SUMMARY
Systemic infusion of bone marrow mesenchymal stem cells (BMMSCs) shows therapeutic benefit for a variety of autoimmune diseases, but the underlying mechanisms are poorly understood. Here we show that in mice systemic infusion of BMMSCs induced transient T-cell apoptosis via the Fas ligand (FasL)-dependent Fas pathway and could ameliorate disease phenotypes in fibrillin-1 mutated systemic sclerosis (SS) and dextran sulfate sodium-induced experimental colitis. FasL−/− BMMSCs did not induce T-cell apoptosis in recipients, and could not ameliorate SS and colitis. Mechanistic analysis revealed that Fas-regulated monocyte chemotactic protein 1 (MCP-1) secretion by BMMSCs recruited T-cells for FasL-mediated apoptosis. The apoptotic T-cells subsequently triggered macrophages to produce high levels of TGFβ which in turn led to the upregulation of Tregs and, ultimately, to immune tolerance. These data therefore demonstrate a previously unrecognized mechanism underlying BMMSC-based immunotherapy involving coupling via Fas/FasL to induce T-cell apoptosis.
INTRODUCTION
Mesenchymal stem cells (MSCs) display profound immunomodulatory properties by inhibiting proliferation and function of several major immune cells, such as dendritic cells, T and B lymphocytes, and natural killer (NK) cells (Nauta and Fibbe, 2007; Uccelli et al., 2007, 2008; Aggarwal and Pittenger, 2005). These unique properties have prompted researchers to investigate mechanisms by which MSCs ameliorate a variety of immune disorders (Nauta and Fibbe, 2007; Bernardo et al., 2009). In fact, MSC-based therapy has been successfully applied in various human diseases, including graft versus host disease (GvHD), systemic lupus erythematosus (SLE), rheumatoid arthritis, autoimmune encephalomyelitis, inflammatory bowel disease, and multiple sclerosis (Aggarwal and Pittenger, 2005; Le Blanc et al., 2004; Chen et al., 2006; Polchert et al., 2008; Sun et al., 2009; Augello et al., 2007; Parekkadan et al., 2008; Zappia et al., 2005; González et al., 2009; Liang et al., 2009). The immunosuppressive properties of MSCs are associated with the production of cytokines, such as interleukin 10 (IL10), nitric oxide (NO), indoleamine 2,3-dioxygenase (IDO), prostaglandin (PG) E2, and TSG-6 (Batten et al., 2006; Zhang et al., 2010; Ren et al., 2008, Sato et al., 2007; Meisel et al., 2004; Aggarwal and Pittenger, 2005; Choi et al., 2011; Roddy et al., 2011). In addition, MSC-induced immune tolerance involves upregulation of CD4+CD25+Foxp3+ regulatory T cells (Tregs) and downregulation of proinflammatory T helper 17 (Th17) cells (Sun et al., 2009; González et al., 2009; Park et al., 2011). However, the detailed mechanism of MSC-based immunotherapy is not fully understood. In this study, we show that MSC-induced T cell apoptosis through Fas signaling is required for MSC-mediated therapeutic effects in SS and experimental colitis in mice.
RESULTS
Fas ligand (FasL) in BMMSCs induces T cell apoptosis
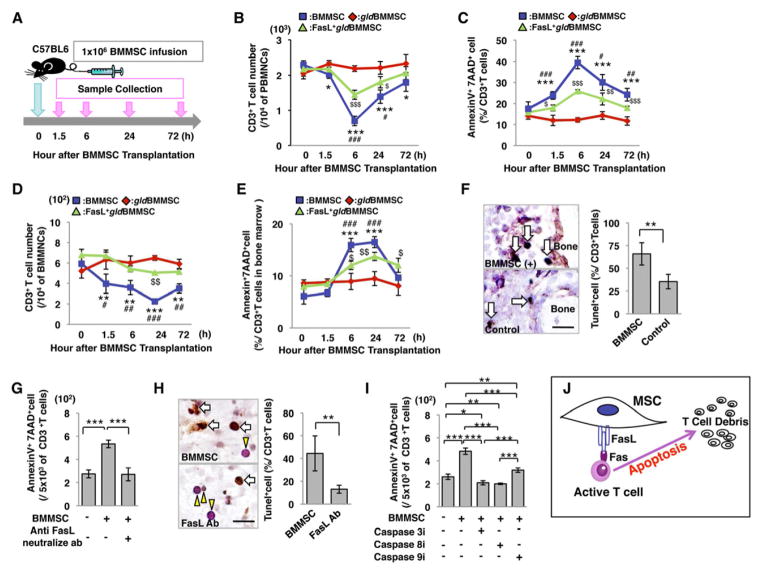
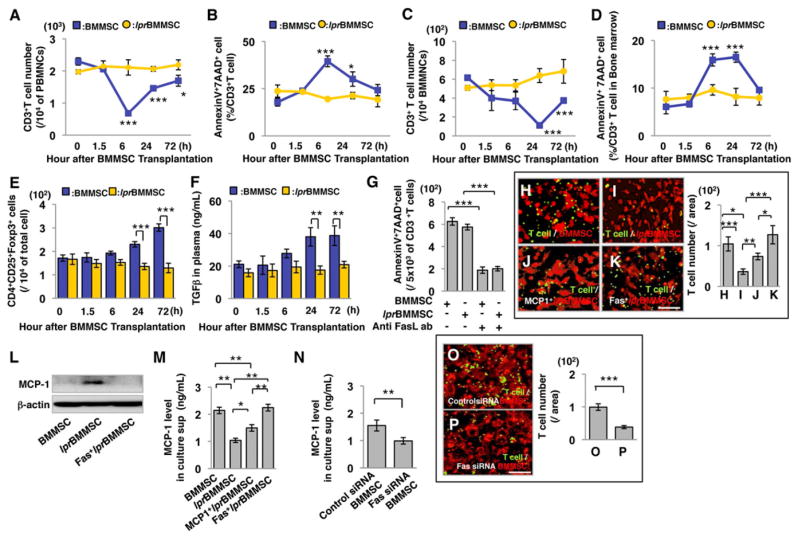
Since BMMSCs express FasL and activated T cells express elevated levels of Fas (Mazar et al., 2009; Figures S1A–1D), we hypothesized that FasL-mediated Fas signaling might play a critical role in BMMSC-based immunomodulation. To test this hypothesis, BMMSCs from C57BL6 mice and FasL-mutated B6Smn.C3-Faslgld/J mice (gldBMMSC), along with FasL transfected gldBMMSCs (FasL+gldBMMSC) were injected into normal C57BL6 mice (Figure 1A). Peripheral blood and bone marrow samples were collected at 0, 1.5, 6, 24, and 72 hours after BMMSC transplantation (Figure 1A). Allogenic BMMSC infusion reduced the number of CD3+ T cells and increased the number of apoptotic CD3+ T cells in peripheral blood and bone marrow, starting at 1.5 hours, reaching the peak at 6 hours and lasting until 72 hours post-transplantation (Figures 1B–1E). In order to compare syngenic and allogenic BMMSCs, we showed that BMMSCs derived from a littermate were the same as allogenic BMMSCs in their capacity to induce T cell apoptosis (Figures S2A–2G). Meanwhile, infusion of FasL−/− gldBMMSCs failed to reduce the number of CD3+ T cells or elevate the number of apoptotic CD3+ T cells in peripheral blood and bone marrow (Figures 1B–1E). However, overexpression of FasL in gldBMMSCs by lentiviral transfection (Figure S1N) partially rescued the capacity to reduce the number of CD3+ T cells and elevate the number of apoptotic CD3+ T cells in peripheral blood, bone marrow, spleen, and lymph node (Figures 1B–1E; S1P–1S). BMMSC infusion also resulted in reducing the number of both CD4+ and CD8+ T cells with correspondingly increased number of apoptotic CD4+ and CD8+ T cells in peripheral blood (Figures S1E and 1F). Interestingly, BMMSC transplantation induced CD4+ T cell apoptosis and Treg upregulation in OT1 TCR TG mice. However, the percentage of CD8+ T cells, which react with OVA-MHC class I antigen, was unchanged after BMMSC transplantation, indicating that transplanted BMMSCs need to be recognized as antigen to initiate CD8+ T cell apoptosis (Figures S1T-1AA). TUNEL staining confirmed that BMMSC infusion elevated the number of apoptotic T cells in bone marrow (Figure 1F). We next verified that BMMSC-induced T cell death was caused by apoptosis based on the in vitro blockage of BMMSC-induced CD3+ T cell apoptosis by neutralizing FasL antibody and caspase 3, 8, and 9 inhibitors (Figures 1G–1I). FasL neutralizing antibody injection could partially block BMMSC-induced CD3+ T cell apoptosis, upregulation of Tregs, and downregulation of Th17 cells in peripheral blood and bone marrow (Figure S1G–M). These data indicate that BMMSCs are capable of inducing T cell apoptosis through the FasL/Fas signaling pathway (Figure 1J). Although BMMSCs failed to induce naïve T cell apoptosis in the co-culture system (data not shown), they were able to induce activated T cell apoptosis in vitro (Figures 1G and 1I).
Figure 1. BMMSCs induce T cell apoptosis via Fas ligand (FasL).
(A) Schema of BMMSC transplantation procedure. 1×106 BMMSCs (n=5), FasL−/− gldBMMSCs (n=4) or FasL-transfected gldBMMSCs (FasL+gldBMMSCs, n=4) were infused into C57BL6 mice through the tail vein. All groups were sacrificed at indicated time points for sample collection. Zero hour represented that mice were immediately sacrificed after BMMSC injection. (B–E) BMMSC transplantation (BMMSC) induced transient reduction in the number of CD3+ T cells and increased AnnexinV+7AAD+ double positive apoptotic CD3+ T cells in peripheral blood mononuclear cells (PBMNCs; B, C) and bone marrow mononuclear cells (BMMNCs; D, E) at indicated time points, while FasL−/− BMMSCs from gld mice (gldBMMSCs) failed to reduce CD3+ T cells or elevate CD3+ T cell apoptosis in peripheral blood (B, C) and bone marrow (D, E). FasL-transfected gldBMMSC transplantation (FasL+gldBMMSC) partially rescued the capacity to reduce the number of CD3+ T cells and induce CD3+ T cell apoptosis in peripheral blood (B, C) and bone marrow (D, E). *P<0.05; **P<0.01; ***P<0.001 vs. gldBMMSC, #P<0.05; ###P<0.001 vs. FasL+gldBMMSC, $P<0.05; $$$P<0.001 vs. gldBMMSC. (F) When BMMSCs were infused into mice, TUNEL and immunohistochemistry staining showed that TUNEL-positive apoptotic cell (brown, white arrow) number in CD3-positive T cells (purple, yellow arrowhead) was higher in the BMMSC-injected group compared to the control group in bone marrow. (G) When BMMSCs were co-cultured with T cells, BMMSC-induced AnnexinV+7AAD+ double positive apoptotic T cells were significantly blocked by anti-FasL neutralizing antibody (1μg/mL) compared to IgG antibody control group. (H) TUNEL and immunohistochemistry staining showed that TUNEL-positive apoptotic T cells (brown, white arrow) were observed in CD3+ T cells (purple, yellow arrowhead) when co-cultured with BMMSCs in vitro. In the presence of anti-FasL neutralizing antibody (FasL Ab), TUNEL-positive cell percentage was significantly less than the untreated control group. (I) In addition, the number of BMMSC-induced AnnexinV+7AAD+ double positive apoptotic T cells was significantly blocked by caspase 3, 8, and 9 inhibitor treatments. The results were representative of three independent experiments. (J) Schematic diagram indicating that BMMSCs induce T cell apoptosis. (*P<0.05; **P<0.01; ***P<0.001. The bar graph represents mean±SD). See also Figures S1 and 2.
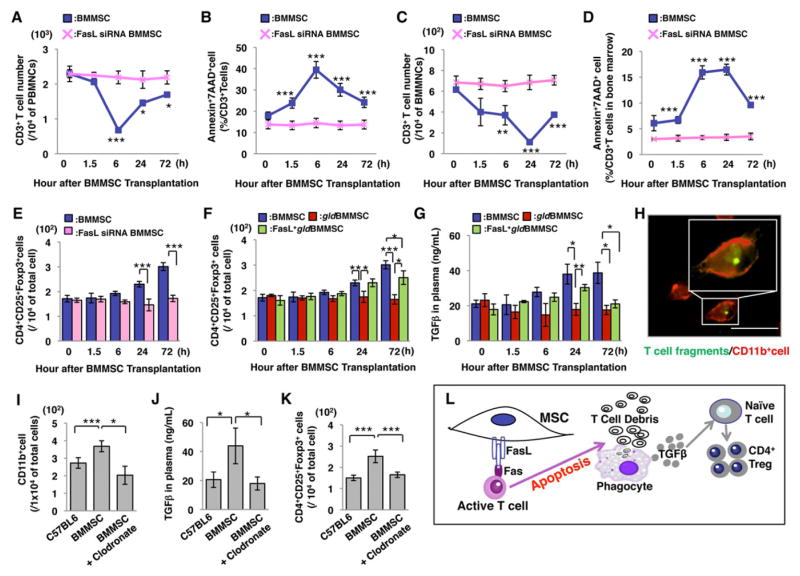
In order to confirm the role of FasL in BMMSC-mediated T cell apoptosis in vivo, we used siRNA to knockdown FasL expression in BMMSCs (Figure S3A) and infused FasL knockdown BMMSCs to C57BL6 mice. As expected, infusion of FasL knockdown BMMSCs (FasL siRNA BMMSCs) failed to reduce the number of CD3+ T cells or induce CD3+ T cell apoptosis in peripheral blood and bone marrow (Figures 2A–2D). Moreover, infusion of FasL knockdown BMMSCs failed to elevate Foxp3+ regulatory T cell (Treg) levels in peripheral blood (Figure 2E). This study confirms that FasL is required for BMMSC-induced T cell apoptosis and Treg upregulation.
Figure 2. FasL is required for BMMSC-induced T cell apoptosis and upregulation of CD4+CD25+Foxp3+ regulatory T cells (Tregs).
(A, B) BMMSC transplantation (BMMSC, n=5) induced a transient reduction in the number of CD3+ T cells (A) and elevation of AnnexinV+7AAD+ double positive apoptotic CD3+ cells (B) in peripheral blood. Transplantation of FasL knockdown BMMSC (FasL siRNA BMMSC, n=3) failed to reduce CD3+ T cells (A) or increase the number of CD3+ apoptotic T cells (B) in peripheral blood. (C, D) BMMSC transplantation (BMMSC, n=5) showed a transient reduction of CD3+ T cells (C) and elevation of AnnexinV+7AAD+ double positive apoptotic CD3+ T cells (D) in bone marrow. Transplantation of FasL knockdown BMMSCs (FasL siRNA BMMSC, n=3) failed to reduce CD3+ T cells (C) or elevate CD3+ apoptotic T cells (D) in bone marrow. (E) BMMSC, but not FasL knockdown BMMSC, transplantation significantly upregulated levels of Tregs at 24 and 72 hours after transplantation in C57BL6 mice. (F) BMMSC transplantation resulted in a significant upregulation of Tregs when compared to the gldBMMSC transplantation group at 24 and 72 hours post-transplantation. FasL-transfected gldBMMSC transplantation (FasL+gldBMMSC) partially rescued BMMSC-induced upregulation of Tregs. (G) TGF-β level in peripheral blood was significantly increased in both BMMSC and FasL+gldBMMSC groups at 24 hours post-transplantation. FasL−/−gldBMMSC transplantation failed to upregulate TGF-β level. (H) Apoptotic pan T cells were engulfed by macrophages in vivo. Green indicates T cells, and red indicates CD11b+ macrophages. Bar=50μm. (I) BMMSC transplantation group increased the number of CD11b+ cells in peripheral blood when compared to the control group (C57BL6). Depletion of macrophages by clodronate liposome treatment showed the effectiveness in reducing CD11b+ cells in the BMMSC transplantation group (BMMSC+clodronate), as assessed by flow cytometric analysis. (J) TGF-β level was significantly increased in peripheral blood after BMMSC transplantation. Clodronate liposome treatment blocked BMMSC-induced upregulation of TGF-β (BMMSC+clodronate). (K) BMMSC transplantation upregulated the level of Tregs in peripheral blood compared to the control group (C57BL6). Clodronate liposome treatment inhibited BMMSC-induced Treg upregulation (BMMSC+clodronate). (L) Schematic diagram indicating that BMMSC-induced T cell apoptosis resulted in immune tolerance as evidenced by upregulation of Tregs. The results were representative of three independent experiments. (*P<0.05, **P<0.01, ***P<0.001. The bar graph represents mean±SD). See also Figure S3.
Since apoptotic T cells trigger TGFβ production by macrophages and upregulate Tregs, which leads to immune tolerance in vivo (Perruche et al., 2008), we examined whether BMMSC-induced T cell apoptosis could also promote the upregulation of Tregs. We found that systemic infusion of BMMSCs did, in fact, elevate Treg levels in peripheral blood at 24 and 72 hours post-transplantation (Figures 2F and S2H–2M), along with elevated TGFβ level and reduced T helper 17 (Th17) cell level in peripheral blood (Figures 2G and S1O). Co-transplantation of BMMSCs and pan T cells resulted in T cell apoptosis at 1.5 and 6 hours post-transplantation. On the other hand, FasL−/− gldBMMSC infusion failed to upregulate the levels of either Tregs or TGFβ (Figures 2F and 2G), suggesting that FasL-mediated T cell apoptosis plays a critical role in Treg upregulation. Indeed, overexpression of FasL in FasL−/− gldBMMSCs rescued BMMSC-induced Treg upregulation and TGFβ production at 24 hours post-transplantation (Figures 2F and 2G).
To examine the mechanism by which BMMSC infusion resulted in TGFβ upregulation in peripheral blood, we used fluorescence analysis to confirm that macrophages engulfed apoptotic T cells in vivo (Perruche et al., 2008; Figure 2H). Then we measured the number of CD11b+ macrophages in spleen cells and found that the number was significantly increased in the BMMSC infusion group (Figure 2I). In contrast, treatment with macrophage inhibitor clodronate liposomes significantly reduced the number of CD11b+ macrophages in spleen cells (Figure 2I) and blocked BMMSC infusion-induced upregulation of TGFβ and Tregs (Figures 2J and 2K). These data suggest that T cell apoptosis, as induced by BMMSC infusion, activates macrophages to produce the TGF-β that results in Treg upregulation (Figure 2L).
We next asked whether apoptosis of infused BMMSCs also triggers macrophages to produce TGFβ to upregulate Tregs. Carboxyfluorescein diacetate N-succinimidyl ester (CFSE)-labeled BMMSCs, gldBMMSCs and FasL knockdown BMMSCs were infused into C57BL6 mice. At 1.5 hours post-infusion, all CFSE+ cells were detected and reached a peak in peripheral blood and bone marrow, after which the cell number was gradually decreased, becoming undetectable at 24 hours post-infusion (Figures S3C and 3D). In contrast, CFSE+ apoptotic cells reached a peak at 6 hours post-infusion and became undetectable at 24 hours post-infusion in peripheral blood and bone marrow (Figures S3E and 3F). The apoptosis of transplanted BMMSCs was also observed by immunofluoresent analysis (Figure S3B). Although apoptosis of the infused FasL-deficient BMMSCs was observed, no corresponding upregulation of TGFβ or Tregs was observed in peripheral blood (Figures 2E, 2F, and 2G). These data suggest that T cell, not BMMSC, apoptosis is required for Treg upregulation (Figure 2L).
FasL is required for BMMSC-based immune therapies in both tight-skin (Tsk/+) systemic sclerosis and inductive experimental colitis mice
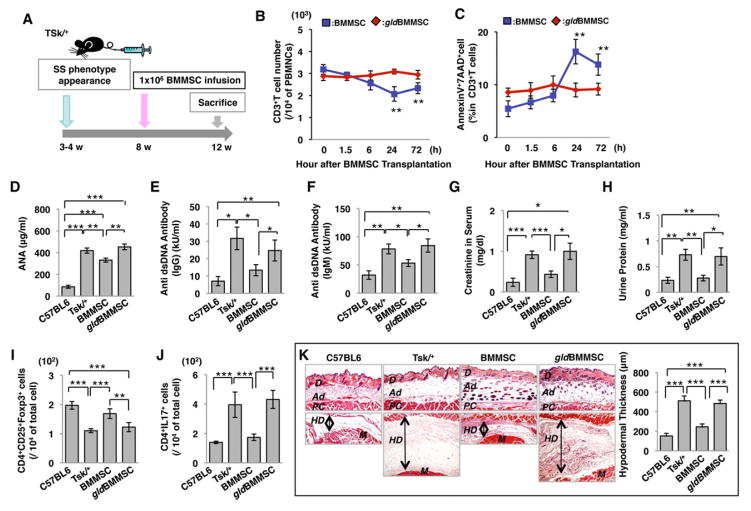
To further study the therapeutic mechanism of BMMSC transplantation, two mouse models, genetic tight-skin (Tsk/+) systemic sclerosis and inductive experimental colitis, were used to evaluate the therapeutic effect of BMMSC transplantation. Allogenic normal BMMSCs or gldBMMSCs (1×106) were systemically transplanted into Tsk/+ systemic sclerosis mice (Green et al., 1976) at 8 weeks of age, and samples were harvested at 12 weeks of age for further evaluation (Figure 3A). The BMMSC-transplanted group showed significant reduction in the number of CD3+ T cells and corresponding elevation in the number of apoptotic CD3+ T cells in peripheral blood from 6 to 72 hours post-transplantation (Figures 3B and 3C). On the other hand, FasL−/− gldBMMSC transplantation failed to induce CD3+ T cell apoptosis (Figures 3B and 3C).
Figure 3. FasL is required for BMMSC-mediated amelioration of systemic sclerosis (SS) phenotypes.
(A) Schema showing how BMMSC transplantation ameliorates SS phenotype. (B, C) BMMSC transplantation (n=6) showed a significantly reduced number of CD3+ T cells (B) and increased number of AnnexinV+7AAD+ double positive apoptotic CD3+ T cells (C) in SS mice as assessed by flow cytometric analysis. However, FasL−/− gldBMMSC (n=6) failed to reduce the number of CD3+ T cells (B) or elevate the number of apoptotic CD3+ T cells (C). (D–F) Tsk/+ SS mice showed elevated levels of antinuclear antibody (ANA, D) and anti-double strand DNA antibodies IgG (E) and IgM (F) when compared to control C57BL6 mice. BMMSC transplantation reduced the levels of ANA (D) and anti-double strand DNA antibodies IgG (E) and IgM (F). In contrast, FasL−/− gldBMMSC transplantation failed to reduce the levels of antinuclear antibody (ANA, D) or anti-double strand DNA IgG (E) and IgM (F) antibodies. (G) Creatinine level in serum was significantly increased in Tsk/+ mice. After BMMSC transplantation, creatinine level was significantly decreased to the level observed in C57BL6 mice. However, gldBMMSC transplantation failed to reduce the creatinine level. (H) The concentration of urine protein was significantly increased in Tsk/+ mice. BMMSC transplantation reduced urine protein to the control level. gldBMMSC transplantation failed to reduce urine protein levels in Tsk/+ mice. (I) Treg level was significantly decreased in Tsk/+ mice compared to C57BL6 mice. After BMMSC transplantation, Treg levels were significantly elevated, whereas gldBMMSC transplantation failed to increase Treg levels in Tsk/+ mice. (J) CD4+IL17+ Th17 cells were significantly increased in Tsk/+ mice compared to C57BL6 mice. Elevated Th17 level was significantly reduced in the BMMSC transplantation group, while gldBMMSC transplantation failed to reduce the Th17 level in Tsk/+ mice. (K) Hyperdermal thickness was significantly increased in Tsk/+ mice (Tsk/+, n=5) compared to control mice (C57BL6, n=5). BMMSC, but not FasL−/− gldBMMSC, transplantation reduced hyperdermal thickness. (*P<0.05, **P<0.01, ***P<0.001. The bar graph represents mean±SD). See also Figure S4.
Tsk/+ mice showed an increase in the levels of antinuclear antibody (ANA), anti-double strand DNA (dsDNA) IgG and IgM antibodies, and creatinine in serum, along with an increase in the level of urine proteins (Figures 3D–3H). Normal BMMSC, but not FasL−/− gldBMMSC, transplantation significantly reduced the levels of ANA, dsDNA IgG and IgM, as well as serum creatinine and urine protein levels (Figures 3D–3H). Moreover, BMMSC transplantation rescued decreased level of Tregs and increased level of Th17 cells in Tsk/+ mice (Figures 3I, 3J, and S4B). As expected, gldBMMSC transplantation failed to regulate the levels of Tregs and Th17 cells in Tsk/+ mice (Figures 3I and 3J). Histological analysis also showed that skin hypodermal (HD) thickness was significantly increased in Tsk/+ mice (Figure 3K). After BMMSC transplantation, HD thickness was reduced to a level equal to that of the control group, whereas gldBMMSC failed to reduce HD thickness (Figure 3K). Additionally, the tightness of skin, as measured by grabbed distance, was significantly improved in the BMMSC, but not the gldBMMSC, transplantation group (Figure S4A).
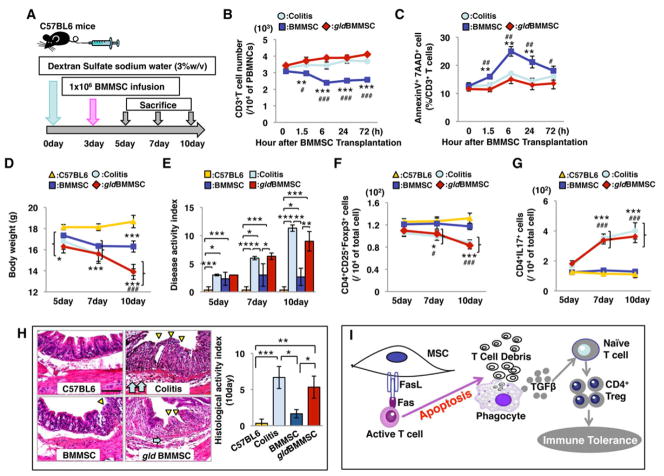
The induced experimental colitis model was generated as previously described (Alex et al., 2009; Zhang et al., 2010). Allogenic normal BMMSCs or FasL−/− gldBMMSCs (1×106) were systemically transplanted into experimental colitis mice at day 3 post 3% dextran sulfate sodium (DSS) induction (Zhang et al., 2010; Figure 4A). Normal BMMSC transplantation reduced the number of CD3+ T cells and elevated the number of AnnexinV+7AAD+ double positive apoptotic CD3+ T cells in peripheral blood starting at 1.5 hours and lasting to 72 hours after transplantation (Figures 4B and 4C). However, the gldBMMSC transplantation group showed no difference from the colitis group in terms of numbers of CD3+ T cells and apoptotic CD3+ T cells (Figures 4B and 4C). The body weight of mice with induced colitis was significantly reduced compared to control C57BL6 mice from day 5 to 10 post-DSS induction (Figure 4D). After normal BMMSC, but not gldBMMSC, transplantation, the body weight was partially restored at day 10 post-DSS induction. The disease activity index (DAI), including body weight loss, diarrhea, and bleeding, was significantly elevated in the colitis mice compared to the control mice. After BMMSC transplantation, the DAI score was decreased, while gldBMMSCs failed to reduce the DAI score (Figure 4E). Both decreased Tregs and elevated Th17 cells were observed in the colitis mice from day 7 to 10 post-DSS induction (Figures 4F and 4G). BMMSC, but not gldBMMSC, transplantation significantly upregulated Tregs and downregulated Th17 cells (Figures 4F and 4G). Furthermore, colon tissue from each group was analyzed (Figure 4H). Both the absence of epithelial layer and infiltration of inflammatory cells were observed in the colitis and gldBMMSC transplantation groups. BMMSC transplantation recovered epithelial structure and eliminated inflammatory cells in colitis mice. Histological activity index (Alex et al., 2009) confirmed that BMMSC transplantation reduced the DAI, while gldBMMSCs failed to improve the DAI (Figure 4H). The data therefore suggest that BMMSC-induced T cell apoptosis with Treg upregulation might offer a potential treatment for the colitis (Figure 4I). Moreover, upregulation of Tregs was required in ameliorating disease phenotype in DSS-induced colitis model (Figures S5A–5F).
Figure 4. FasL plays a critical role in BMMSC-mediated immune therapy for Dextran sulfate sodium (DSS)-induced experimental colitis.
(A) Schema showing BMMSC transplantation in DSS-induced experimental colitis mice. (B, C) BMMSC transplantation (n=6) showed a significantly reduced number of CD3+ T cells at 24 hours post-transplantation (B) and increased number of AnnexinV+7AAD+ double positive apoptotic CD3+ T cells at 24–72 hours post-transplantation (C) in colitis mice as assessed by flow cytometric analysis. However, FasL−/− gldBMMSC (n=6) failed to reduce the number of CD3+ T cells (B) or elevate the number of apoptotic CD3+ T cells (C). (D) Colitis mice (colitis, n=5), BMMSC transplanted group, and gldBMMSC showed significantly reduced body weight from 5 to 10 days after DSS induction. The BMMSC transplantation group showed inhibition of body weight loss compared to the colitis and gldBMMSC transplantation groups at 10 days after DSS induction. (E) Disease activity index (DAI) was significantly increased in colitis mice compared to C57BL6 mice from 5 days to 10 days after DSS induction. BMMSC transplantation significantly reduced DAI score, but it was still higher than that observed in C57BL6 mice. FasL−/− gldBMMSC transplantation failed to reduce DAI score at all time points. (F) Treg level was significantly reduced in colitis mice compared to C57BL6 mice at 7days after DSS induction. BMMSC, but not FasL−/−gldBMMSC, transplantation upregulated the Treg levels in colitis mice. (G) Th17 cell level was significantly elevated in colitis mice compared to C57BL6 mice at 7 days after DSS induction. BMMSC, but not FasL−/−gldBMMSC, transplantation reduced the levels of Th17 cells in colitis mice from 7 to 10 days after DSS induction. (H) Hematoxylin and eosin staining showed the infiltration of inflammatory cells (blue arrows) in colon with destruction of epithelial layer (yellow triangles) in colitis mice. BMMSC, but not FasL−/−gldBMMSC, transplantation rescued disease phenotype in colon and reduced histological activity index. (I) Schematic diagram of BMMSC transplantation for immunotherapies. (Bar= 200μm; *P<0.05, **P<0.01, ***P<0.001. The bar graph represents mean±SD). See also Figure S5.
Fas is required for BMMSC-mediated therapy by recruitment of T cells
In addition to the production of FasL, BMMSCs also express Fas (Figure S6A). To examine whether Fas plays a role in BMMSC-based immunotherapies, we infused Fas−/−BMMSCs, derived from C3MRL-Faslpr/J mice (lprBMMSCs), to C57BL6 mice and found that Fas−/− lprBMMSCs failed to reduce the number of CD3+ T cells or elevate the number of apoptotic CD3+ T cells in peripheral blood and bone marrow (Figures 5A–5D). In addition, we revealed that lprBMMSC transplantation failed to upregulate the levels of Tregs and TGFβ and downregulate Th17 cell level in peripheral blood (Figures 5E, 5F, and S6X). Moreover, Fas knockdown BMMSCs using siRNA showed the same effect as observed in Fas null lprBMMSC (Figure S6Y-6EE). When transplanted into DSS-induced colitis mice, lprBMMSCs neither provided therapeutic effects on body weight, disease activity index, histological activity index nor balanced the levels of Tregs and Th17 cells (Figures S6B–6G). In addition, lprBMMSC transplantation failed to treat Tsk/+ SS mice, showing no rescue of the levels of ANA, anti-dsDNA antibodies IgG and IgM antibodies, creatinine, urine protein, Grabbed distance, Tregs, or Th17 cells (Figures S6H–6Q). Taken together, these data suggest that Fas−/− lprBMMSCs, like FasL−/− gldBMMSCs, were unable to ameliorate disease phenotypes in SS and colitis mouse models.
Figure 5. Fas plays an essential role in BMMSC-mediated CD3+ T cell apoptosis and up-regulation of Tregs via regulating monocyte chemotactic protein 1 (MCP-1) secretion.
(AD) BMMSC transplantation (BMMSC) induced transient reduction in the number of CD3+ T cells and increase in the number of AnnexinV+7AAD+ double positive apoptotic CD3+ T cells in peripheral blood mononuclear cells (PBMNCs; A, B) and bone marrow mononuclear cells (BMMNCs, n=5; C, D) at indicated time points, while Fas−/− BMMSC from lpr mice (lprBMMSC, n=5) failed to reduce the number of CD3+ T cells or increase the number of CD3+ apoptotic T cells in peripheral blood (A, B) and bone marrow (C, D). (E, F) lprBMMSC transplantation failed to elevate Treg levels (E) and TGF-β (F) in C57BL6 mice compared to the BMMSC transplantation group at indicated time points. (G) lprBMMSC induced activated T cell apoptosis in a BMMSC/T cell in vitro co-cultured system, which was blocked by anti-FasL neutralizing antibody (1μg/mL). (H–K) Activated T cells (green) migrate to BMMSCs (red) in a transwell co-culture system (H). lprBMMSCs showed a significantly reduced capacity to induce activated T cell migration (I), which could be partially rescued by overexpression of MCP-1 (J) and totally rescued by overexpression of Fas (K) in lprBMMSCs. The results were representative of three independent experiments. (L) Quantitative RT-PCR analysis showed no significant difference between BMMSC and lprBMMSC in terms of MCP-1 expression level. However, overexpression of MCP-1 and Fas in lprBMMSC significantly elevated gene expression level of MCP-1. (M) Western blot showed that lprBMMSCs express a higher cytoplasm level of MCP-1 than BMMSC. Overexpression of Fas in lprBMMSC reduced the expression level of MCP-1 in cytoplasm. (N) ELISA analysis showed that MCP-1 secretion in culture supernatant was significantly reduced in lprBMMSCs compared to BMMSC. Overexpression of MCP-1 and Fas in lprBMMSCs significantly elevated MCP-1 secretion in culture supernatant. (O) ELISA data showed that knockdown Fas expression using siRNA resulted in reduction of MCP-1 level in culture medium compared to control siRNA group. (P–Q) Fas siRNA-treated BMMSCs (Q) showed reduced T cell migration in transwell co-culture system compared to control siRNA group (P). (*P<0.05, **P<0.01, ***P<0.001. The bar graph represents mean±SD). See also Figure S6.
Next, we investigated the underlying mechanisms by which lprBMMSC transplantation failed to treat the diseases. We showed that lprBMMSCs expressed a normal level of FasL by Western blot analysis (Figure S6R) and induced CD3+ T cell apoptosis in a co-culture system (Figure 5G). This was blocked by anti-FasL neutralizing antibody (Figure 5G), suggesting that the failure to induce in vivo T cell apoptosis by lprBMMSCs does not result from the lack of expression of functional FasL. We therefore hypothesized that Fas expression affects the BMMSC immunomodulatory property via a non-FasL-related mechanism, such as regulating the recruitment of T cells. To test this, we used an in vitro transwell co-culture system to show that activated T cells migrate to BMMSCs to initiate cell-cell contact (Figure 5H). However, lprBMMSCs showed a significantly reduced capacity to recruit activated T cells in the co-culture system when compared to control BMMSCs (Figures 5H and 5I). We then used a cytokine array analysis to determine that lprBMMSCs express a low level of monocyte chemotactic protein 1 (MCP-1), a member of the C-C motif chemokine family and a T cell chemoattractant cytokine (Carr et al. 1994; Figure S6S). Interestingly, overexpression of MCP-1 in lprBMMSCs partially rescued their capacity to recruit T cells (Figures 5H–5J). Overexpression of Fas in lprBMMSCs showed that the secretion level of multiple cytokine was restored (Figures S6S and S6U) and fully rescued their capacity to recruit T cells (Figures 5H, 5I, 5K). However, the expression level of MCP-1 protein in lprBMMSCs was higher than that in control BMMSCs, and overexpression of Fas reduced MCP-1 cytoplasm protein level in lprBMMSCs (Figure 5L), indicating that Fas regulates MCP-1 secretion, but not expression. Next, we examined MCP-1 level in the culture supernatant, and we found that the MCP-1 level in lprBMMSCs was significantly lower than BMMSCs (Figure 5M). Overexpression of MCP-1 and Fas in lprBMMSCs rescued MCP-1 levels in culture supernatant (Figure 5M). We next confirmed that Fas regulated MCP-1 secretion using the siRNA knockdown approach (Figure S6T). Downregulation of Fas expression in BMMSCs resulted in a reduced MCP-1 secretion (Figure 5N), with a corresponding reduction in the capacity to recruit activated T cells in the co-culture system (Figures 5O and 5P).
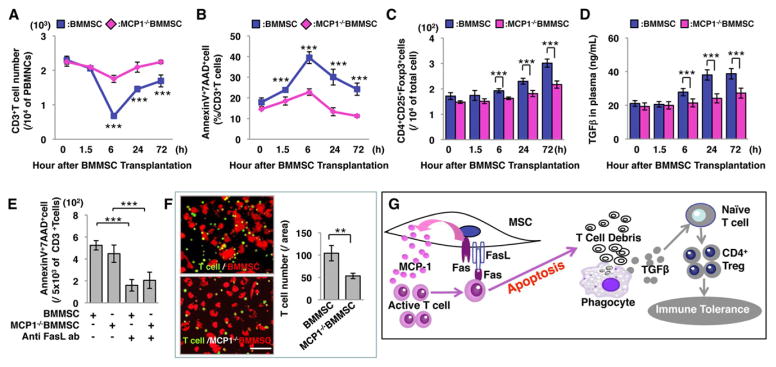
In order to confirm that MCP-1 contributes to BMMSC-based immunoregulation, we isolated BMMSCs from MCP-1 mutant B6.129S4-Ccl2tm1Rol/J mice and showed that MCP-1−/− BMMSCs were defective in reducing the number of CD3+ T cells or elevating apoptotic CD3+ T cells in C57BL6 mice when compared to control BMMSCs (Figures 6A and 6B). Also, MCP-1−/− BMMSCs failed to upregulate the levels of Tregs and TGFβ within 72 hours post-transplantation (Figures 6C and 6D). The deficiency of inducing T cell apoptosis and Treg upregulation by MCP-1−/−BMMSCs was not associated with FasL function (Figure 6E). When MCP-1−/− BMMSCs were co-cultured with activated T cells in a transwell culture system, the number of T cells migrating to BMMSCs was significantly reduced compared to control BMMSCs (Figure 6F). Also, Fas and MCP-1 play an important role in attracting B cells, NK cells, and immature dendritic cells (iDCs) in an in vitro culture system (Figure S7A–7C). These data indicate that MCP-1 secretion regulates BMMSC-induced T cell migration (Figure 6G). Moreover, we showed that Fas also regulated the secretion of other cytokines, such as C-X-C motif chemokine 10 (CXCL-10) and tissue inhibitor of matrix metalloprotease-1 (TIMP-1) (Figures S6V and 6W).
Figure 6. MCP-1 plays an important role in T cell recruitment.
(A) MCP-1−/−BMMSC transplantation showed a slightly reduced number of CD3+ T cells in peripheral blood, but the level of reduction was significantly less than that of the BMMSC transplantation group. (B) AnnexinV+7AAD+ double positive apoptotic CD3+T cell percentage was slightly increased in the MCP-1−/− BMMSC transplant group. (C) Treg level was slightly increased in the MCP-1−/− BMMSC-transplanted group at 72 hours post-transplantation, but significantly lower than the BMMSC transplantation group. (D) TGF-β level in serum was slightly increased in the MCP-1−/− BMMSC-transplanted group at 72 hours after transplantation compared to 0 hour, but the elevation level was lower than the BMMSC transplantation group. (E) When T cells were stimulated with CD3 and CD28 antibody and co-cultured with BMMSC or MCP-1−/− BMMSC in a transwell culture system, the number of migrated T cells was significantly higher in the BMMSC group (E) than the MCP-1−/− BMMSC group. (F) Schematic diagram showing the mechanism of BMMSC-induced immunotherapies. (**P<0.01, ***P<0.005; The bar graph represents mean±SD). See also Figure S7.
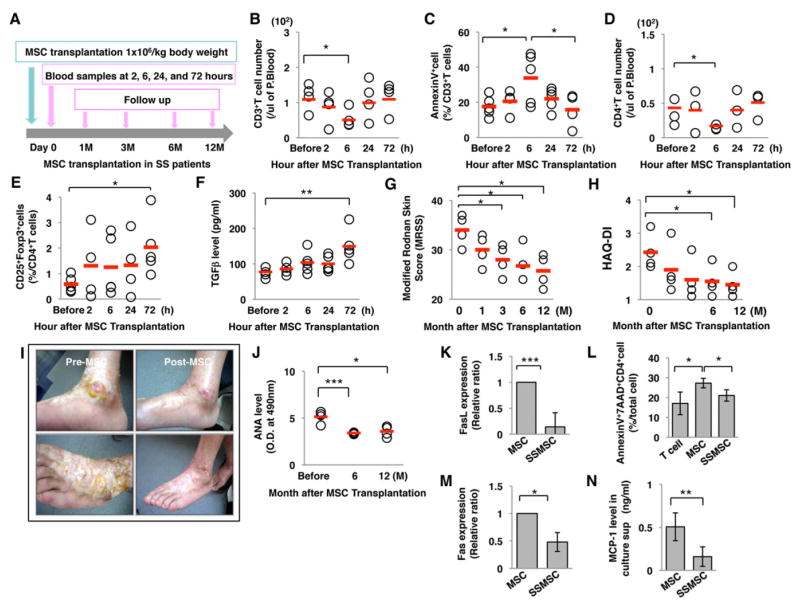
Allogenic MSC transplantation (MSCT) induced CD3+ T cell apoptosis and Treg up-regulation in patients with systemic sclerosis (SS)
Based on the above results in experimental animal models, we conducted a pilot clinical investigation to assess whether T cell apoptosis and Treg upregulation occurred in SS patients treated with MSCT. Five patients (4 females and 1 male, Table S1), ranging in age from 44 to 61 years old (average 51.2±7.8 years old) and having SS for a duration of 48–480 months (average 163.2±182.1 months) were enrolled for allogenic MSCT (Sun et al., 2009; Liang et al., 2009) and peripheral blood was collected at indicated time points (Figure 7A). Allogenic MSC transplantation induced a significant reduced number of CD3+ T cells and upregulated number of AnnexinV-positive apoptotic CD3+ T cells at 6 hours post-MSCT, followed by a decrease in CD3+ T cell number and apoptotic rate to baseline level by 72 hours (Figure 7B and 7C). A reduced number of CD4+ T cells was also observed at 6 hours post-MSCT (Figure 7D). Importantly, the frequency of Tregs in peripheral blood was significantly upregulated at 72 hours post-MSCT (Figure 7E), along with elevated level of TGFβ (Figure 7F). Assessment of Modified Rodnan Skin Score (MRSS) and Health Assessment Questionnaire (HAQ-DI) indicated that MSCT provided optimal treatment for SS patients at follow-up period (Figure 7G and 7H). Furthermore, reduced level of ANA was observed in SS patients at the 12-month follow-up period (Figure 7J). Interestingly, the MSC derived from SS patients (SSMSC) showed a deficiency in FasL and Fas expression when compared to MSC derived from healthy donors (MSC) (Figures 7K and 7M). SSMSCs showed a reduced capacity to induce T cell apoptosis (Figure 7L) and secrete MCP-1 (Figure 7N) by the reduced expression levels of FasL and Fas. In addition, we found that MSCT significantly improved skin ulcers in a patient (Figure 7I). These early clinical data demonstrate safety and efficacy of MSCT in SS patients and improvement of disease activities at post-allogenic MSCT. However, the long-term effects of MSCT on SS patients will require further investigation.
Figure 7. Allogenic MSC transplantation induces CD3+ T cell apoptosis and Treg upregulation in systemic sclerosis (SS) patients.
(A) Schema of MSC transplantation in SS patients. (B) Flow cytometric analysis showed reduced number of CD3+ T cells from 2 to 72 hours post-transplantation. (C) AnnexinV+-positive apoptotic CD3+ T cells were significantly increased at 6 hours after MSC transplantation. (D) Flow cytometric analysis showed reduced number of CD4+ T cells from 2 to 72 hours post-transplantation. (E) Treg levels in peripheral blood were significantly increased at 72 hours after allogenic MSC transplantation. (F) Serum level of TGFβ was significantly increased in MSC transplantation group at 72 hours post-transplantation. (G, H) Modified Rodnan Skin Score (MRSS, G) and Health assessment Questionnaire disease activity index (HAQ-DI, H) were significantly reduced after allogenic MSC transplantation. (I) Representative images of skin ulcers prior to MSC transplantation (pre-MSC) and at 6 months post-transplantation (post-MSC). (J) The reduced ANA level was maintained at 12 months after MSC transplantation. (K) Real-time PCR analysis showed significantly decreased FasL expression in SS patient MSCs (SSMSC) compared to MSC from healthy donor (MSC). (L) SSMSC showed a significantly decreased capacity to induce T cell apoptosis compared to normal MSC in vitro. (M) SSMSC showed a reduced expression of Fas by real-time PCR analysis. (N) MCP-1 secretion level in SSMSC was significantly lower than that in MSC culture supernatant. (*P<0.05, **P<0.01, ***P<0.005; The bar graph represents mean±SD). See also Table S1.
DISCUSSION
The FasL/Fas-mediated cell death pathway represents typical apoptotic signaling in many cell types (Hohlbaum et al., 2000; Pluchino et al., 2005; Zhang et al., 2008). MSCs derived from bone marrow (BMMSCs) express FasL and induce tumor cell apoptosis in vitro (Mazar et al., 2009). However, it is unknown that whether BMMSCs induce T cell apoptosis via Fas/FasL pathway leading to immune tolerance. Therefore, we transplanted BMMSCs into C57BL6 mice and demonstrated that BMMSCs, but not FasL-deficient BMMSCs, induced transient T cell apoptosis. Furthermore, we found a reduced number of T cells in multiple organs, including peripheral blood, bone marrow, spleen, and lymph node. It appears that alteration of T cell number, owing to T cell redistribution, is not supported by the experimental evidence. Since CD3 antibody-induced T cell apoptosis resulted in immune tolerance (Chatenoud et al., 1994 and 1997), we confirm here that BMMSC-induced T cell apoptosis upregulates Tregs via high levels of macrophage-released TGF-β (Kleinclauss et al., 2006; Perruche et al., 2008). Although transplanted FasL−/− gldBMMSCs and FasL knockdown BMMSCs undergo apoptosis in vivo, they failed to induce upregulation of Tregs. This evidence further confirms that T cell, but not transplanted BMMSC, apoptosis is required for inductive upregulation of Tregs (Perruche et al., 2008).
Despite the expression of functional FasL by Fas−/− lprBMMSCs, they failed to induce T cell apoptosis and upregulate Tregs in vivo. Mechanistically, Fas controls chemoattractant cytokine MCP-1 secretion in BMMSCs. Decreased MCP-1 secretion from lprBMMSC results in the failure to recruit activated T cells to BMMSCs (Carr et al., 1994; Xu et al., 1996) and, hence, infusion of Fas−/− lprBMMSCs failed to induce T cell apoptosis in vivo. However, when lprBMMSCs were directly co-cultured with CD3+ T cells, they could induce T cell apoptosis, suggesting that lprBMMSC may not able to initiate cell-cell contact with T cells in vivo. Moreover, Fas−/− lprBMMSCs show a higher cytoplasm level of MCP-1 than control BMMSCs, suggesting that Fas regulates MCP-1 secretion, but not MCP-1 production. When MCP-1−/− BMMSCs were systemically transplanted into C57BL6 mice, CD3+ T cell apoptosis and Treg upregulation were significantly reduced compared to control BMMSC group, suggesting that MCP-1 is one of the factors regulating MSC-based immune tolerance. It was reported that BMMSCs could inhibit CD4/Th17 T cells with MCP-1 paracrine conversion from agonist to antagonist (Rafei et al., 2009). Here we showed that MCP-1 helped to recruit T cells to up-regulate Tregs. Prospectively, it will be important to dissect the mechanism by which Fas regulates MCP-1 secretion. In addition to MCP-1 secretion, Fas also regulates multiple cytokine and chemokine secretions in BMMSCs, which may extensively affect the recruitment of T cells. Therefore, MCP-1 may only represent one of those chemokines contributing to BMMSC-mediated T cell recruitment.
Significantly, our primary clinical investigation showed that MSC infusion induced CD3+ T cell apoptosis and Treg upregulation in allogenic MSC-infused SS patients. In our 1–12 month follow-up period, we did not find any clinical sign of side effects, including cardiovascular and pulmonary insufficiencies, infection, malignancy, or metabolic disturbances, suggesting the safety of the MSC therapy in SS patients. The therapeutic effects of allogenic MSC transplantation were significant as shown by the reduction of MRSS, HAQDI, in addition to improved quality of life. Furthermore, we demonstrated that MSC transplantation dramatically improved treatment-refractory skin ulcers.
Thus, we have uncovered a previously unrecognized BMMSC-mediated therapeutic mechanism by which BMMSCs use Fas to regulate MCP-1 secretion for T cell recruitment and subsequently use FasL to induce T cell apoptosis. Macrophages subsequently take the debris of apoptotic T cells to release a high level of TGF-β, leading to upregulation of Tregs and, ultimately, immune tolerance for immunotherapies. Collaborative execution of therapeutic effect between Fas and FasL may therefore represent a new functional role of receptor/ligand in cell-based therapies.
EXPERIMENTAL PROCEDURES
Animals and antibodies
Female C57BL/6J (BL6), B6CgFblnTSK+/+PldnPa/J, C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT1TCRTG), B6Smn.C3-Faslgld/J (BL6 gld), C3MRL-Faslpr/J (C3H lpr), and B6.129S4-Ccl2tm1Rol/J mice were purchased from the Jackson Lab. gld and lpr strain have spontaneous mutation in FasL (Faslgld) and Fas (Faslpr), respectively, with no other spontaneous mutation. Female immunocompromised mice (Beige nude/nude XIDIII) were purchased from Harlan. All animal experiments were performed under the institutionally approved protocols for the use of animal research (USC #10941 and 11327). The antibodies used in this study are described in the Supplementary Experimental Procedures.
Isolation of mouse bone marrow mesenchymal stem cells (BMMSCs)
The mouse BMMSCs were isolated from femurs and tibias and maintained. Detailed methods are described in the Supplementary Experimental Procedures.
Isolation of CD11b-positive cells
To isolate CD11b-positive phagocytes, mouse splenocytes were isolated and incubated with PE-conjugated anti-CD11b antibody (BD). After 30 min incubation on ice, CD11b-positive cells were sorted out using anti-PE magnetic beads (Miltenyi Biotech) according to manufacturer’s instructions.
Flow cytometry analysis
Whole peripheral blood was stained with anti-CD45, anti-CD3, anti-CD4, and CD8a antibodies and treated with BD FACS™ Lysing Solution (BD Bioscience) to get mononuclear cells (MNCs). The apoptotic T cells were detected by staining with CD3 antibody, followed by Annexin-V Apoptosis Detection Kit I (BD Pharmingen). For fluorescent labeling of cells, BMMSCs or T cells were incubated with Carboxyfluorescein diacetate N-succinimidyl ester (CFSE, SIGMA) for 15 min or PKH-26 (Invitrogen) for 5min, according to manufacturer’s instructions. For Foxp3 intercellular staining, T cells were stained with anti-CD4, CD8a, and CD25 antibodies (1 μg each) for 30 min on ice. Next, cells were stained with anti-Foxp3 antibody using Foxp3 staining buffer kit (eBioscience). For IL17 staining, T cells were stained with anti-CD4 antibody and then stained with anti-IL17antibody using Foxp3 staining buffer kit. All samples were analyzed with FACScalibur (BD Bioscience).
Western blot analysis
20μg of protein were used and SDS-PAGE and Western blotting were performed according to standard procedures. β-actin on the same membrane served as the loading control. Detailed procedures are described in Supplementary Experimental Procedures.
Co-culture of T cells with BMMSCs
BMMSCs (0.2×106) were seeded on a 24-well culture plate (Corning) and incubated 24 hours. The prestimulated T cells were directly loaded onto BMMSCs and co-cultured for 2 days. In some experiments, anti-Fas ligand neutralizing antibody (BD) or caspase 3, 8 or 9 inhibitors (R&D systems) were added in the co-culture. Apoptotic T cells were detected as described above.
T cell migration assay
For T cell migration assay, a transwell system was used. PKH26-stained BMMSCs (0.2×106) were seeded on the lower chamber of a 12-well culture plate (Corning) with transwell and incubated 24 hours. The prestimulated T cells with anti-CD3 and anti-CD28 antibodies for 48 hours were loaded onto the upper chamber of transwell and co-cultured for 48 hours, followed by observation under a fluorescent microscope. Green-labeled cell number was counted and normalized by red-labeled number of MSCs in five representative images.
Overexpression of Fas ligand
293T cells for lentivirus production were seeded in a 10 cm culture dish (Corning) until 80% confluence. Plasmids with proper proportion, FasL gene expression vector: psPAX:pCMV-VSV-G (all from Addgene) =5:3:2, were mixed in opti-MEM (Invitrogen) with Lipofectamin LTX (Invitrogen) according to the protocol of the manufacturer. EGFP expression plasmid (Addgene) was used as control. The supernatant was collected 24h and 48h after transfection and filtered through a 0.45μm filter to remove cell debris. For infection, the supernatant containing lentivirus was added into target cell culture in the presence of 4μg/ml polybrene (SIGMA), and the transgene expression was validated by GFP under microscopic observation.
Overexpression of Fas and MCP-1
To generate Fas and MCP-1 overexpression vectors, a pCMV6-AC-GFP TrueORF mammalian expression vector system (Origene) was used. Fas and MCP-1 cDNA clones generated from C57BL/6J strain mice were purchased from Open Biosystems (Huntsville) and amplified by PCR with Sgf I and Mlu I restriction cutting sites. The PCR products were directly subcloned into pCR-Blunt II-TOPO vector using Zero Blunt® TOPO® PCR Cloning Kit (Invitrogen). After sequencing, Fas and MCP-1 cDNAs with SgfI/MluI sites were subcloned into pCMV6-AC-GFP expression vector. All constructs were verified by sequencing before transfection into cells. After construction, lprBMMSCs were transfected with cDNAs using LIPOFECTAMINE PLUS reagent (LIFE TECHNOLOGIES), according to manufacturer’s instructions for 48 hours.
Inhibition of Fas and FasL
Expression levels of Fas and FasL on BMMSCs were knocked down using siRNA transfection according to manufacturer’s instructions. Fluorescein-conjugated control siRNA was used as control and as a method of evaluating transfection efficacy. All siRNA products were purchased from Santa Cruz.
Allogenic BMMSC transplantation into acute colitis mice
Acute colitis was induced by administering 3% (w/v) dextran sulfate sodium (DSS, molecular mass 36,000 –50,000 Da; MP Biochemicals) through drinking water, which was fed ad libitum for 10 days (Zhang et al., 2010). Passage one BMMSCs, gldBMMSCs or lprBMMSCs were infused (1×106 cells) into disease model mice (n=6) intravenously at day 3 after feeding DSS water. In control group, mice received PBS (n=6). All mice were harvested at day 10 after feeding DSS water and analyzed. Induced colitis was evaluated as previously described (Alex et al., 2009).
Allogenic BMMSC transplantation into systemic sclerosis (SS) mice
Passage one BMMSCs, gldBMMSCs or lprBMMSCs were infused (1×106 cells) into SS mice intravenously at 8 weeks of age (n=6). In control group, SS mice received PBS (n=5). All mice were sacrificed at 12 weeks of age for further analysis. The protein concentration in urine was measured using Bio-Rad Protein Assay (Bio-Rad).
Allogenic MSC transplantation into systemic sclerosis (SS) patients
MSCs from umbilical cord were sorted out and expanded, following a previous report (Liang et al., 2009). Expanded MSCs were intravenously infused into the SS recipients (1×106/kg body weight). The trial was conducted in compliance with current Good Clinical Practice standards and in accordance with the principles set forth under the Declaration of Helsinki, 1989. This protocol was approved by the IRB of the Drum Tower Hospital of Nanjing, University Medical School, China. Informed consent was obtained from each patient.
Statistical analysis
Student’s t-test was used to analyze statistical difference. The p values less than 0.05 were considered significant.
Supplementary Material
Highlights.
FasL is required for MSC-based immune therapies via induction of T cell apoptosis.
Fas-regulated MCP-1 secretion for the recruitment of T cells.
Apoptotic T cells trigger macrophages producing TGF-β to upregulate Tregs.
Acknowledgments
Basic research parts of this work were supported by grants from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services (R01DE017449, R01 DE019932, and R01 DE019413 to S.S.), a grant from the California Institute for Regenerative Medicine (RN1-00572 for S.S.), and the Intramural Research Program of NIDCR, NIH (for W.J.C. and T.C.). Clinical studies were supported by a grant from the China Major International (Regional) Joint Research Project (81120108021). ClinicalTrials.gov Identifier: NCT00962923.
Abbreviations used in this study
- BMMSCs
bone marrow mesenchymal stem cells
- BMMSCT
bone marrow mesenchymal stem cell transplantation
- FasL
Fas ligand
- SS
systemic sclerosis
- Tregs
CD4+CD25+Foxp3+ regulatory T cells
Footnotes
Supplemental information, including 7 figures, 1 table, and experimental procedures can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- Batten P, Sarathchandra P, Antoniw JW, Tay SS, Lowdell MW, Taylor PM, Yacoub MH. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: Relevance to tissue engineering human heart valves. Tissue Eng. 2006;12:2263–2273. doi: 10.1089/ten.2006.12.2263. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-{kappa}B signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976;82:493–512. [PMC free article] [PubMed] [Google Scholar]
- Hohlbaum AM, Moe S, Marshak-Rothstein A. Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. J Exp Med. 2000;191:1209–1219. doi: 10.1084/jem.191.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinclauss F, Perruche S, Masson E, Carvalho-Bittencourt M, Biichle S, Remy-Martin JP, Ferrand C, Martin M, Bittard H, Chalopin JM, Seilles E, Tiberghien P, Saas P. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13:41–52. doi: 10.1038/sj.cdd.4401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang H, Hua B, Wang H, Wang J, Han Z, Sun L. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler. 2009;15:644–646. doi: 10.1177/1352458509104590. [DOI] [PubMed] [Google Scholar]
- Mazar J, Thomas M, Bezrukov L, Chanturia A, Pekkurnaz G, Yin S, Kuznetsov S, Robey PG, Zimmerberg J. Cytotoxicity Mediated by the Fas Ligand (FasL)-activated Apoptotic Pathway in Stem Cells. J Biol Chem. 2009;284:22022–22028. doi: 10.1074/jbc.M109.032235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Gö bel U, Dä ubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Parekkadan B, Tilles AW, Yarmush ML. Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independent of regulatory T cells. Stem Cells. 2008;26:1913–1919. doi: 10.1634/stemcells.2007-0790. [DOI] [PubMed] [Google Scholar]
- Park MJ, Park HS, Cho ML, Oh HJ, Cho YG, Min SY, Chung BH, Lee JW, Kim HY, Cho SG. Transforming growth factor β-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum. 2011;63:1668–1680. doi: 10.1002/art.30326. [DOI] [PubMed] [Google Scholar]
- Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;5:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Polchert D, Sobinsky J, Douglas GW, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan PW, Birman E, Yuan S, Young YK, Boivin MN, Former K, Basik M, Galipeau J. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chmokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH, Jr, Prockop DJ. Action at a Distance: Systemically Administered Adult Stem/Progenitor Cells (MSCs) Reduce Inflammatory Damage to the Cornea Without Engraftment and Primarily by Secretion of TSG-6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal Stem Cell Transplantation Reverses Multi-Organ Dysfunction in Systemic Lupus Erythematosus Mice and Humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol. 1996;60:365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2010;184:1656–1662. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu G, Zhang L, Roberts AI, Shi Y. Th17 cells undergo Fas-mediated activation-induced cell death independent of IFN-gamma. J Immunol. 2008;181:190–196. doi: 10.4049/jimmunol.181.1.190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.