Abstract
Female receptivity including the immobile hormone-dependent lordosis posture is essential for successful reproduction in rodents. It is well documented that lordosis is organized during the perinatal period when the actions of androgens decrease the males’ ability to display this behavior in adulthood. Conversely the absence of androgens, and the presence of low levels of prepubertal estrogens, preserves circuitry that regulates this behavior in females. The current study set out to determine whether sex chromosomal genes are involved in the differentiation of this behavior. An agonadal mouse model was used to test this hypothesis. The SF-1 gene (Nr5a1) is required for development of gonads and adrenal glands, and knockout mice are consequently not exposed to endogenous gonadal steroids. Thus contributions of sex chromosome genes can be disassociated from the actions of estrogens. Use of this model reveals a direct genetic contribution from sex chromosomes in the display of lordosis and other female-typical sexual behavior patterns. It is likely that the concentrations of gonadal steroids present during normal male development modify the actions of sex chromosome genes on the potential to display female sexual behavior.
Keywords: Mice, Steroidogenic factor 1, sex difference, female sex behavior, progesterone receptor
Introduction
Female sexual behaviors facilitate and enable copulation and therefore ensure successful mating and consequently reproductive success. One of the best documented indicators of receptivity in rodents is lordosis, an arched back immobile reflex posture exhibited when males attempt to mount and mate. This posture has been used to map the reflexive circuits in the central nervous system that are regulated by the steroid hormones required for its expression (Kow and Pfaff, 1977; Pfaff and Sakuma, 1979). The lordosis reflex, is sexually differentiated and is displayed to a much greater extent by females as the male brain is normally defeminized (Blaustein and Erskine, 2002). The sex differences in circulating androgens during the perinatal period mold brain sex differences via a number of factors, including steroidogenic enzymes, receptors and neurotransmitters including but not limited to; testosterone, aromatase enzyme, estradiol, estrogen receptors, and glutamate receptors (Kudwa et al., 2005; Schwarz et al., 2008). The ability of neonatal testosterone to block adult expression of these “female-typical” behavior patterns in rodents is one of the cornerstone arguments for the primary role that gonadal steroids play in brain sexual differentiation (Phoenix et al., 1959).
In addition to the organizing roles that steroid hormones play, sex differences can also be influenced in the brain by genes on the sex chromosomes that are expressed at different levels in male and female brains. For example, genes on the Y-chromosome that are not represented on X-chromosome, and/or X-chromosome genes that escape X-inactivation might favor differentiation in male or female directions (De Vries et al., 2002). There are several mouse models currently available that can be used to test this hypothesis (Majdic and Tobet, 2011).
Steroidogenic factor 1 (SF-1) is a transcription factor that regulates the expression of many genes involved in development and function of the reproductive axis (Parker and Schimmer, 1997). Mice lacking the Nr5a1 gene (SF-1 KO) are born without gonads and adrenal glands and are therefore not exposed to endogenous sex steroid hormones (Ingraham et al., 1994). As such, sex differences between chromosomal males (XY) and females (XX) may be caused by genes located on sex chromosomes in contrast to differences in gonadal steroid hormones during development (Budefeld et al., 2008). In addition to gonadal and adrenal agenesis, these mice have a disorganized ventromedial hypothalamus (VMH) and the male knockouts have feminine external genitalia (Dellovade et al., 2000; Parker and Schimmer, 1997). The SF-1 KO model has provided evidence that developmental exposure to gonadal steroids is not required for adult display of aggression (Grgurevic et al., 2008) while brain specific female knockout mice have reduced levels of lordosis (Kim et al., 2010).
The aim of the present study was therefore to examine female-typical sexual behaviors and immunoreactive progesterone receptors in SF-1 KO mice to determine a contribution from sex chromosomal genes to the development of female biased brain circuitry and behavior. It is well established that progesterone, acting through the estrogen-induced receptors in the VMH area are an important component of the functional circuitry needed for the appropriate display of female sex behavior (Rubin and Barfield, 1983). In the current study, immunoreactive progesterone receptors were examined to assess the functional capacity of this component of the behavioral circuitry.
Material and Methods
Animals
Mice with the disrupted SF-1 allele were backcrossed for more than 10 generations to C57BL/6J mice to produce a congenic line. All mice were housed under standard laboratory conditions at the University of Ljubljana Veterinary School in a 12:12 light/dark cycle (lights off at 1600 h) with phytoestrogen free food (Harlan mouse chow 2919, Harlan Teklad, Milano, Italy) and water ad libitum. All animal experiments were done according to ethical principles and in accordance with EU directive (86/609/EEC). Animal experiments were approved by the Veterinary commission of Slovenia and the Animal Care and Use Committee at Colorado State University.
SF-1 heterozygous mice were mated to produce homozygous SF-1 KO and wild type (WT) offspring. To ensure survival of SF1 KO mice, all newborn pups were injected daily for 6-7 days with 50 μl of a corticosteroid cocktail in corn oil (s.c. 400 ng/ml hydrocortisone, 40 ng/ml dexamethasone, and 25 ng/ml fludrocortisone acetate; all from Sigma, Steinheim, Germany). Mice were genotyped by PCR assay of tail DNA on day 6 or 7 after birth as previously described (Ingraham et al., 1994). Female WT littermates or female pups from other C57BL/6J litters born within 3 days were used as a source of adrenal transplants; these techniques have been published (Majdic et al., 2002). After weaning, mice were initially group housed and then separated at 30 days of age and remained isolated until behavioral tests. C57BL/6J mice were used for controls, which were gonadectomized at 21-25 days of age and housed under the same conditions as SF-1 KO animals. We tested mice in each of these four groups; SF1KOF (n=7), SF1WTF (n=7), SF1KOM (n=6), and SF1WTM (n=7). Behavioral testing started at the age of 65 days, and mice were sacrificed by perfusion fixation with 4 % paraformaldehyde 7 days after the last test, around 90 days of age.
Behavioral Testing
All behavioral tests were conducted by NG who was blinded to genotype and chromosomal sex of the experimental mice at the time of the testing. For stimulus animals, sexually experienced C57BL/6J males were used. All subject mice received estradiol benzoate (EB; 0.5 μg/0.15 ml s.c.) 48 hours prior to test followed by progesterone (P; 1.6mg/0.2 ml s.c.) approximately 4-9 hours prior to testing. Tests were started at least one hour after the dark portion of the light/dark cycle under the red light illumination.
Testing was performed in a clear glass aquarium (26 × 42 cm) without bedding, food and water. A mirror was placed under the aquarium to facilitate counting intromissions. Tested aninmals were placed in the aquaria for at least 1 hour to habituate before male partners were added. Behavior was evaluated during 20 min tests or until ejaculation by a stimulus male. If no receptivity was observed, the test ended after 15 mount attempts. If the stimulus males did not try to mount the subject, they were replaced with different stimulus males after 5 minutes of testing. Each mouse was tested 7 successive times with at least 3 days and no more than 6 days between tests to mimic the natural female cycle.
During testing, the numbers of attempted and successful mounts, and the numbers of times the subject stood during these mounts were scored. Mounts were counted when the tested mice had all four limbs on the floor. Lordosis was scored from 1 to 5 regarding to dismounts, movement and vocalization of the tested animal (Dominguez-Salazar et al., 2004). These data were used to calculate lordosis quotients (LQ) by the following formula: number of times the female stood for mounts /total number of mounts and mount attempts x 100. In addition, number of intromissions, number of thrusts, latency to intromit and number of ejaculations were scored.
Immunocytochemistry on floating sections
Brains were embedded in 5% agarose (Sigma) and sectioned at 50 m in cold 0.05M PBS using a vibrating microtome (Integraslice 7550 MM, Campden Instruments, UK). Sections were incubated in 0.1M glycine (Sigma) in 0.05M PBS for 30 min followed by incubation in 0.5% sodium borohydride (Sigma) for 15 min at 4°C. Glycine and sodium borohydride were washed out with 15 min and 20 min washes in 0.05M PBS. Sections were blocked in 5% normal goat serum (Chemicon, Temecula, CA, USA) containing 0.5% Triton X-100 (Sigma) and 1% H2O2 (Merck, Darmstadt, Germany) for 30 min at 4°C. Rabbit primary antibodies against progesterone receptor (PR, 1:1000, Dako, Glostrup, Denmark) were diluted in 0.05M PBS containing 1% bovine serum albumin (Sigma) and 0.5% Triton X-100. Sections were incubated with primary antibodies over 3 nights at 4°C with shaking. Sections were then washed in 0.05M PBS containing 1% normal goat serum and 0.02% Triton X-100 four times 15 minutes at room temperature. Biotinylated secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) against primary rabbit antibodies were diluted 1:500 in 0.05M PBS containing 1% normal goat serum and 0.5% Triton X-100. Sections were incubated with secondary antibodies for two hours, followed by 4 washes (15 minutes each) in 0.05M PBS buffer containing 0.02% Triton X-100. Streptavidin – HRP complex (Jackson Immunoresearch) was diluted 1:2000 in 0.05M PBS solution containing 0.5% TritonX-100. Sections were incubated with Streptavidin – HRP for 1 hour at room temperature and then washed in Tris-buffered saline (0.05M Tris-HCl/0.9% NaCl; pH 7.5; Sigma) for 1 hour at room temperature. Antigen-antibody complexes were visualized as a black reaction product by incubating sections in 0.025% 3′3′-Diaminobenzidine/ammonium nickel (II) sulfate substrate (Sigma) in Tris-buffered saline (pH 7.5) containing 0.02% H2O2 for 5 min at room temperature. After mounting, sections were dried and coverslipped using hydrophobic medium (Pertex, Burgdorf, Germany). Immunocytochemical controls included omission of the primary antiserum and validation of immunoreactivity with patterns of distribution from prior publications.
Data collection and analyses
Digital images of ventromedial region of hypothalamus were obtained using a Nikon Eclipse 80i microscope with Nikon DS-Fi1 camera. Images were enhanced for contrast using an Adobe Photoshop software package (Version 8.0). The number of neurons that were immunoreactive for progesterone receptor (PR) was analyzed in two coronal sections containing the ventrolateral part of the VMH 1.55 mm and 1.7 mm caudal from bregma according to stereotaxic coordinates (Paxinos and Franklin, 2001) in WT gonadectomized mice and the corresponding ventromedial hypothalamic region in SF-1 KO mice. Digital images were taken under 100x magnification and the third ventricle and base of the brain were considered as reference boundaries. Due to the possibility of asymmetry in antigen detection between the left and right sides of the brain, the side with more immunoreactive cells or fibers was always chosen for analysis. As the sections were run free-floating and the brains were not notched ahead of time, the actual left or right side of the brain was unknown.
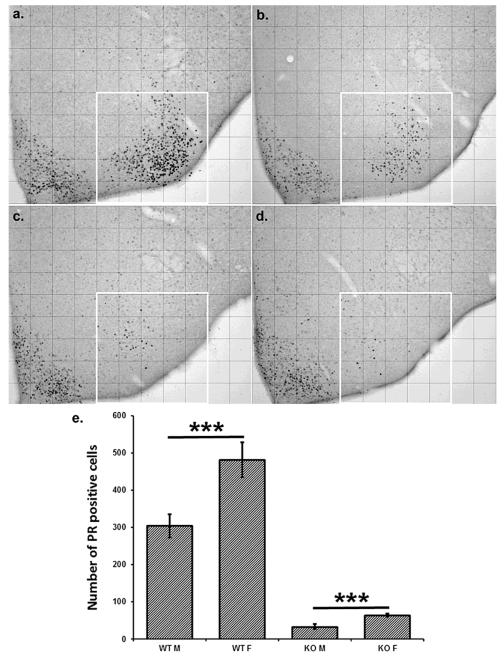
The number of immunoreactive cells in the VMH was evaluated using Image J software (NIH, Bethesda, MD, USA). Analyzed regions were divided into grid squares to provide a mechanism to discern objective changes in the positions of cells. For the PR immunoreactive cells in the VMH we used a grid of 12×9 squares, measuring 104×104 m each when taken with 10x objective lens. The number of immunoreactive cells was counted in each box in the designated region shown in figure 2. The boxes were then collapsed into columns for the purpose of statistical analysis.
Figure 2.
The number of progesterone receptor immunopositive cells was reduced in SF-1 KO mice of both sexes (c - female, d - male) in comparison to WT mice (a - female, b - male). However, there was a sex difference present in both genotypes, suggesting sex chromosome effect in addition to sex hormone effect for progesterone receptor expression in the ventromedial hypothalamus (e; SF1-KOF n=5, SF1-WTF n=6, SF1-KOM n=5, SF1-WTM n=6; data are mean ± SEM; *** p < 0.001).
Statistics
All statistical analyses were performed using the NCSS software package (NCSS 2007, Kaysville, UT). Differences in behaviors between groups were examined by repeated measure ANOVA with sex and genotype as independent variables, and trial as a within factor repeated measure. Differences in the number of progesterone receptor immunopositive cells were examined by 3-way ANOVA with sex and genotype as independent variables and columns as a repeated measure to account for location. Statistical differences were considered significant with p < 0.05.
Results
Sex chromosomes and female receptivity
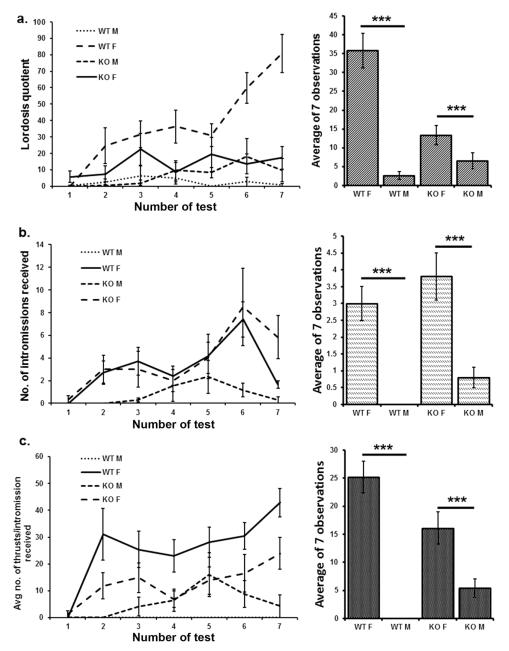
As expected, lordosis levels were greatest in WT female mice tested with stud males. Lordoses as measured quantitatively by LQ’s were reduced in SF-1 KO mice in comparison to WT females. However, there was still a significant sex difference in LQ with SF-1 KO females (XX genotype) displaying almost double the LQ of SF-1 KO males (XY genotype) through all seven tests. For LQ, main effects for chromosomal sex (F(1,23)=31.7, p<0.001), presence or absence of the functional SF-1 (F(1,23)=7.3, p<0.001), and an interaction (F(1,23)=14.5, p<0.001), were all highly significant (Figure 1a). When other measures of receptivity/attractiveness were analyzed such as numbers of intromissions, thrusts per intromission, and ejaculations received by tested mice, there was a striking sex difference between genetic males and females in both WT and SF-1 KO mice with no genotype effect for the number of intromissions received (F(1,23)=42.3, p<0.001), the number of thrusts per intromission (F(1,23)=49.3, p<0.001) and for the number of ejaculations received (F(1,23)=21.13, p<0.001; Figure 1b, c). Even though SF-1 KO males and females were indistinguishably phenotypic females to the investigators, the data suggest that WT stud males were more attracted to SF-1 KO females and/or that SF-1 KO females were more receptive.
Figure 1.
Lordosis quotients (a) were lower in SF-1 KO mice than in WT, and males in comparison to females regardless of the SF-1 allele. The number of intromissions (b) and number of thrusts per intromission (c) were strongly dependent on genetic sex with female mice of both SF-1 genotypes receiving significantly more intromissions than males. This is in spite of the fact that the SF1 KO males were phenotypic females (SF1-KOF n=7, SF1-WTF n=6, SF1-KOM n=7, SF1-WTM n=7; data are mean ± SEM; *** p < 0.001).
Sex chromosomes and immunoreactive progesterone receptor
As expected, WT females had significantly more immunoreactive cells in the ventrolateral part of the VMH (but not in a neighboring control region; the arcuate nucleus) than WT males. For both males and females the greatest number of cells was located greater than 208 μm from the ventricle and up to 520μm. In SF-1 KO mice, the number of immunopositive PR cells was reduced in the VMH (but not in the arcuate nucleus), although some immunopositive cells were still detected in the region of the VMH (Figure 2a). Statistical analyses revealed a strong effect of sex (F(1,95) = 22.9, p < 0.001) and genotype (F(1,95) = 223.8, p < 0.001) with female WT or KO having about 50% more immunoreactive cells than male WT or Kos, respectively (Figure 2). Interestingly, of the few PR immunopositive cells that remained in KO mice, the majority were located less than 312μm from the ventricle in SF-1 KO mice (genotype x location interaction; F(1, 95) = 37.1, p < 0.001).
Discussion
This study examined the potential influence of sex chromosomal genes on the ability to display female receptivity and up-regulate the number of cells containing immunoreactive progesterone receptors in adult mice. Lordosis quality as measured by LQ was the highest in WT females and lowest in WT males with a large sex difference present as expected for WT. Interestingly, despite reduced LQ levels in SF-1 KO mice, a sex difference persisted in these mice that were not exposed to endogenous gonadal steroids during development. The difference between genetic males and females developing without gonads (SF1 KO) was even stronger for other measures of receptivity such as the incidence of receiving intromissions and thrusts from stud males. All of these measures were similar in XX WT and SF1 KO females, but notably greater than in XY WT and SF1 KO males, suggesting that sex chromosomal genes may be involved in the differentiation of these behaviors. This is further suggested by data for latency to intromission and number of ejaculations received, which also differed significantly between genetic males and females (data not shown). This suggests that testis differentiation blocked adult expression of lordosis and male receptivity in WT XY mice. Fundamentally low receptivity in SF-1 KO mice of both sexes is likely the consequence of the disruption of cell positions in the region of the VMH, which are important for the expression of lordosis behavior (Rubin and Barfield, 1983).
Studies in neonatal global SF1 KO mice and adult brain specific SF1 KO mice (Davis et al., 2004; Dellovade et al., 2000) indicate that VMH cells are present in the area but are no longer organized into a Nissl-defined nucleus. Data in the current study show that the number of immunoreactive progesterone receptor containing cells in the VMH area was reduced in the VMH area in SF-1 KO mice. Interestingly, the position of these remaining cells was primarily medial relative to the primary positions of these cells in WT mice. Given the significant decrease in the number of cells, it is not possible to differentiate whether the cells in the medial region were more robust to the influence of the KO or whether the medial positions were the by-product of changes in cell positions for cells that should have been more lateral. Alterations in cell positions has been described for several other cells and/or nerve fibers (Budefeld et al., 2008; Budefeld et al., 2011) including estrogen receptor alpha (Dellovade et al., 2000; Majdic et al., 2002) that may be necessary for the induction of immunoreactive PR. Given the importance of PR induction in cells of this region for lordosis behavior (Rubin and Barfield, 1983), it is likely that this is a critical biochemical basis for reduced lordosis behavior observed in this study. However, despite low LQ scores, once males mounted female KO mice they were able to attain as many intromissions as they achieved with WT females. Because female mice do not tend to become completely immobile during lordosis (Bonthuis et al., 2010) the fact that males attained intromissions can be taken as an additional measure of female receptivity. In addition, the fact that the SF1 KO males did not receive as many intromissions or ejaculations from males as the KO females again reinforces that view that genetic females were more prone to display functional receptivity than genetic males.
Another major model for examining sex chromosomal gene contributions to sexual differentiation is the four-core-genotype (FCG) mouse (Arnold and Chen, 2009). To this point examination of female sex behavior in FCG mice have not yielded significant results forgenetic contributions (Emilie Rissman, personal communication), although newer genetic models may be needed to more fully explore the issue (Bonthuis et al., 2012). However, it is important to consider that FCG mice are exposed to significant gonadal steroid exposure during development as both XX and XY female mice have functional gonads (even if reversed relative to the sex chromosomal complement). Thus, it is reasonable to speculate, especially knowing that sex steroid hormones are important for the appropriate display of female sex behavior, that sex steroid hormones from the gonads override genetic influences on this behavior. It is also worth noting that sex chromosomal effects also were not seen for immunoreactive PR in the VMH of neonatal FCGmice (Wagner et al., 2004), although this assessment was done at birth when the regulation of immunoreactive PR may be different in females (Brock et al., 2010).
In the current study, there was a sex difference in immunoreactive PR in the region of the VMH and this difference was maintained, albeit at much lower levels, among adult SF-1 KO mice. Sex differences in immunoreactive PR have been noted in the region of the VMH in both neonatal (Wagner et al., 2001) and adult (Temple et al., 2001) mice. While the sex difference may be detectable at birth (Wagner et al., 2001), it is not regulated by estradiol similarly across development. Sex differences in immunoreactive PR in the VMH were found from P0 to P20 in WT, but were hormone (i.e., aromatase gene knockout) dependent only in males (Brock et al., 2010). At P25 the sex difference became less apparent as immunoreactive PR became aromatase-dependent in females (Brock et al., 2010). These data taken together with the current results are consistent with suggestion that there are age-related changes in the estrogen-inducibility of immunoreactive PR in females that occur during development that may relate to a sexually differentiated process.
One question arising from the current study is the determination of the circuitry for activating lordosis behavior in SF-1 KO mice that have a vastly different neuronal cytoarchitecture than WT mice. Previous studies have failed to detect many (if any) neurons containing immunoreactive estrogen receptor alpha in the ventrolateral part of the VMH in SF-1 KO mice (Dellovade et al., 2000; Majdic et al., 2002). Nevertheless, the current study demonstrates low levels of detection for cells containing immunoreactive PR in this area in SF-1 KO mice. A previous report (Kudwa and Rissman, 2003) indicated that some PR immunopositive cells are present in the most ventrolateral part of the VMH region in WT mice without estradiol treatment, and in estrogen receptor alpha and beta knockout mice after estradiol treatment (Moffatt et al., 1998). This may suggest that some cells in this region do not need estradiol stimulation to express immunoreactive PR and could therefore represent an estradiol independent pathway for induction of PR expression. Interestingly, it has been suggested that some PR positive cells reside close to, but outside the VMH and are involved in the regulation of lordosis behavior (Blaustein and Erskine, 2002). PR immunopositive cells detected in the present study could therefore represent these cells that normally reside lateral to the VMH boundary and have perhaps a supplemental role in the regulation of lordosis. The question is whether in the reorganized region of the VMH in SF1 KO mice these cells remain to help regulate lordosis behavior.
It has been generally believed for several decades that the development of female behavior patterns in adult life is a default pattern that is not dependent on organizational actions of sex steroid hormones, despite occasional studies to the contrary (reviewed by Bakker and Baum, 2008). This includes a report that ovaries only start to produce sex steroids around P7 (Mannan and O’Shaughnessy, 1991). A recent study is particularly striking in providing data for the importance of estrogenic stimulation for the development of female behavioral potentials (Brock et al., 2011). In this study it was reported that mice with genetic disruption of the aromatase gene and thereby lacking estrogens displayed lower levels of LQ in comparison to WT females even when primed with estradiol prior to testing. A previous study with brain specific SF-1 KO mice (Kim et al., 2010) showed that female sex behavior was drastically reduced when gonadal function in development was relatively normal but the organization of the region of the VMH was notably altered. While lack of developmental exposure to estrogens can lead to lower levels of female sexual behaviors (Brock et al., 2011) and could account for sex differences in this behavior, in the current study males and female were exposed to the same lack of estrogens during development when SF-1 was disrupted. Yet, genetic females could still display higher levels of female behaviors than males. The extent to which the lack of estrogens during the pubertal period could perhaps contribute to low LQ levels will have to be confirmed in the future studies.
In conclusion, this study demonstrates that sex differences in the expression of female sex behavior and the immunoexpression of PR in the region of the VMH may be due in part to contributions from sex chromosomal genes in addition to sex steroid hormones derived from gonadal sources.
Highlights.
SF-1 KO mice are born without gonads, male and female mice are thus phenotypically females Female SF-1 KO mice display more robust female sex behavior than SF-1 KO male mice
Female SF-1 KO mice have more progesterone receptor immunopositive cells than male SF-1 KO mice
Sex chromosome genes contribute to the development of sex difference in female sex behavior
Acknowledgements
This work was supported by NIH R01 MH61376 (SAT, GM) and ARRS P4-0053 (GM, NG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine Sexual Behavior: Cellular Integration of Hormonal and Afferent Information in the Rodent Forebrain. In: Pfaff DW, editor. Hormones, brain and behavior. Academic press; San Diego, CA: 2002. pp. 139–214. [Google Scholar]
- Bonthuis PJ, Cox KH, Rissman EF. X-chromosome dosage affects male sexual behavior. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol. 2010;31:341–358. doi: 10.1016/j.yfrne.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 2011;31:5574–5578. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O, Douhard Q, Baum MJ, Bakker J. Reduced prepubertal expression of progesterone receptor in the hypothalamus of female aromatase knockout mice. Endocrinology. 2010;151:1814–1821. doi: 10.1210/en.2009-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budefeld T, Grgurevic N, Tobet SA, Majdic G. Sex differences in brain developing in the presence or absence of gonads. Dev Neurobiol. 2008;68:981–995. doi: 10.1002/dneu.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budefeld T, Tobet SA, Majdic G. Altered position of cell bodies and fibers in the ventromedial region in SF-1 knockout mice. Exp Neurol. 2011;232:176–184. doi: 10.1016/j.expneurol.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Stallings NR, Zhao L, Parker KL, Tobet SA. Loss of steroidogenic factor 1 alters cellular topography in the mouse ventromedial nucleus of the hypothalamus. J Neurobiol. 2004;60:424–436. doi: 10.1002/neu.20030. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dominguez-Salazar E, Bateman HL, Rissman EF. Background matters: the effects of estrogen receptor alpha gene disruption on male sexual behavior are modified by background strain. Horm Behav. 2004;46:482–490. doi: 10.1016/j.yhbeh.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Grgurevic N, Budefeld T, Rissman EF, Tobet SA, Majdic G. Aggressive behaviors in adult SF-1 knockout mice that are not exposed to gonadal steroids during development. Behav Neurosci. 2008;122:876–884. doi: 10.1037/0735-7044.122.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen W, Nachtigal MW, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes and Development. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- Kim KW, Li S, Zhao H, Peng B, Tobet SA, Elmquist JK, Parker KL, Zhao L. CNS-specific ablation of steroidogenic factor 1 results in impaired female reproductive function. Mol Endocrinol. 2010;24:1240–1250. doi: 10.1210/me.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Sensory control of reproductive behavior in female rodents. Ann N Y Acad Sci. 1977;290:72–97. doi: 10.1111/j.1749-6632.1977.tb39718.x. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2005 doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Majdic G, Tobet S. Cooperation of sex chromosomal genes and endocrine influences for hypothalamic sexual differentiation. Front Neuroendocrinol. 2011;32:137–145. doi: 10.1016/j.yfrne.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- Mannan MA, O’Shaughnessy PJ. Steroidogenesis during postnatal development in the mouse ovary. J Endocrinol. 1991;130:101–106. doi: 10.1677/joe.0.1300101. [DOI] [PubMed] [Google Scholar]
- Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-alpha gene-disrupted mice. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18:361–377. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. second edition Academic press; San Diego, San Francisco, New York, Boston, London, Sydney, Tokyo: 2001. [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37:218–224. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Fugger HN, Li X, Shetty SJ, Gustafsson J, Rissman EF. Estrogen receptor beta regulates sexually dimorphic neural responses to estradiol. Endocrinology. 2001;142:510–513. doi: 10.1210/endo.142.1.8054. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ. Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor alpha expression. J Neurobiol. 2001;47:176–182. doi: 10.1002/neu.1025. [DOI] [PubMed] [Google Scholar]