Abstract
The differentiation of T helper (Th) cells is critically dependent on cytokine milieu. The innate immune monocytes produce IL-1β which can affect the development of Th17 and Th1 cells that predominantly produce IL-17 and IFN-γ, respectively. Oligosaccharides from microorganisms, crops and mushrooms can stimulate innate immune cells. Active Hexose Correlated Compound (AHCC) that contains a large amount of oligosaccharides is a natural extract prepared from the mycelium of the edible Basidiomycete fungus. This compound is reported to modulate immune responses against pathogens although the mechanisms for this effect are largely unknown. Here we show that AHCC could induce high levels of IL-1β production from human monocytes. Furthermore, AHCC-treated monocytes increased the production of IL-17 and IFN-γ from autologous CD4+ T cells, which was blocked by adding IL-1 receptor antagonist. These finding provide new insight into how food supplements like AHCC could enhance human immunity by modulating monocytes and Th cells.
Keywords: Cytokines, T helper cells, monocytes
1. Introduction
CD4+ T helper (Th) cells are an essential component of the immune system. Th cells are heterogeneous and can be divided into Th1, Th2 and Th17 cells based on the cytokines they dominantly produce [1]. While Th1 and Th17 cells produce IFN-γ and IL-17, respectively, and Th2 cells produce IL-4 and IL-13. Each Th cell subset has a unique role in host defense against pathogens [2]. Th1 cells effectively restrain intracellular microorganisms such as mycobacteria and virus by activating macrophages with IFN-γ. Th17 cells play a major role in eliminating extracellular fungi and bacteria by activating and expanding neutrophils with IL-17. Parasites are controlled by Th2 cells that produce IL-5 and IL-13 which can activate eosinophils. The development of the different Th cell subsets is critically dependent on cytokine milieu [1, 3]. IFN-γ and IL-12 induce Th1 cell differentiation while IL-4 promotes Th2 cell development. Innate immune cytokines including IL-1 and IL-23 can enhance Th17 cell polarization.
Monocytes are large circulating leukocytes of the myeloid lineage. Monocytes have essential functions of innate immunity including phagocytosis and cytokine production [4]. Monocytes are armed with the receptors that recognize pathogen-associated molecular patterns (PAMPs). These receptors include Toll-like receptors (TLRs) that recognize microbial molecules such as lipopolysaccharide (LPS). Activation of these receptors results in the production of an array of inflammatory molecules such as IL-1β. The production of such molecules is essential for initiating and directing the development of immune responses via recruiting and providing activation signals to immune cells. With the capacity to produce cytokines, monocytes can promote the development of adaptive immune responses including differentiations of T helper (Th) cells [4, 5].
Oligosaccharides including α-glucans and β-glucans from various microorganisms, crops and mushrooms are known to stimulate immune cells such as macrophages and dendritic cells [6-10]. Active Hexose Correlated Compound (AHCC) is an extract from the mycelium of the edible Basidiomycete fungus that contains oligosaccharides, amino acids, lipids and minerals [11]. Oligosaccharides are the most abundant component of AHCC comprising about 74% of the dry weight [11]. Of these oligosaccharides, nearly 20% are partially acetylated α-1,4-glucans with a mean molecular weight under 5000 Daltons [11]. Both in vivo and in vitro studies reported the immunomodulatory effects of AHCC that included increased numbers and function of myeloid dendritic cells (mDCs) [12] as well as increased activity of natural killer (NK) cells, CD8+ T cells and γδ T cells [12-14]. Also, a recent study showed an increased frequency of IFN-γ-producing CD4+ and CD8+ T cells in healthy individuals who took AHCC for 30 days [15]. However, it is still largely unknown about the exact molecular mechanism(s) involved in such effect. Thus, we investigated whether AHCC could promote Th1, 2 and 17 cell responses by stimulating monocytes in humans in that the latter cells are abundant in human blood and have the potent capacity to produce cytokines such as IL-1β. The results of our study show that AHCC-treated monocytes enhance IL-17 and IFN-γ production from human CD4+ T cells in an IL-1β-depedent manner, providing new insight into the immunomodulatory mechanisms of the dietary supplement AHCC.
2. Materials and Methods
2.1. Human cells
This work was approved by the institutional review committee of Yale University. Human peripheral blood was obtained from the New York Blood Center or drawn from healthy adult donors after obtaining informed consent. Mononuclear cells were prepared from blood on FicollPAQUE gradients. Monocytes and memory CD4+ T cells were purified from mononuclear cells using negative cell purification kits (Stem cell Technologies Inc, Canada and Miltenyi Biotec Inc, Auburn, CA, respectively). Purified cells were resuspeded in RPMI 1640 media supplemented with 10% FCS, penicillin and streptomycin
2.2. Monocyte stimulation, flow cytometry and ELISA
Monocytes were incubated at 37°C, 5% CO2 for 5 or 18 hours in the presence of AHCC freeze dried powder (AHCC FD, 20 – 500 μg/ml), α-glucan (100 - 500 μg/ml) purified from AHCC, lipopolysaccharide (LPS, 100 ng/ml) or PBS (control). In some experiments, polymyxin B (50 μg/ml) was added to cells treated with AHCC or LPS. Golgiplug (BD Bioscience) was added during the last 4 hours of stimulation for cells which were stimulated for 5 hours. Stimulated cells were fixed, permeabilized and stained with antibodies to IL-1β followed by analyzing on a flow cytometer. Culture supernatants from 18 hour stimulation were analyzed for IL-1β and IL-6 by ELISA (ebioscience, San Diego, CA).
2.3. CD4+ T cell stimulation and cytokine assay
Purified CD4+ T cells were stimulated at 37°C, 5% CO2 for 7 days with anti-CD3/CD28 antibody-coated beads in the presence or absence of autologous monocytes pretreated for 5 hours with AHCC FD (100 or 500 μg/ml), LPS (100 ng/ml) or PBS. In some experiments, recombinant human IL-1 receptor antagonist (125 ng/ml, R&D Systems) was added at days 0 and 2 of cell culture [16]. IFN-γ, IL-13 and IL-17 in culture supernatants were analyzed using a Bio-plex Pro human cytokine assay kit (BioRad, Hercules, CA) or ELISA (ebioscience).
2.4. Statistical Analysis
A paired t-test and ANOVA were done to analyze data as appropriate using GraphPad. P values of less than 0.05 were considered statistically significant. Post hoc analysis was done by the Bonferroni test.
3. Results
3.1. AHCC induces IL-1β production from human monocytes
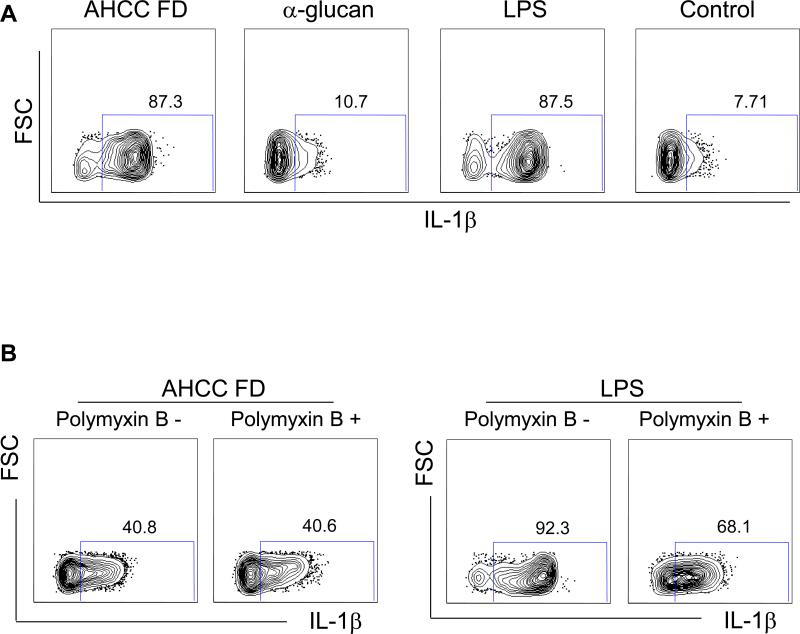
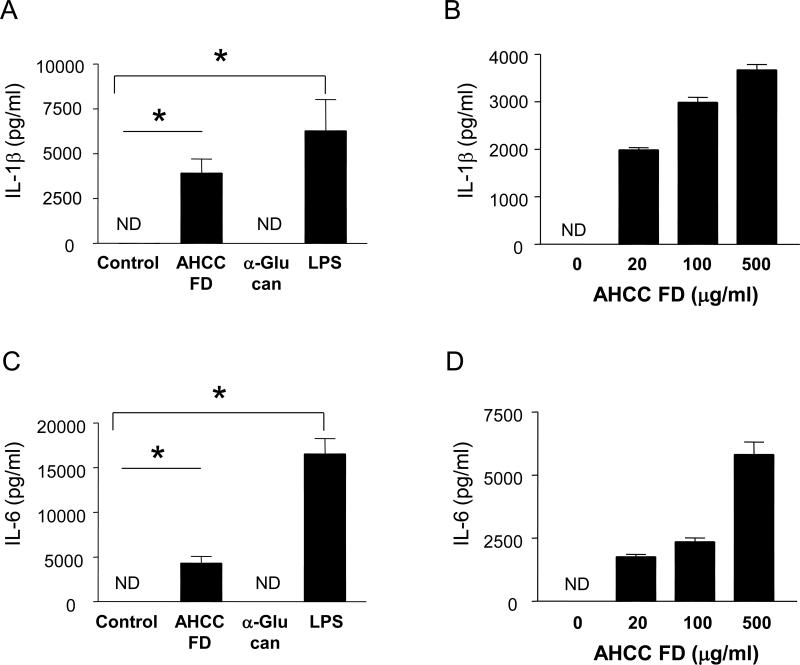
We analyzed IL-1β production from human monocytes in the presence or absence of AHCC FD, α-glucan or LPS. Intracellular flow cytometric analysis showed that monocytes expressed this cytokine in response to AHCC or LPS but not α-glucan or vehicle control (PBS) (Fig. 1A). This phenomenon by the AHCC was not secondary to any LPS contamination since adding polymyxin B, a known LPS neutralizer, did not alter the expression of IL-1β although the effect of LPS was blocked by this chemical (Fig. 1B). We also measured IL-1β production from the stimulated monocytes using ELISA. Analogous to the results of the flow cytometric analysis, monocytes produced substantial amounts of IL-1β in response to AHCC but not α-glucan in a dose-dependent manner (Fig. 2). In fact, low-dose AHCC (20 μg/ml) could induce the production of the cytokines from human monocytes (Fig. 2B). We also found that AHCC but not a-glucan-treated human monocytes produced high levels of IL-6 in a dose-dependent manner (Fig. 2C and D).
Fig 1. AHCC induces expression of IL-1β in human monocytes.
Monocytes purified from peripheral blood of healthy individuals were incubated for 5 hours with AHCC freeze dried powder (AHCC FD, 500 μg/ml for A and B), α-glucan fraction (500 μg/ml) of AHCC, LPS (100 ng/ml) or vehicle control (control) in the presence or absence of the LPS neutralizer polymyxin B (50 μg/ml). Cells were then fixed, permeabilized and stained with antibodies to IL-1β. Stained cells were analyzed on a flow cytometer. (A) Representative dot plots showing IL-1β expression by monocytes. (B) Polymyxin B (50 μg/ml) inhibited IL-1β expression by monocytes treated with LPS but not AHCC FD (500 μg/ml). Representative data from 6 (A) and 5 (B) independent experiments.
Fig 2. IL-1β and IL-6 are secreted from human monocytes in response to AHCC.
Purified human monocytes were treated for 18 hours with AHCC FD (500 μg/ml for A, C; 20 – 500 μg/ml for B, D), α-glucan (500 μg/ml), LPS (100 ng/ml) or vehicle control (control). Cell culture supernatants were collected and analyzed for IL-1β and IL-6 using ELISA. (A, C) Bars and error bars indicate mean (n = 4) and standard error of mean, respectively. *P < 0.05. (B, D) Dose kinetics of AHCC for IL-1β secretion by monocytes. Bars and error bars indicate mean and standard deviation of triplicates, respectively. Representative data from 3 independent experiments.
3.2. AHCC-treated monocytes increase IL-17 and IFN-γ production from human CD4+ T cells dependently of IL-1β
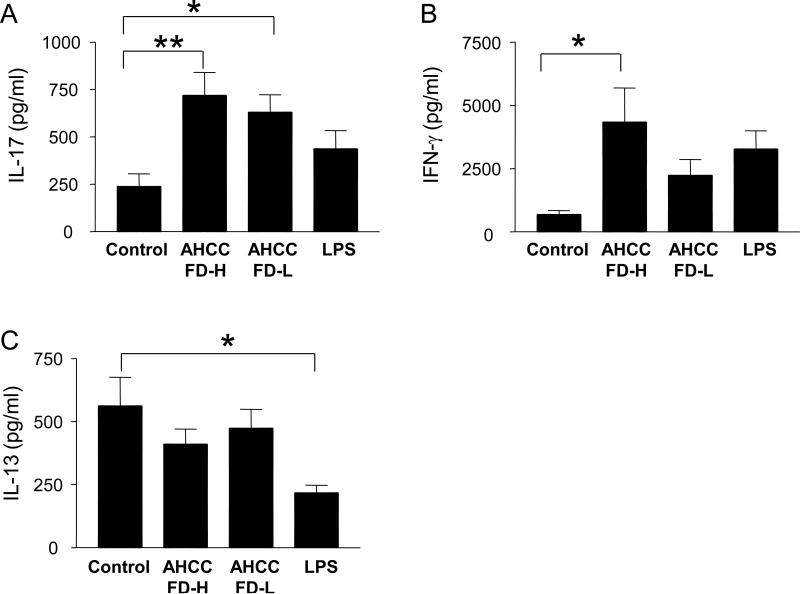
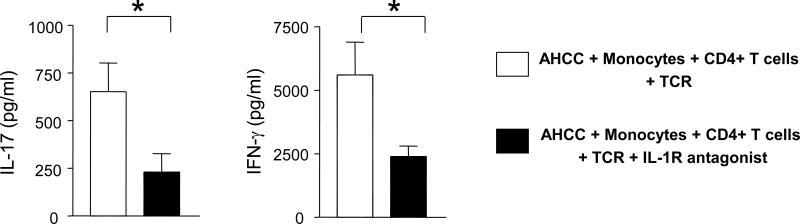
We next determined whether IL-1β produced from AHCC-treated monocytes could promote cytokine production from CD4+ T cells. We first stimulated monocytes for 5 hours with AHCC at high or low doses or LPS. Autologous memory CD4+ T cells were then added to the monocytes and cultured in the presence of anti-CD3/CD28 antibodies. In the presence of monocytes treated with the high-dose AHCC, CD4+ T cells had increased production of IL-17 and IFN-γ but not IL-13 (Fig. 3). In the presence of monocytes treated with low-dose AHCC, only IL-17 production was increased. To address whether this phenomenon was dependent on IL-1β, we added IL-1 receptor antagonist (IL-1Ra), which is known to interrupt IL-1β binding to IL-1 receptor, during cell culture [16]. Indeed, adding IL-1Ra suppressed the production of IL-17 and IFN-γ from CD4+ T cells that were stimulated with anti-CD3/CD28 antibodies in the presence of AHCC-treated autologous monocytes (Fig. 4).
Fig 3. AHCC-treated monocytes induce high levels of IL-17 and IFN-γ production from CD4+ T cells in the presence of anti-CD3 and -CD28 antibodies.
Purified human monocytes that had been treated for 5 hours with AHCC FD at high (AHCC FD-H, 500 μg/ml) or low (AHCC FD-L, 100 μg/ml) concentrations, LPS (100 ng/ml) or vehicle control were added to autologous memory CD4+ T cells. Cells were then cultured for 7 days in the presence of anti-CD3 and –CD28 antibodies. Culture supernatants were collected and analyzed for IL-17, IFN-γ and IL-13 by multiplex cytokine assay. Bars and error bars indicate mean (n = 8) and standard error of mean, respectively. *P < 0.05.
Fig 4. IL-1 receptor antagonist (IL-1Ra) suppresses IL-17 and IFN-γ production from monocytes stimulated with anti-CD3 and -CD28 antibodies in the presence of AHCC-treated monocytes.
Purified human monocytes that had been treated for 5 hours with AHCC FD (500 μg/ml) were added to autologous memory CD4+ T cells. Cells were then cultured for 7 days with anti-CD3 and -CD28 antibodies. Recombinant human IL-1Ra (125 ng/ml) was added at days 0 and 2 of cell culture. Culture supernatants were collected and analyzed for IL-17 and IFN-γ by ELISA. Bars and error bars indicate mean (n = 7) and standard error of mean, respectively. *P < 0.05.
4. Discussion
Monocytes are potent innate immune cells that produce cytokines including IL-1β [4]. These cytokines can affect the function of CD4+ Th cells which are critically involved in host defense against infection and malignancy by producing cytokines such as IL-17 and IFN-γ [1-3]. In fact, IL-1β is known to promote Th17 cell response in humans [16-18]. Active Hexose Correlated Compound (AHCC) is a natural product originating from the mycelium of edible Basidiomycete fungi [11]. The most abundant component of AHCC is oligosaccharides which account for about 74% of the dry weight [11]. The immune boosting effect of AHCC has been reported in animal and humans [11-15, 19, 20]. However, it is still largely unknown how AHCC could promote immune responses. Here we demonstrated that human monocytes produced high levels of IL-1β in response to AHCC, leading to increased IL-17 and IFN-γ production from human CD4+ T cells.
Oligosaccharides including α-glucans and β-glucans from various microorganisms, crops and mushrooms are known to stimulate innate immune cells. For instance, myeloid DCs that were treated with β-glucans from yeast produced IL-23, leading to the development of Th17 cells [6]. In fact, α-glucans from Streptococci and Pseudoallescheria boydii, a saprophytic fungus, [7] as well as from edible mushroom Tricholoma matsutake [10] were reported to stimulate the production of inflammatory cytokines from macrophages and DCs. Oligosaccharides including α-glucans are the predominant component of AHCC, an enzyme-fermented extract of the mycelia of Basidiomycetes mushroom, which is used as a dietary supplement [11]. In our study, human monocytes produced IL-1β and IL-6 in response to AHCC but not α-glucans purified from AHCC, suggesting that immune modulating effect of AHCC on human monocytes may stem from non-α-glucan oligosaccharides of AHCC.
The importance of Th1 and Th17 cells in host defense is well documented. In particular, Th17 cells produce IL-17 that promotes neutrophil-mediated immune responses against extracellular microorganisms by inducing the neutrophil activating cytokines IL-8 and G-CSF from epithelial cells and fibroblasts [21]. Mice deficient of IL-17 receptor A had increased susceptibility to microorganisms such as Candida albicans and Klebsiella pneumoniae [22-24]. Of interest, oral administration of AHCC improved survival and bacterial load in mice intramuscularly infected with Klebsiella pneumoniae [19, 20]. Similarly, mice with chemotherapy-induced neutropenia had better survival in the presence of Candida infection when AHCC was administered orally or intraperitoneally [25]. In our study, we also noticed increased IFN-γ production from CD4+ T cells in the presence of AHCC-treated monocytes. This effect was dependent on IL-1β. Although recent studies indicated the role for IL-1β in promoting Th17 cell responses in humans [16-18], several studies reported the possible augmentation of Th1 response by IL-1β [26, 27]. Th1 cells play a major role in controlling intracellular microorganism such as virus and Mycobacterium through activating macrophages and cytotoxic CD8+ T cells by producing IFN-γ. Oral supplementation of AHCC increased survival and decreased severity in mice intranasally infected with influenza virus [13]. Also, oral administration of AHCC to mice infected with West Nile virus, a virus causing encephalitis in humans, improved viremia and survival [28]. The results of our study offer a possible explanation for the clinical benefits of AHCC in these animal studies by showing enhanced Th1 and Th17 cell responses through IL-1β production from AHCC-treated monocytes in humans.
Taken together, we demonstrated that AHCC, an extract prepared from mycelia of the Basidiomycete mushroom, could induce IL-1β production from human monocytes, leading to the promotion of Th1 and Th17 cell responses. These finding provide new insight into how food supplements like AHCC could enhance human immunity.
Highlights.
AHCC, an extract from the edible Basidiomycete fungus, can affect immune responses. We found high levels of IL-1β in supernatants of AHCC-treated human monocytes. Such supernatants promoted Th17 and Th1 responses dependently of IL-1β. Our findings suggest that AHCC could promote immune responses via inducing IL-1β.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (AG028069, AT 005241 all to IK) and an unrestricted research fund from Amino Up Chemical Co, Sapporo, Japan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Insoo Kang received an unrestricted research fund from Amino Up Chemical Co, Sapporo, Japan, the manufacturer of AHCC that was studied in this work. Hajime Fujii is an employee of Amino Up Chemical Co.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 5.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 6.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, Consolaro MR, De Marchi M, Giachino D, Robbiano A, Astegiano M, Sambataro A, Kastelein RA, Carra G, Trinchieri G. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittencourt VC, Figueiredo RT, da Silva RB, Mourao-Sa DS, Fernandez PL, Sassaki GL, Mulloy B, Bozza MT, Barreto-Bergter E. An alpha-glucan of Pseudallescheria boydii is involved in fungal phagocytosis and Toll-like receptor activation. J Biol Chem. 2006;281:22614–22623. doi: 10.1074/jbc.M511417200. [DOI] [PubMed] [Google Scholar]
- 8.Estrada A, Yun CH, Van Kessel A, Li B, Hauta S, Laarveld B. Immunomodulatory activities of oat beta-glucan in vitro and in vivo. Microbiol Immunol. 1997;41:991–998. doi: 10.1111/j.1348-0421.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 9.Nisini R, Torosantucci A, Romagnoli G, Chiani P, Donati S, Gagliardi MC, Teloni R, Sargentini V, Mariotti S, Iorio E, Cassone A. beta-Glucan of Candida albicans cell wall causes the subversion of human monocyte differentiation into dendritic cells. J Leukoc Biol. 2007;82:1136–1142. doi: 10.1189/jlb.0307160. [DOI] [PubMed] [Google Scholar]
- 10.Hoshi H, Yagi Y, Iijima H, Matsunaga K, Ishihara Y, Yasuhara T. Isolation and characterization of a novel immunomodulatory alpha-glucan-protein complex from the mycelium of Tricholoma matsutake in basidiomycetes. J Agric Food Chem. 2005;53:8948–8956. doi: 10.1021/jf0510743. [DOI] [PubMed] [Google Scholar]
- 11.Ritz BW. Supplementation with active hexose correlated compound increases survival following infectious challenge in mice. Nutr Rev. 2008;66:526–531. doi: 10.1111/j.1753-4887.2008.00085.x. [DOI] [PubMed] [Google Scholar]
- 12.Terakawa N, Matsui Y, Satoi S, Yanagimoto H, Takahashi K, Yamamoto T, Yamao J, Takai S, Kwon AH, Kamiyama Y. Immunological effect of active hexose correlated compound (AHCC) in healthy volunteers: a double-blind, placebo-controlled trial. Nutr Cancer. 2008;60:643–651. doi: 10.1080/01635580801993280. [DOI] [PubMed] [Google Scholar]
- 13.Ritz BW, Nogusa S, Ackerman EA, Gardner EM. Supplementation with active hexose correlated compound increases the innate immune response of young mice to primary influenza infection. J Nutr. 2006;136:2868–2873. doi: 10.1093/jn/136.11.2868. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Zhang D, Sun B, Fujii H, Kosuna K, Yin Z. Active hexose correlated compound enhances tumor surveillance through regulating both innate and adaptive immune responses. Cancer Immunol Immunother. 2006;55:1258–1266. doi: 10.1007/s00262-005-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin Z, Fujii H, Walshe T. Effects of active hexose correlated compound on frequency of CD4+ and CD8+ T cells producing interferon-gamma and/or tumor necrosis factor-alpha in healthy adults. Hum Immunol. 2010;71:1187–1190. doi: 10.1016/j.humimm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 18.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 19.Aviles H, O'Donnell P, Orshal J, Fujii H, Sun B, Sonnenfeld G. Active hexose correlated compound activates immune function to decrease bacterial load in a murine model of intramuscular infection. Am J Surg. 2008;195:537–545. doi: 10.1016/j.amjsurg.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 20.Aviles H, Belay T, Vance M, Sun B, Sonnenfeld G. Active hexose correlated compound enhances the immune function of mice in the hindlimb-unloading model of spaceflight conditions. J Appl Physiol. 2004;97:1437–1444. doi: 10.1152/japplphysiol.00259.2004. [DOI] [PubMed] [Google Scholar]
- 21.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye P. Requirement of interleukin-17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi H, Ikeda T, Tansho S, Ono Y, Yamazaki M, Sato A, Yamaoka K, Yamaguchi H, Abe S. [Prophylactic efficacy of a basidiomycetes preparation AHCC against lethal opportunistic infections in mice] Yakugaku Zasshi. 2000;120:715–719. doi: 10.1248/yakushi1947.120.8_715. [DOI] [PubMed] [Google Scholar]
- 26.Joseph SB, Miner KT, Croft M. Augmentation of naive, Th1 and Th2 effector CD4 responses by IL-6, IL-1 and TNF. Eur J Immunol. 1998;28:277–289. doi: 10.1002/(SICI)1521-4141(199801)28:01<277::AID-IMMU277>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Lamacchia C, Palmer G, Seemayer CA, Talabot-Ayer D, Gabay C. Enhanced Th1 and Th17 responses and arthritis severity in mice with a deficiency of myeloid cell-specific interleukin-1 receptor antagonist. Arthritis Rheum. 2010;62:452–462. doi: 10.1002/art.27235. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Welte T, Fang H, Chang GJ, Born WK, O'Brien RL, Sun B, Fujii H, Kosuna K, Wang T. Oral administration of active hexose correlated compound enhances host resistance to West Nile encephalitis in mice. J Nutr. 2009;139:598–602. doi: 10.3945/jn.108.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]