Abstract
To visualize and isolate live dopamine (DA)-producing neurons in the embryonic ventral mesencephalon, we generated transgenic mice expressing green fluorescent protein (GFP) under the control of the rat tyrosine hydroxylase gene promoter. In the transgenic mice, GFP expression was observed in the developing DA neurons containing tyrosine hydroxylase. The outgrowth and cue-dependent guidance of GFP-labeled axons was monitored in vitro with brain culture systems. To isolate DA neurons expressing GFP from brain tissue, cells with GFP fluorescence were sorted by fluorescence-activated cell sorting. More than 60% of the sorted GFP+ cells were positive for tyrosine hydroxylase, confirming that the population had been successfully enriched with DA neurons. The sorted GFP+ cells were transplanted into a rat model of Parkinson's disease. Some of these cells survived and innervated the host striatum, resulting in a recovery from Parkinsonian behavioral defects. This strategy for isolating an enriched population of DA neurons should be useful for cellular and molecular studies of these neurons and for clinical applications in the treatment of Parkinson's disease.
Dopamine (DA)-producing neurons, the cell bodies of which are located in the ventral midbrain, play a central role in a variety of brain functions, such as motor control, cognition, memory processing, and emotion (1, 2). The development of midbrain DA neurons provides a good model system for the investigation of molecular mechanisms underlying the determination of specific neuronal fates and axonal guidance pathways. During embryonic development, the midbrain DA neurons appear in the intermediate zone of the ventral neural tube and extend their fibers en masse to target regions, including the striatum, through selective axonal pathways (3, 4). Recent studies have identified several factors involved in the development of DA neurons (5). The axonal guidance of DA neurons toward the forebrain is regulated by chemorepulsive factors secreted from the mesencephalic floor plate and directional cues associated with the midbrain substrate (6, 7). However, the precise mechanisms controlling the development of DA neurons are not fully understood.
The gradual loss of DA neurons in the substantia nigra is responsible for most of the symptoms of Parkinson's disease (8). Intrastriatal grafting of DA-secreting cells has been reported to result in the amelioration of the motor syndrome in Parkinsonian patients and in animal models of the disease (9). Although transplantation is considered a promising therapy for Parkinson's disease, its clinical use is still restricted to a very few cases. The major limiting factors of this therapy are the difficulty in obtaining sufficient viable embryonic mesencephalic tissue and the controversial ethical and legal issues raised by the use of human fetal allografts.
In the present study, we successfully obtained the expression of green fluorescent protein (GFP) in midbrain DA neurons under the control of a tissue-specific gene promoter in transgenic mice. We show that the labeling of DA neurons with GFP is useful for their dynamic imaging in living tissue and cell culture systems. Furthermore, we isolated GFP-labeled DA neurons by fluorescence-activated cell sorting (FACS). Transplantation of the sorted cells permitted the recovery of rotation behavior in a rat model of Parkinson's disease. This strategy should be useful for cellular and molecular studies of DA neurons and the development of donor cells for transplantation into patients with Parkinson's disease.
Experimental Procedures
Generation of Transgenic Mice.
A 0.7-kb DNA fragment encoding a GFP variant from the pEGFP-N1 plasmid (CLONTECH) was inserted into the EcoRI site of the pBST-N plasmid (10). The 9-kb 5′-flanking region of the rat tyrosine hydroxylase (TH) gene from the pTH/9-kb plasmid (11) was inserted upstream of the resulting plasmid to generate pTH-GFP. The transgene was microinjected into fertilized (C57BL/6J × DBA/2J) F2 mouse eggs, which were then implanted in pseudopregnant females. We used the transgenic line termed TH-GFP 6–7 for all of the experiments presented in this paper.
Dissociated Cell Culture.
Pregnancies were dated by inspection for the vaginal plug, and the day of the plug was defined as embryonic day 0 (E0). Ventral mesencephalons (VMs) were dissected from embryos (E12.5), then digested in 0.25% trypsin at 37°C for 5 min. After washing with fresh medium, cells were triturated with a fire-polished Pasteur pipette and cultured as described (12–14).
Hemibrain Culture.
Brains were removed from embryos (E11.5), cut in half along the dorsal and ventral midlines, and placed on a collagen-coated membrane with the ventricular side down. The ventral region of the mesencephalon of transgenic embryos was resected and used as a graft. The graft was transplanted into the corresponding region in the nontransgenic brain, the VM of which had been removed. The hemibrains were cultured for 24 h in chemically defined DMEM/F12 medium containing 10% FBS, as described (7).
Cell Sorting.
FACS sorting of GFP+ cells was performed on a FACS Vantage flow cytometer/cell sorter (Becton Dickinson), essentially as described (13–16). Dead cells were excluded by gating on forward and side scatter and by elimination of cells stained with propidium iodide, a fluorescent dye labeling only dead cells. Cells harvested from wild-type mice were used to set the background fluorescence.
Immunofluorescence Analyses for Cryosections and Cultured Cells.
Cryosections and cultured cells were prepared for immunofluorescence analyses as described (14). Mouse anti-TH mAb (17) diluted by 1:500 or rabbit anti-TH antibody (18) diluted by 1:10,000 was used as the primary antibody in combination with a Cy3-conjugated secondary antibody (Jackson ImmunoResearch), diluted by 1:1,000.
Unilateral 6-Hydroxydopamine Lesions and Amphetamine-Induced Circling Behavior.
Young adult male Sprague—Dawley rats, weighing 180–200 g at the start of the experiment, were used. Unilateral lesions of the ascending mesostriatal DA pathway were made by intracerebral injections of 6-hydroxydopamine as described (19). Two weeks after the lesion surgery, amphetamine (0.25 mg/kg i.p.)-induced turning behavior was monitored over a period of 60 min. The rats that exhibited a net rotational asymmetry of at least six full turns per min toward the lesioned side were selected for transplantation surgery, because this extent of amphetamine-induced rotational asymmetry corresponds, on average, to a 99% depletion of striatal DA neurons (20). The drug-induced rotational behavior was tested again 5 weeks after grafting to examine the functional effects of the implanted GFP+ cells.
Transplantation Surgery.
The sorted GFP+ cells were spun and resuspended in a final volume of 100–150 μl Hanks' balanced salt solution. The cell viability, assessed with a trypan blue dye exclusion test, was over 95% just before grafting, and the concentration was 10,000–13,000 cells per μl. Recipient rats were divided into two groups: lesion control (n = 4) and transplantation (n = 7). In the transplantation group, two stereotaxic deposits, of 2 μl of cell suspension each, were injected into the lesioned striatum as described (19, 21). All of the engrafted rats were given i.p. injections of cyclosporin A daily (Novartis Pharma, Tokyo, Japan; 10 mg/kg), starting on the day of transplantation surgery.
Tissue Processing.
Sections of the brain with grafts were cut and processed for TH immunohistochemistry as described (19, 21). The number of TH-immunoreactive neurons in each graft was counted under bright-field illumination on every third section. The raw counts of TH-immunoreactive cells were corrected according to the formula of Abercrombie (22).
Statistical Analyses.
All data were expressed as the mean ± SEM. A paired two-tailed Student's t test was used to compare amphetamine-induced rotation scores before and after grafting. A P value of less than 0.05 was considered significant.
Results
Expression of GFP in Midbrain DA Neurons.
Transgenic mice expressing GFP in the midbrain DA neurons under the control of the 9-kb 5′ upstream sequence of the rat gene encoding TH, which is the initial and rate-limiting enzyme of catecholamine biosynthesis, were generated (Fig. 1A). This promoter region has been reported to confer cell type- and stage-specific expression of the TH gene or a reporter in catecholamine-producing cells (23, 24).
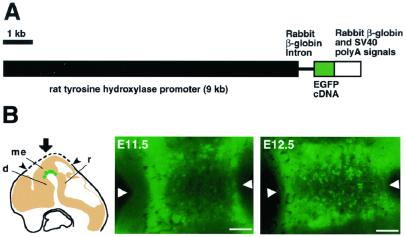
Figure 1.
TH-GFP transgenic mouse. (A) Structure of the TH-GFP transgene. (B) Early development of midbrain DA neurons labeled with GFP. Embryos were dissected at various developmental stages. As illustrated (Left), the dorsal surface of the head was cut with a razor along the dotted line, and vertical stereomicroscopic images of the VM were taken from above (arrow). d, diencephalon; me, mesencephalon; r, rhombencephalon. The position of the midline is shown by arrowheads, oriented with rostral to the left. (Scale bars = 100 μm.)
During mouse development, midbrain DA neurons are generated around the midline in the VM at E11–E13 and migrate ventrolaterally to form the primordia of the substantia nigra and ventral tegmental area (25). Therefore, we first observed whole-mounted live TH-GFP embryos at E11.5 and E12.5 with a fluorescence stereomicroscope. We could detect weak GFP fluorescence in the VM region; however, it was difficult to identify DA neurons because of the thickness of the neural tube (data not shown). To observe GFP-labeled DA neurons in the VM more clearly, we cut off the dorsal surface of the head and viewed the ventral neural tube from the ventricular side (see Fig. 1B Left). Fig. 1B Center and Right show fluorescence stereomicroscopic images of the VM region. At E11.5, a small number of GFP-labeled cell bodies were seen around the midline (Fig. 1B Center). At E12.5, the number of labeled cells increased, and they appeared to be aligned along the midline (Fig. 1B Right). The characteristic arrangement of GFP+ cells along the ventral midline and the timing of their appearance strongly suggest that the GFP was expressed in developing DA neurons in the VM of the TH-GFP mice.
To evaluate the GFP expression site in the developing mesencephalon, frozen sections were prepared from embryos at E12.5 and immunostained with an anti-TH antibody. GFP fluorescence colocalized well with TH immunoreactivity in the VM (Fig. 2 A–C), where TH is expressed only in the DA neurons (3). We found a small number of cells that expressed GFP but not TH along the midline of VM (Fig. 2C). It is possible that these GFP+TH− cells were, at least in part, precursor cells for DA neurons, because they were located near the ventricular zone neighboring the TH+ cells.
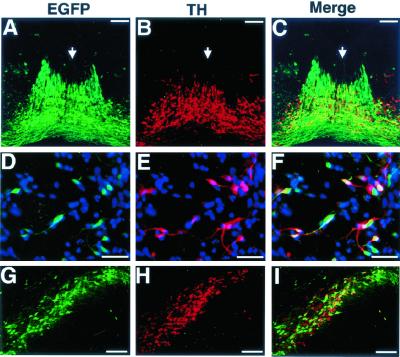
Figure 2.
Expression pattern of GFP in DA neurons from TH-GFP transgenic mice in the embryonic VM and adult substantia nigra. (A–C) Frontal frozen sections of the mesencephalon from an E12.5 TH-GFP embryo were stained with anti-TH and Cy3-conjugated secondary antibodies. (A) GFP fluorescence (green). (B) Anti-TH staining (red). (C) Merged image. (D–F) VM cells from TH-GFP mouse embryos (E12.5) were cultured for 24 h, fixed, and stained with anti-TH and Cy3-conjugated secondary antibodies. All of the nuclei were labeled with Hoechst 33342 (blue). (D) GFP fluorescence (green). (E) Anti-TH staining (red). (F) Merged image. (G–I) Section of the substantia nigra from an adult TH-GFP mouse stained with anti-TH and Cy3-conjugated secondary antibodies. (G) GFP fluorescence (green). (H) Anti-TH staining (red). (I) Merged image. (Scale bars: A–C, 100 μm; D–F, 25 μm; G–I, 150 μm.)
To examine the nature of the GFP+ cells in more detail, we cultured dissociated VM cells from TH-GFP embryos at E12.5. After 24 h in vitro, we observed GFP fluorescence in live cells with neuron-like morphology, under an inverted fluorescence microscope (data not shown). To examine the expression of TH in the GFP+ cells, the culture was fixed and stained with the anti-TH antibody (Fig. 2 D–F). Of the total number of cells counted (n = 2,045), GFP fluorescence was detected in 16% (328 cells) and TH immunoreactivity, in 11.8% (242 cells). Of the GFP+ cells (328 cells), 61.9% (203 cells) were also TH+, and 83.9% (203 cells) of the TH+ cells (242 cells) showed detectable GFP fluorescence.
We next immunostained sections taken from adult TH-GFP mouse brains (Fig. 2 G–I). We observed a number of GFP+ cells with neuronal morphology in the midbrain substantia nigra, where DA neurons are localized. Of the total number of GFP+ cells counted (n = 1287), 87.1% (1,121 cells) were TH+, and of the total TH+ cells (n = 1,210), 89.8% (1,087 cells) expressed GFP. These data indicate that in the TH-GFP transgenic mice GFP is predominantly localized in both developing and mature DA neurons.
Monitoring the Behavior of Axons from the GFP+ Neurons in Vitro.
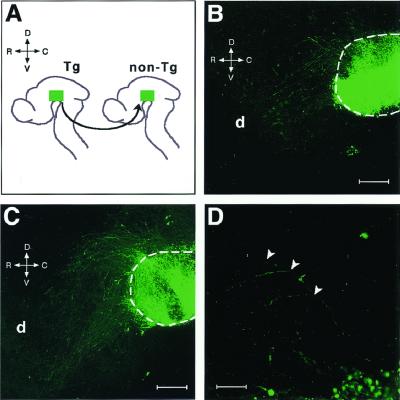
Midbrain DA neurons begin to extend their axons between E11 and E12 during mouse development. To monitor the outgrowth of axons from the GFP+ DA neurons, we prepared hemibrain cultures on a collagen-coated membrane (7). We dissected the basal plate from E11.5 transgenic embryos and transplanted the transgenic VM into the corresponding region of the nontransgenic brain (see Fig. 3A). After 18 h in culture, the neurons expressing GFP in the graft began to extend their axons, and some labeled axons entered the nontransgenic host brain across the boundary (Fig. 3B). After 24 h, the labeled axons grew toward the diencephalon along the rostrocaudal axis of the host brain (Fig. 3 C and D). These results indicate that the GFP+ axons followed the guidance cues for DA neurons in the host brain and that GFP labeling is feasible for visualizing cue-dependent axon outgrowth in this brain culture system.
Figure 3.
Rostral innervation of GFP+ axons in a flat-mounted hemibrain preparation. (A) Transplantation procedure. The basal plate explant (green) was taken from a transgenic mouse brain (Tg) and grafted into the corresponding region of a nontransgenic brain (non-Tg). The rostrocaudal (R-C) and dorsoventral (D-V) axes of the brain are indicated. After 18 h in culture (B), the labeled axons grew across the boundary between the graft and host brain (dotted line). After 24 h (C), they further extended toward the diencephalon (d) in the host brain. (D) A 4-fold magnified view of C. The arrowheads indicate the points at which the labeled axons turned in the rostral direction. (Scale bars: B and C, 200 μm; D, 50 μm.)
Isolation of DA Neurons by FACS.
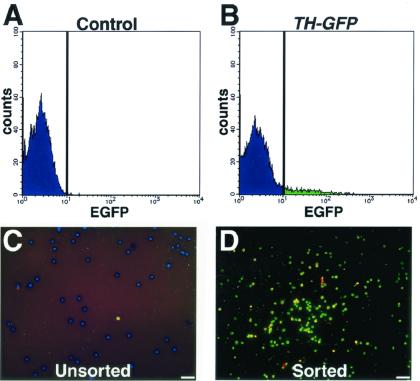
The data presented above established that the TH-GFP transgene was expressed in DA neurons in the VM. We previously established methods for using FACS to isolate directly from tissue transfected neural progenitor cells that express fluorescent reporter genes placed under the control of neural regulatory elements (26). Here we used TH-GFP mice to isolate in vivo differentiated DA neurons by FACS for further analysis of their functional properties. We enzymatically dissociated VMs from wild-type or TH-GFP embryos (E11.5–E13.5) to prepare cell suspensions and then analyzed for GFP fluorescence by flow cytometry (Fig. 4). Dead cells were excluded by gating on forward and side scatter as well as by the elimination of propidium iodide-positive events. To obtain a highly purified population of GFP+ DA neurons, cells were considered positive only if their fluorescence was brighter than that of any of the control cells, which were derived from the VMs of age- and strain-matched nontransgenic mice. By these criteria, ≈10% of the live cells were isolated as GFP+ cells. Reanalysis of the sorted cell population by flow cytometry confirmed that all of the sorted cells expressed GFP (data not shown). The sorted GFP+ cells were plated onto coverslips and cultured in DMEM/F-12 medium supplemented with 10% FBS. Almost all of the cells died within 24 h after plating, suggesting that other cells in the mesencephalon are required for the survival of DA neurons in this culture condition. We then examined the attached cells 1 h after plating, with an inverted fluorescence microscope. Virtually all of the cells showed the fluorescence of GFP (data not shown), confirming that we could obtain a pure population of cells that expressed the TH-GFP transgene. The cells were then fixed and stained with an anti-TH antibody (Fig. 4 C and D). Over 60% of the sorted cells were TH+. In contrast, control cultures of an unsorted cell population contained 10% TH+ cells, as described above (Fig. 2 D–F). Thus, sorting based on TH-GFP fluorescence yielded a population enriched for DA neurons from the embryonic VM.
Figure 4.
Isolation of GFP+ cells by FACS. Cells from wild-type (A) and TH-GFP (B) mice were analyzed with a flow cytometer/cell sorter. Unsorted (C) and sorted GFP+ (D) cells were plated onto polyethyleneimine-coated coverslips, cultured for 1 h, and then fixed and stained with an antibody against TH (red) and Hoechst 33342 (blue). The photographs are merged images of GFP fluorescence (green), anti-TH staining (red), and Hoechst 33342 (blue). (Scale bars = 25 μm.)
Functional Recovery of a Rat Model of Parkinson's Disease by Transplantation of the Sorted DA Neurons.
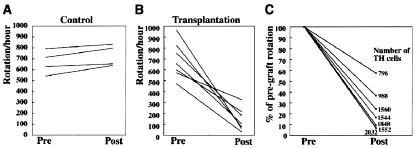
To study the function of the sorted GFP+ cells in vivo, we transplanted these cells into the brain of a rat model of Parkinson's disease (1). All of the animals with unilateral 6-hydroxydopamine lesions displayed a robust rotation response to amphetamine before transplantation, and there was no significant difference in rotational scores between the lesion control and transplantation groups (P > 0.05, unpaired two-tailed Student's t test). In the transplantation group, each rat received a total of ≈50,000 grafted GFP+ cells in the lesioned striatum. Five weeks after the GFP+ cells were grafted, although the lesion control animals did not show any improvement (P > 0.05) (Fig. 5A), a significant reduction in rotational asymmetry was observed in the grafted rats (P < 0.01) (Fig. 5B). The rotation scores of the grafted animals were reduced by 77.3% on average, compared with the pretransplantation scores.
Figure 5.
Transplanted GFP+ cells cause functional recovery in a rat model of Parkinson's disease. (A and B) Net ipsilateral amphetamine-induced rotation asymmetry over the 60-min test session. (A) Control animals (n = 4) showed no improvement. (B) All of the animals with grafts (n = 7) showed a steady improvement. (C) Reduction of the rotation scores compared with pregraft rotation. The total number of surviving TH+ cells in the striatum of each animal is also shown.
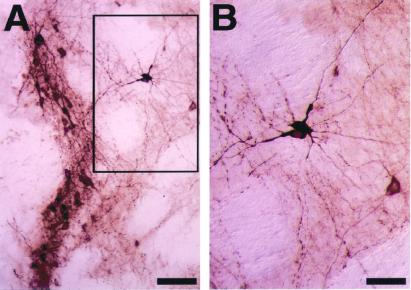
The 6-hydroxydopamine lesions of the mesostriatal bundle produced a virtually complete loss of TH+ DA neurons in the substantia nigra, with a concomitant depletion of TH-immunoreactive fibers in the lesioned striatum (data not shown). In all of the rats receiving the GFP+ cells, two grafts with TH+ cells were seen in the rostral part of the host striatum. Individual TH+ cells within the grafts displayed morphological features suggestive of mature neurons and were found to extend numerous TH+ fibers into the DA-depleted striatum (Fig. 6). The mean number of TH+ cells in the two grafts combined was 1,474 ± 167. [Cells on every third section were counted, and the value was corrected according to the formula of Abercrombie (22).] The percentage reduction in the net rotational asymmetry of individual animals was plotted against the number of surviving TH+ neurons (Fig. 5C), and linear regression analysis was performed. These two parameters were significantly correlated (P < 0.005, r2 = 0.847). Taken together, these results showed that DA neurons that are sorted based on TH-GFP expression are functionally sufficient to improve amphetamine-induced rotational asymmetry in a rat model of Parkinson's disease.
Figure 6.
Morphology of TH+ neurons in the graft. Brain sections of the 6-hydroxydopamine-lesioned animals that received sorted GFP+ cells were immunostained for TH. (A) Low-power magnification. (B) High-power magnification views of three neurons displaying the morphology of mature DA neurons in the rectangle in A. (Scale bars: A, 80 μm; B, 40 μm.)
Discussion
Here we report the generation of TH-GFP transgenic mice that express GFP in DA neurons in the embryonic VM and in the substantia nigra of the adult midbrain. With the use of a hemibrain culture system, we monitored the outgrowth and cue-dependent guidance of the labeled axons extending from these DA neurons. We also developed a protocol for the direct isolation of live DA neurons.
In this study, we used a 9-kb 5′ flanking sequence of the rat TH gene to direct the expression of GFP in the DA neurons. As expected, GFP expression was observed in a group of cells located in the VM in early embryos. GFP fluorescence was also detected in other nonmesencephalic brain regions such as the olfactory bulb that contain DA neurons (data not shown). We extensively examined the identity of the GFP-expressing cells by anti-TH immunostaining of sections and cultured cells prepared from the VM. We confirmed that most of the TH+ DA neurons expressed GFP. Moreover, we found that a population of GFP+ cells that were negative for TH exists near the ventricular zone neighboring the DA neurons in the VM. DA neurons are known to be generated in the ventricular zone along the midline in the VM from E11 to E13; they then migrate ventrally from the ventricular zone to the pial surface (25). Therefore, there is a possibility that the GFP+TH− cells near the ventricular zone are immature DA neurons that do not yet express detectable levels of TH protein. Consistent with this view, an increased percentage of the GFP+ cells expressed TH in the substantia nigra of the adult midbrain compared with the embryonic VM. Thus, the TH-GFP transgene is useful for identifying both young and mature DA neurons in vivo.
In the TH-GFP mice, GFP could be seen by fluorescence microscopy without fixation. This procedure is much simpler than the standard methods for detecting catecholaminergic neurons, such as immunohistochemistry with anti-TH antibodies and catecholamine fluorescence (3). In the neurons expressing GFP, the fluorescence was detected in the soma, dendrites, and axon. Thus, GFP imaging enabled us to monitor the development of DA neurons in live preparations.
The innervation of the forebrain by DA neurons is an excellent model for studying the mechanisms of axon guidance along the longitudinal axis. DA neurons start to extend their axons rostrally to innervate the striatum, limbic system, and neocortex at around E12 (3). The whole-mount culture system developed by Nakamura et al. (7) can mimic the development of DA axons in vivo. Using TH-GFP embryos for this culture method, we were able to monitor the real-time rostral innervation of DA neurons. These observations imply that the expression of GFP in transgenic mice is nontoxic and has no effect on the developmental processes of these neurons, which has also been discussed recently (27). Our data show that GFP labeling of DA neurons provides a simple and useful system for the study of the dynamic processes of their development.
The TH-GFP mice provided us with a means for prospective identification and direct isolation of live DA neurons. The fluorescence of GFP expressed in the DA neurons was easily detected by flow cytometry with an argon-ion laser tuned to 488 nm. Cell sorting based on TH-GFP expression yielded a population that was enriched by at least 6-fold in TH+ cells. The physiological function of the sorted cell population was then analyzed by transplanting the cells into a rat model of Parkinson's disease. Considering both the proportion of DA neurons in the sorted cells and the cell count in the graft, the survival rate of the DA neurons after intrastriatal transplantation can be estimated to be 6–10%, which is similar to those found in xenograft experiments (28, 29). Furthermore, the surviving DA neurons extensively innervated the host striatum. Correspondingly, VM tissues from the TH-GFP mice showed a massive outgrowth of axons in a collagen gel culture (data not shown). These results imply that developing DA neurons have the potential to extend their axons independently of attractive factors in their surrounding environment. The morphological features and strong TH immunoreactivity of the sorted and grafted GFP+ neurons were similar to those found in grafts after transplantation of whole VM tissue (30). All of the animals that received the sorted DA neurons in their striatum showed significant recovery in the amphetamine-induced rotation behavior. A good correlation was seen between the DA neuron cell number in the grafts and the degree of recovery (Fig. 5C), suggesting that dopamine secretion from the grafted DA neurons was responsible for the improvement of the rotational phenotype in this rat model of Parkinson's disease. Taking these observations together, we conclude that the sorted GFP neurons are functional DA neurons that can survive and innervate the adult striatum. This involves an isolation of primary DA neurons from the embryonic VM. The sorted cells should be useful material for biological analyses of DA neurons and clinical studies of neurological disorders caused by defects in DA neurons.
Fetal nigral transplantation is a clinically promising treatment for Parkinson's disease. In clinical transplantation, the number of surviving DA neurons is a crucial factor that influences the extent of functional recovery (9). Consequently, the best available strategy has been to implant mesencephalic tissues from up to seven embryos into one hemisphere of a single patient (31). The ethical and technical problems associated with obtaining such large quantities of fresh embryonic tissue mean that neural transplantation based on present techniques is unlikely to become a widely available therapy. To overcome these ethical and technical limitations, a variety of strategies have been proposed. A technique for converting neural progenitor cells whose population has been expanded in vitro into DA neurons has been reported (32). Furthermore, protocols to induce DA neuronal differentiation from embryonic stem cells have also been reported (33, 34). However, these cultures are likely to contain a variety of unidentified cells. By introducing the TH-GFP transgene into these cultures, it is possible to identify live DA neurons prospectively and isolate them by FACS (T.Y., T. Shimazaki, K.S., and H.O., unpublished results). This approach will enable us to isolate DA neurons from mixed culture populations, which are useful in transplantation therapy for Parkinson's disease.
Acknowledgments
We thank I. Nagatsu and H. Hatanaka for providing the anti-TH antibody, K. Kiuchi for preparing the primary cultures of DA neurons, Y. Hattori and M. Saito for generating transgenic mice, K. Takahashi for animal care and breeding, and S. A. Goldman for valuable discussion. This work was supported by grants from the Ministry of Education, Science, Sports, Culture, and Technology of Japan, and CREST of the Japan Science and Technology Corporation.
Abbreviations
- DA
dopamine
- FACS
fluorescence-activated cell sorting
- GFP
green fluorescent protein
- TH
tyrosine hydroxylase
- VM
ventral mesencephalon
- E
embryonic day
References
- 1.Gerfen C R. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 2.Le Moal M, Simon H. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 3.Kalsbeek A, Voorn P, Buijs R M. In: Handbook of Chemical Neuroanatomy. Björklund A, Hökfelt T, Tohyama T, editors. Vol. 10. Amsterdam: Elsevier; 1992. pp. 63–112. [Google Scholar]
- 4.Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen H J. Neuroscience. 1988;25:857–887. doi: 10.1016/0306-4522(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 5.Hynes M, Rosenthal A. Curr Opin Neurobiol. 1999;9:26–36. doi: 10.1016/s0959-4388(99)80004-x. [DOI] [PubMed] [Google Scholar]
- 6.Tamada A, Shirasaki R, Murakami F. Neuron. 1995;14:1083–1093. doi: 10.1016/0896-6273(95)90347-x. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura S, Ito Y, Shirasaki R, Murakami F. J Neurosci. 2000;20:4112–4119. doi: 10.1523/JNEUROSCI.20-11-04112.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagatsu T, Yamaguchi T, Rahman M K, Trocewicz J, Oka K, Hirata Y, Nagatsu I, Narabayashi H, Kondo T, Iizuka R. In: Advances in Neurology. Hasseler RG, Christ J F, editors. Vol. 40. New York: Raven; 1984. pp. 467–473. [PubMed] [Google Scholar]
- 9.Björklund A, Lindvall O. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, Sasaoka T, Morita S, Nagatsu I, Iguchi A, Kurosawa Y, Fujita K, Nomura T, Kimura M, Katsuki M, Nagatsu T. Proc Natl Acad Sci USA. 1992;89:1631–1635. doi: 10.1073/pnas.89.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwawaki T, Kohno K, Kobayashi K. Biochem Biophys Res Commun. 2000;274:590–595. doi: 10.1006/bbrc.2000.3204. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko Y, Sakakibara S, Imai T, Suzuki A, Nakamura Y, Sawamoto K, Ogawa Y, Toyama Y, Miyata T, Okano H. Dev Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi A, Miyata T, Sawamoto K, Takashita N, Murayama A, Akamatsu W, Ogawa M, Okabe M, Tano Y, Goldman S A, Okano H. Mol Cell Neurosci. 2001;17:259–273. doi: 10.1006/mcne.2000.0925. [DOI] [PubMed] [Google Scholar]
- 14.Sawamoto, K., Nakao, N., Kakishita, K., Ogawa, Y., Toyama, Y., Yamamoto, A., Yamaguchi, M., Mori, K., Goldman, S. A., Itakura, T., et al. (2001) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 15.Sawamoto, K., Yamamoto, A., Kawaguchi, A., Yamaguchi, M., Mori, K., Goldman, S. A. & Okano, H. (2001) J. Neurosci. Res., in press. [DOI] [PubMed]
- 16.Roy N S, Wang S, Jiang L, Kang J, Restelli C, Fraser R A R, Couldwell W T, Kawaguchi A, Okano H, Nedergaard M, et al. Nat Med. 2000;6:271–278. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 17.Hatanaka H, Arimatsu Y. Neurosci Res. 1984;1:253–263. doi: 10.1016/s0168-0102(84)80004-8. [DOI] [PubMed] [Google Scholar]
- 18.Nagatsu I, Komori K, Miura K, Sakai M, Karasawa N, Yamada K. Biomed Res. 1989;10:277–286. [Google Scholar]
- 19.Nakao N, Ogura M, Nakai K, Itakura T. J Neurosci. 1998;18:1806–1817. doi: 10.1523/JNEUROSCI.18-05-01806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt R H, Björklund A, Stenevi U, Dunnett S B, Gage F H. Acta Physiol Scand Suppl. 1983;522:19–28. [PubMed] [Google Scholar]
- 21.Nakao N, Frodl E M, Duan W M, Widner H, Brundin P. Proc Natl Acad Sci USA. 1994;91:12408–12412. doi: 10.1073/pnas.91.26.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abercrombie M. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 23.Kim S J, Lee J W, Chun H S, Joh T H, Son J H. Mol Cells. 1997;7:394–398. [PubMed] [Google Scholar]
- 24.Min N, Joh T H, Kim K S, Peng C, Son J H. Mol Brain Res. 1994;27:281–289. doi: 10.1016/0169-328x(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 25.Kawano H, Ohyama K, Kawamura K, Nagatsu I. Dev Brain Res. 1995;86:101–113. doi: 10.1016/0165-3806(95)00018-9. [DOI] [PubMed] [Google Scholar]
- 26.Okano H, Goldman S A. NeuroSci News. 2000;3:27–31. [Google Scholar]
- 27.Goldman S A, Roy N. Nat Med. 2000;6:483–484. doi: 10.1038/74920. [DOI] [PubMed] [Google Scholar]
- 28.Frodl E M, Duan W M, Sauer H, Kupsch A, Brundin P. Brain Res. 1994;647:286–298. doi: 10.1016/0006-8993(94)91328-5. [DOI] [PubMed] [Google Scholar]
- 29.Nakao N, Frodl E M, Widner H, Carlson E, Eggerding F A, Epstein C J, Brundin P. Nat Med. 1995;1:226–231. doi: 10.1038/nm0395-226. [DOI] [PubMed] [Google Scholar]
- 30.Spenger C, Haque N S, Studer L, Evtouchenko L, Wagner B, Buhler B, Lendahl U, Dunnett S B, Seiler R W. Exp Brain Res. 1996;112:47–57. doi: 10.1007/BF00227177. [DOI] [PubMed] [Google Scholar]
- 31.Kordower J H, Freeman T B, Snow B J, Vingerhoets F J G, Mufson E J, Sanberg P R, Hauser R A, Smith D A, Nauert G M, Peri D P, et al. N Engl J Med. 1995;332:1118–1123. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 32.Studer L, Tabar V, McKay R D. Nat Neurosci. 1998;1:290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa S, Sasai Y. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 34.Lee S H, Lumelsky N, Studer L, Auerbach J M, McKay R D. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]