Abstract
Objective
This study examined cancer survivors’ experience of and responses to challenges and stressors associated with every-day living. The impact of daily stressors on quality of life concerns and cortisol patterns was also investigated.
Design
Participants were 111 cancer survivors who participated in a national telephone diary study of daily experiences (NSDE). Their responses were compared with those of 111 sociodemographically-matched participants with no cancer history using a multilevel modeling approach.
Main Outcome Measures
Over an 8-day period, participants completed a daily inventory of the occurrence and impact of stressful events, affect, and physical symptoms. Salivary cortisol was sampled 4 times per day, and indices of awakening response (CAR), diurnal slope, and overall output (AUC) were examined.
Results
Cancer survivors experienced similar numbers and types of stressful events as the comparison group. While appraisals were largely comparable, cancer survivors showed a modest tendency to perceive stressors as more severe and disruptive, particularly those involving interpersonal tensions. The occurrence of stressors was associated with increased negative affect, decreased positive affect, and increased physical symptoms, but little change in cortisol. Relative to the comparison group, cancer survivors showed less pronounced changes in positive affect and cortisol output when stressors occurred, but a greater increase in negative affect in response to interpersonal conflicts.
Conclusions
Findings indicate that cancer survivors show a resilient ability to respond to day-to-day stressors and challenges. However, daily stressors can have a significant impact on survivors’ mood and physical symptoms and therefore may be an important intervention target.
Keywords: cancer, stress, affect, cortisol, quality of life
Due to advances in detection and treatment, approximately 2 in 3 adults diagnosed with cancer today can be expected to survive more than 5 years (Ries et al., 2006). This improved survival rate, combined with the aging population, has led to a substantial increase in the number of cancer survivors in the U.S., now numbering more than 10.5 million individuals (Ries et al., 2006). While the medical concerns of cancer survivors are gaining attention (Hewitt, Greenfield, & Stovall, 2005; President’s Cancer Panel, 2005–2006), there is also growing interest in understanding their psychosocial concerns and quality of life.
Most research has focused on overall emotional well-being or psychological symptoms among cancer survivors. Results are generally encouraging, indicating that cancer survivors show comparable levels of psychological functioning to age-matched comparison groups (Bradley, Lutgendorf, Rose, Costanzo, & Anderson, 2006; Dorval, Maunsell, Deschenes, Brisson, & Masse, 1998; Ganz, Rowland, Desmond, Meyerowitz, & Wyatt, 1998; Helgeson & Tomich, 2005; Wenzel et al., 2002). However, several large population-based studies have found that cancer survivors report greater distress and more mental health symptoms relative to those with no cancer history (Arndt, Merx, Stegmaier, Ziegler, & Brenner, 2004, 2005; Baker, Haffer, & Denniston, 2003; Hewitt, Rowland, & Yancik, 2003; Rabin et al., 2007), but comparable functioning in positive domains such as psychological and social well-being (Costanzo, Ryff, & Singer, 2009).
The measures used in prior studies capture how cancer survivors respond and adjust to the major life challenge of a cancer diagnosis, treatment, and sequelae. However, it is not known how survivors respond to the routine challenges of every day living, such as day-to-day stressors and hassles related to work or caring for others, for example. If cancer survivors are thought to be a psychologically vulnerable group, as suggested by the aforementioned population-based studies, perhaps they are also more sensitive than others to the stress associated with daily hassles. For example, it is not uncommon for cancer survivors to report posttraumatic stress symptoms, including heightened responses to stress (Jim & Jacobsen, 2008). An alternate hypothesis stems from the transactional model of coping, which posits that stress occurs when appraisal of the demands of a situation exceeds resources for meeting demands (Lazarus & Folkman, 1984), which has subsequently been applied in the context of cancer (e.g., Heim, Valach, & Schaffner, 1997; Stanton & Snider, 1993; Costanzo, Lutgendorf, Rothrock & Anderson, 2006). Specifically, after successfully coping with the demands associated with a serious illness and its treatment, cancer survivors may appraise every-day stressors as less severe and bothersome. In the present study, we investigated cancer survivors’ well-being at the level of day-to-day experiences to test these hypotheses. Specifically, we examined appraisals of daily stressors as well as the affective, somatic, and physiological sequelae of these experiences.
While prior research has focused on global emotional functioning or distress related to the cancer experience, we argue that responses to daily stressors may also play a salient role in cancer survivors’ quality of life. Daily stress processes have been shown to contribute to both psychological and physical symptoms in other populations (Almeida, Neupert, Banks, & Serido, 2005; Almeida, Wethington, & Kessler, 2002; Neupert, Almeida, & Charles, 2007). Among cancer survivors, depressed mood, fatigue, pain, and other somatic and affective symptoms are commonly-reported quality of life concerns (Arndt et al., 2005; Arndt, Merx, Sturmer, et al., 2004; Baker et al., 2003; Bower et al., 2006; Carlson et al., 2004; Cella, Davis, Breitbart, & Curt, 2001; Hewitt et al., 2003). It may be that daily stressors could exacerbate these already prominent concerns, and if so, responses to day-to-day hassles could be an important intervention target in this population.
Daily stress processes have also been linked to neuroendocrine activity, especially the hormones of the HPA axis, including increased cortisol and disruptions in the daily cortisol rhythm (Jacobs et al., 2007; Smyth et al., 1998; Seltzer et al., 2009; van Eck, Berkhof, Nicolson, & Sulon, 1996). These relationships may have particular relevance for cancer patients and survivors given well-documented associations between cortisol, disrupted circadian rhythms, and cancer development, progression, and survival (Filipski, et al., 2002; Fu & Lee, 2003; Fu, Pelicano, Liu, Huang, & Lee, 2002; Mormont et al., 2000; Schernhammer et al., 2003; Sephton & Spiegel, 2003; Sephton, Sapolsky, Kraemer, & Spiegel, 2000).
The present study examined affective, somatic, and cortisol responses to daily stressors in a sample of cancer survivors who were participants in the National Survey of Midlife Development in the United States (MIDUS), designed to study health and well-being during midlife. A subset of MIDUS respondents completed the National Study of Daily Experiences (NDSE), a unique eight-day diary study that assessed participants’ responses to daily events. The large, population-based sample afforded the opportunity to compare respondents with a history of cancer to those with no cancer history. The overall goal was to understand the day-to-day stressful experiences of cancer survivors and their relationships to important biobehavioral sequelae. Stressors involving interpersonal tensions were of particular interest due to the well-documented relationships between perceptions of social relationships and both quality of life and health outcomes among cancer patients (Carpenter, Fowler, Maxwell, & Andersen, 2010; Costanzo et al., 2005; Frick, Motzke, Fischer, Busch, & Bumeder, 2005; Hann et al., 2002; Karnell, Christensen, Rosenthal, Magnuson, & Funk, 2007; Kroenke, Kubzansky, Schernhammer, Holmes, & Kawachi, 2006; Lutgendorf et al., 2005) as well as the ability of social stressors to reliably evoke affective and physiological responses (Birditt, Fingerman, & Almeida, 2005; Dickerson & Kemeny, 2004). The specific objectives were as follows:
The first objective was to describe both the frequency and perceptions of daily stressors among cancer survivors as compared to individuals with no history of cancer. We were interested in determining whether cancer survivors differed with respect to appraisal of the severity and impact of stressors that occurred during the study period, with a special focus on those events involving interpersonal tensions. The second objective was to explore relationships between the experience of daily stressful experiences and both quality of life concerns (mood disturbance and physical symptoms) and diurnal cortisol patterns. Specifically, we sought to determine whether affective, somatic, and physiological reactivity to stress differed between cancer survivors and individuals with no cancer history. The goal was to understand whether daily stressors had a disproportionate impact for cancer survivors. We also examined whether the length of time since the initial cancer diagnosis affected cancer survivors’ responses to stress.
Method
MIDUS and NSDE
Data were drawn from MIDUS, a national survey of 7,108 adults ages 25 to 74 years completed in 1995–96 (Wave 1) and 2004–06 (Wave 2). MIDUS is comprised of four subsamples: a national random digit dialing (RDD) sample (n = 3,487); oversamples from five metropolitan areas (n = 757); siblings of individuals from the RDD sample (n = 950); and a national RDD sample of twin pairs (n = 1,914). The main RDD sample was selected from working telephone banks. For each household contacted, a random respondent between 25 and 74 years of age was selected. Of those contacted, 70% agreed to participate.
The National Study of Daily Experiences (NSDE) examined daily stressful experiences in a subsample of Wave 2 MIDUS respondents (n = 2,022) who were recruited after participating in Wave 2 of MIDUS. Of those contacted for NSDE, 63% agreed to participate. Participants completed 10–15 minute telephone interviews on eight consecutive evenings at approximately the same time each day (Almeida, McGonagle, & King, 2009). Of NSDE participants, 72% completed all eight interview days, with 96% completing at least six interview days. Additional details regarding the NSDE can be found in Almeida, McGonagle, and King (2009).
The present study focused on participants in the NSDE study, all of whom also participated in the larger MIDUS survey. While most data reported herein are derived specifically from the NSDE, data from Wave 2 of MIDUS were used to determine cancer status, other relevant health history, and demographic information.
Sample
Cancer survivors
Individuals who participated in NSDE and responded affirmatively to the question: “Have you ever had cancer?” in Wave 2 of the larger MIDUS study were selected for the analysis. In the present study, we follow the National Cancer Institute’s and National Coalition for Cancer Survivorship’s definition of a cancer survivor to include individuals diagnosed with cancer from the time of diagnosis through the remainder of life (National Cancer Institute, 2011).
As part of the Wave 2 MIDUS survey, participants were queried about the type of cancer and their age at diagnosis. Those who reported a diagnosis of skin cancer only were excluded from the present analyses. Overall, 111 individuals met eligibility criteria. These cancer survivors were a median of 11 years post-diagnosis (range 1–59 years). Cancer sites included breast (29.7%), prostate (20.7%), colon (14.4%), cervical (9.0%), leukemia or lymphoma (9.0%), uterine (2.7%), ovarian (2.7%), lung (1.8%), other (15.3%), and unknown (0.9%).
Comparison group
A computerized algorithm was used to select a comparison group of NSDE participants matched on age (within 3 years), gender, and education level. Matches were randomly selected for each cancer survivor (n = 111) from the pool of all individuals with no cancer history meeting matching criteria. Participants with other health conditions were not excluded; the comparison group was intended to be a sample of peers with no cancer history.
Participants’ ages ranged from 35 to 83 years with a mean age of 65 years for both groups. Full demographic data were drawn from the larger MIDUS survey and are provided in Table 1. Chi-square analyses indicated that the comparison group did not differ significantly from the cancer survivors on any demographic variables, including ethnicity, region of residence, or employment status (all p values exceeded .10). There was also no difference in income, p = .50.
Table 1.
Demographic Characteristics of Cancer Survivors and the Comparison Group
| Cancer Survivors n = 111 % |
Comparison Group n = 111 % |
|
|---|---|---|
| Sex | ||
| Female | 63.1 | 63.1 |
| Male | 36.9 | 36.9 |
| Ethnicity | ||
| Caucasian | 92.8 | 95.5 |
| African American | 1.8 | 0.9 |
| Native American | 1.8 | 0.9 |
| Asian | 0.0 | 0.9 |
| Other | 3.6 | 1.8 |
| Relationship status | ||
| Married | 68.5 | 76.6 |
| Divorced or separated | 14.4 | 9.9 |
| Widowed | 13.5 | 9.9 |
| Never married | 3.6 | 3.6 |
| Education | ||
| Less than 12 years | 5.4 | 5.4 |
| High school graduate | 27.0 | 27.0 |
| Some college or trade school | 25.2 | 25.2 |
| College graduate/advanced degree | 42.3 | 42.3 |
| Employment status | ||
| Employed | 32.4 | 34.2 |
| Retired | 57.7 | 54.1 |
| Homemaker | 8.1 | 8.1 |
| Disabled | 1.9 | 0.9 |
| Other | 0.0 | 2.7 |
| Income | ||
| Mean (SD) | $52,177 ($46,013) | $56,660 ($52,305) |
NSDE interviewers were not provided with participants’ health history information from the MIDUS survey and thus were blind to the participants’ cancer status.
Measures
Daily Inventory of Stressful Experiences (DISE)
The DISE is a semi-structured telephone interview that documents the occurrence of stressful events, as well as descriptive information related to their duration, timing, and the impact on the participant (Almeida et al., 2002). The DISE was administered daily across the eight-day study period at approximately the same time each day. All questions were administered by telephone.
Participants were asked about the occurrence of stressful events during the past day, including interpersonal tensions (having an argument or disagreement and situations in which the participant let something pass in order to avoided a disagreement), overload events (other stressful events occurring at home or at work/school), and network stressors (events that happened to close friends or relatives). In the present study, analyses examined overall occurrence of stressful events but also focused specifically on interpersonal tensions.
For any events reported, participants were asked to rate how stressful the experience was from “not at all” to “very”. They were also asked to rate their perception of control on a 4-point scale from “none” to “a lot.” Finally, participants were asked to appraise the extent to which the stressor disrupted their daily routine on a 4-point scale from “none at all” to “a lot.”
Positive and negative affect
During the same telephone interview, participants completed a daily inventory assessing negative and positive affect. Participants were asked to rate how often they experienced 14 negative mood states (e.g., nervous, hopeless, irritable) and 13 positive mood states (e.g., cheerful, full of life, confident) on a 5-point scale from “none of the time” to “all of the time” from the time of awakening to the time of the telephone interview. The scale demonstrated excellent reliability in the NSDE sample; Cronbach’s alpha was .91 for negative affect and .96 for positive affect.
Physical symptoms
Each day, participants were asked how often they experienced 26 symptoms, including fatigue, aches/pains, muscle weakness, gastrointestinal symptoms, chest pain, dizziness, menstrual and menopausal symptoms, and cold and flu symptoms. They were asked to rate the severity of any symptoms experienced on a 10-point scale. This information was also reported as part of the daily telephone interview.
Cortisol
Salivary cortisol samples were collected on days 2 through 5 of the eight-day interview period using salivette collection containers (Sarstedt; Numbrecht, Germany). Day 1 served as an “instruction day” regarding the salivary collection method, and Days 6–8 were used to assist the participants with sending the samples back to the laboratory.
On each of the 4 collection days, participants were asked to provide saliva samples at 4 times: upon awakening (before getting out of bed), 30 minutes later, just before lunch, and just before bed. Participants were instructed to wait at least 1 hour after a major meal to provide samples. The timing of the “before lunch” sample was selected to allow for assessment of the cortisol awakening response (CAR) recovery in the larger NSDE study and to avoid potential contamination of food or beverages. While the participants were asked to provide samples based on their personal schedule rather than specific clock times, 90% of the “before lunch” samples were taken between 11 AM and 3 PM (SD = 79 minutes), and 90% of the “before bed” samples were taken between 9 PM and 12:30 AM (SD = 75 minutes).
Cortisol concentrations were determined by luminescence immunbioassay (IBL; Hamburg, Germany). Cortisol slopes were calculated by regressing log transformed cortisol values on the time that elapsed between samples for each individual. The cortisol awakening response (CAR) reflects the change in cortisol between the waking and 30-minutes post-waking samples, and the diurnal slope reflects the linear change across the 30-minutes post-waking, before lunch, and before bed samples. Flatter slopes are thought to be indicative of greater dysregulation and less deactivation of the HPA response toward the end of the day. We also examined area under the curve (AUC; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) as an estimate of total cortisol output.
More detailed descriptions of cortisol collection and analysis procedures can be found in Almeida, McGonagle, and King (2009a) and Almeida, Piazza, and Stawski (2009b).
Health conditions and medication use
On a self-administered questionnaire that was given to all respondents at Wave 2 of the larger MIDUS study, participants were asked to report whether they had experienced any of 30 different sets of chronic health conditions over the past 12 months (e.g., “asthma, bronchitis, or emphysema,” “arthritis, rheumatism, or other bone or joint diseases,” “high blood pressure or hypertension”). Excluding cancer, cancer survivors reported significantly more chronic conditions (M = 3.6, SD = 2.9) than did the comparison group (M = 2.6, SD = 2.3), p <.01.
Participants were also queried about current use of medications. A composite variable was created of medications known to influence cortisol, including allergy medication, steroid inhalers, other steroid medications (e.g., prednisone), medications containing cortisone, oral contraceptives, other hormonal medications, and psychotropic medications. There were no significant differences between groups in the use of classes of medications with the potential to affect cortisol values. The most frequently endorsed classes included allergy (18% of cancer survivors, 15% of the comparison group), psychotropic (17% of cancer survivors, 15% of the comparison group), and hormonal medications (8% of cancer survivors, 12% of the comparison group).
Analyses
All variables were examined for outliers. Cancer survivors were compared to the matched comparison group on the occurrence and appraisal (severity, disruption, and perceived control) of daily stressors using two-tailed t-tests.
To examine group differences in affective, somatic, and physiological responses to stressors, we used multilevel modeling (Snidjers & Bosker, 2002), following models described by Stawski, Sliwinski, Almeida, and Smyth (2008) and Hoffman and Stawski (2009). Because positive and negative affect, physical symptoms, cortisol, and information on daily stressors were collected across multiple days, we used linear multilevel models to model each of these indices as a function of (a) whether or not a stressor was reported on that day, (b) group (cancer survivors versus comparison group), and (c) their interaction. We opted to use a simple dichotomization for daily stressors (“stressor day” if one or more stressors occurred versus “non-stressor day” if no stressors occurred) as this provides a clear test of how affect, physical symptoms, and cortisol vary as a function of the presence or absence of stressors. Furthermore, multiple stressor days were very rare (~8% of all days across all participants), and preliminary analyses indicated that the influence of multiple stressors over and above the dichotomization of stressor days was negligible. Occurrence of daily stressors was treated as a time-varying covariate using grand-mean centering, such that the resulting estimate for this time-varying effect reflected the change in each dependent variable (affect, physical symptoms, cortisol) associated with the experience of a stressor (i.e., level of the dependent variable on a day when no stressors were reported compared to a day during which stressors were reported). The changes in each dependent variable served as indices of stress reactivity (i.e., changes in the dependent variable associated with the reported experience of a stressor). The interaction between group and the daily stressor effect was the critical test of whether cancer survivors differed from the comparison group in their affective, somatic, and physiological reactivity to daily stressors. Given the potential influence of medications on cortisol (Granger, Hibel, Fortunato, & Kapelewski, 2009), the composite index of medication use was included as a covariate in all analyses involving cortisol parameters.
We conducted additional analyses considering whether, among cancer survivors, time since diagnosis moderated occurrence and appraisal of daily stressors, as well as affective, somatic, and physiological responses to daily stressors. These analyses were restricted to cancer survivors, and time since diagnosis was included as an additional predictor in the models examining occurrence and appraisal of daily stressors. For models examining responses to daily stressors, both main effects of time since diagnosis as well as its interaction with the daily stressor effect were tested to determine variation in response as a function of time since diagnosis. Additional follow-up analyses examined the effects of chronic health conditions and current or recent cancer treatment on the study results.
Results
Experience and Appraisal of Daily Stressors
Differences between cancer survivors and the comparison group with respect to occurrence and perceived impact of daily stressors are reported in Table 2. There were no significant differences in the number of stressful life events experienced; both groups reported an average of four events over the eight-day interview period. There were also no significant differences between groups in the number of different types of stressors occurring, including interpersonal tensions, overload stressors, or network stressors, all p values > .05. Interpersonal tensions (arguments/disagreements or avoided arguments/disagreements) were the most common type of stressful occurrence in both groups.
Table 2.
Daily Stressor Profile of Cancer Survivors Relative to the Comparison Group
| Cancer Survivors n = 111 | Comparison Group n = 111 | |
|---|---|---|
| M and SD | M and SD | |
| Number of stressors | ||
| Total | 3.81 (2.96) | 4.02 (3.34) |
| Arguments | 0.67 (1.18) | 0.76 (1.07) |
| Avoided arguments | 0.96 (1.00) | 1.12 (1.20) |
| Home overload | 0.83 (1.09) | 0.67 (1.08) |
| Work overload | 0.32 (0.80) | 0.42 (1.07) |
| Network | 0.43 (0.72) | 0.43 (0.86) |
| Appraisal: Any Stressor | ||
| Severity | 1.74 (0.76) | 1.76 (0.67) |
| Perceived control | 1.36 (0.95) | 1.39 (0.95) |
| Disruption to daily routine* | 0.99 (0.74) | 0.77 (0.67) |
| Appraisal: Arguments | ||
| Severity* | 2.16 (0.71) | 1.84 (0.80) |
| Perceived control | 1.78 (1.10) | 1.59 (1.11) |
| Disruption to daily routine | 0.97 (0.95) | 0.74 (0.87) |
| Appraisal: Avoided Arguments | ||
| Severity | 1.31 (0.86) | 1.49 (0.77) |
| Perceived control | 1.69 (1.16) | 1.70 (1.14) |
| Disruption to daily routine* | 0.76 (0.82) | 0.48 (0.70) |
Note. Appraisal domains were rated on a scale of 0 to 3.
Cancer survivors differ from the comparison group at p < .05.
Cancer survivors showed a modest tendency to appraise stressors as more severe or disruptive. Specifically, cancer survivors rated arguments and disagreements as more stressful than did the comparison group, p = .045; however, there were no differences in severity ratings for avoided arguments or overall stressors. Cancer survivors also perceived stressors to be more disruptive to their daily routine, p = .033, and this difference also held specifically for avoided arguments, p = .049. However, there were no differences between groups in perceptions of control over stressors. Moreover, the number of years since cancer diagnosis was not related to the experience or appraisal of daily stressors.
Affective, Somatic, and Physiological Responses to Daily Stressors
Average scores on mood, physical symptoms, and cortisol parameters across the eight-day study period are summarized in Table 3. Cancer survivors reported slightly lower positive affect than did the comparison group, p = .049. Survivors experienced an average 16 symptoms during the eight-day study period, a similar number and severity compared to their peers with no cancer history. There were also no group differences in cortisol patterns. Within the sample of cancer survivors, a longer time since initial diagnosis was associated with greater positive affect (p = .029) and lower cortisol output, as measured by AUC (p = .044).
Table 3.
Mood, Physical Symptoms, and Cortisol Patterns of Across the Eight-Day Study Period
| Cancer Survivors n = 111 | Comparison Group n = 111 | |
|---|---|---|
| M and SD | M and SD | |
| Mood | ||
| Negative affect | 2.70 (3.25) | 2.29 (2.93) |
| Positive affect* | 34.47 (10.12) | 36.85 (7.63) |
| Physical symptoms | ||
| Occurrence (number of symptoms) | 15.62 (12.42) | 17.53 (15.90) |
| Severity | 3.72 (1.58) | 3.41 (1.40) |
| Cortisol (nmol/l) | ||
| Awakening Response (CAR) | 0.44 (0.81) | 0.21 (0.90) |
| Daily decline | −0.14 (0.06) | −0.13 (0.06) |
| Area under curve (AUC) | 79.96 (19.41) | 84.59 (19.15) |
Cancer survivors differ from the comparison group at p < .05.
As anticipated, both the cancer survivors and the comparison group experienced increased negative affect, decreased positive affect, and an increased number of physical symptoms on stressor days. Coefficients from these models representing the relationship between occurrence of a stressor and responses for each group (i.e., stress reactivity slopes) are provided in Table 4.
Table 4.
Multilevel Model Estimates of Reactivity Slopes
| (A) Mood and Physical Symptoms | |||||
|---|---|---|---|---|---|
| Negative Affect | Positive Affect | Number of Symptoms | Symptom Severity | ||
| All Stressors | Cancer survivors | .15 (.02)** | −.09 (.03)** | .30 (.12)** | .08 (.11) |
| Comparison group | .15 (.02)** | −.18 (.03)** | .31 (.11)** | .20 (.11) | |
| Difference | .00 (.02) | .10 (.05)* | −.01 (.16) | −.13 (.15) | |
| Arguments | Cancer survivors | .28 (.03)** | −.23 (.05)** | .59 (.10)** | .07 (.19) |
| Comparison group | .20 (.03)** | −.22 (.05)** | .58 (.21)** | .24 (.17) | |
| Difference | .08 (.04)* | −.01 (.08) | −.01 (.28) | −.16 (.26) | |
| Avoided Arguments | Cancer survivors | .06 (.02)** | .02 (.05) | .26 (.16) | .15 (.16) |
| Comparison group | .13 (.02)** | −.09 (.04)* | .02 (.16) | −.01 (.14) | |
| Difference | −.07 (.03)* | .11 (.06) | −.24 (.23) | .17 (.21) | |
| (B) Cortisol | |||||
| Awakening Response | Diurnal Slope | Area Under the Curve | |||
| All Stressors | Cancer survivors | −.02 (.14) | .01 (.01) | −7.37 (15.97) | |
| Comparison group | .00 (.12) | .00 (.01) | 42.49 (15.04)** | ||
| Difference | −.02 (.18) | .01 (.01) | −49.87 (21.94)* | ||
| Arguments | Cancer survivors | .17 (.24) | .01 (.01) | −31.29 (25.59) | |
| Comparison group | −.11 (.20) | .01 (.01) | 65.42 (23.76)** | ||
| Difference | .27 (.32) | .00 (.01) | −96.73 (34.92)** | ||
| Avoided Arguments | Cancer survivors | .15 (.19) | .01 (.01) | −10.71 (25.02) | |
| Comparison group | .20 (.18) | .00 (.01) | 13.68 (22.98) | ||
| Difference | −.05 (.26) | .01 (.01) | −24.39 (21.23) | ||
Note. Standard errors are provided in parentheses. Estimates reflect the change in outcome from a non-stressor day to a stressor day. Positive slopes indicate levels were higher on stressor days compared to non-stressor days and negative slopes indicate levels were lower on stressor days compared to non-stressor days. Difference reflects the difference in reactivity slopes between groups.
p < .05.
p < .01.
Among the cancer survivors, we investigated whether length of time since diagnosis moderated any of these effects. Time since diagnosis was not associated with the magnitude of the daily stressor effect on positive or negative affect, physical symptoms, or cortisol parameters (all ps>.15).
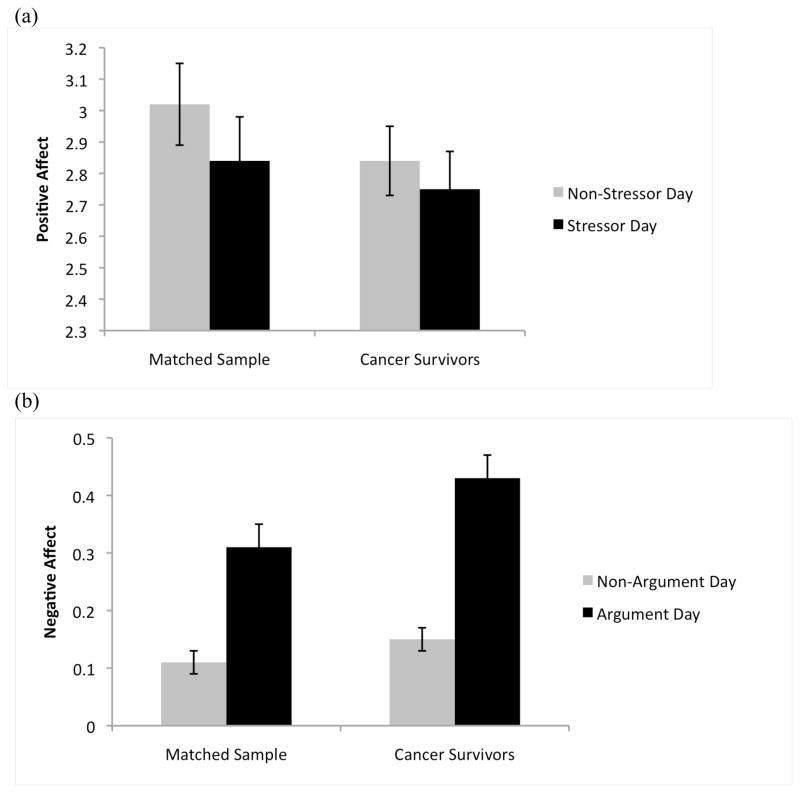
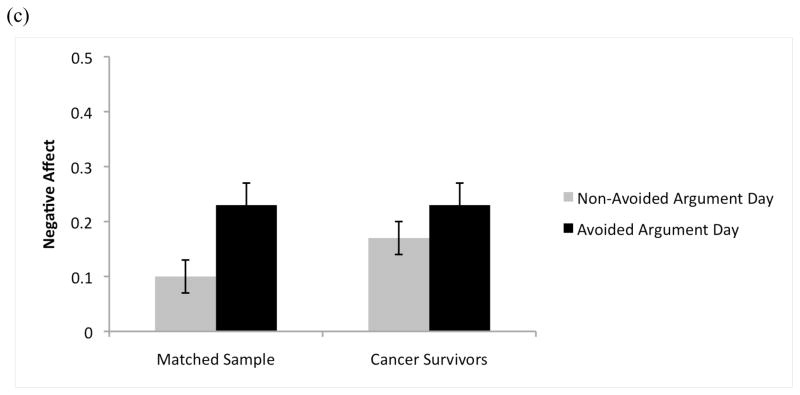
Finally, we examined whether cancer survivors differed from the comparison group in their affective, somatic, and cortisol reactivity to daily stressors (see Table 4). With respect to affective responses, cancer survivors showed a less pronounced decline in positive affect on stressor days relative to the comparison group, p = .022 (see Figure 1a). An examination of events involving interpersonal tensions suggested that this difference was stronger for avoided arguments, although it was not significant (p = .091), with both groups showing comparable declines in positive affect in response to arguments or disagreements. Both groups showed a similar increase in negative affect on stressor days. When considering stressors involving interpersonal tensions, cancer survivors were more reactive to arguments or disagreements but less reactive to avoided disagreements. Specifically, on days in which an argument or disagreement occurred, cancer survivors showed a larger increase in negative affect than did the comparison group, p = .046 (Figure 1b). However, on days in which arguments or disagreements were deliberately avoided, cancer survivors reported a smaller increase in negative affect than did those with no cancer history, p = .030 (Figure 1c).
Figure 1.
Group differences in affective responses to daily stressors are illustrated. Compared to those with no cancer history, cancer survivors showed (a) a smaller decline in positive affect on stressor days, p = .022; (b) a larger increase in negative affect on days in which an argument or disagreement occurred, p = .05; and (c) a smaller decline in negative affect on days in which an argument was avoided, p = .030.
Although both cancer survivors and the comparison group reported increased physical symptoms on stressor days, there were no differences between groups on symptom occurrence or severity in response to daily stress.
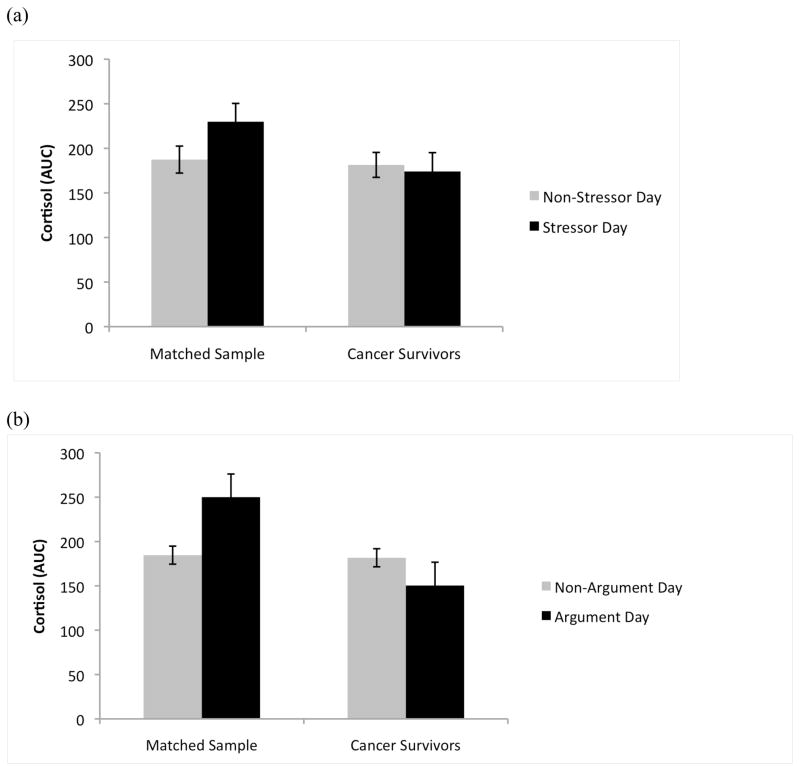
With respect to cortisol responses, neither group exhibited a significant change in either awakening response (CAR) or diurnal slope on stressor days compared to non-stressor days. Those with no cancer history showed the expected increase in cortisol output (AUC) on stressor days, but cancer survivors did not show a significant change, p = .003 (Figure 2a). This was true when all stressors were considered as well as on days in which an argument or disagreement occurred, p = .006 (Figure 2b).
Figure 2.
Group differences in cortisol output (AUC) are illustrated. While participants with no cancer history showed the expected increase in cortisol output in response to stress, cancer survivors showed no significant change in cortisol output on stressor days, both (a) when all stressors were considered, p = .003, and (b) when only arguments were considered, p = .006.
Effects of Health-Related Variables
Because cancer survivors reported significantly more chronic conditions (M = 3.6, SD = 2.9) than did the comparison group (M = 2.6, SD = 2.3), p <.01, all analyses comparing groups were re-run covarying for number of chronic conditions. Adjusting for chronic conditions did not affect the significance of any of the study results.
Three cancer survivors reported undergoing chemotherapy or radiation therapy at the time of, or during the months prior, to participation in the study. All analyses were re-run excluding these participants and the matched comparison respondents. Eliminating these participants did not affect the significance of any of the study results.
Discussion
Findings from the present study suggest that cancer survivors experienced similar numbers and types of daily stressful events as those with no cancer history. Further, the results largely paint a picture of resilience among cancer survivors at the level of responses to daily experiences. There was not a robust pattern of group differences suggesting that cancer survivors are more vulnerable to the effects of daily stressors. Specifically, results indicate that survivors generally make similar appraisals of day-to-day-hassles as their same-age peers and show comparable affective, somatic, and physiological responses to stress. The findings are consistent with literature focusing on global emotional functioning indicating that cancer survivors demonstrate comparable levels of psychological functioning to their peers (Bradley et al., 2006; Dorval et al., 1998; Ganz et al., 1998; Helgeson & Tomich, 2005; Wenzel et al., 2002). Results also did not support the hypothesis that the experience of contending with the significant stress associated with cancer diagnosis and treatment would inoculate cancer survivors against minor, every-day stressors by altering their appraisals. However, this phenomenon cannot be discounted. Members of the comparison group may have also experienced significant life stressors, particularly given the relatively older ages of participants, which could in turn affect their appraisals of everyday stress. For the most part, both groups perceived stressful events occurring during the study period to be controllable and mild in severity.
Alongside the overall portrait of resilience, there were group differences that bear further discussion. First, there was a modest tendency for cancer survivors to appraise daily stressors involving interpersonal tensions or disagreements as more severe and disruptive. It may be that cancer survivors are more sensitive to relationship stresses. Many cancer survivors report closer interpersonal relationships and an increased sense of importance of their relationships as a result of their experience with cancer (Bishop & Wingard, 2004; Cordova & Andrykowski, 2003; Manne et al., 2004; Sears, Stanton, & Danoff-Burg, 2003; Stanton, Bower, & Low, 2006). The enhanced importance of relationships may make even routine tensions more stressful. Alternately, it may be that cancer survivors experience objectively more severe and disruptive interpersonal stressors. The difficulties associated with cancer can certainly cause strain in relationships and stress for family caregivers, increasing day-to-day relationship stress.
Consistent with their appraisals, cancer survivors also experienced a more substantial increase in negative affect on the days in which they had an argument or disagreement with someone. This is also consistent with prior work from the NSDE sample suggesting that individuals with chronic health conditions experience greater emotional reactivity to daily stressors, particularly among older participants (Piazza, Charles, & Almeida, 2007). In contrast, deliberately avoiding an argument or disagreement was associated with a very modest increase in negative affect for the cancer survivors compared to a more substantial increase in negative affect for the comparison group participants. The pattern of results suggests that cancer survivors may be most sensitive to overt disagreements but are less reactive to more covert tensions.
Another notable finding was that cancer survivors displayed a less pronounced decline in positive affect than did the comparison group in response to stressors when all types of stressors were included. This may have in part been due to the finding that cancer survivors reported lower positive affect overall than the comparison group, leaving less room for a decline. An examination of events involving interpersonal tensions clarified that this pattern emerged for avoided arguments, but not overt arguments or disagreements. Consistent with the findings for negative affect, the pattern of findings suggests a less marked affective response to avoided arguments or disagreements. These findings stand in contrast to the aforementioned research showing greater affective responses to stressors among individuals with chronic health conditions (Piazza et al., 2007). The reason for this difference is not clear. Perhaps survivors who find they prioritize their relationships with friends and family to a greater extent after a cancer diagnosis similarly value maintaining harmonious relationships or “keeping the peace” and are more easily able to let go of interpersonal frustrations.
An additional important finding was that cancer survivors in this study showed no significant change in cortisol output on days in which stressors occurred. In contrast, the comparison group showed the expected increase in cortisol, consistent with prior research showing links between daily stress and cortisol output (Jacobs et al., 2007; Smyth et al., 1998; Seltzer et al., 2009; van Eck et al., 1996). The findings suggest that cancer survivors may have a somewhat blunted physiological response to daily stressors. Prior research has documented cortisol hyporesponsiveness to acute stressors among metastatic breast cancer patients (Giese-Davis et al., 2006; van der Pompe, Antoni, & Heijnen, 1996). Of greater relevance to the present sample, our results align with those from a prior study indicating that breast cancer survivors showed blunted cortisol responses to the stress associated with undergoing mammography as compared to a sample of women with no cancer history (Porter et al., 2003).
While dysregulation in circadian rhythms and cortisol patterns are thought to play a role in tumor development and progression and have been linked with mortality (Filipski, et al., 2002; Fu & Lee, 2003; Fu, Pelicano, Liu, Huang, & Lee, 2002; Mormont, et al., 2000; Schernhammer, et al., 2003; Sephton & Spiegel, 2003; Sephton, Sapolsky, Kraemer, & Spiegel, 2000), the clinical significance of the cortisol hyporeactivity to day-to-day stressors observed in this study of cancer survivors is not clear. The cancer survivors did not show alterations in diurnal patterns relative to the comparison group, including cortisol decline over the day, in contrast to prior findings with metastatic breast cancer patients (e.g., Abercrombie et al., 2004). Moreover, there were no significant changes in cortisol slopes for either group on days in which a stressor occurred.
It should be emphasized that the group differences were modest in magnitude and the general pattern of findings suggest that cancer survivors respond similarly to their peers when encountering daily stressors. Moreover, we did not find that those who were closer in time to their cancer diagnosis were any more vulnerable to the effects of daily stressors than those who were many years beyond the initial diagnosis.
While cancer survivors appear to be managing daily stressors well, findings indicate that day-to-day stressors can nonetheless have a significant impact on important dimensions of cancer survivors’ quality of life, including mood and physical symptoms. On days in which a stressor occurred, particularly an argument or disagreement, cancer survivors experienced increased negative affect, decreased positive affect, and an increased number of physical symptoms. These findings are consistent with the broader body of daily stress literature indicating that daily stressors reliably evoke increased negative affect and decreased positive affect (Stawski et al., 2008; Stawski, Almeida, Lachman, Tun, & Rosnick, 2010) and more physical symptoms (Charles & Almeida, 2006; Hoffman & Stawski, 2009), particularly when interpersonal stressors are considered (Birditt et al., 2005; Dickerson & Kemeny, 2004). Our results replicate and extend these findings to cancer survivors, suggesting that cancer survivors are unfortunately not immune to the effects of daily stressors even after facing the significant challenges associated with a cancer diagnosis and treatment.
With respect to the cancer survivorship literature, most prior work has focused on the impact of the emotional and physical sequelae of cancer on quality of life, and our findings highlight the significant role of every-day stressors in affecting cancer survivors’ well-being and suggest that daily stress processes can exacerbate quality of life concerns. Moreover, the effects of daily stressors on well-being did not differ between cancer survivors who had been more recently diagnosed and those who were long-term survivors, suggesting that daily stressors have a relatively consistent effect on quality of life throughout the cancer survivorship continuum.
Despite the benefits of this unique dataset and value of being able to obtain data from a matched comparison sample, there are a number of limitations that should be acknowledged. Because MIDUS is a national survey of aging, rather than a specific study of cancer survivors, there was a relative paucity of disease- and treatment-related information, such as initial disease stage, treatment history, end date of treatment, current disease status, or ongoing sequelae available in this dataset. These factors likely play an important role in the quality of life dimensions examined, and may also affect survivors’ responses to daily stressors. While there was a range in time since diagnosis, a large proportion of participants were many years past their cancer diagnosis: a median of 11 years post-diagnosis. Although we did not find that time since diagnosis moderated any of the relationships examined, results may not generalize to a population of more recently diagnosed cancer survivors or those undergoing active treatment.
This is the first study to our knowledge to examine cancer survivors’ well-being at the level of responses to daily experiences. We have previously reported that, following a cancer diagnosis, participants in the larger MIDUS study experienced elevated anxiety and depressive symptomatology relative to their peers. However, cancer survivors also showed resilience in a number of domains, including social well-being, personal growth, and spirituality (Costanzo, et al., 2009). Findings from the present study add another dimension, indicating that cancer survivors also show a resilient ability to weather day-to-day stressors and challenges both emotionally and physiologically. While the data suggest that cancer survivors are somewhat more sensitive to arguments or disagreements, they appear to face other interpersonal challenges with greater equanimity.
Results also highlight the importance for researchers and clinicians to focus not only on the distress associated with the experience of cancer, but also to attend to other more modest stressors in understanding the well-being of cancer survivors. Particularly after cancer survivors move beyond active treatment, every-day stressors and challenges may increase in salience, particularly those involving interpersonal tensions. Therapeutic interventions with stress management components that target strategies for coping with every-day life demands may have an added benefit of optimizing cancer survivors’ quality of life.
Acknowledgments
This research was supported by grants P01 AG020166 and R01 AG019239 from the National Institute on Aging to conduct a longitudinal follow-up of the MIDUS (Midlife in the United States) investigation. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. The research was also supported by grant KL2 RR0205012 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources.
References
- Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009a;55(2):219–237. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Neupert SD, Banks SR, Serido J. Do daily stress processes account for socioeconomic health disparities? The Journals of Gerontology Series B Psychoogical Sciences and Social Sciences. 2005;60(Spec No 2):34–39. doi: 10.1093/geronb/60.special_issue_2.s34. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Inter-individual differences and inra-individual variability in the cortisol awakening response: An examination of age and gender. Psychology and Aging. 2009b;24:819–827. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events: an interview-based approach for measuring daily stressors. Assessment. 2002;9(1):41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: A population-based study. Journal of Clinical Oncology. 2004;22:4829–4836. doi: 10.1200/JCO.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Persistence of restriction in quality of life from the first to the third year after diagnosis in women with breast cancer. Journal of Clinical Oncology. 2005;23:4945–4953. doi: 10.1200/JCO.2005.03.475. [DOI] [PubMed] [Google Scholar]
- Arndt V, Merx H, Sturmer T, Stegmaier C, Ziegler H, Brenner H. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. European Journal of Cancer. 2004;40:673–680. doi: 10.1016/j.ejca.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97:674–681. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- Birditt KS, Fingerman KL, Almeida DM. Age differences in exposure and reactions to interpersonal tensions: A daily diary study. Psychology and Aging. 2005;20:330–340. doi: 10.1037/0882-7974.20.2.330. [DOI] [PubMed] [Google Scholar]
- Bishop MM, Wingard JR. Thriving after hematopoietic stem cell transplant: a focus on positive changes in quality of life. Expert Review of Pharmacoeconomics and Outcomes Research. 2004;4(1):111–123. doi: 10.1586/14737167.4.1.111. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- Bradley SL, Lutgendorf SK, Rose S, Costanzo ES, Anderson B. Quality of life and mental health in cervical and endometrial cancer survivors. Gynecologic Oncology. 2006;100:479–486. doi: 10.1016/j.ygyno.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Angen M, Cullum J, Goodey E, Koopmans J, Lamont L, et al. High levels of untreated distress and fatigue in cancer patients. British Journal of Cancer. 2004;90(12):2297–2304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Fowler JM, Maxwell GL, Andersen BL. Direct and buffering effects of social support among gynecologic cancer survivors. Annals of Behavioral Medicine. 2010;39(1):79–90. doi: 10.1007/s12160-010-9160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Davis K, Breitbart W, Curt G. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United Stated sample of cancer survivors. Journal of Clinical Oncology. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- Charles ST, Piazza JR, Luong G, Almeida DM. Now you see it, now you don’t: Age differences in affective reactivity to social tensions. Psychology and Aging. 2009;24:645–653. doi: 10.1037/a0016673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova MJ, Andrykowski MA. Responses to cancer diagnosis and treatment: posttraumatic stress and posttraumatic growth. Seminars in Clinical Neuropsychiatry. 2003;8(4):286–296. [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Rothrock NE, Anderson B. Coping and quality of life among women extensively treated for gynecologic cancer. Psycho-Oncology. 2006;15:132–142. doi: 10.1002/pon.930. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Ryff CD, Singer BH. Psychosocial adjustment among cancer survivors: findings from a national survey of health and well-being. Health Psychology. 2009;28(2):147–156. doi: 10.1037/a0013221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dorval M, Maunsell E, Deschenes L, Brisson J, Masse B. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. Journal of Clinical Oncology. 1998;16:487–494. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- Filipski E, King VM, Li X, Granda TG, Mormont MC, Liu X, et al. Host circadian clock as a control point in tumor progression. Journal of the National Cancer Institute. 2002;94(9):690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- Frick E, Motzke C, Fischer N, Busch R, Bumeder I. Is perceived social support a predictor of survival for patients undergoing autologous peripheral blood stem cell transplantation? Psychooncology. 2005;14(9):759–770. doi: 10.1002/pon.908. [DOI] [PubMed] [Google Scholar]
- Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nature Reviews Cancer. 2003;3(5):350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111(1):41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. Journal of Clinical Oncology. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- Giese-Davis J, Wilhelm FH, Congrad A, Abercrombie HC, Sephton S, Yutsis M, Neri E, Taylor B, Kraemer HC, Spiegel D. Depression and stress reactivity in metastatic breast cancer. Psychosomatic Medicine. 2006;68:675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Hann D, Baker F, Denniston M, Gesme D, Reding D, Flynn T, et al. The influence of social support on depressive symptoms in cancer patients: age and gender differences. Journal of Psychosomatic Research. 2002;52(5):279–283. doi: 10.1016/s0022-3999(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Heim E, Valach L, Schaffner L. Coping and psychosocial adaptation: Longitudinal effects over time and stages in breast cancer. Psychosomatic Medicine. 1997;59:408–418. doi: 10.1097/00006842-199707000-00011. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Tomich PL. Surviving cancer: a comparison of 5-year disease-free breast cancer survivors with healthy women. Psycho-Oncology. 2005;14:307–317. doi: 10.1002/pon.848. [DOI] [PubMed] [Google Scholar]
- Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C: Institute of Medicine and National Research Council; 2005. [Google Scholar]
- Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: Age, health, and disability. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Stawski RS. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6:97–120. [Google Scholar]
- Jacobs N, Myin-Germeys I, Derom C, Delespaul P, van Os J, Nicolson NA. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biological Psychology. 2007;74(1):60–66. doi: 10.1016/j.biopsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Jim HSL, Jacobsen PB. Posttraumatic stress and posttraumatic growth in cancer survivorship: A review. The Cancer Journal. 2008;14:414–419. doi: 10.1097/PPO.0b013e31818d8963. [DOI] [PubMed] [Google Scholar]
- Karnell LH, Christensen AJ, Rosenthal EL, Magnuson JS, Funk GF. Influence of social support on health-related quality of life outcomes in head and neck cancer. Head & Neck. 2007;29(2):143–146. doi: 10.1002/hed.20501. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. Journal of Clinical Oncology. 2006;24(7):1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer Publishing Company, Inc; 1984. [Google Scholar]
- Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. Journal of Clinical Oncology. 2005;23(28):7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Manne S, Ostroff J, Winkel G, Goldstein L, Fox K, Grana G. Posttraumatic growth after breast cancer: Patient, partner, and couple perspectives. Psychosomatic Medicine. 2004;66:442–454. doi: 10.1097/01.psy.0000127689.38525.7d. [DOI] [PubMed] [Google Scholar]
- Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000;6(8):3038–3045. [PubMed] [Google Scholar]
- National Cancer Institute. Estimated US cancer prevalence counts: Definitions. 2011 Retrieved from http://cancercontrol.cancer.gov/ocs/definitions.html.
- Neupert SD, Almeida DM, Charles ST. Age differences in reactivity to daily stressors: the role of personal control. J Gerontol B Psychol Sci Soc Sci. 2007;62(4):216–225. doi: 10.1093/geronb/62.4.p216. [DOI] [PubMed] [Google Scholar]
- Piazza JR, Charles ST, Almeida DM. Living with chronic conditions: Age differences in affective well-being. Journals of Gerontology: Psychological Science. 2007;62B:313–321. doi: 10.1093/geronb/62.6.p313. [DOI] [PubMed] [Google Scholar]
- Porter LS, Mishel M, Neelson V, Belyea M, Pisano E, Soo MS. Cortisol levels and responses to mammography screening in breast cancer survivors: A pilot study. Psychosomatic Medicine. 2003;65:842–848. doi: 10.1097/01.psy.0000088595.91705.c5. [DOI] [PubMed] [Google Scholar]
- President’s Cancer Panel. Assessing Progress, Advancing Change. Washington, D.C: U.S. Department of Health and Human Services; 2005–2006. [Google Scholar]
- Rabin C, Rogers ML, Pinto BM, Nash JM, Frierson GM, Trask PC. Effect of personal cancer history and family cancer history on levels of psychological distress. Social Science and Medicine. 2007;64:411–416. doi: 10.1016/j.socscimed.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al., editors. SEER Cancer Statistics Review, 1975–2003. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. Journal of the National Cancer Institute. 2003;95(11):825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- Sears SR, Stanton AL, Danoff-Burg S. The yellow brick road and the emerald city: Benefit finding, postive reappraisal coping, and posttraumatic growth in women with early-stage breast cancer. Health Psychology. 2003;22:487–497. doi: 10.1037/0278-6133.22.5.487. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Almeida DM, Greenberg JS, Savla J, Stawski RS, Hong J, Lounds JJ. Daily stress and dysregulatiuon of salivary cortisol in parents of children with disabilities: A report from the MIDUS study. Journal of Health and Social Behavior. 2009;50:1–15. doi: 10.1177/002214650905000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain, Behavior, & Immunity. 2003;17(5):321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23(4):353–370. doi: 10.1016/s0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Snidjers TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London: Sage Publications; 1999. [Google Scholar]
- Stanton AL, Bower JE, Low CA. Posttraumatic growth after cancer. In: Calhoun LG, Tedeschi RG, editors. Handbook of Posttraumatic Growth: Research and Practice. Mahwah, NJ: Erlbaum; 2006. pp. 138–175. [Google Scholar]
- Stanton AL, Snider PR. Coping with a breast cancer diagnosis: A prospective study. Health Psychology. 1993;12:16–23. doi: 10.1037//0278-6133.12.1.16. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Almeida DM, Lachman ME, Tun P, Rosnick CB. Fluid cognitive ability is associated with greater exposure and smaller emotional reactions to daily stressors. Psychology and Aging. 2010;25:330–342. doi: 10.1037/a0018246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski RS, Sliwinski MJ, Almeida DM, Smyth JM. Reported exposure and emotional reactivity to daily stressors: The roles of adult age and global perceived stress. Psychology and Aging. 2008;23:52–61. doi: 10.1037/0882-7974.23.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pompe G, Antoni MH, Heijnen CJ. Elevated basal cortisol levels and attenuated ACTH and cortisol responses to a behavioral challenge in women with metastatic breast cancer. Psychoneuroendocrinology. 1996;21:361–374. doi: 10.1016/0306-4530(96)00009-1. [DOI] [PubMed] [Google Scholar]
- van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58(5):447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Wenzel LB, Donnelly JP, Fowler JM, Habbal R, Taylor TH, Aziz N, et al. Resilience, reflection, and residual stress in ovarian cancer survivorship: a gynecologic oncology group study. Psycho-Oncology. 2002;11:142–153. doi: 10.1002/pon.567. [DOI] [PubMed] [Google Scholar]