Abstract
Background
Ghrelin and glucagon-like peptide-1 (GLP-1) are gut hormones known to induce hunger and satiety, respectively. Current knowledge about the effects of different macronutrients on circulating ghrelin and GLP-1 comes mainly from acute test meals, whereas little is known about the effects of chronic dietary intake on gut hormone secretion. This study was designed to examine whether 8-week habituation to diets with different percentages of carbohydrate and fat would affect serum ghrelin, GLP-1, and subjective hunger in a postabsorptive state and in response to a standard liquid mixed meal.
Methods
Sixty-one overweight men and women were provided all food for 8 weeks of either a higher-carbohydrate/lower-fat diet (High-CHO/Low-FAT; 55% CHO, 18% PRO, 27% FAT) or a lower-carbohydrate/higher-fat diet (Low-CHO/High-FAT; 43% CHO, 18% PRO, 39% FAT). After overnight fasts at baseline and week 8, participants consumed a standard liquid meal (7 kcals/kg, 58.6% CHO, 17.4% PRO, 24% FAT). Blood was sampled before the meal and at 15, 60, 90, 120, 180, and 240 minutes to determine total serum ghrelin and active GLP-1. Hunger was assessed by a visual analog scale. Mixed models were used to evaluate whether the temporal patterns of total serum ghrelin and active GLP-1 differed with diet.
Results
Although both diet groups reported greater hunger after 8 weeks (p=0.03), circulating ghrelin and GLP-1 were not affected by acclimation to different macronutrients.
Conclusion
Habituation to different diets does not appear to influence fasting ghrelin, fasting GLP-1, or responses of these gut hormones to a standard meal.
Keywords: Macronutrients, ghrelin, glucagon-like peptide-1 (GLP-1)
1. Introduction
With rising obesity rates worldwide, research over the past decade has generated much interest about how gut hormones influence food intake and satiety, and in turn, how food intake influences gut hormones. If manipulating macronutrients of one's diet can influence food intake via gut hormones among overweight individuals, this may offer a novel strategy for diet interventions to enhance satiety and facilitate weight loss.
Ghrelin is one such gut hormone. Numerous reports have confirmed ghrelin as an “orexigen,” acting on the hypothalamus to stimulate food intake [1-3]. Circulating concentrations of total ghrelin increase prior to meals and decline within 15-20 minutes of initiating food consumption [4, 5]. Cross-sectional data from single test meals indicate that carbohydrate acutely suppresses ghrelin most potently while dietary fat has the least impact on ghrelin suppression [6-10]. However, longitudinal effects of chronic macronutrient intake on ghrelin are less clear.
Glucagon-like peptide-1 (GLP-1) is a satiety hormone that is secreted from L-cells of the distal small intestine in response to food intake [11, 12]. Studies have demonstrated that GLP-1 acts at the brainstem and hypothalamus to decrease food intake and increase subjective satiety [13, 14]. Although little is known about the effects of specific macronutrients on GLP-1 secretion, limited data suggest that dietary fat may induce the greatest increase in acute GLP-1 secretion [15, 16]. Like ghrelin, longitudinal effects of chronic macronutrient intake on GLP-1 are unclear.
Thus, the first primary aim of this study was to determine whether 8-week habituation to diets differing in carbohydrate and fat would affect fasting concentrations of total ghrelin and active GLP-1. Another primary aim was to determine whether diet habituation would affect gut hormone responses to a standard liquid mixed meal. A secondary aim was to determine whether subjective hunger would change with diet habituation and gut hormone concentrations.
2. Methods
2.1 Participants
Sixty-one sedentary, overweight men and women, ages 21-49 yr, completed the study. By self-report, 49% were African American (AA) and 51% were European American (EA). Inclusion required body mass index (BMI) >25 kg/m2 and body weight <136.3kg, with stable weight within 2.3kg over the previous 6 months. Exclusion criteria included diabetes, polycystic ovary syndrome, any disorder or medication known to affect body composition or glucose metabolism (including oral contraceptives, cholesterol medications, or blood pressure medications), regular exercise >2 hours per week, tobacco use, and irregular menstrual cycles. All participants provided written consent, and the study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB).
2.2 Diet Habituation
After a 3-day run-in phase on a standard diet (55% CHO, 18% PRO, 27% FAT), subjects were assigned to either continue this higher-carbohydrate/lower-fat diet (High-CHO/Low-FAT; 55% CHO, 18% PRO, 27% FAT) or begin a lower-carbohydrate/higher-fat diet (Low-CHO/High-FAT; 43% CHO, 18% PRO, 39% FAT) for 8 weeks as previously described [17]. Because other studies have shown protein to be the most satiating macronutrient [1, 7, 18], protein content of both diets was held constant in order to tease apart effects of moderate carbohydrate reduction concomitant with increased dietary fat. All food was provided by the UAB General Clinical Research Center (GCRC) with energy calculated for weight maintenance using the Harris Benedict formula [19] with an activity factor of 1.35 for females and 1.5 for males. Each weekday morning, participants reported to the GCRC to pick up food and obtain body weight measurements.
2.3 Liquid Meal Test
At baseline and week 8, each participant completed a liquid meal test following a 12-hour overnight fast. Blood samples collected at -15 and -5 minutes prior to the test meal were averaged to determine fasting concentrations of ghrelin and GLP-1. Participants consumed a standard liquid meal [Carnation Instant Breakfast (Nestle; Vevey, Switzerland) and whole milk; 7 kcals/kg, 58.6% CHO, 17.4% PRO, 24% FAT] within a 5-minute period, and blood was sampled at 15, 60, 90, 120, 180, and 240 minutes. At each blood sample, participants were asked to rate subjective hunger by marking a visual analog scale (VAS). The VAS consisted of a 100mm line anchored at both ends with the statements “I am not hungry at all” and “I am as hungry as I have ever felt.” Hunger was quantified by measuring the distance in mm from the left anchor to the mark [20].
2.4 Assays
All analyses were conducted in the Core Laboratory of UAB's Nutrition Obesity Research Center, Diabetes Research and Training Center, and GCRC. Total ghrelin was measured in duplicate 20 μl aliquots by enzyme-linked immunoabsorbent assay (ELISA; Millipore Corporation; Billerica, MA). In the Core laboratory, mean intra-assay c.v. was 7.10%, and mean interassay c.v. was 5.98%. GLP-1 was measured in duplicate 100-μL aliquots by ELISA (Millipore Corporation; Billerica, MA). This ELISA is highly specific for the immunologic measurement of the active form of GLP-1 (7–36 amide). In the Core laboratory, mean intra-assay c.v. was 7.32% and mean interassay c.v. was 6.25%.
2.5 Statistical Analysis
Continuous variables with non-normal distributions were log transformed for analyses. Differences between diet groups with respect to demographic characteristics and body habitus were evaluated using t- and chi-square tests for continuous and categorical variables, respectively. The Mann-Whitney U test was used to identify differences in age as this variable was non-normally distributed after log transformation. Because each meal test included multiple observations per participant, mixed models were used to test whether the patterns of the 4-hour postprandial meal responses differed according to pre- vs. post-intervention and the type of diet. Incremental area under the curve (AUC) was calculated by the trapezoidal method [21] for ghrelin, GLP-1, insulin, and hunger. Paired t-tests were used to examine changes within diet groups from week 0 to week 8 for each AUC composite. Spearman correlation coefficients were calculated for the relationship between subjective hunger and gut hormones. All analyses were two-sided with a Type I error rate of 0.05 and were performed using SAS version 9.2 (SAS Institute, Cary, NC), SPSS version 19.0 (Chicago, IL), and GraphPad Prism version 5.0 (La Jolla, CA).
3. Results
Participant characteristics are displayed in Table 1. Despite provision of eucaloric diets, mild weight change was observed over the 8-week study period (-1.04 ± 1.51kg). Weight change did not differ between diet groups nor was weight change correlated with any variable of interest.
Table 1.
Baseline characteristics (mean ± SD)
| All (n=61) | 55% CHO (n=27) | 43% CHO (n=34) | P-value | |
|---|---|---|---|---|
| Sex (% male) | 47.5 | 48.3 | 51.7 | 0.611 |
| Ethnicity (% AA) | 49.2 | 46.7 | 53.3 | 0.799 |
| Age (y) | 35.1 ± 8.3 | 34.3 ± 8.3 | 35.8 ± 8.4 | 0.495 |
| Weight (kg) | 98.7 ± 19.1 | 97.1 ± 20.6 | 99.8 ± 18.0 | 0.585 |
| BMI (kg/m2) | 32.3 ± 4.2 | 31.3 ± 4.4 | 33.0 ± 3.9 | 0.123 |
Table 2 shows average values of ghrelin and GLP-1 across the meal test. AUC within each diet group did not significantly differ from week 0 to week 8 for either hormone.
Table 2.
Ghrelin and GLP-1 concentrations across the meal test
| Ghrelin (pg/mL) | Fasting | 15 min | 60 min | 90 min | 120 min | 180 min | 240 min | AUC* |
|---|---|---|---|---|---|---|---|---|
| High-CHO/Low-FAT Week 0 |
592 ± 305 | 565 ± 324 | 481 ± 282 | 523 ± 253 | 491 ± 241 | 492 ± 273 | 553 ± 272 | 122850 ± 60693 |
| High-CHO/Low-FAT Week 8 |
656 ± 322 | 637 ± 314 | 554 ± 286 | 562 ± 290 | 527 ± 286 | 549 ± 309 | 573 ± 311 | 134227.9 ± 69409.1 |
| Low-CHO/High-FAT Week 0 |
762.6 ± 296 | 732 ± 285 | 651 ± 254 | 693 ± 261 | 676 ± 313 | 694 ± 284 | 735 ± 331 | 166995 ± 64306 |
| Low-CHO/High-FAT Week 8 |
779 ± 337 | 756 ± 298 | 681 ± 276 | 704 ± 298 | 700 ± 300 | 684 ± 297 | 698 ± 335 | 168641 ± 69784 |
| GLP-1 (pM) | Fasting | 15 min | 60 min | 90 min | 120 min | 180 min | 240 min | AUC* |
| High-CHO/Low-FAT Week 0 |
3.4 ± 3.8 | 8.1 ± 10.1 | 5.5 ± 4.7 | 5.6 ± 4.8 | 5.0 ± 4.2 | 4.8 ± 4.2 | 4.5 ± 4.0 | 1290.8 ± 1038.5 |
| High-CHO/Low-FAT Week 8 |
4.2 ± 4.5 | 9.2 ± 9.6 | 6.7 ± 5.0 | 7.0 ± 5.4 | 6.4 ± 5.1 | 7.6 ± 11.1 | 5.9 ± 7.2 | 1687.7 ± 1470.0 |
| Low-CHO/High-FAT Week 0 |
4.0 ± 4.2 | 7.4 ± 5.6 | 5.5 ± 4.3 | 6.5 ± 4.3 | 6.0 ± 4.2 | 4.8 ± 4.1 | 4.6 ± 3.9 | 1341.2 ± 997.4 |
| Low-CHO/High-FAT Week 8 |
4.2 ± 4.8 | 8.3 ± 7.3 | 6.4 ± 5.7 | 6.8 ± 6.2 | 6.1 ± 5.1 | 5.2 ± 4.9 | 5.9 ± 5.8 | 1453.1 ± 1304.5 |
Data are displayed as mean ± SD.
AUC = area under the curve; changes in AUC within diet groups did not differ from week 0 to week 8 at a significance level of p=0.05.
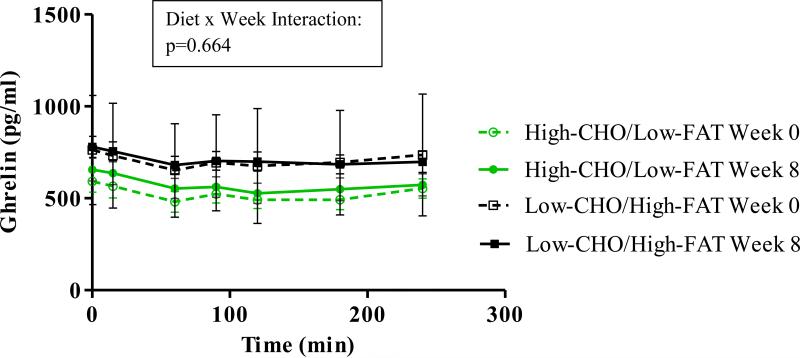
Figure 1 displays time courses for total serum ghrelin. Although ghrelin concentrations changed over the 4 hours of testing (p<0.001 for both meal tests), both diet groups showed similar patterns at both meal tests (p = 0.664 for diet x week). On average, the Higher-CHO/Lower-FAT diet group had lower circulating ghrelin (p<0.001) prior to the intervention, and this group difference persisted despite diet habituation.
Figure 1.
Time courses for total serum ghrelin. Ghrelin changed across the minutes of each meal test (p<0.001 for both). The higher-CHO/lower-FAT group had lower ghrelin at baseline and week 8 (p<0.001 for Diet Group), but p-values were not significant for change over 8 weeks or Diet x Week interaction.
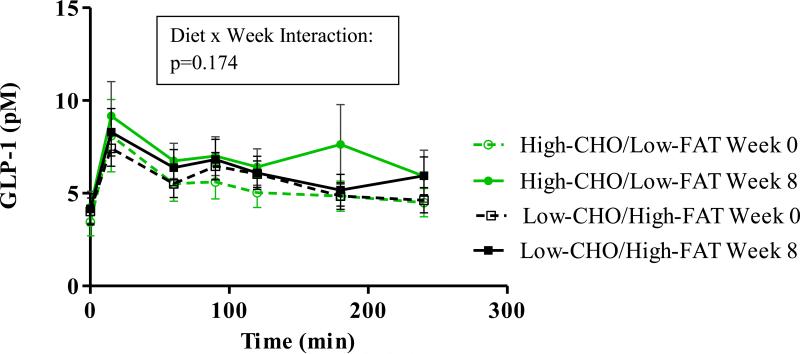
Figure 2 shows time courses for serum GLP-1. Changes from fasting values across 4 hours of testing were greater after 8 weeks compared to pre-intervention (p=0.03). However, both diet groups showed similar patterns at both meal tests (p = 0.174 for diet x week).
Figure 2.
Time courses for serum GLP-1. Changes from fasting values across the 4 hours of testing were greater at the 8-week test (p=0.030) compared to the 0-week test (p=0.155). There were no significant differences for Diet Group, Week, or Diet x Week interaction.
Figure 3 shows time courses for subjective hunger. At both meal tests, hunger changed across the test period (p<0.001), but the patterns of change were similar for both diet groups with lack of significance for the diet x week interaction (p = 0.244) indicating no effect of the diet intervention on hunger response to the test meal. Although there were no significant differences between diet groups, participants reported greater hunger at week 8 compared to week 0 (p=0.03). Fasting GLP-1, postprandial GLP-1, and fasting ghrelin were not related to subjective hunger at either meal test. Postprandial ghrelin was significantly correlated with hunger during minutes 15-120 of the baseline meal test (r-values ranged from 0.355 to 0.447; p-values ranged from <0.001 to 0.005). However, these associations were no longer significant at week 8.
Figure 3.
Time courses for subjective hunger. Hunger changed across the minutes of each meal test (p<0.001 for both). There were no significant differences for Diet Group or Diet x Week interaction, but both diet groups reported greater hunger at week 8 compared to week 0 (p=0.032 for Week).
4. Discussion
Whereas most studies have considered only acute effects of macronutrients on gut hormones, this study was designed to examine whether chronic changes in dietary macronutrient profile would affect fasting ghrelin and GLP-1 as well as gut hormone responses to a standard mixed meal. Our results indicated that 8-week habituation to Higher-CHO/Lower-FAT vs. Lower-CHO/Higher-FAT diets did not appreciably affect postabsorptive or postprandial ghrelin or GLP-1.
The first major finding of this study is that habituation to diets of different macronutrient profiles did not influence fasting ghrelin or ghrelin response to a standard liquid mixed meal. Two earlier studies concluded that the previous day's dietary intake had no effect on fasting ghrelin [22, 23]. The current study expands those findings by demonstrating that 8-week diet acclimation likewise showed no effect on fasting ghrelin. Patterns for postprandial ghrelin response to the test meal were similar for both diet groups at both 0 and 8-weeks. Ghrelin concentrations declined within 15 minutes and continued to decline until 1 hour after ingestion of the meal. Current knowledge of how dietary carbohydrate and fat affect circulating ghrelin comes mainly from cross-sectional studies with test diets of differing macronutrients. Three such studies [7, 15, 24] provide evidence that lipids have the mildest effect on ghrelin suppression, while carbohydrate ingestion results in the most rapid suppression of ghrelin, with carbohydrate also inducing a sharper and more rapid rebound of ghrelin back to basal levels. Taken together, our findings indicate that although macronutrient composition of one's diet may affect circulating ghrelin on an acute basis, these responses are not conditioned over time, and chronic macronutrient intake has little influence over acute responses of ghrelin to individual meals.
Likewise, fasting GLP-1 and GLP-1 response to the test meal were not significantly affected by the different macronutrient profiles. Patterns of GLP-1 response were similar for both groups at both meal tests, with GLP-1 increasing within 15 minutes and declining thereafter. Although previous studies have noted a positive association between caloric content of a meal and change in GLP-1 [25], little is known about how specific macronutrients affect GLP-1 secretion. One previous study among healthy men and women reported that GLP-1 increased dramatically after ingestion of fat alone, but the GLP-1 response was greatly reduced with the addition of carbohydrate [15]. Similarly, in a crossover study investigating different macronutrient preloads in dogs, dietary fat produced the greatest increase in circulating GLP-1 [16]. Our results suggest that while dietary lipid may increase GLP-1 concentrations on an acute basis, acclimation to a higher-fat diet over an 8-week period did not appear to affect GLP-1 response to a standard test meal. Although GLP-1 concentrations showed considerable variance, and GLP-1 response did not significantly differ by diet group, the total cohort demonstrated greater increases in GLP-1 at week 8 compared to baseline. Both diet groups experienced a similar, slight weight loss across the intervention period despite careful efforts to provide appropriate energy for weight maintenance. Though weight change was not correlated with changes in gut hormone concentrations, it is plausible that the mild weight loss influenced GLP-1 response, as previous studies have demonstrated increases in postprandial GLP-1 following weight reduction by gastric bypass surgery [26, 27].
Profiles of subjective hunger were similar for both diet groups, with hunger declining within 15 minutes of food intake and then increasing thereafter. Hunger did not differ between diets following habituation; however, despite greater GLP-1 response at week 8, both diet groups reported greater hunger at week 8 compared to baseline. We can only speculate concerning causative factors for increased hunger following the intervention. Hunger was not associated with GLP-1 at either meal test, and postprandial correlations between ghrelin and hunger that were observed at the first meal test were no longer significant at week 8. These results suggest that factors other than the two gut hormones predominate in perceptions of hunger. Food availability as well as various environmental and emotional stimuli have been shown to impact perceived hunger and fullness [11, 28], so perhaps increased hunger at the post-intervention assessment reflected a psychological effect of having all food provided by an outside source. In any case, macronutrient profiles of the diets did not seem to influence subjective hunger, and other factors associated with increased hunger at week 8 appeared to override any influence of ghrelin or GLP-1.
Strengths of this study included longitudinal design; matching of participants in the two diet groups for age, BMI, gender, and race; and careful control of food intake with all food provided. Macronutrient compositions of the 2 diet arms were selected to be practical and applicable in a “real world” setting [29], but is possible that diets with more dramatic differences in carbohydrate and fat may have produced stronger changes in ghrelin, similar to those observed in studies involving acute administration [7, 15, 24]. Because the study was designed to look at gross macronutrient composition, we are unable to conclude whether specific types of carbohydrate or fatty acids may differentially influence gut hormones or hunger. Also, because we only measured total ghrelin concentrations, we are unable to comment on differences between acylated vs. unacylated forms. A limitation of the study was that the High-CHO/Low-FAT group had lower ghrelin both before and after the intervention; however, this difference was considered by the statistical mixed models analysis. By study design, all participants in this cohort were overweight. Given that fasting levels of ghrelin [8, 30-32] and GLP-1 [33, 34] tend to be lower among overweight individuals, and obesity has been associated with blunted postprandial responses of both hormones [30-32, 35, 36], it is possible that a normal-weight cohort would display different results.
In conclusion, although previous studies indicate that acute carbohydrate intake induces sharper decreases in circulating ghrelin compared to other macronutrients while dietary fat leads to greater increases in GLP-1, we demonstrated that 8-week habituation to diets of different carbohydrate/fat content had little influence on ghrelin, GLP-1, or subjective hunger responses to a standard test meal among overweight adults. This knowledge that acute responses of these gut hormones to specific macronutrients are not conditioned over time will be important for the design of future intervention studies examining other influences on these gut hormones. Indeed, future studies are indicated to confirm whether consistent consumption of specific dietary macronutrients may impact obesity treatments by affecting gut hormones.
Highlights.
We examine effects of 8-week diet habituation on circulating gut hormones.
Diets differing in carbohydrate and fat content did not affect total ghrelin.
Diet habituation did not affect glucagon-like peptide-1 (GLP-1).
Habitual diet does not appear to influence circulating ghrelin or GLP-1.
Acknowledgements
All authors read and approved the final manuscript. The authors are grateful to Maryellen Williams and Cindy Zeng for laboratory analyses and Betty Darnell and Suzanne Choquette for menu development and GCRC coordination.
Sources of support
This work was supported by the National Institute of Health and National Institute of Diabetes and Digestive and Kidney Disease (R01DK67538). Core laboratory support, nursing, and inpatient/outpatient facilities were provided by M01-RR-00032 (GCRC), UL1RR025777 (CTSA), P30-DK56336 (NORC), P60DK079626 (DRTC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
All authors participated in the design of the research project; LLG, ACE, PCL, and KC conducted data collection; GM, ACE, and BAG analyzed data; ACE and BAG wrote the paper and had primary responsibility for final content.
Competing interests
The authors have no conflicts of interest to disclose.
References
- 1.Potier M, Darcel N, Tomé D. Protein, amino acids and the control of food intake. Curr Opin Clin Nutr Metab Care. 2009;12:54–8. doi: 10.1097/MCO.0b013e32831b9e01. [DOI] [PubMed] [Google Scholar]
- 2.Ueno H, Shiiya T, Nakazato M. Translational research of ghrelin. Ann N Y Acad Sci. 2010;1200:120–7. doi: 10.1111/j.1749-6632.2010.05509.x. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Kangawa K. Ghrelin: more than endogenous growth hormone secretagogue. Ann N Y Acad Sci. 2010;1200:140–8. doi: 10.1111/j.1749-6632.2010.05516.x. [DOI] [PubMed] [Google Scholar]
- 4.Cummings D, Purnell J, Frayo R, Schmidova K, Wisse B, Weigle D. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 5.Bowen J, Noakes M, Trenerry C, Clifton PM. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab. 2006;91:1477–83. doi: 10.1210/jc.2005-1856. [DOI] [PubMed] [Google Scholar]
- 6.Overduin J, Frayo R, Grill H, Kaplan J, Cummings D. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146:845–50. doi: 10.1210/en.2004-0609. [DOI] [PubMed] [Google Scholar]
- 7.Foster-Schubert K, Overduin J, Prudom C, Liu J, Callahan H, Gaylinn B, Thorner M, Cummings D. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–9. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin X, Li Y, Xu G, An W, Zhang W. Ghrelin fluctuation, what determines its production? Acta Biochim Biophys Sin (Shanghai) 2009;41:188–97. doi: 10.1093/abbs/gmp001. [DOI] [PubMed] [Google Scholar]
- 9.Blom WA, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HF. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr. 2005;81:367–75. doi: 10.1093/ajcn.81.2.367. [DOI] [PubMed] [Google Scholar]
- 10.Erdmann J, Töpsch R, Lippl F, Gussmann P, Schusdziarra V. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab. 2004;89:3048–54. doi: 10.1210/jc.2003-031610. [DOI] [PubMed] [Google Scholar]
- 11.Moran TH. Gut peptides in the control of food intake. Int J Obes (Lond) 2009;33(Suppl 1):S7–10. doi: 10.1038/ijo.2009.9. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol. 2008;70:239–55. doi: 10.1146/annurev.physiol.70.113006.100506. [DOI] [PubMed] [Google Scholar]
- 13.Jayasena CN, Bloom SR. Role of gut hormones in obesity. Endocrinol Metab Clin North Am. 2008;37:769–87. xi. doi: 10.1016/j.ecl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Gardiner JV, Jayasena CN, Bloom SR. Gut hormones: a weight off your mind. J Neuroendocrinol. 2008;20:834–41. doi: 10.1111/j.1365-2826.2008.01729.x. [DOI] [PubMed] [Google Scholar]
- 15.Radulescu A, Gannon MC, Nuttall FQ. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. J Clin Endocrinol Metab. 2010;95:3385–91. doi: 10.1210/jc.2009-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubbs DC, Vester Boler BM, Ridge TK, Spears JK, Graves TK, Swanson KS. Dietary macronutrients and feeding frequency affect fasting and postprandial concentrations of hormones involved in appetite regulation in adult dogs. J Anim Sci. 2010;88:3945–53. doi: 10.2527/jas.2010-2938. [DOI] [PubMed] [Google Scholar]
- 17.Goree LL, Chandler-Laney P, Ellis AC, Casazza K, Granger WM, Gower BA. Dietary macronutrient composition affects {beta} cell responsiveness but not insulin sensitivity. Am J Clin Nutr. 2011;94:120–7. doi: 10.3945/ajcn.110.002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldhorst M, Smeets A, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K, Lejeune M, Luscombe-Marsh N, Westerterp-Plantenga M. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94:300–7. doi: 10.1016/j.physbeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Harris J, Benedict F. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918;4:370–3. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 21.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Votruba S, Kirchner H, Tschöp M, Salbe A, Krakoff J. Morning ghrelin concentrations are not affected by short-term overfeeding and do not predict ad libitum food intake in humans. Am J Clin Nutr. 2009;89:801–6. doi: 10.3945/ajcn.2008.27011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandarana K, Drew ME, Emmanuel J, Karra E, Gelegen C, Chan P, Cron NJ, Batterham RL. Subject standardization, acclimatization, and sample processing affect gut hormone levels and appetite in humans. Gastroenterology. 2009;136:2115–26. doi: 10.1053/j.gastro.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 24.Tannous dit El Khoury D, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab. 2006;50:260–9. doi: 10.1159/000091684. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhri OB, Wynne K, Bloom SR. Can gut hormones control appetite and prevent obesity? Diabetes Care. 2008;31(Suppl 2):S284–9. doi: 10.2337/dc08-s269. [DOI] [PubMed] [Google Scholar]
- 26.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valverde I, Puente J, Martín-Duce A, Molina L, Lozano O, Sancho V, Malaisse WJ, Villanueva-Peñacarrillo ML. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg. 2005;15:387–97. doi: 10.1381/0960892053576613. [DOI] [PubMed] [Google Scholar]
- 28.Woods SC, D'Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37–50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States Department of Agriculture [January 2012];Diet Quality and Food Consumption: Food and Nutrient Intake Tables 2011. available at http://www.ers.usda.gov/Briefing/DietQuality/Data.
- 30.Vincent RP, Ashrafian H, le Roux CW. Mechanisms of disease: the role of gastrointestinal hormones in appetite and obesity. Nat Clin Pract Gastroenterol Hepatol. 2008;5:268–77. doi: 10.1038/ncpgasthep1118. [DOI] [PubMed] [Google Scholar]
- 31.Pavlatos S, Kokkinos A, Tentolouris N, Doupis J, Kyriaki D, Katsilambros N. Acute effects of high-protein and high-fat isoenergetic meals on total ghrelin plasma concentrations in lean and obese women. Horm Metab Res. 2005;37:773–5. doi: 10.1055/s-2005-921101. [DOI] [PubMed] [Google Scholar]
- 32.Tentolouris N, Kokkinos A, Tsigos C, Kyriaki D, Doupis J, Raptis SA, Katsilambros N. Differential effects of high-fat and high-carbohydrate content isoenergetic meals on plasma active ghrelin concentrations in lean and obese women. Horm Metab Res. 2004;36:559–63. doi: 10.1055/s-2004-825761. [DOI] [PubMed] [Google Scholar]
- 33.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 34.Chanoine JP, Mackelvie KJ, Barr SI, Wong AC, Meneilly GS, Elahi DH. GLP-1 and appetite responses to a meal in lean and overweight adolescents following exercise. Obesity (Silver Spring) 2008;16:202–4. doi: 10.1038/oby.2007.39. [DOI] [PubMed] [Google Scholar]
- 35.Cummings D. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38:916–9. doi: 10.1136/gut.38.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]