Abstract
MHC class II-derived recombinant T cell receptor ligands (RTLs) modulate the behavior of pathogenic T cells and can reverse clinical and histological signs of autoimmune disease in experimental autoimmune encephalomyelitis (EAE), experimental autoimmune uveitis (EAU) and collagen-induced arthritis (CIA), and are currently in clinical trials for treatment of multiple sclerosis (MS). To expand the utility of these rationally-designed biologics and explore their mechanism(s) of activity in vivo, we have engineered RTL constructs bearing cysteine-tethered antigenic peptides and demonstrate that the appropriate cysteine-tethered RTLs effectively treat EAE. The data presented here suggests that the mechanism by which antigen-specific tolerance induction by RTLs bearing cysteine-tethered antigenic peptides in vivo involves delivery of RTL/antigen to endosomal compartments for processing and re-presentation by full-length MHC class II, with RTLs bearing cysteine-tethered antigenic peptides requiring gamma-interferon-inducible lysosomal thiol-reductase (GILT) for therapeutic activity.
Keywords: EAE, GILT mice, RTL550-CYS-Mog, MHC Class II
INTRODUCTION
We have previously described recombinant TCR ligands (RTLs) derived from the beta-1 and alpha-1 domains of MHC class II molecules bearing covalently-tethered Ags (Burrows et al. 1998; Burrows et al. 1999; Burrows et al. 2001; Chang et al. 2001). These molecules effectively tolerize Ag-specific CD4+ T cells (Fontenot et al. 2006; Huan et al. 2008) and are being used as molecular tools for probing the earliest TCR signal transduction events in T cells (Burrows et al. 2001 ; Wang et al. 2003; Burrows et al. 2001) and for dissecting the processing of antigens within the endosomal compartments of antigen presenting cells (Sinha et al. 2010; Offner et al. 2011). MHC class II molecules present 9–15 aa peptides with amino- & carboxy-termini that can extend outside of the peptide binding groove, and we have previously described work that takes advantage of these features by engineering MHC class II-derived RTLs bearing genetically encoded peptide antigens as amino-terminal extensions of the recombinant molecules, with the covalently–tethered peptides folding back into the peptide binding groove (Chang et al. 2001). While this strategy has proven extremely useful for developing tolerogenic RTLs bearing peptide antigens that consist of only the twenty naturally occurring amino acids, we sought to develop methods for covalently tethering peptide antigens that cannot be genetically encoded within the DNA sequence of the vectors used for expressing the recombinant molecules, such as peptide antigens that contain post-translational modifications (PTMs). Here we present data documenting the utility of cysteine chemistry to covalently tether peptide antigens to recombinant b1a1 MHC class II molecules. The beta-1 domains of all MHC class II molecules have a conserved C17-C81 disulfide bond (IAb-derived RTL550 numbering). Peptide antigens with single cysteine substitutions in the P4 anchor position can be efficiently covalently tethered to recombinant MHC class II molecules by inserting into the conserved beta-1 domain disulfide bond under appropriate conditions. Cysteine-tethered MOG-35-55 recombinant I-Ab (RTL550-Cys-MOG-35-55) effectively treated experimental autoimmune encephalomyelitis (EAE) in WT C57BL/6 mice, but could not treat EAE in gamma-interferon-inducible lysosomal thiol-reductase (GILT) KO mice. The data presented here suggests that the mechanism by which antigen-specific tolerance induction by RTLs bearing cysteine-tethered antigenic peptides in vivo involves delivery of RTL/antigen to endosomal compartments for processing and re-presentation by full-length MHC class II, with RTLs bearing cysteine-tethered antigenic peptides requiring GILT for therapeutic activity.
METHODS
Design, expression, purification & biophysical characterization of recombinant MHC molecules
General methods for the design, cloning and expression of recombinant b1 and a1 domains of MHC molecules, termed recombinant T cell receptor ligands (RTLs), have been previously described (Burrows et al. 1999; Chang et al. 2001; Huan et al. 2005; Fontenot et al. 2006).
Redox capture conditions
Reactions (10:1 peptide:protein molar ratio) were incubated at 37 C for 60 hours, with I-Ab-derived RTL550, 200 ug/ml in 100 mM NaPO4, pH 6.5, 150 mM NaCl, 0.05% SDS, 0.01% NaN3. For sample analysis 20 μl aliquots at various time points were mixed with an equal volume of a 2X electrophoresis sample buffer (1% glycerol. 500mM Tris, 0.2% SDS and bromophenol blue at pH 8.0). Samples were then placed on ice for 30 min, heated at 90 C for 6 min +/− β-mercaptoethanol and separated by 10–20% SDS-PAGE followed by coomassie blue staining to visualize the proteins. Proteins were quantified by densitometry scanning using BioRad’s Molecular Imager FX and Quantity One software.
Animals
C57BL/6 male mice were obtained from Jackson Laboratories at 7–8 weeks of age. Gamma interferon-inducible lysosomal thiol reductase knockout (GILT KO) animals on the C57BL/6 background were obtained from Dr. Peter Cresswell, Yale University/Howard Hughes Medical Institute (New Haven CT). C57BL/6 and GILT KO mice were housed in the Animal Resource Facility at the Portland Veterans Affairs Medical Center (Portland, OR, USA) in accordance with institutional guidelines. The study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals, and the protocols were approved by the Institutional Animal Care and Use Committee.
Antigens
Synthetic mouse MOG (mMOG) (MEVGWYRPPFSRVVHLYRNGK) as well as shortened and cysteine-substituted variants were synthesized by Genescript, Inc., (Piscataway, NJ, USA).
Induction of active EAE and treatment with RTL550-Cys-MOG
Mice immunized with mMOG-35-55 peptide received 200 μg of mMOG-35-55 peptide in an equal volume of CFA containing 4 mg/ml heat-killed Mycobacterium tuberculosis (MTb). All mice were also injected with 75 and 200 ng pertussis toxin (Ptx) intraperitoneally on days 0 and 2 relative to immunization. The mice were assessed for signs of EAE according to the following scale: 0, normal; 1, limp tail or mild hind limb weakness; 2, moderate hind limb weakness or mild ataxia; 3, moderately severe hind limb weakness; 4, severe hind limb weakness or mild forelimb weakness or moderate ataxia; 5, paraplegia with no more than moderate forelimb weakness; and 6, paraplegia with severe forelimb weakness or severe ataxia or moribund condition. At the onset of clinical signs of EAE (days 10–13 when the clinical scores were ≥1.5), mice were divided into two groups and treated with 100 μl of 1 mg/ml RTL550; “empty”), RTL551 bearing genetically encoded mMOG-35-55, or RTL550-Cys-MOG-35-55, i.v. for 5 consecutive days. Mice were monitored for changes in disease score until they were euthanized for ex vivo analyses. Statistical differences between disease scores of vehicle, RTL550, RTL551 and RTL550-Cys-MOG-35-55 treatment groups were determined by Mann-Whitney U test.
RESULTS
The design and characterization of human DR-, DP- & DQ-, murine I-Ab- & I-As-, and Lewis rat RT1.B-derived single chain constructs consisting of the β1 and α1 domains of the heterodimeric MHC class II molecules expressed as single polypeptide chains has been previously described (Chang et al. 2001; Fontenot et al. 2006; Burrows et al. 1999). These molecules are referred to as recombinant T cell receptor ligands (RTLs). “Empty” RTL550 derived from murine I-Ab (RTL550; RTL550-series) efficiently captured MOG-35-55 (S42C) peptide (Figure 1).
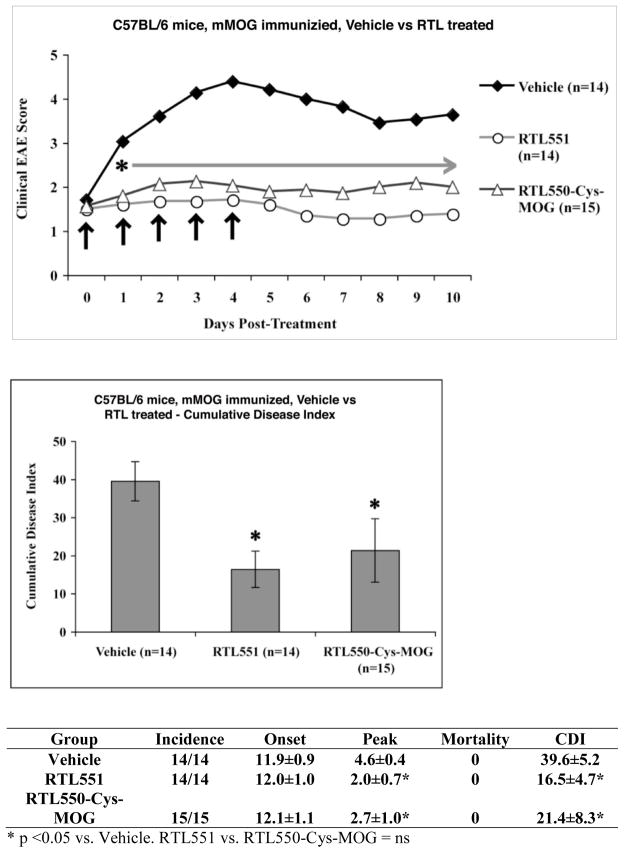
Fig. 1. Disulfide tethering of recombinant MHC class II-derived RTLs.
Primary amino acid sequence of (A) RTL550 and RTL551, derived from murine H2-A & H2-B primary sequences, and the MOG-35-55 (S42C) peptide alone. Non-native amino-terminal methionine of RTL550 is indicated by italics, cysteine residues are highlighted in bold font and the beta 1/alpha 1 junction is indicated (↓). The covalently-tethered MOG-35-55 peptide in RTL551 is underlined and the linker used is double-underlined. (B) Coomassie blue stained 10–20% SDS-PAGE showing disulfide-tethering of RTL550 and the MOG-35-55 (S42C) peptide. The disulfide-tethered RTL550/peptide complex migrates as a higher molecular weight species (~ 31 kD) than the “empty” RTL550. Samples under reducing (Red) and non-reducing (NR) conditions as indicated.
RTL550-Cys-MOG-35-55 (S42C) treats EAE in WT C57BL/6 but not GILT KO mice
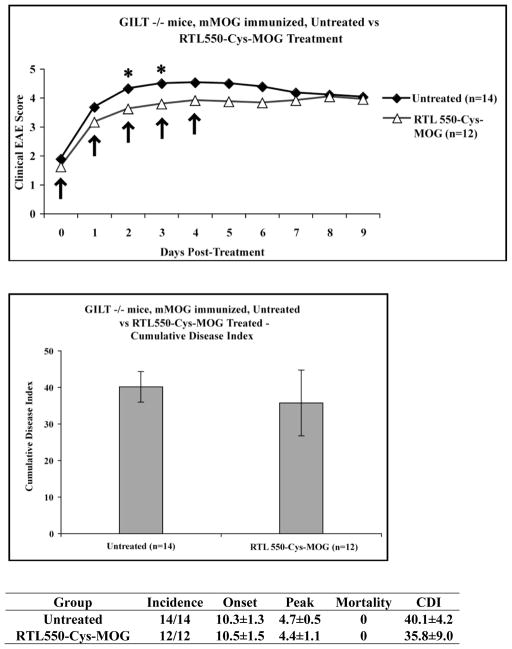
Treatment of WT C57BL/6 EAE male mice with vehicle, RTL551 or 550-Cys-MOG-35-55 (S42C) was initiated at onset of clinical signs of disease followed by 4 additional daily injections. RTL551 has the MOG-35-55 peptide genetically engineered into the expression construct, with the Ag peptide linked at the amino-terminus of the primary sequence of the molecule (Figure 1A). In RTL550-Cys-MOG-35-55 (S42C) the peptide is cysteine-tethered to the RTL550 “empty” construct. Clinical scores of EAE in WT C57BL/6 mice are shown in Figure 2. EAE progression was completely halted in the mice as early as one treatment with RTL551 or RTL550-Cys-MOG-35-55 (S42C), and both treatment groups had significantly reduced clinical disease scores at all the time points as compared to the vehicle alone control group of mice which showed a worsening of disease score over time, with similar clinical efficacy of both RTL551 bearing genetically encoded MOG-35-55, or RTL550 with cysteine-tethered MOG-35-55 (S42C) (Figure 2). We used gamma-interferon-inducible lysosomal thiol-reductase (GILT) KO mice to examine how important processing and re-presentation of Ag peptide is for the therapeutic efficacy of the compounds. We have previously demonstrated that RTLs bearing antigenic peptides as amino-terminal extensions are processed with the antigenic peptide re-presented in the context of full-length MHC class II (Dahan et al. 2011). Proteins that are internalized and processed by antigen processing cells (APCs) for presentation of MHC class II-restricted peptide antigens to CD4+ T cells frequently contain disulfide bonds, and their reduction in the endocytic pathway facilitates processing and access to peptides that would otherwise be inaccessible for MHC class II loading. GILT is constitutively expressed in late endocytic compartments of APCs and it has been shown to be a prerequisite for presentation of particular antigenic peptides (Maric et al. 2001; Sealy et al. 2008; Norton and Haque 2009; Bogunovic et al. 2010). While RTL551 was effective in treating GILT KO mice (Cumulative disease index (CDI) of untreated GILT KO mice was 39±1 and CDI of GILT KO treated with RTL551 was 19±5, p < 0.05.), the cysteine-tethered RTL550-Cys-MOG-35-55 (S42C) did not treat the GILT KO mice (Figure 3), suggesting that GILT is required for therapeutic efficacy of the cysteine-tethered, but not the genetically-encoded antigen constructs. The data presented here suggests that at least part of the mechanism for tolerance induction in vivo is receptor-mediated delivery of RTLs bearing MOG-35-55 to endosomal compartments for processing and re-presentation by full-length MHC class II, and that in the absence of GILT, reduction and re-presentation of cysteine-tethered peptide does not occur.
Fig. 2. RTL-550-Cys-MOG-35-55 (S42P) treats EAE.
Male C57BL/6 mice were immunized with MOG-35-55/CFA/Ptx. Mice were scored for clinical signs of EAE as outlined in Materials and Methods. EAE mice treated with RTL-550-Cys-MOG-35-55 (S42P) at onset had significantly reduced clinical scores as compared to vehicle treated mice. Data presented are the mean ± SD disease scores of 14–15 mice per group. Significant differences between the groups daily mean EAE score were determined using Mann Whitney U test. Cumulative Disease Index was determined by student’s T test.
Fig. 3. GILT is required for treatment of EAE.
Male GILT KO mice on the C57BL/6 background were immunized with MOG-35-55/CFA/Ptx. Mice were scored for clinical signs of EAE as outlined in Materials and Methods. EAE mice treated with RTL-550-Cys-MOG-35-55 (S42P) at onset did not have clinical scores significantly different then vehicle alone treated mice. Data presented are the mean ± SD disease scores of 12–14 mice per group. Significant differences between the groups daily mean EAE score were determined using Mann Whitney U test. Cumulative Disease Index was determined by Student’s t test.
DISCUSSION
Here we present data demonstrating that cysteine-tethered RTL550-Cys-MOG effectively treats EAE. Testing of cysteine-tethered IAb-derived RTLs in the C57BL/6 MOG-induced EAE model allowed us to test the broader applicability of the RTL approach to treatment of autoimmunity involving antigens that cannot be simply encoded into the primary sequence of the recombinant molecule, and our data support the therapeutic efficacy of cysteine-tethered RTL550-Cys-MOG-35-55 (S42C) as being equivalent to RTL551 bearing genetically encoded MOG-35-55 in wild-type mice (Figure 2). The use of GILT KO mice on the same background allowed us to gauge the importance of processing & re-presentation of the cysteine-tethered MOG-35-55 peptide. While RTL551 was effective in treating GILT KO mice, the cysteine-tethered RTL550-Cys-MOG-35-55 (S42C) did not treat the GILT KO mice (Figure 3). This suggests that GILT is required for therapeutic efficacy of the cysteine-tethered, but not the genetically-encoded antigen constructs, and helps clarify the multiple potential in vivo mechanism of RTL therapy. Previous studies using RTLs bearing genetically encoded cognate or non-cognate peptide antigen documented that RTLs bind directly to cognate TCR with low avidity in vitro (McMahan et al. 2003). Signal transduction studies using T cell hybridomas and primary human T cell lines further documented that RTLs bind directly to the TCR, with RTL in vitro serving as partial TCR agonists and triggering specific downstream signaling events that deplete intracellular calcium stores without fully activating T cells and without triggering an influx of extracellular calcium (Wang et al. 2003). The potent clinical efficacy of RTLs in various animal disease models led to the successful initiation of a Phase I clinical trial for human HLA-DR2-derived RTLs for treatment of multiple sclerosis, which we have recently successfully completed (Offner et al. 2011; Yadav et al. 2012). As part of these clinical trials, pharmacokinetic analysis of DR2-derived RTL bearing genetically encoded MOG-35-55 (RTL1000) was performed on a subset of patients. This study revealed a dose-dependent increase in exposure and a short half-life in blood plasma of ~5min for subjects receiving RTL1000 (Yadav et al. 2012). The rapid half-life and clearance values and the high non-physiological volume of distribution suggested that RTL1000 binds to cellular components in blood. Consistent with these observations, pre-clinical studies in mice demonstrated a half-life of ~10 minutes (unpublished data). RTL551 binding to mouse APCs was shown to inhibit T-cell activation and transfer of EAE (Sinha et al. 2010), and RTL1000 binding to human platelets reduced platelet aggregation and prolonged occlusive thrombus formation in blood (Itakura et al. 2010). Taken together these findings suggest that RTLs bind to a high affinity receptor distinct from the TCR on T cells, enabling both antigen-specific and more general mechanisms for modulating autoimmune disease and neuroinflammation. We have recently identified and characterized this high affinity receptor on CD11b+ cells (Vandenbark et al. 2012). Our data is consistent with an in vivo model of therapeutic efficacy that includes 1) cell-surface-bound RTLs interacting directly with TCR on cognate T cells, 2) direct modulation of CD11b+ cells toward a tolerogenic phenotype, and, based on the work presented here, 3) receptor-mediated delivery of RTLs bearing antigen to endosomal compartments for processing and re-presentation by full-length MHC class II, delivering an antigen-specific tolerogenic signal to cognate T cells. The data presented here suggests that the efficacy of RTLs bearing cysteine-tethered antigen requires GILT, because RTL550-Cys-MOG-35-55 S42C does not treat EAE in GILT KO mice (Figure 3).
The chemistry involved in constructing RTLs with cysteine-tethered antigenic peptides is a straightforward approach toward expanding the practical utility of RTL treatment of autoimmune disease to situations that cannot be accomplished using RTLs bearing genetically encoded peptide antigens. We have previously described the utility of conventional RTL therapy to collagen-induced arthritis, myelin basic protein (MBP) induced EAE and uveitis using genetically encoded collagen or MBP peptides (Huan et al. 2008; Burrows et al. 2000; Adamus et al. 2006). While successful in the experimental setting, in both rheumatoid arthritis (RA) and human multiple sclerosis (MS) there is evidence for pathology driven by T cells that recognize peptides bearing PTMs (Cantaert et al. 2006; Quirke et al. 2011; Moscarello et al. 2007). In human RA, a key target of the aberrant immune response are proteins that have undergone PTM by citrullination, an inflammation-driven process that is thought to break immune tolerance because T cells that recognize the citrullinated epitopes generated in the joints as a result of inflammation are not expressed in the thymus and are thus deleted during lymphocyte selection (Vossenaar et al. 2004; Chapuy-Regaud et al. 2005; Makrygiannakis et al. 2006; Cantaert et al. 2006; Quirke et al. 2011). Similarly, citrillination of MBP has been suggested to play an important role in the pathology of multiple sclerosis, with six sites on human MBP citrullinated in pathological settings, with the change of the positively charged arginine to neutral citrulline compromising the ability of MBP to interact with the lipid bilayer (Moscarello et al. 2007). These post-translational modifications occur during inflammation and cell death and cannot simply be genetically encoded into the RTLs. The chemistry described here provides a practical solution to this problem and is currently being used to develop DR4- and DR2-derived cysteine-tethered peptide constructs to test their efficacy for targeted tolerance induction in models of disease that involve citrillinated epitopes.
Acknowledgments
The authors would like to thank Dr. Maja Maric (Georgetown University) for advice, Dr. Peter Cresswell (Yale University) for providing the GILT KO animals, and Ms. Eva Niehaus for assistance in preparing the manuscript. This work was supported by NIH grants AI43960 and NS047661 and National Multiple Sclerosis Society grant RG3794-B-6. This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
The abbreviations used are
- Ag
antigen
- APC
antigen presenting cell
- HLA
histocompatibility leukocyte antigen
- MHC
major histocompatibility antigen
- RTL
Recombinant TCR Ligand
- TCR
T cell receptor
Footnotes
Dr. Burrows, Dr. Offner, Dr. Vandenbark, and OHSU have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAMC Conflict of Interest in Research Committees.
References
- Adamus G, Burrows GG, Vandenbark AA, Offner H. Treatment of autoimmune anterior uveitis with recombinant TCR ligands. Invest Ophthalmol Vis Sci. 2006;47(6):2555–2561. doi: 10.1167/iovs.05–1242. 47/6/2555. [DOI] [PubMed] [Google Scholar]
- Arthur A, Vandenbark RM-R, Andrew Shayne, Gil Benedek JH, Chou Yuan K, Buenafe Abigail C, Dahan Rony, Yoram Reiter JM, Offner Halina, Burrows Gregory G. Binding of partial MHC class II constructs to monocytes reduces CD74 expression and induces both specific and bystander T-cell tolerance. Nature Immunology. 2012 doi: 10.1016/j.jaut.2012.08.004. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic B, Srinivasan P, Ueda Y, Tomita Y, Maric M. Comparative quantitative mass spectrometry analysis of MHC class II-associated peptides reveals a role of GILT in formation of self-peptide repertoire. PLoS One. 5(5):e10599. doi: 10.1371/journal.pone.0010599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows GG, Adlard KL, Bebo BF, Jr, Chang JW, Tenditnyy K, Vandenbark AA, Offner H. Regulation of encephalitogenic T cells with recombinant TCR ligands. J Immunol. 2000;164 (12):6366–6371. doi: 10.4049/jimmunol.164.12.6366. [DOI] [PubMed] [Google Scholar]
- Burrows GG, Bebo BF, Jr, Adlard KL, Vandenbark AA, Offner H. Two-domain MHC class II molecules form stable complexes with myelin basic protein 69–89 peptide that detect and inhibit rat encephalitogenic T cells and treat experimental autoimmune encephalomyelitis. J Immunol. 1998;161 (11):5987–5996. [PubMed] [Google Scholar]
- Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark AA. Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12 (9):771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- Burrows GG, Chou YK, Wang C, Chang JW, Finn TP, Culbertson NE, Kim J, Bourdette DN, Lewinsohn DA, Lewinsohn DM, Ikeda M, Yoshioka T, Allen CN, Offner H, Vandenbark AA. Rudimentary TCR signaling triggers default IL-10 secretion by human Th1 cells. J Immunol. 2001;167 (8):4386–4395. doi: 10.4049/jimmunol.167.8.4386. [DOI] [PubMed] [Google Scholar]
- Cantaert T, De Rycke L, Bongartz T, Matteson EL, Tak PP, Nicholas AP, Baeten D. Citrullinated proteins in rheumatoid arthritis: crucial...but not sufficient! Arthritis Rheum. 2006;54(11):3381–3389. doi: 10.1002/art.22206. [DOI] [PubMed] [Google Scholar]
- Chang JW, Mechling DE, Bachinger HP, Burrows GG. Design, engineering, and production of human recombinant t cell receptor ligands derived from human leukocyte antigen DR2. J Biol Chem. 2001;276(26):24170–24176. doi: 10.1074/jbc.M101808200. [pii] [DOI] [PubMed] [Google Scholar]
- Chapuy-Regaud S, Nogueira L, Clavel C, Sebbag M, Vincent C, Serre G. IgG subclass distribution of the rheumatoid arthritis-specific autoantibodies to citrullinated fibrin. Clin Exp Immunol. 2005;139(3):542–550. doi: 10.1111/j.1365-2249.2004.02708.x. CEI2708 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan R, Tabul M, Chou YK, Meza-Romero R, Andrew S, Ferro AJ, Burrows GG, Offner H, Vandenbark AA, Reiter Y. TCR-like antibodies distinguish conformational and functional differences in two- versus four-domain auto reactive MHC class II-peptide complexes. Eur J Immunol. 41(5):1465–1479. doi: 10.1002/eji.201041241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot AP, Keizer TS, McCleskey M, Mack DG, Meza-Romero R, Huan J, Edwards DM, Chou YK, Vandenbark AA, Scott B, Burrows GG. Recombinant HLA-DP2 binds beryllium and tolerizes beryllium-specific pathogenic CD4+ T cells. J Immunol. 2006;177(6):3874–3883. doi: 10.4049/jimmunol.177.6.3874. 177/6/3874 [pii] [DOI] [PubMed] [Google Scholar]
- Huan J, Kaler LJ, Mooney JL, Subramanian S, Hopke C, Vandenbark AA, Rosloniec EF, Burrows GG, Offner H. MHC class II derived recombinant T cell receptor ligands protect DBA/1LacJ mice from collagen-induced arthritis. J Immunol. 2008;180(2):1249–1257. doi: 10.4049/jimmunol.180.2.1249. 180/2/1249 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan JY, Meza-Romero R, Mooney JL, Chou YK, Vandenbark AA, Bachinger H-P, Burrows GG. Rationally designed mutations convert complexes of human recombinant T cell receptor ligands into monomers that retain biological activity. J Chem Tech & Biotech. 2005;80:2–12. doi: 10.1002/jctb.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura A, Aslan JE, Sinha S, White-Adams TC, Patel IA, Meza-Romero R, Vandenbark AA, Burrows GG, Offner H, McCarty OJ. Characterization of human platelet binding of recombinant T cell receptor ligand. J Neuroinflammation. 7:75. doi: 10.1186/1742-2094-7-75. 1742–2094-7-75 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrygiannakis D, af Klint E, Lundberg IE, Lofberg R, Ulfgren AK, Klareskog L, Catrina AI. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65(9):1219–1222. doi: 10.1136/ard.2005.049403. ard.2005.049403 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, Cannon KS, Alfonso C, Karlsson L, Flavell RA, Cresswell P. Defective antigen processing in GILT-free mice. Science. 2001;294(5545):1361–1365. doi: 10.1126/science.1065500294/5545/1361. [pii] [DOI] [PubMed] [Google Scholar]
- McMahan RH, Watson L, Meza-Romero R, Burrows GG, Bourdette DN, Buenafe AC. Production, characterization, and immunogenicity of a soluble rat single chain T cell receptor specific for an encephalitogenic peptide. J Biol Chem. 2003;278 (33):30961–30970. doi: 10.1074/jbc.M300628200. [DOI] [PubMed] [Google Scholar]
- Moscarello MA, Mastronardi FG, Wood DD. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res. 2007;32(2):251–256. doi: 10.1007/s11064-006-9144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton DL, Haque A. Insights into the Role of GILT in HLA Class II Antigen Processing and Presentation by Melanoma. J Oncol. 2009;2009:142959. doi: 10.1155/2009/142959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Sinha S, Burrows GG, Ferro AJ, Vandenbark AA. RTL therapy for multiple sclerosis: a Phase I clinical study. J Neuroimmunol. 2011;231(1–2):7–14. doi: 10.1016/j.jneuroim.2010.09.013. S0165-5728(10)00432-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirke AM, Fisher BA, Kinloch AJ, Venables PJ. Citrullination of autoantigens: upstream of TNFalpha in the pathogenesis of rheumatoid arthritis. FEBS Lett. 585(23):3681–3688. doi: 10.1016/j.febslet.2011.06.006. S0014-5793(11)00454-6 [pii] [DOI] [PubMed] [Google Scholar]
- Sealy R, Chaka W, Surman S, Brown SA, Cresswell P, Hurwitz JL. Target peptide sequence within infectious human immunodeficiency virus type 1 does not ensure envelope-specific T-helper cell reactivation: influences of cysteine protease and gamma interferon-induced thiol reductase activities. Clin Vaccine Immunol. 2008;15(4):713–719. doi: 10.1128/CVI.00412-07. CVI.00412-07 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Miller L, Subramanian S, McCarty OJ, Proctor T, Meza-Romero R, Huan J, Burrows GG, Vandenbark AA, Offner H. Binding of recombinant T cell receptor ligands (RTL) to antigen presenting cells prevents upregulation of CD11b and inhibits T cell activation and transfer of experimental autoimmune encephalomyelitis. J Neuroimmunol. 225(1–2):52–61. doi: 10.1016/j.jneuroim.2010.04.013. S0165-5728(10)00173-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayshree Yadav DNB, Bowen James, Lynch Sharon G, Mattson David, Preiningerova Jana, Bever Christopher, Simon Jack H, Goldstein Andrew, Burrows G, Offner Halina, Ferro Al, Vandenbark Arthur. Recombinant T-Cell Receptor Ligand (RTL) for treatment of multiple sclerosis: A double-blind, placebo-controlled, phase 1, dose-escalation study. Autoimmune Diseases. 2012 doi: 10.1155/2012/954739. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenaar ER, Zendman AJ, Van Venrooij WJ. Citrullination, a possible functional link between susceptibility genes and rheumatoid arthritis. Arthritis Res Ther. 2004;6(1):1–5. doi: 10.1186/ar1027. ar1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Mooney JL, Meza-Romero R, Chou YK, Huan J, Vandenbark AA, Offner H, Burrows GG. Recombinant TCR ligand induces early TCR signaling and a unique pattern of downstream activation. J Immunol. 2003;171 (4):1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]