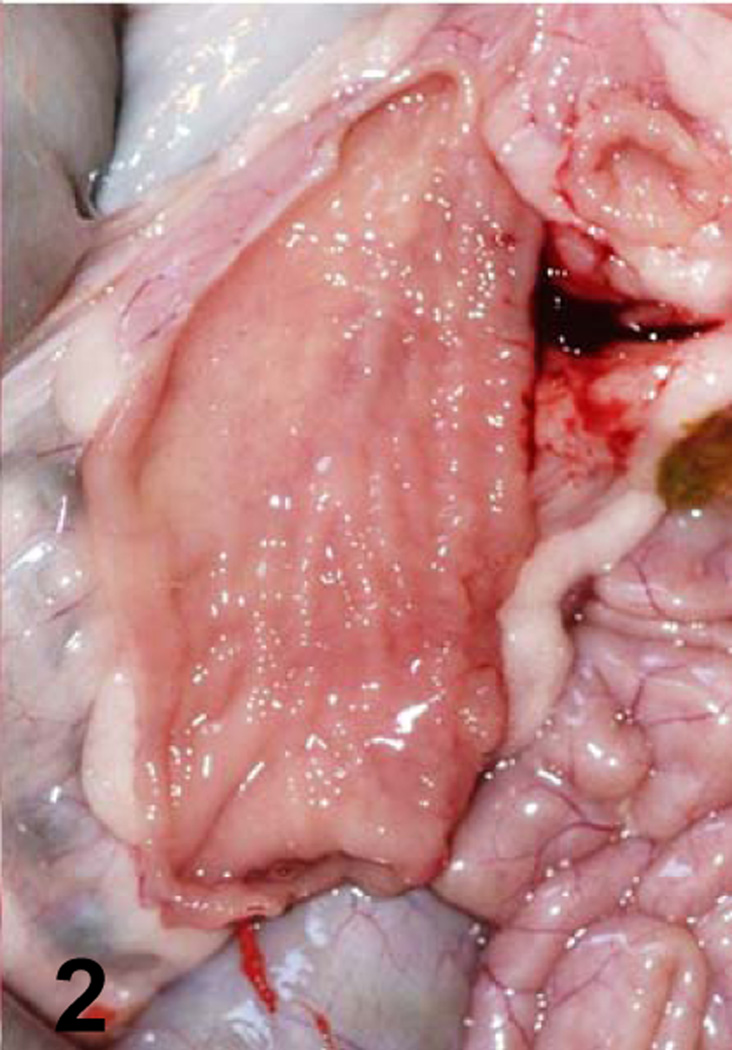
Jejunum of a goat inoculated with C. perfringens type C CPB null mutant. No gross lesions are observed.
Acknowledgements
National Institute of Allergy and Infectious Diseases grant AI056177 supported this research. The authors thank Dr. P. Hauer for supplying monoclonal antibodies against CPB. We thank Jackie Parker and Jim Cravotta for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors express that there are no conflicts of interest that could bias the work presented in this manuscript.
References
- Bueschel D, Jost B, Billington S, Trinh H, Songer G. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: correlation of genotype with phenotype. Vet. Microbiol. 2003;94:121–129. doi: 10.1016/s0378-1135(03)00081-6. [DOI] [PubMed] [Google Scholar]
- Diab SS, Kinde H, Moore J, Shahriar MF, Odani J, Anthenill L, Songer G, Uzal FA. Pathology of Clostridium perfringens type C enterotoxemia in horses. Vet. Path. 2011 doi: 10.1177/0300985811404710. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Fernandez Miyakawa ME, Sayeed S, Poon R, Adams V, Rood JI, Uzal FA, McClane BA. Dissecting the contributions of Clostridium perfringens type C toxins to lethality in the mouse intravenous injection model. Infect. Immun. 2006;74:5200–5210. doi: 10.1128/IAI.00534-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner C, Popescu F, Wyder M, Sutter E, Zeeh F, Frey J, von Schubert C, Posthaus H. Rapid cytopathic effects of Clostridium perfringens beta-toxin on porcine endothelial cells. Infect. Immun. 2010;78:2966–2973. doi: 10.1128/IAI.01284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence G, Cooke R. Experimental pigbel: the production and pathology of necrotizing enteritis due to Clostridium welchii type C in the guinea-pig. Br. J. Exp. Pathol. 1980;61:261–271. [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Okada Y, Inagi E, Tanabe Y, Shimizu Y, Nagashima K, Sakurai J, Nagahama M, Tanaka S. Enteritis necroticans 'pigbel' in a Japanese diabetic adult. Pathol. Int. 2007;57:622–626. doi: 10.1111/j.1440-1827.2007.02149.x. [DOI] [PubMed] [Google Scholar]
- Ma M, Vidal J, Saputo J, McClane BA, Uzal FA. The VirS/VirR two-component system regulates the anaerobic cytotoxicity, intestinal pathogenicity, and enterotoxemic lethality of Clostridium perfringens type C isolate CN3685. Mbio. 2011;2:1–9. doi: 10.1128/mBio.00338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miclard J, Jäggi M, Sutter E, Wyder M, Grabscheid B, Posthaus H. Clostridium perfringens beta-toxin targets endothelial cells in necrotizing enteritis in piglets. Vet. Microbiol. 2009;137:320–325. doi: 10.1016/j.vetmic.2009.01.025. [DOI] [PubMed] [Google Scholar]
- McClane BA, Uzal FA, Fernandez Miyakawa ME, Lyerly D, Wilkins TD. In: The Enterotoxigenic Clostridia. Dworkin SMF, Rosenburg E, Schleifer KF, Stackebrandt E, editors. New York: The Prokaryotes, Springer-Verlag; 2004. pp. 698–752. [Google Scholar]
- Popescu F, Wyder M, Gurtner C, Frey J, Cooke RA, Greenhill AR, Posthaus H. Susceptibility of primary human endothelial cells to Clostridium perfringens beta-toxin suggesting similar pathogenesis in human and porcine necrotizing enteritis. Vet Microbiol. 2011 doi: 10.1016/j.vetmic.2011.02.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sakurai J, Duncan CL. Some properties of beta-toxin produced by Clostridium perfringens type C. Infect. Immun. 1978;21:678–680. doi: 10.1128/iai.21.2.678-680.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, Chen Y, Gupta P, Rood JI, McClane BA. Beta toxin is essential for the intestinal virulence of Clostridium perfringens C. perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 2008;67:15–30. doi: 10.1111/j.1365-2958.2007.06007.x. [DOI] [PubMed] [Google Scholar]
- Smith MC, Sherman DM. Goat medicine. Pennsylvania: Lea and Febiger; 1996. pp. 407–412.pp. 474–478. [Google Scholar]
- Songer JG, Uzal FA. Clostridial enteric infections in pigs. J. Vet. Diagn. Invest. 2005;17:528–536. doi: 10.1177/104063870501700602. [DOI] [PubMed] [Google Scholar]
- Uzal FA, Saputo J, Vidal JE. Development and application of new mouse models to study the pathogenesis of Clostridium perfringens type C enterotoxemias. Infect. Immun. 2009;12:5291–5299. doi: 10.1128/IAI.00825-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzal FA, McClane BA. Recent progress in understanding the pathogenesis of Clostridium perfringens type C infections. Vet. Microbiol. 2011 doi: 10.1016/j.vetmic.2011.02.048. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JE, McClane BA, Saputo J, Parker J, Uzal FA. Effects of Clostridium perfringens beta-toxins on the rabbit small intestine and colon. Infect. Immun. 2008;76:4396–4404. doi: 10.1128/IAI.00547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]