Abstract
Innate or acquired resistance to cancer therapeutics remains an important area of biomedical investigation that has clear ramifications for improving cancer specific death rates. Importantly, clues to key resistance mechanisms may lie in the well-orchestrated and highly conserved cellular and systemic responses to injury and stress. Many anti-neoplastic therapies typically rely on DNA damage, which engages potent DNA damage response signaling pathways that culminate in apoptosis or growth arrest at checkpoints to allow for damage repair. However, an alternative cellular response, senescence, can also be initiated when challenged with these internal/external pressures and in ideal situations acts as a self-protecting mechanism. Senescence-induction therapies are an attractive concept in that they represent a normal, highly conserved and commonly-invoked tumor-suppressing response to overwhelming genotoxic stress or oncogene activation. Yet, such approaches should ensure that senescence by-pass or senescence re-emergence does not occur, as emergent cells appear to have highly drug resistant phenotypes. Further, cell non-autonomous senescence responses may contribute to therapy-resistance in certain circumstances. Here we provide an overview of mechanisms by which cellular senescence plausibly contributes to therapy resistance and concepts by which senescence responses can be influenced to improve cancer treatment outcomes.
Keywords: DNA damage response, oncogene induced senescence, microenvironment
1. Introduction
Cancer is one of the most prevalent diseases diagnosed in developed countries and one of the leading causes of mortality in the United States. According to the National Cancer Institute approximately 1.6 million people were diagnosed with some form of cancer in 2010 and 570,000 lives were lost to neoplastic diseases (Howlader, 2011). Historically cancer was treated via surgical removal with some of the earliest indications of surgical intervention dating back to ancient Egypt with depictions of breast cancer surgeries (Mansfield, 1976). While surgical removal or radiotherapy of a solid tumor can be effective in treating localized primary disease, it has limited effectiveness in situations where tumor cells have spread outside of their tissue of origin.
The post-world war one era saw the dawning of systematic, scientifically-based approaches for treating cancer patients. Experiments by Goodman and Gilman using nitrogen mustard for non-Hodgkin’s lymphoma are representative of these early efforts that met with success. The evolution of systemic therapeutics and the science underlying their applications has resulted in complete cures for a subset of advanced tumor types and improved survival for most others. However, the ability of tumor cells to acquire resistance to cytotoxic and cytostatic agents can drastically reduce the efficacy of current interventions. It is interesting to note that the advent of chemotherapy was paralleled by the recognition of acquired therapy resistance (Chabner and Roberts, 2005). For patients with metastatic disease, drug resistance contributes to the majority of treatment failures (Longley and Johnston, 2005; Mahon et al., 2011). Despite initial responses, many tumors will relapse and progress regardless of repeated exposures to anti-neoplastic therapeutics. This naturally begs the question as to why? In this review we discuss the role of cellular senescence as a contributing factor in therapy resistance. We provide an overview of cellular damage responses to cancer treatments with a focus on signaling pathways leading to senescence, a state generally associated with tumor suppression. We describe evidence supporting contrary roles whereby senescence can enhance responses or promote resistance to cancer-directed therapeutics, and discuss opportunities to exploit senescence in the context of clinical care.
2. Chemotherapeutics, Cellular Damage and Damage Responses
Pharmacological agents designed to induce tumor cell death and/or suppress growth fall into several classes based on mechanism of action. Whereas recent advances in anti-cancer therapeutics have focused on developing inhibitors that exploit specific oncogenic mutations that hyper-activate growth regulatory pathways, the most widely used agents are poorly selective for neoplastic cells and rely on marginal differences between benign cells and tumor cells that involve proliferation rates, DNA replication, genome instability, redox states, angiogenesis, and other features to provide a favorable therapeutic index. For instance, alkylating agents can cause DNA interstrand cross-links, which lead to DNA double strand breaks and cellular arrest (Knox et al., 1988). Topoisomerase inhibitors work by stabilizing DNA which prevents proper cellular replication and in turn initiates a damage response (Pommier, 1993). In addition to the stabilization of DNA, some topoisomerase inhibitors also induce interstrand cross-linking and free radical production (Minotti et al., 2004). Platinum-based drugs form adducts with DNA and to some extent double strand breaks (Graham et al., 2004), while the glycopeptide antibiotic bleomycin induces direct double strand breaks (Povirk et al., 1989). Spindle poisons bind to β-tubulin stabilizing microtubules and depending on the class they either block or promote depolymerization of microtubules leading to cellular growth arrest and apoptosis (Kennedy et al., 2004). While the mode of action may vary among therapies, most have substantial effects on benign cells, and their incorporation into treatment regimens are based on schedules that minimizing collateral damage to the patient while maximizing the impact upon the cancer.
There are numerous hypothesized and proven mechanisms that contribute to therapy resistance that span cellular, tumor, and host dynamics. These include principles of cancer stem cells, enrichment and selection for natively resistance cells, induction of efflux pumps and alterations in the tumor microenvironment. Importantly, clues to key resistance mechanisms may lie in the well-orchestrated and highly conserved cellular and systemic responses to injury and stress. Many anti-neoplastic therapies typically rely on DNA damage, which engages potent DNA damage response (DDR) signaling pathways that culminate in apoptosis or growth arrest at checkpoints to allow for damage repair. However, given the nature of treatment regimens, doses high enough to kill rapidly dividing cells but low enough to minimize the damage to normal tissues and organs, may not achieve complete tumor ablation. It is the DDR which is activated following treatment in cells failing to succumb to apoptosis which can induce a terminal growth arrest state termed cellular senescence. Once senesced, these cells may have an intrinsic resistance to future treatment attempts, but also, by definition, they are no longer proliferating.
3. The DNA Damage Response
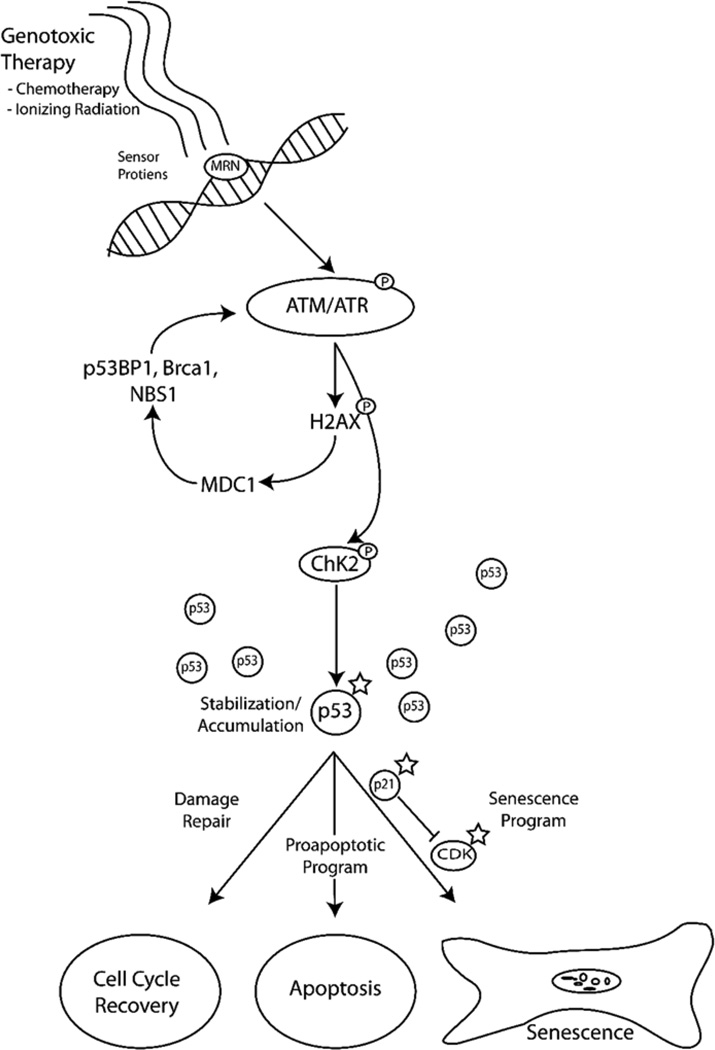
The DNA Damage Response (DDR) is a complex developmentally conserved process which is initiated following injury to the integrity of DNA. This response is set in motion to protect cells from irreversible damage following exposure to exogenous/endogenous genotoxins, and to eliminate those cells with damage too extreme to repair fully. There are several factors that can induce the damage response ranging from UV exposure to commonly used cancer treatments such as γ-radiation and chemotherapeutics (reviewed by (Jackson and Bartek, 2009)). Common environmental exposures and cellular metabolism routinely result in DNA damage that results in 1 million individual lesions per cell per day (Lodish, 2004). Thus, repair processes are in constant activity and the rate of damage approximates the rate of repair. Following exposure to DNA damaging agents, the rate of damage far exceeds the capacity of the repair process to efficiently reinstate a functional DNA structure, resulting in permanent growth arrest or the execution of cell death programs. The initiation of the DDR is mediated by sensor proteins (Zhou and Elledge, 2000), such as the Mre11-Rad50-Nbs1 (MRN) mediator complex (Lee and Paull, 2005; Paull and Lee, 2005). These proteins identify the sites of damage and target them for either repair or elimination (FIGURE 1).
Figure 1. DNA Damage Response (DDR) Pathway.
Following exposure to DNA damaging agents, the DDR signaling cascade is activated by sensor proteins such as MRN. Once damage is identified, ATM/ATR are recruited to the site of damage and undergo autophosphorylation resulting in activation. This subsequently results in the activation of H2AX by phosphorylation, triggering the accumulation of MDC1 to the site of damage. MDC1 accumulation promotes the recruitment of checkpoint proteins and the enhancement of the DDR. Concurrent with the DDR enhancement, ATM activates CHK2 which results in both the stabilization and accumulation of p53. Depending on the extent of damage several avenues can be followed including cell-cycle-recovery, apoptosis or the initiation of senescence.
**A star indicates proposed targets to pro-senescence therapies
The second phase of the DDR is the signaling cascade that results after damage is identified. Key signaling components in mammalian cells are the protein kinases ATR and ATM, which are recruited in response to single and double strand breaks, respectively (Durocher and Jackson, 2001). For the purpose of this review we will provide a simplified overview on the DDR to double strand breaks with respect to their role in the response to chemotherapeutics (for a comprehensive review see (Ciccia and Elledge, 2010)). Upon recruitment to the site of DNA damage, ATM autophosphorylates at multiple sites resulting in its activation (Kozlov et al., 2006). The activation of ATM has several effects, one of which leads to the phosphorylation of serine 139 on H2AX, which plays a role in the accumulation of MDC1 at the site of damage (Ciccia and Elledge, 2010; Harper and Elledge, 2007). This in turn promotes the retention of several checkpoint proteins such as p53BP1, BRCA1 and NBS1 at the break site providing a matrix for enhancing the DDR and promoting damage repair (Huen and Chen, 2008; Mailand et al., 2007; Stucki and Jackson, 2006).
Concurrent with the DDR enhancement, ATM activation leads to an activation of CHK2 through direct phosphorylation which results in homodimerization at the FHA domains (Ahn et al., 2004). The newly activated CHK2 and previously active ATM directly regulate the downstream target p53 by blocking its ubiquitination by MDM2, leading to p53 stabilization and accumulation (Ahn et al., 2004). This stabilization/accumulation can have several results depending on the desired fate of the cell. The first of which, if feasible is that it can lead to the activation of the repair mechanisms which if achieved can allow the cell to attain normal functions (Ciccia and Elledge, 2010; Riley et al., 2008). However, if the cell is beyond repair, other avenues following p53 activation such as the upregulation of proapoptotic proteins or the activation of p21 which then interferes with cyclin-dependent kinase activity resulting in permanent cell cycle arrest and a state of senescence, will be engaged (Gewirtz et al., 2008; Riley et al., 2008). Interestingly, in the absence of DNA damage several of these pathways can be initiated to promote senescence, a process referred to as a pseudo-DDR (Pospelova et al., 2009). As discussed below, cellular senescence is increasingly recognized as an important component of tumor suppressing mechanisms that also may influence responses and outcomes to cancer therapeutics.
4. Cellular Senescence: Mechanisms and Outcomes
The word senescence is derived from the Latin word senex, meaning old age or advanced in age. In addition to describing an organismal state that is associated with advanced chronological age, the term senescence has also been applied to a phenotype observed in individual cells, also associated with chronological age—often measured by numbers of cell divisions. Cellular senescence is also influenced by other factors that modify DNA and the functions of other organelles to phenocopy the growth-arrested phenotype that accompanies replicative exhaustion. The concept of cellular senescence was first introduced by Hayflick and Moorehead (1961) who reported that normal human diploid cells had a limited capacity for proliferation—usually approximately 50 cell divisions in vitro, at which time cells lose the ability to further divide. These senescent cells are morphologically distinct from normal cells; they tend to be enlarged, flattened and display increased cytoplasmic granularity. In addition to the visible physical changes there is an increased activity of senescence associated β-galactosidase present within the cells (Collado and Serrano, 2010). These senescent cells remain viable, metabolically active and are distinguishably different from a quiescent cell which is in a temporary state of growth arrest (Shelton et al., 1999).
There are several different mechanisms capable of inducing cellular senescence. The earliest described initiator was replicative exhaustion (Hayflick and Moorhead, 1961). The concept of replicative exhaustion describes the general rule that cells have a finite number of cellular divisions. This limited proliferative capacity contributes not only to the aging process but the ability of tissues to maintain proper function. The mechanism behind replicative exhaustion involves the progressive erosion of the telomeres after many replication cycles along with or without elevated expression of cyclin dependent kinase inhibitors (Choudhury et al., 2007). As a direct result of telomere erosion cells elicit a response similar to the DDR which activates the signaling cascade driving the cell to a permanently growth arrested senescent state (Collado et al., 2007). This signaling cascade includes the activation of ATM and ATR kinases which leads to the phosphorylation of histone H2AX (d'Adda di Fagagna et al., 2003). There is also an activation of Chk1 and Chk2, modifications of p53, induction of p21 and p16 and the consequential arrest of cell cycle progression (d'Adda di Fagagna et al., 2003; Gewirtz et al., 2008).
The consequence of excessive oxidative stress exposure upon a cell appears to elicit a similar response to that was observed with replicative exhaustion (Chen et al., 2007). Early studies showed that it was critical to maintain the cellular redox balance and to detoxify reactive oxygen species (ROS) in order to prevent a senescent phenotype (Ho et al., 2000). This was followed by the finding that glutathione-dependent redox homeostasis plays a role in maintaining telomere function and integrity (Kurz et al., 2004). Later, chronic exposure to low doses of H2O2 was found to lead to an accumulation of DNA damage and a decreased capacity for DNA repair resulting in telomere shortening and induction of the senescence process (Duan et al., 2005). The mechanisms mediating the response to oxidative stress were investigated further and it was found that ATM signaling plays a central role in the induction of senescence (Zhan et al., 2010).
Another critical role of cellular senescence is its action as a tumor suppressor mechanism, which is often termed oncogene induced senescence (OIS). In response to the activation of an oncogene or a loss of a tumor suppressor activity, the senescence signaling cascade is initiated to protect the integrity of the cell and restrain neoplastic growth (Campisi, 2001). Early cell based assays found that enhanced expression of the oncogene RAS resulted in stable cellular arrest and that this state was achieved via accumulation of p53 and p16 (Serrano et al., 1997). This was confirmed in lymphocytes when the induction of RAS invoked a cellular senescence response through the RB pathway (Braig et al., 2005). Upregulated expression or activating mutations of BRAF in melanocytes also results in a shift to a senescence phenotype (Michaloglou et al., 2005). This appears to be mediated by an activation of p16, a known cyclin dependent kinase inhibitor (Gewirtz et al., 2008; Michaloglou et al., 2005). However, BRAF mutations can also result in senescence independent of p16 or p53 status when C-MYC expression is suppressed (Zhuang et al., 2008). The ATM pathway has also been implicated in initiating a senescence response following perturbation of the oncogenes mos, cdc6 and Cyclin E (Bartkova et al., 2006). Additionally, in certain situations overexpression of oncogenes can lead to hyper proliferation and as a result, activation of the cellular senescence response (Di Micco et al., 2006). Furthermore, inactivation of tumor suppressors, such as PTEN and NF1 appear to also initiate a senescence response (Chen et al., 2005; Courtois-Cox et al., 2006). One mechanism for the PTEN induced senescence may be through a codependent relationship with p53; upon loss of PTEN there is an activation of p19ARF which induces p53 function and activation of p21 (Chen et al., 2005). Moreover, recent evidence has suggested that this transition to a senescent state may be in part mediated through autophagic responses to acute oncogenic stress (Young et al., 2009).
While there are many different oncogenes that can induce senescence, most appear to utilize a similar signaling cascade via activation of ATM/p53/RB pathway (reviewed in (Caino et al., 2009; Courtois-Cox et al., 2008)). This cascade is hypothesized to have evolved as a protective mechanism to inhibit oncogenesis by arresting premalignant lesions or blocking mutagenic processes. This ‘fail-safe’ mechanism is of particular importance in long-lived organisms that harbor renewable tissues requiring multiple rounds of cell division and resultant errors that accompany DNA replication.
Though many premalignant cells are halted from further progression by the engagement of senescence, the frequency of overtly malignant tumors indicates that many cells either do not have fully active senescence programs or develop bypass mechanisms to regain proliferation capabilities. With all cancer therapeutics, the primary intent is to either remove the malignancy, promote an apoptotic response, or permanently arrest the continued growth and spread of cancerous cells. While tumor cells do not have the same replicative limits as normal tissues, many still retain the capacity to senesce, particularly following exposure to DNA damaging agents. In solid tumors the damage response tends to mimic the previously described senescence responses. In brief, following exposure to cytotoxic agents some cells will undergo an apoptotic response while others may not receive enough damage to initiate this pathway. The damage received is handled in a similar manner as reactions to critical shortened telomeres (Chang et al., 1999; Suzuki and Boothman, 2008) even in the absence of telomere shortening (Elmore et al., 2002). In cell-based assays, the treatment with cytoxic chemotherapy agents/ionizing radiation promotes senescence through the functions of p21 and p53 (Chang et al., 2002; Mirzayans et al., 2005). In glioblastoma cells the radiation-induced senescence response appears to also rely signaling through p53 (Quick and Gewirtz, 2006). Additionally, even treatments such as microtubule poisons, which do not directly target DNA, have been shown to induce the senescence phenotype (Gewirtz et al., 2008). This is thought to be through the generation of ROS which can cause genotoxic damage (Gewirtz et al., 2008).
A DDR culminating in senescence has clearly been observed in many different cancer types in-vitro and evidence suggests that this response is relevant in-vivo as well. In fact, one of the earliest reports of treatment induced senescence in patients came from a neoadjuvant chemotherapy study in breast carcinoma in which approximately 42% of resected tumors stained positive for senescence markers (te Poele et al., 2002). This has since been verified in evaluations of lung cancer patients receiving neoadjuvant therapy (Roberson et al., 2005); in a study of non-small cell lung cancer, patients were randomized to either a neoadjuvant chemotherapy regimen or no treatment prior to surgery. Patients receiving the chemotherapy had a marked increase in the expression of senescence markers suggesting chemotherapy can indeed induce cellular arrest via this mechanism. Additionally, evaluations of prostate tumors before and after chemotherapy treatment with mitoxantrone, revealed an increase in senescent markers such as p16 and p21 (Coppe et al., 2008). In a recent study, patients with malignant pleural mesothelioma treated with neoadjuvant therapy were evaluated for evidence of tumor cell senescence (Sidi et al., 2011). Patients whose tumors showed evidence of senescence, measured by elevated p21 and PAI-1, appeared to have little response to treatment. This observation appears consistent with previous cell based experiments using malignant pleural mesothelioma that found that chemotherapy resistance is p21 dependent (Lazzarini et al., 2008). In the subset of patients that did not exhibit a change in senescence markers, a substantially higher apoptosis rate was observed in response to therapy. Furthermore, Sidi et al also had access to survival and progression data from which they concluded that an induction of tumor cell senescence following neoadjuvant therapy was associated with a poor clinical outcome. This latter study suggests that chemotherapy resistance may be in part explained by this intrinsic tumor response involving senescence.
Cellular senescence can be induced through a multitude of internal/external pressures and in ideal situations acts as a self-protecting mechanism. However, if senescence is bypassed, cells can become immortalized and potentially undergo a malignant transformation (Wright et al., 1989). Senescence bypass appears to be a relatively infrequent process (Elmore et al., 2005) but could have serious implications in regards to the health of an organism. Early studies looked at the role of p21 and found that following inactivation, cells were able to bypass senescence after being challenged with DNA damage (Brown et al., 1997). Cell based experiments found that after knockout of one copy of RB, cells experienced a loss of heterozygosity and were eventually able to bypass senescence. Additionally, it was identified that alterations in both p21 and p53 promote the bypass of senescence (Wei et al., 2003). This was confirmed in a set of experiments which initially showed that the loss of the tumor suppressor PTEN induced senescence. However, if p53 was inactivated as well, senescence was bypassed and lethal prostate cancer developed in mice (Chen et al., 2005). The p53 pathway was further implicated when Elmore et al. (2005) found that following treatment with chemotherapy and radiation, a senescence-resistant breast cancer clone emerged. In these cells, levels of cdc-2 were elevated, levels of MDR1 were undetectable, and p53 remained intact. This suggested that senescence resistance may be mediated through the ability to block the down-regulation of cdc-2. There is also evidence that mutations in RAS family members, leading to their altered expression, can aid in the evasion of p53-mediated senescence signaling cascades (Sarkisian et al., 2007). In melanocytes a bypass of senescence can be reached through the loss of p16 expression, which is a result of genomic deletions in the CDKN2A locus (Chin et al., 1997). Further studies of senescence in melanocytes and melanoma found that the common BRAF valine-to-glutamic acid mutation is involved melanoma development, and along with p53 disruption, may encourage tumorgenesis (Yu et al., 2009). In addition, a BRAF mutant mouse was generated which can both develop melanoma and undergo senescence even without the loss of p16 expression (Dhomen et al., 2009). These studies suggest that BRAF mutations may play an alternate mechanism contributing to the evasion of senescence.
There have been numerous reports across several cancer types that senescence is associated with poor a therapeutic index to cancer therapeutics. When tumors are driven away from the desired response of cellular death to an unresponsive state, the ability to target the differential characteristics of the tumor is lost. This is further complicated by the fact that senescent cells still appear to be metabolically active retaining the potential to secrete paracrine acting factors (Kahlem et al., 2004). Furthermore, reports of arrested tumor cells regaining their capacity to proliferate (Beausejour et al., 2003; Dirac and Bernards, 2003) suggest that chemotherapy resistance may in part be driven by the ability of subsets of tumor cells to emerge from a senescence state. This concept and how it contributes to chemotherapy resistance is discussed more fully below.
5. The Pro: Promoting Tumor Senescence to Overcome Drug Resistance
It is important to recognize that divergent views exist regarding the dynamics of senescence in the clinical setting, and the ramifications of senescence as a desirable or adverse contributor to therapeutic responses. There have been an increasing number of reports indicating that initiating a senescence program in solid tumors could be a potential therapeutic treatment to overcome drug resistance. This is based on the idea that tumor cells remain prone to senescence and that they are readily induced into this phenotype following treatment with chemotherapeutics and ionizing radiation (Roninson, 2003; Shay and Roninson, 2004). One of the seminal papers suggesting the potential of senescence based treatments proposed two therapeutic strategies (Roninson, 2003). The first approach involves targeting and interfering with the activity of CDK inhibitors, key mediators of the senescence phenotype. With the evidence that the CDK inhibitor, p21 may have an adverse role in cancer progression and associate with relapse (Fizazi et al., 2002; Lacombe et al., 2001), targeting its activity while administering traditional treatments may improve patient outcomes.
The second strategy involves the development of chemical agents that induce senescence independent of p21or p21-inducible genes. Given that the reasoning for this approach is to induce senescence without the associated systemic side-effects of genotoxic drugs, theoretically a senescence inducer could be used in conjunction with standard cytotoxic therapeutics (Roberson et al., 2005; te Poele et al., 2002). Subsequent reports have suggested that p53 activation/stabilization could be used as a potential mechanism to push tumors into a senescent state (Brown et al., 2009; Kortlever et al., 2006). In cell based assays the artificial activation of p53 triggered tumor-specific apoptosis and senescence (Ventura et al., 2007). These and other results have led to the development of drugs which attempt to restore p53 activity (Efeyan et al., 2007; Kumamoto et al., 2008). Another potential avenue for treatment involves targeting cell cycle machinery (Nardella et al., 2011). The aim of these therapeutics would be to stabilize the CDK inhibitor p27 (Lin et al., 2010) or to directly target CDK2 itself and induce senescence (Senderowicz, 2003a, b). Targeting oncogenes directly as a senescence therapy has also been pursued as an option and such inhibitors are already in clinical testing. For example Quarfloxin, an inhibitor of c-MYC, has been tested in a phase II trial (NCT00780663) (Gomez-Curet et al., 2006; Huang et al., 2006; Wang et al., 2007). Additionally, temporary and selective inactivation of tumor suppressor PTEN has been proposed as a way to induce hyper-activation of cellular processes resulting in senescence (Alimonti et al., 2010). In this context, a recent study found that distinct programs regulated by p53 promoted acute DNA damage responses separately from tumor suppressor functions, suggesting that selective management of p53 inhibition could mitigate oncogenic roles while preserving signaling aspects that would enhance treatment responses (Brady et al., 2011).
Importantly, senescence-induction therapy hinges on the principle that senescent cells are not detrimental, or do not accumulate overtime but are eventually cleared by the host’s immune system or other mechanisms. Additionally, this idea is firmly centered on the concept that senescence is an irreversible process. While shifting damaged or diseased cells into a senescent state may ultimately prove to be a protective mechanism, at this point in time the evidence is not entirely clear whether this would be clinically useful. In addition if cells are truly capable of emerging from senescence post therapy and regaining their capacity to proliferate then this therapeutic course may be detrimental to an individual’s long term survivability.
6. The Con: Senescence and the Tumor Microenvironment Promote Resistance
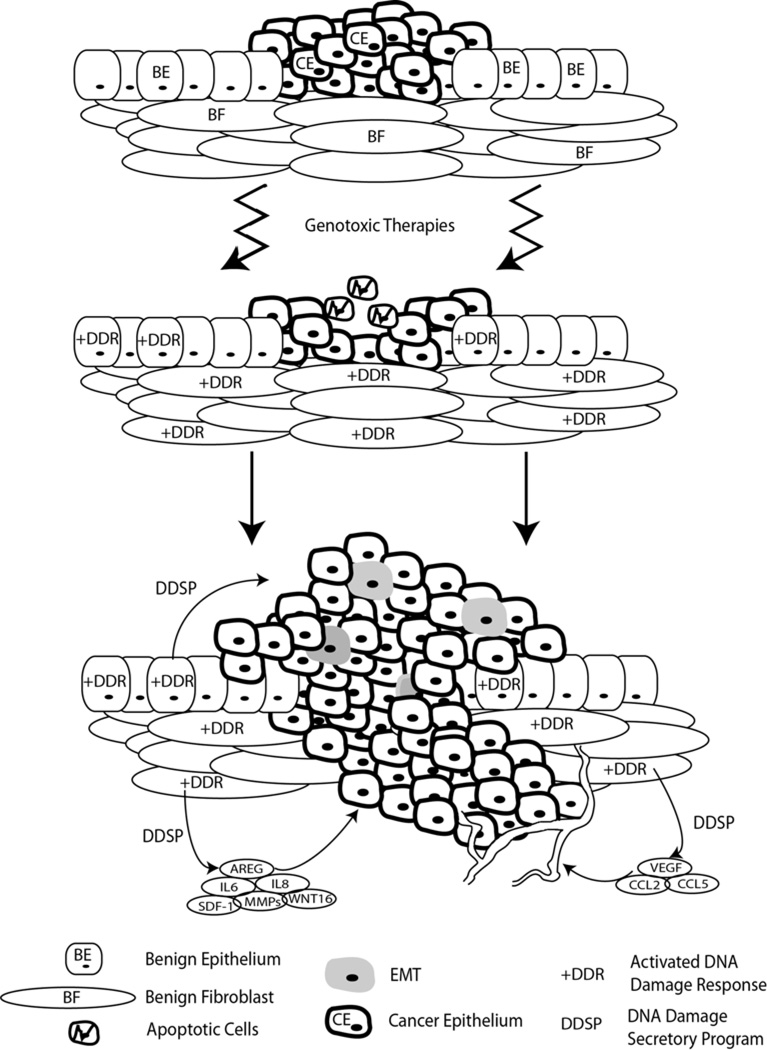
The importance of the microenvironment and its role in therapy response is increasingly recognized. A patient treated with cytotoxic agents receives systemic damage impacting normal constituents of tissue and organ systems as well as the neoplastic cells. The resulting damage may elicit a response from the tumor microenvironment (TME) which potentially can have a dramatic impact upon treatment efficacy. Several studies have documented that the DDR induces a remarkable spectrum of secreted growth factors, proteases, and cytokines, many of which have known roles in promoting detrimental tumor cell phenotypes (Bavik et al., 2006; Krtolica et al., 2001; Rodier et al., 2009). Collectively, this attribute of senescent cells involving secreted factors has been termed a Senescence Associated Secretory Phenotype (SASP) (Freund et al., 2011), Senescence-Messaging Secretome (SMS) (Kuilman and Peeper, 2009), or DNA Damage associated Secretory Program (DDSP). Comprehensive studies of transcriptional responses to genotoxic stress induced by cancer therapeutics have identified a spectrum of highly-induced secreted proteins originating in the tumor microenvironment that comprise several hundred factors (Bavik et al., 2006; Coppe et al., 2008; Kuilman et al., 2008). The composition of the DDSP is complex, may vary by tissue and cell type, and includes pro-inflammatory proteins such as IL6 and IL8, a number of matrix metalloproteinases, pro-neurogenic factors, pro-angiogenic factors and epithelial mitogens including EGFR agonists such as amphiregulin and epiregulin (FIGURE 2). This response likely evolved to signal tissue damage and invoke repair mechanisms, but in the context of a malignancy where tumor cells are poised to take advantage of such microenvironment signals, the cumulative effects may have adverse consequences. For example, the SASP and DDSP have been shown to promote the proliferation of premalignant and malignant epithelium (Krtolica et al., 2001), enhance invasion, and induce an epithelial to mesenchymal transition in carcinoma cells (Coppe et al., 2008), a phenotype shown to be resistant to chemotherapy and radiation (McConkey et al., 2009).
Figure 2. Microenvironment Derived DNA Damage Secretory Program.
Damaging agents impact normal constituents of tissue and organ systems as well as the neoplastic cells. The resulting damage suppresses tumor cell proliferation and induces cell death, but may also elicit a response from the tumor microenvironment which potentially can have a dramatic impact upon treatment efficacy. This damage response comprises several hundred proteins termed the DNA Damage Secretory Program (DDSP) that can promote the proliferation of premalignant and malignant epithelium, enhance invasion, promote angiogenesis and inflammation, and induce an epithelial to mesenchymal transition (EMT) in carcinoma cell. Individually and collectively these events have the potential to influence therapy resistance.
Interactions with components of the microenvironment can clearly impact tumor cell responses to chemotherapy. In a recent study evaluating estrogen receptor-negative breast cancer, tumors enriched with populations of reactive stroma were more resistant to a chemotherapy cocktail of 5-flourouracil, epirubicin and cyclophosphamide (Farmer et al., 2009). This finding supports the influence of crosstalk between stomal cells and tumor cells which can enhance resistance to cytotoxic drugs. A striking example of this effect involves the recovery of imatinib sensitivity of xenografted leukemia cells following their removal from a pro-resistant microenvironment (Williams et al., 2007). Using a mammary chimera model Nguyen et al. (2011) were able to support the hypothesis that ionizing radiation acts upon the microenvironment and in turn promotes breast cancer growth.
Of importance, it appears that not all microenvironments exhibit the same degree or diversity of damage responses to genotoxic stress, and thus may afford differential resistance microenvironments to tumor cells. A recent study using a transplantable lymphoma system found that after systemic treatment with doxorubicin, lymphoma cells were able to survive and proliferate in the thymus, but not other organs such as lymph nodes. Tumor cell resistance was shown to be due to the treatment-induced production of paracrine-acting factors, IL6 and Timp1, from the thymic endothelium, which were not induced in cell types comprising other organs. Suppression of these pro-survival factors resulted in enhanced tumor cell killing (Gilbert and Hemann, 2010). Secreted cytokines emanating from the microenvironment in the bone marrow may also contribute to chemotherapy resistance as well, but the full extent of this mechanism still remains unclear (Chantrain et al., 2008).
An interesting set of experiments tested the effects of tumor microenvironments exposed to ionizing radiation on the progression of carcinomas (Barcellos-Hoff et al., 2005; Nguyen et al., 2011). Initially host animals were either left untreated or were irradiated prior to implantation with p53 mutant mammary epithelial cells. Cells implanted in the mammary fat pads of irradiated host had a significantly higher tumor formation rate and the resulting tumors were both larger and histologically distinct compared with unirradiated controls. The investigators concluded that the differential growth responses resulted from an altered, damaged, stromal microenvironment. Comparable findings were observed in a similar set of experiments in which immortal myogenic cells were implanted into both irradiated and untreated muscle (Morgan et al., 2002). Whether these damaged microenvironments would also promote therapy resistance has not been tested.
The regimens currently used to apply anti-cancer therapeutics represent an ideal scenario for microenvironment damage responses---resulting in senescence---to promote resistance. Most chemotherapeutics are administered in cycles, scheduled to allow normal host cells and tissues to recover and avoid irreversible damage and host lethality. Similarly, radiotherapy is usually given in fractions spread over days to weeks. Induction of a damage response by the initial cycle would then expose the tumor cells surviving the first treatments to high local concentrations of pro-survival factors that fortify the remaining tumor cells against subsequent cycles of treatment. The logical therapeutic opportunity is to either inhibit individual components of the SASP or DDSP, or target master regulators of the response that transduce the DDR signal to promote the production of these effector proteins. To date, several key regulators have been identified and include NFkB (Chien et al., 2011; Janssens and Tschopp, 2006), p53 (Marion et al., 2009), IL-1alpha (Orjalo et al., 2009), and p38MAPK (Freund et al., 2011). In support of this concept, a recent study found that inhibiting NFkB signaling suppressed the senescence-associated secretory phenotype and enhanced tumor responses to chemotherapy in a mouse model of lymphoma (Chien et al., 2011).
7. Therapy Resistance and Breaking Senescence
While senescence is generally considered an irreversible state of cellular arrest, the potential dangers of an emergent phenotype should be considered. If cells in the arrested state are not cleared via processes such as phagocytosis they remain in the organism harboring the potential to recapture the proliferative state, often with damaged genomes. There have been reports both supporting immune mediated attrition (Xue et al., 2007) and the continued accretion of senescent cells in vivo (Dimri et al., 1995). These varied findings suggest that the evidence supporting the clearance/accumulation of senescent cells still remains inconclusive. However, it is important to keep in mind that an accumulation of arrested cells could provide a potential repository for future carcinogenesis if the concept of emergence from senescence is accurate.
Several of the earliest studies evaluating the emergent phenotype focused the transcriptional activities of two major tumor suppressors, p53 and RB1. While it was known that the expression of both genes was critical for establishing the senescent state their roles involving the maintenance of senescence was not fully understood. However, when the suppression of p53 expression in senescent fibroblast was found to lead to rapid re-entry into cellular proliferation and loss of expression of senescent associated genes, the picture became clearer (Dirac and Bernards, 2003). These findings suggested that senescence in these cells was reversible and that both initiation and maintenance of this arrest was p53-dependent. Furthermore, other investigators found that acute loss of RB1 in senescent cells promoted a reversal of the senescent phenotype (Sage et al., 2003). Additional findings suggest that it may be a combination of these tumor suppressors’ activities that maintain senescence. This mechanism was highlighted by a report suggesting that the RB regulator p16 may be involved as well, given that only in the absence of p16 expression was senescence arrest reversed via inactivation of p53 (Beausejour et al., 2003).
More recently it was found that lung cancer cells can escape senescence through the up regulation of Cdc2/Cdk1 (Roberson et al., 2005). While it appears that this is a rare population of cells, the fact that this occurs suggests that alternate senescence escape mechanisms exist and need to be addressed. Further evaluation of the escape response through the elevation of Cdc2/Cdk1 revealed that the downstream effector of this paradigm may be survivin (Wang et al., 2011). Of interest, survivin expression improved cell resistance to paclitaxel, therefore not only promoting senescence escape but post-escape drug resistance as well. This appears to be consistent with the findings by Puig et al. (Puig et al., 2008) demonstrating that DNA damaging agents can induce a senescent phenotype. The investigators then showed that a subset of cells were able to escape senescence and that those escaping cells were more resistant to chemotherapeutics than the parental cells. Further support for this idea was reported by Chao et al. (Chao et al., 2011) when they found that resistance to the microtubule-stabilizing agent Discodermolide in a subset of lung carcinoma cells was linked to the emergence from senescence and possibly mediated by alterations in the expression of 4E-BP1. The different escape routes observed might suggest that each cell population invokes unique mechanisms that allow for breaking senescence. Furthermore, these variable mechanisms may also be present among different cancer types and in turn partly explain treatment failures observed in the clinical setting.
8. Conclusions and Future Directions
Innate or acquired resistance to cancer therapeutics remains an important area of biomedical investigation that has clear ramifications for improving cancer specific death rates. In this context, manipulating cellular senescence may improve therapy responses via several mechanisms. The promotion of ‘intrinsic’ tumor cell senescence is an attractive concept in that it represents a normal, highly conserved and commonly-invoked tumor-suppressing response to overwhelming genotoxic stress or oncogene activation. This powerful program is often subverted in tumor cells, providing a strong rationale for re-engaging this key tumor suppressing response. Activating a senescence program within tumor cells could represent a means to arrest tumor growth through pathways that are not cross-reactive with other cancer therapeutics. However, such an approach should ensure that senescence by-pass does not occur, as emergent cells appear to have highly drug resistant phenotypes. Selectively inhibiting the senescence-associated secretory phenotype in benign cells comprising the tumor microenvironment may also serve to enhance the therapeutic index of currently deployed genotoxic therapies by eliminating pro-survival pro-resistance signals and enhancing the vulnerabilities of tumor cells through extrinsic mechanisms.
Acknowledgments
Financial Support: This work was supported by NIH CAP50CA097186
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahn J, Urist M, Prives C. The Chk2 protein kinase. DNA Repair (Amst) 2004;3:1039–1047. doi: 10.1016/j.dnarep.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, Rosivatz E, Woscholski R, Cognetti F, Scher HI, Pandolfi PP. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J. Clin. Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat. Rev. Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, Kenzelmann Broz D, Basak S, Park EJ, McLaughlin ME, Karnezis AN, Attardi LD. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell. 2011;145:571–583. doi: 10.1016/j.cell.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat. Rev. Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- Caino MC, Meshki J, Kazanietz MG. Hallmarks for senescence in carcinogenesis: novel signaling players. Apoptosis. 2009;14:392–408. doi: 10.1007/s10495-009-0316-z. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- Chabner BA, Roberts TG., Jr Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- Chang BD, Broude EV, Dokmanovic M, Zhu H, Ruth A, Xuan Y, Kandel ES, Lausch E, Christov K, Roninson IB. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59:3761–3767. [PubMed] [Google Scholar]
- Chang BD, Swift ME, Shen M, Fang J, Broude EV, Roninson IB. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc. Natl. Acad. Sci. U. S. A. 2002;99:389–394. doi: 10.1073/pnas.012602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantrain CF, Feron O, Marbaix E, DeClerck YA. Bone marrow microenvironment and tumor progression. Cancer Microenviron. 2008;1:23–35. doi: 10.1007/s12307-008-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SK, Lin J, Brouwer-Visser J, Smith AB, 3rd, Horwitz SB, McDaid HM. Resistance to discodermolide, a microtubule-stabilizing agent and senescence inducer, is 4E-BP1-dependent. Proc. Natl. Acad. Sci. U. S. A. 2011;108:391–396. doi: 10.1073/pnas.1016962108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Hales CN, Ozanne SE. DNA damage, cellular senescence and organismal ageing: causal or correlative. Nucleic Acids Res. 2007;35:7417–7428. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–2136. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner JW, 2nd, DePinho RA. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, Buer J, Lee HW, von Zglinicki T, Ganser A, Schirmacher P, Nakauchi H, Rudolph KL. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat. Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol. Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein PE, MacCollin M, Cichowski K. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, Larue L, Pritchard C, Marais R. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d'Adda di Fagagna F. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirac AM, Bernards R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J. Biol. Chem. 2003;278:11731–11734. doi: 10.1074/jbc.C300023200. [DOI] [PubMed] [Google Scholar]
- Duan J, Zhang Z, Tong T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int. J. Biochem. Cell. Biol. 2005;37:1407–1420. doi: 10.1016/j.biocel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Durocher D, Jackson SP. DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr. Opin. Cell. Biol. 2001;13:225–231. doi: 10.1016/s0955-0674(00)00201-5. [DOI] [PubMed] [Google Scholar]
- Efeyan A, Ortega-Molina A, Velasco-Miguel S, Herranz D, Vassilev LT, Serrano M. Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin. Cancer Res. 2007;67:7350–7357. doi: 10.1158/0008-5472.CAN-07-0200. [DOI] [PubMed] [Google Scholar]
- Elmore LW, Di X, Dumur C, Holt SE, Gewirtz DA. Evasion of a single-step, chemotherapy-induced senescence in breast cancer cells: implications for treatment response. Clin. Cancer Res. 2005;11:2637–2643. doi: 10.1158/1078-0432.CCR-04-1462. [DOI] [PubMed] [Google Scholar]
- Elmore LW, Rehder CW, Di X, McChesney PA, Jackson-Cook CK, Gewirtz DA, Holt SE. Adriamycin-induced senescence in breast tumor cells involves functional p53 and telomere dysfunction. J. Biol. Chem. 2002;277:35509–35515. doi: 10.1074/jbc.M205477200. [DOI] [PubMed] [Google Scholar]
- Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, Andre S, Piccart M, Campone M, Brain E, Macgrogan G, Petit T, Jassem J, Bibeau F, Blot E, Bogaerts J, Aguet M, Bergh J, Iggo R, Delorenzi M. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat. Med. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- Fizazi K, Martinez LA, Sikes CR, Johnston DA, Stephens LC, McDonnell TJ, Logothetis CJ, Trapman J, Pisters LL, Ordonez NG, Troncoso P, Navone NM. The association of p21((WAF-1/CIP1)) with progression to androgen-independent prostate cancer. Clin. Cancer Res. 2002;8:775–781. [PubMed] [Google Scholar]
- Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem. Pharmacol. 2008;76:947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Curet I, Perkins RS, Bennett R, Feidler KL, Dunn SP, Krueger LJ. c-Myc inhibition negatively impacts lymphoma growth. J. Pediatr. Surg. 2006;41:207–211. doi: 10.1016/j.jpedsurg.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Graham J, Mushin M, Kirkpatrick P. Oxaliplatin. Nat. Rev. Drug Discov. 2004;3:11–12. doi: 10.1038/nrd1287. [DOI] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol. Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Ho HY, Cheng ML, Lu FJ, Chou YH, Stern A, Liang CM, Chiu DT. Enhanced oxidative stress and accelerated cellular senescence in glucose-6-phosphate dehydrogenase (G6PD)-deficient human fibroblasts. Free Radic. Biol. Med. 2000;29:156–169. doi: 10.1016/s0891-5849(00)00331-2. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, et al. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975–2008. based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- Huang MJ, Cheng YC, Liu CR, Lin S, Liu HE. A small-molecule c-Myc inhibitor, 10058-F4, induces cell-cycle arrest, apoptosis, and myeloid differentiation of human acute myeloid leukemia. Exp. Hematol. 2006;34:1480–1489. doi: 10.1016/j.exphem.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Huen MS, Chen J. The DNA damage response pathways: at the crossroad of protein modifications. Cell Res. 2008;18:8–16. doi: 10.1038/cr.2007.109. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S, Tschopp J. Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death. Differ. 2006;13:773–784. doi: 10.1038/sj.cdd.4401843. [DOI] [PubMed] [Google Scholar]
- Kahlem P, Dorken B, Schmitt CA. Cellular senescence in cancer treatment: friend or foe? J. Clin. Invest. 2004;113:169–174. doi: 10.1172/JCI20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J. Natl. Cancer Inst. 2004;96:1659–1668. doi: 10.1093/jnci/djh312. [DOI] [PubMed] [Google Scholar]
- Knox RJ, Friedlos F, Jarman M, Roberts JJ. A new cytotoxic, DNA interstrand crosslinking agent, 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide, is formed from 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by a nitroreductase enzyme in Walker carcinoma cells. Biochem. Pharmacol. 1988;37:4661–4669. doi: 10.1016/0006-2952(88)90335-8. [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov SV, Graham ME, Peng C, Chen P, Robinson PJ, Lavin MF. Involvement of novel autophosphorylation sites in ATM activation. EMBO J. 2006;25:3504–3514. doi: 10.1038/sj.emboj.7601231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- Kumamoto K, Spillare EA, Fujita K, Horikawa I, Yamashita T, Appella E, Nagashima M, Takenoshita S, Yokota J, Harris CC. Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence. Cancer Res. 2008;68:3193–3203. doi: 10.1158/0008-5472.CAN-07-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- Lacombe L, Maillette A, Meyer F, Veilleux C, Moore L, Fradet Y. Expression of p21 predicts PSA failure in locally advanced prostate cancer treated by prostatectomy. Int. J. Cancer. 2001;95:135–139. doi: 10.1002/1097-0215(20010520)95:3<135::aid-ijc1023>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lazzarini R, Moretti S, Orecchia S, Betta PG, Procopio A, Catalano A. Enhanced antitumor therapy by inhibition of p21waf1 in human malignant mesothelioma. Clin. Cancer Res. 2008;14:5099–5107. doi: 10.1158/1078-0432.CCR-08-0255. [DOI] [PubMed] [Google Scholar]
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, Cordon-Cardo C, Teruya-Feldstein J, Pandolfi PP. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell J. Molecular Biology of the Cell. 5th Ed. New York, NY: WH Freeman; 2004. p. 963. [Google Scholar]
- Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J. Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- Mahon KL, Henshall SM, Sutherland RL, Horvath LG. Pathways of chemotherapy resistance in castration-resistant prostate cancer. Endocr. Relat. Cancer. 2011;18:R103–R123. doi: 10.1530/ERC-10-0343. [DOI] [PubMed] [Google Scholar]
- Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- Mansfield CM. Early breast cancer its history and results of treatment. Exp Biol Med. 1976;5:1–129. [PubMed] [Google Scholar]
- Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, Siefker-Radtke A, Dinney C. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Mirzayans R, Scott A, Cameron M, Murray D. Induction of accelerated senescence by gamma radiation in human solid tumor-derived cell lines expressing wild-type TP53. Radiat. Res. 2005;163:53–62. doi: 10.1667/rr3280. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Gross JG, Pagel CN, Beauchamp JR, Fassati A, Thrasher AJ, Di Santo JP, Fisher IB, Shiwen X, Abraham DJ, Partridge TA. Myogenic cell proliferation and generation of a reversible tumorigenic phenotype are triggered by preirradiation of the recipient site. J. Cell Biol. 2002;157:693–702. doi: 10.1083/jcb.200108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardella C, Clohessy JG, Alimonti A, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nat. Rev. Cancer. 2011;11:503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Oketch-Rabah HA, Illa-Bochaca I, Geyer FC, Reis-Filho JS, Mao JH, Ravani SA, Zavadil J, Borowsky AD, Jerry DJ, Dunphy KA, Seo JH, Haslam S, Medina D, Barcellos-Hoff MH. Radiation acts on the microenvironment to affect breast carcinogenesis by distinct mechanisms that decrease cancer latency and affect tumor type. Cancer Cell. 2011;19:640–651. doi: 10.1016/j.ccr.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Lee JH. The Mre11/Rad50/Nbs1 complex and its role as a DNA double-strand break sensor for ATM. Cell Cycle. 2005;4:737–740. doi: 10.4161/cc.4.6.1715. [DOI] [PubMed] [Google Scholar]
- Pommier Y. DNA topoisomerase I and II in cancer chemotherapy: update and perspectives. Cancer Chemother. Pharmacol. 1993;32:103–108. doi: 10.1007/BF00685611. [DOI] [PubMed] [Google Scholar]
- Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle. 2009;8:4112–4118. doi: 10.4161/cc.8.24.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk LF, Han YH, Steighner RJ. Structure of bleomycin-induced DNA double-strand breaks: predominance of blunt ends and single-base 5' extensions. Biochemistry. 1989;28:5808–5814. doi: 10.1021/bi00440a016. [DOI] [PubMed] [Google Scholar]
- Puig PE, Guilly MN, Bouchot A, Droin N, Cathelin D, Bouyer F, Favier L, Ghiringhelli F, Kroemer G, Solary E, Martin F, Chauffert B. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol. Int. 2008;32:1031–1043. doi: 10.1016/j.cellbi.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Quick QA, Gewirtz DA. An accelerated senescence response to radiation in wild-type p53 glioblastoma multiforme cells. J. Neurosurg. 2006;105:111–118. doi: 10.3171/jns.2006.105.1.111. [DOI] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Roberson RS, Kussick SJ, Vallieres E, Chen SY, Wu DY. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 2005;65:2795–2803. doi: 10.1158/0008-5472.CAN-04-1270. [DOI] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424:223–228. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- Senderowicz AM. Novel direct and indirect cyclin-dependent kinase modulators for the prevention and treatment of human neoplasms. Cancer Chemother. Pharmacol. 2003a;52(Suppl 1):S61–S73. doi: 10.1007/s00280-003-0624-x. [DOI] [PubMed] [Google Scholar]
- Senderowicz AM. Novel small molecule cyclin-dependent kinases modulators in human clinical trials. Cancer Biol. Ther. 2003b;2:S84–S95. [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23:2919–2933. doi: 10.1038/sj.onc.1207518. [DOI] [PubMed] [Google Scholar]
- Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr. Biol. 1999;9:939–945. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- Sidi R, Pasello G, Opitz I, Soltermann A, Tutic M, Rehrauer H, Weder W, Stahel RA, Felley-Bosco E. Induction of senescence markers after neo-adjuvant chemotherapy of malignant pleural mesothelioma and association with clinical outcome: an exploratory analysis. Eur. J. Cancer. 2011;47:326–332. doi: 10.1016/j.ejca.2010.09.044. [DOI] [PubMed] [Google Scholar]
- Stucki M, Jackson SP. gammaH2AX and MDC1: anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair (Amst) 2006;5:534–543. doi: 10.1016/j.dnarep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Boothman DA. Stress-induced premature senescence (SIPS)--influence of SIPS on radiotherapy. J. Radiat. Res. (Tokyo) 2008;49:105–112. doi: 10.1269/jrr.07081. [DOI] [PubMed] [Google Scholar]
- te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–1883. [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- Wang H, Hammoudeh DI, Follis AV, Reese BE, Lazo JS, Metallo SJ, Prochownik EV. Improved low molecular weight Myc-Max inhibitors. Mol. Cancer Ther. 2007;6:2399–2408. doi: 10.1158/1535-7163.MCT-07-0005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wu PC, Roberson RS, Luk BV, Ivanova I, Chu E, Wu DY. Survivin and escaping in therapy-induced cellular senescence. Int. J. Cancer. 2011;128:1546–1558. doi: 10.1002/ijc.25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Herbig U, Wei S, Dutriaux A, Sedivy JM. Loss of retinoblastoma but not p16 function allows bypass of replicative senescence in human fibroblasts. EMBO Rep. 2003;4:1061–1066. doi: 10.1038/sj.embor.7400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, den Besten W, Sherr CJ. Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes Dev. 2007;21:2283–2287. doi: 10.1101/gad.1588607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE, Pereira-Smith OM, Shay JW. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol. Cell. Biol. 1989;9:3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavare S, Arakawa S, Shimizu S, Watt FM. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, McDaid R, Lee J, Possik P, Li L, Kumar SM, Elder DE, Van Belle P, Gimotty P, Guerra M, Hammond R, Nathanson KL, Dalla Palma M, Herlyn M, Xu X. The role of BRAF mutation and p53 inactivation during transformation of a subpopulation of primary human melanocytes. Am. J. Pathol. 2009;174:2367–2377. doi: 10.2353/ajpath.2009.081057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H, Suzuki T, Aizawa K, Miyagawa K, Nagai R. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J. Biol. Chem. 2010;285:29662–29670. doi: 10.1074/jbc.M110.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- Zhuang D, Mannava S, Grachtchouk V, Tang WH, Patil S, Wawrzyniak JA, Berman AE, Giordano TJ, Prochownik EV, Soengas MS, Nikiforov MA. C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells. Oncogene. 2008;27:6623–6634. doi: 10.1038/onc.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]