Abstract
Life and societies would significantly change if memory capacity or persistence in health and disease could be enhanced. It has been known for many years that memory can be improved and strengthened. Substances known to enhance memory include hormones, neurotransmitters, neuropeptides, and metabolic substrates. Recently, attention has been given to identifying the molecular mechanisms and targets whereby memory enhancement can be achieved. One approach would be to target the physiological changes that are induced by learning and naturally required for memory strengthening via consolidation and reconsolidation. Here we will review approaches that boost memories by targeting the cAMP response element binding protein-CCAAT enhancer binding protein (CREB-C/EBP) pathway and/or its recently identified target gene insulin-like growth factor 2 (IGF-2).
Keywords: cognitive enhancer, memory consolidation, reconsolidation, growth factor
Introduction
The continuing increase in life span is unfortunately associated with an increased prevalence of cognitive dysfunctions, including memory loss and diminished ability to form new long-term memories. Aging-related memory impairment is a common condition characterized by mild symptoms of cognitive decline. The aged population becomes slower in processing, storing, and recalling new information, and shows impairments in cognitive functioning including memory, concentration, and organization. “I forget names so easily now” or “I forget where I put things all the time” are common complaints of the aged. More dramatic and devastating are the cognitive and memory losses in neurodegenerative diseases such as Alzheimer’s Disease (AD), in which the impairments interfere with normal life functioning. The economic impact of Alzheimer’s disease in the U.S. alone is estimated to be more than $100 billion annually 1. Thus, it is imperative to identify or develop approaches that can restore or prevent memory impairments. Furthermore, ethical debates aside, the possibility of speeding normal learning and boosting memory retention is appealing to the healthy population. Hence, identifying memory enhancers is of great importance and interest.
Numerous studies have indicated that memory can be enhanced with many types of strategies or drugs that can either boost the baseline of the biological systems or modulate memory strengthening or retrieval. These drugs modulate neurotransmission, neurotransmitter receptors, neuropeptide 2–4, stress and relative hormones 5, 6 and metabolism 7, 8. The literature on this topic and the list of effective drugs is vast. Here, we focus our discussions on an ensemble of mechanisms that can be targeted to boost memories because they underlie the memory processes known as consolidation and reconsolidation. Indeed one approach that can be employed to search for mechanisms and targets of memory enhancement is to identify and exploit the biological mechanisms by which learned information transforms into long-lasting memory. In humans, temporal lobe-dependent memories of facts, places, and time undergo a process known as consolidation by which newly learned information that is in a labile state becomes a long-lasting, stable memory 9. Consolidation requires a phase of de-novo gene expression that lasts for more than 24 hours; its interruption results in memory loss 10, 11. Moreover, once stable and resilient to disruption, memories can again become labile and sensitive to interference if they are reactivated by, for example, recalling the memory. Memories then regain their resilience to interference by undergoing a process known as reconsolidation 12–15. In several types of memories, reconsolidation has been found to be temporally limited. In fact, in rodents only young memories (weeks-old), not old (months-old) ones are lost after amnesic agents are given together with memory reactivation 16–20. It has been proposed that, in animal models, reconsolidation is a phase of lingering consolidation that can last for weeks 15, 21. If memory is retrieved during this sensitive phase, it undergoes reconsolidation to strengthen the memory itself, indicating that an important function of the postretrieval reconsolidation process is to promote memory strengthening and persistence 15, 22. Thus, in principle, a strategy that potentiates either the initial consolidation or the reconsolidation phases may be successfully exploited as an effective cognitive enhancer. In agreement with this hypothesis, various gene mutations targeting the pathways known to be involved in long-term plasticity or memory formation result in memory enhancement. Excellent reviews have summarized and discussed these mutants 23, 24.
In this review, we discuss the hypothesis that memory can be enhanced by targeting a variety of steps of the cAMP - cAMP response element binding protein – CCAAT enhancer binding protein (CREB-C/EBP)-dependent gene cascade, which has been shown to be required for memory consolidation in numerous species 25. After a brief summary of current knowledge, we will expand on the effect of a target gene, insulin-like growth factor 2 (IGF-2, also known as IGF-II), which is regulated by the CREB-C/EBP pathway 26. We will also discuss memory enhancement in reconsolidation and relative temporal constraints and conclude with some unresolved questions about memory enhancement and its potential clinical applications.
Promoting memory enhancement by targeting the cAMP-CREB-C/EBP pathway
Studies of the molecular bases of long-term memory formation started with the findings in simple invertebrates that cAMP and the induction of CREB- and C/EBP-dependent gene expression have a key function in synaptic plasticity and memory formation 27–29 particularly during the temporal window of consolidation, when memory is still labile 9, 30, 31. Specifically, in invertebrates, including Aplysia californica and Drosophila melanogaster, the molecular disruption of CREB or C/EBP isoforms leads to memory impairment 25. Similarly, molecular and genetic disruptions of either CREB or C/EBP isoforms in rodents impair different types of long-term memories, including spatial, contextual appetitive and aversive, leading to the conclusion that the cAMP-CREB-C/EBP pathway has an evolutionarily conserved, fundamental role in long-term memory formation 25, 32–34. Some authors have found that the essential function of CREB in memory formation may be overcome by genetic backgrounds and that CREB-independent forms of long-term plasticity and memory exist 35. Despite the fact that, indeed, some form of memory and/or long-term potentiation (LTP) may be CREB-independent, these contrasting effects may be due to protein compensation of the transgene that can overcome the requirement for CREB. Hence, in summary, numerous studies across species, brain regions and types of learning paradigms indicate that CREB is critical for memory formation. Despite the possiblity that memory consolidation may also be enhanced independently of the CREB pathway, in this review, we will focus on discussing nodes of the CREB-C/EBP pathway that represent important targets for promoting memory enhancement and persistence.
Several intracellular signaling pathways have been shown to activate the CREB-C/EBP cascade 25 (Figure 1), which include important targets for both memory disruption and strengthening. Selective modulation of specific components of this pathway may lead to the enhancement of memories, and furthermore, make them more persistent. Indeed, studies have demonstrated that this is the case.
Figure 1.
Schematic representation of the cAMP response element binding protein (CREB)-CCAAT enhancer binding protein (C/EBP) pathway activated during memory formation. Neurotransmitters, growth factors, and membrane depolarization are examples of the stimuli that activate intracellular signal transduction pathways that can lead to the activation of the CREB-dependent pathway. Growth factors, signal via receptor tyrosine kinase (RTK), which upon ligand binding and dimerization, induces activation of two pathways: the Ras/Raf/ mitogen-activated protein kinase (MAPK)/ MAP kinase kinase (MEK) and the phosphoinositide 3-kinase (PI3K)-dependent pathways. Activations of these pathways recruit additional protein kinases including p90 ribosomal S6 kinase (RSK2) and mitogen- and stress-activated protein kinase (MSK) for the MAPK-dependent pathway and Akt and p70S6 kinase(p70S6K) for the PI3K-dependent pathway to catalyze phosphoryation of CREB (pCREB) in its Ser-133 residue, which is an important step for its activation 25. CREB phosphoryation can also be achieved by many neurotransmitters binding to their receptors. Via these receptors, neurotransmitters can couple of cyclic adenosine monophosphate (cAMP) by regulating adenylyl cyclase activity. cAMP recruits protein kinase A (PKA) as the main kinase for CREB phosphorylation. Phosphodiesterase (PDE) can catalyze the hydrolysis of cAMP and inhibit its signaling. Additionally, increases in intracellular Ca2+ influx through voltage- or ligand-gated cation channels such as voltage-sensitive calcium channels (VSCCs) or NMDA receptors (NMDARs) can also lead to CREB phosphorylation via different calcium-dependent protein kinases 25. Once phosphorylated, CREB recruits its transcription coactivator CREB-binding protein (CBP) to promote transcription 62. The functional activation of CREB leads to the expression of target genes, among which are immediate early genes (IEGs), such as the transcription factor C/EBP 27, 108, which, in turn, regulates the expression of late response genes including insulin-like growth factor 2 (IGF-2) 26. Transcriptional regulation is further regulated by the chromatin state. In general, histone acetyl transferases (HATs) acetylate histone tails and promote a relaxed chromatin state and transcription. Histone deacetylases (HDACs), on the other hand, de-acetylate histones and promote a condensed chromatin state and genes silencing 61. Abbreviations: AC,, adenylyl cyclase; ATP, adenosine triphosphate.
The expression of an activated form of CREB in both Drosophila melanogaster and neuronal cultures of Aplysia californica was found to lead to long-term memory and/or plasticity from learning or stimulation paradigms that evoke only short-term memory or plasticity under control conditions 28, 36 (but see 37). Similarly, overexpression of C/EBP in Aplysia neurons converted short-term plasticity into long-term plasticity 38. These findings were the first evidence that, if the molecular cascade involved in long-term plasticity and memory formation is overstimulated, memory retention can be enhanced or made more persistent.
Similar results were found in mammalian systems. Overexpression of a neuronal-specific adenylyl cyclase 1 (AC1), an enzyme that catalyzes the synthesis of cAMP, in the forebrain of mice, resulted in enhanced object recognition and contextual fear conditioning memories 39. Injections of a protein kinase A (PKA) activator, Sp-cAMPs, into the amygdala enhanced reward-related learning in a dose-dependent manner 40. Furthermore, rolipram, an inhibitor of the phosphodiesterase 4 (PDE4) family of enzymes, which catalyze cAMP hydrolysis and thereby increases cAMP concentrations and CREB phosphorylation, significantly enhanced memory retention in rodent contextual fear conditioning 41, object recognition 42, and several spatial tasks 43. In agreement with these results, recent studies have shown that both PDE4 subtype D (PDE4D) deficient mice, and mice in which PDE4D in the dorsal hippocampus was knocked down by lentiviral vectors have enhanced spatial and object recognition memories 44. Inhibitors of other PDEs, for example, the selective PDE5 inhibitors sidenafil, zaprinast, and vardenafil, which can lead to an increase in CREB phosphorylation, have also shown enhancing effects in spatial object recognition and active avoidance memories in rodents 45, 46, although others have reported contrasting findings 47.
Memory enhancing effects have also been found by directly targeting the expression of CREB. Viral vector-mediated overexpression of CREB in the rat amygdala or mouse hippocampus respectively enhanced cued and contextual fear conditioning 48, 49. Transgenic overexpression of dominant active forms of CREB in the forebrain resulted in enhanced memory retention in a variety of tasks, including fear conditioning, social recognition, the Morris water maze, and passive avoidance 50.
How does boosting CREB-dependent transcription activity promote memory enhancement? Probably by targeting many mechanistic levels. For example, CREB transcribes proteins that are necessary for the stabilization of memory after learning. Thus, enhancing CREB activity may augment the levels of these critical proteins, resulting in stronger memories. A series of elegant studies has increased our understanding of how CREB regulates memory formation, thereby mediating memory retention. In the lateral amygdala, neurons with viral-mediated overexpression of CREB are more likely to be active after memory retrieval than are their neighboring neurons, and selective ablation of these CREB-overexpressing neurons leads to memory impairment 51, 52, suggesting that auditory fear conditioning can recruit CREB-expressing neurons in the lateral amygdala to establish fear memory formation. Furthermore, viral-mediated overexpression of CREB in the amgydala enhances synaptic transmission and increases neuronal excitability 53. Similar increases in neuronal excitability are seen in the dorsal hippocampus of transgenic mice expressing a constitutively active form of CREB 54, leading to the conclusion that memory enhancement correlates with increased neuronal excitability that is mediated, at least in part, by increased CREB expression and/or its functions.
Furthermore, activation of the CREB-C/EBP cascade is regulated by inhibitory constraints 55–58, and the removal of which results in stronger or more persistent memories. For example, mutant mice with reduced expression of the general control nonrepressed 2 (GCN2) kinase, an eukaryotic initiation factor 2 α (eIF2α) kinase, have suppressed expression of activating transcription factor 4 (ATF4) mRNA, a repressor of CREB-mediated gene expression, and display enhanced spatial memory, as assessed in the Morris water maze 59. Likewise, mutant mice with reduced phosphorylation of eIF2α exhibit enhanced memory in a variety of tasks 60. Similarly, transgenic mice with forebrain expression of a general dominant negative inhibitor of the C/EBP/ATF family (EGFP-AZIP) have enhanced spatial memory retention. EGFP-AZIP expression presumably relieves the inhibitory regulators and results in a lower threshold for LTP induction as well as increased spatial memory retention 57. In summary, any molecular step that regulates induction of the CREB- and/or C/EBP-dependent gene expression may, in principle, be a good target for promoting memory enhancement.
As with all transcription factors, CREB and C/EBP functions on gene expression are controlled by the state and regulation of chromatin. Chromatin modification can modulate the transcription necessary for memory formation by regulating access of transcriptional regulatory proteins to deoxyribonucleic acid (DNA). For example, histone acetylation is a chromatin modification that regulates DNA-histone interactions via two opposing classes of enzymes: histone acetyltransferases (HATs), which acetylate histone tails and promote transcription, and histone deacetylases (HDACs), which de-acetylate histones and promote genes silencing 61. One important function of CREB is to recruit CREB-binding protein (CBP), a cofactor with intrinsic histone acetyl transferase activity, which results in increased gene transcription 62. Genetic mutations in CBP are associated with Rubinstein-Taybi syndrome, which is characterized by facial abnormalities, broad thumbs and mental retardation 63. Mutant mice lacking one functional copy of CBP exhibit impaired long-term inhibitory avoidance and contextual fear conditioning memories 64. More specifically, transgenic mice with a functional loss of CBP’s HAT activity have impaired long-term memory formation 65, while injections of HDAC inhibitors, which would compensate for deficits in CBP activity in CBP mutant mice, rescue the memory impairment 65, 66. Similarly, PDE4 inhibitors, which enhance CREB signaling, also dose-dependently rescue the long-term memory defects in CBP mutants 67. These studies suggest that modulating CREB-dependent signaling or the state of chromatin via HDAC inhibitors may be potential therapeutic options for cognitive disorders associated with CBP dysfunction. In agreement with this hypothesis, in wild-type mice, HDAC inhibitors have been found to enhance long-term memory in a variety of tasks, including contextual fear conditioning 68, water maze 69, fear-potentiated startle 70, extinction of fear memory 71, and novel object recognition 72. Several extensive reviews have been published on HDAC inhibitors and their potential applications to treat cognitive disorders 73–75.
One step downstream of the activation of CREBs and C/EBPs is regulation of the expression of their target genes. Some of these target genes may include secreted factors or membrane receptors that can be more easily targeted by pharmacological approaches. One of these genes, IGF-2, was recently identified 26.
IGF-2, a C/EBP target gene required for memory consolidation, promotes memory enhancement
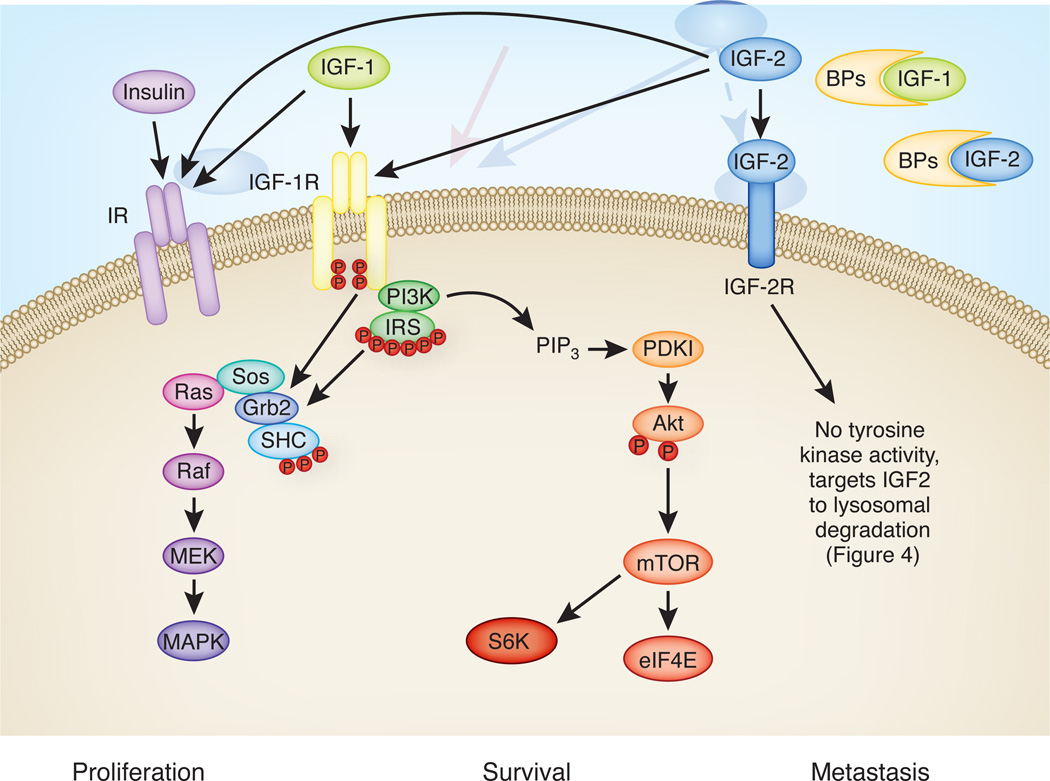
IGF-2 has been recently identified as a target gene of C/EBPβ during memory consolidation in rats 26. IGF-2 is a mitogenic polypeptide that is structurally similar to insulin and IGF-1. Insulin, IGFs, and their respective receptors, together with IGF-binding proteins (IGFBP), constitute the IGF-related system (Figure 2). This protein system is important for normal somatic growth and development, tissue repair and regeneration 76. IGF-2, as compared to the other family members, is more abundantly expressed in the adult brain and is found in regions that are critically involved in memory consolidation, such as the hippocampus and cortex 77. The mechanism of action of IGF-2 is not clear and may be multifaceted 78. The highest affinity of IGF-2 is for IGF-2 receptor (IGF- 2R). Beside IGF-2, IGF-2R binds a large number of ligands including lysosomal enzymes, IGF-2, transforming growth factor-β (TGF-β), granzyme B, plasminogen, glycosylated leychemia inhibitory factor and retinoids 79. The majority of IGF-2Rs are located intracellularly and are also known as cation-independent mannose 6 phosphate receptors, whereby they facilitates the trafficking of lysosomal enzymes between the trans-Golgi network, endosomes and lysosomes 79. Cell surface IGF-2Rs sequester circulating and local levels of IGF-2, thereby leading to IGF-2 degradation. IGF-2Rs can also mediate the endocytosis of extracellular lysosomal enzymes 80. IGF-2 can also bind with lower affinity to IGF-1 receptors (IGF-1Rs), which resulted in the downstream activation of typical tyrosine kinase-mediated growth pathways 78.
Figure 2.
The insulin-like growth factor (IGF)-related system components. The IGF-related system is composed of three ligands: insulin, IGF-1 and IGF-2. IGF-1 and IGF-2 levels in circulation are regulated by IGF binding proteins (BPs). There are multiple receptor conformations. Insulin and IGF-1 receptor (IR and IGF-1R) are tyrosine kinase receptors. Two isoforms of IR, IR-A and IR-B, are found in the brain 109. For simplicity, only signaling initiated by the activated IGF-1R is shown. Activation of IR or IGF-1R leads to phosphorylation of adaptor proteins belonging to the insulin receptor substrate (IRS) family or Src homology 2 domain containing transforming protein (SHC) 110. Activation of IRS and SHC leads to activation of Raf/Ras/ mitogen-activated protein kinase (MAPK)/ MAP kinase kinase (MEK) and phosphoinositide 3-kinase (PI3K)/Akt pathways. Phosphorylation of Akt leads to subsequent activation of mammalian target of rapamycin (mTOR), eukaryotic translation initiation factor 4E (eIF4E), and p70S6 kinase (S6K). Activation of these pathways leads to enhanced proliferation, survival and metastasis in cancer cells 110. IGF-2 binds IR-A 111, IGF-1R, and IGF-2R 110, with the highest affinity to IGF-2R. IGF-2R is structurally distinct from IR and IGF-1R and is not a receptor tyrosine kinase. Once IGF-2 binds, IGF-2R targets IGF-2 to endocytosis-mediated lysosomal degradation as well as effecting signal transduction 85 (see Figure 4). Abbreviations: Grb2, growth factor receptor-bound protein 2; PDK1, phosphoinositide-dependent kinase-1; PIP3, Phosphatidylinositol (3,4,5)-trisphosphate.
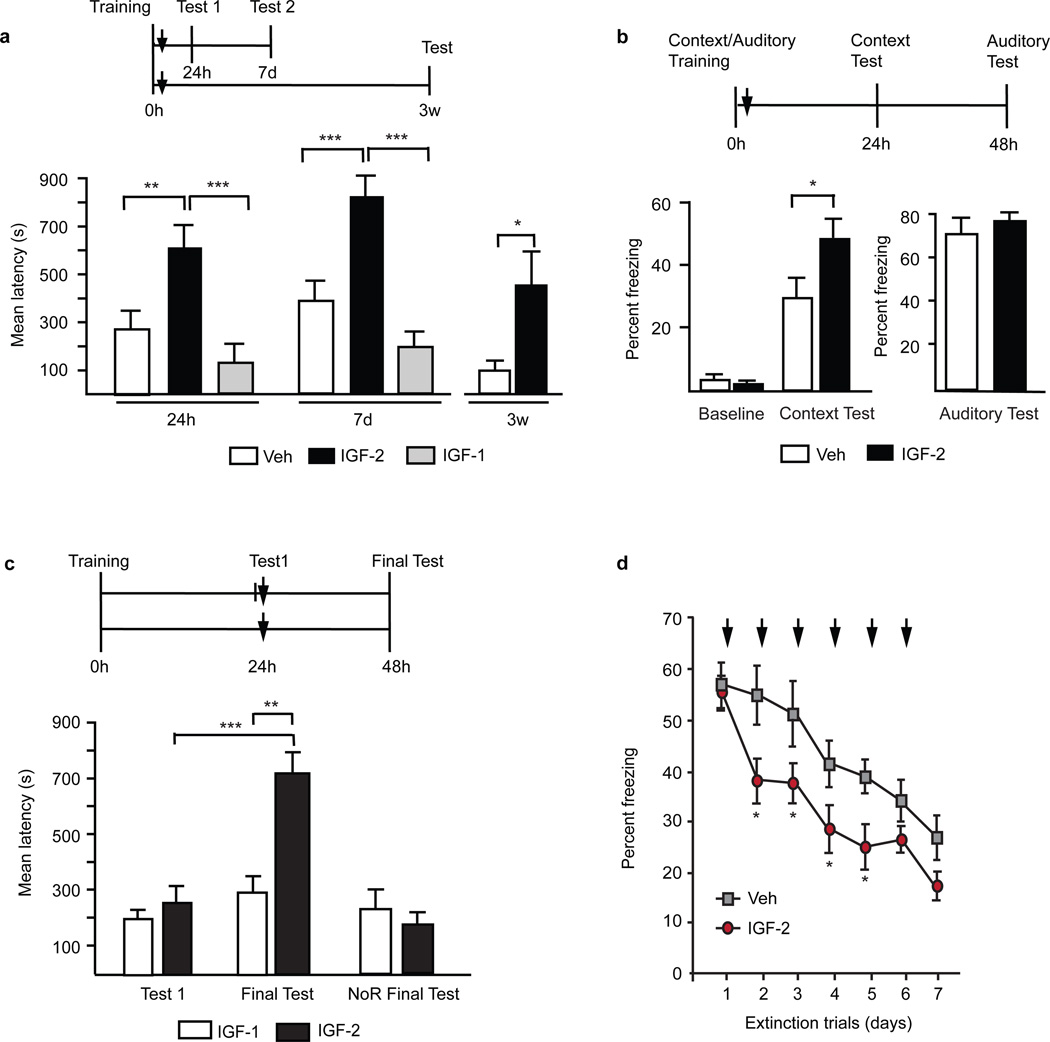
IGF-2 mRNA and protein levels were found to be upregulated in the rat hippocampus 20 hours after inhibitory avoidance training 26. Inhibitory avoidance is a contextual fear conditioning based task that requires an intact hippocampus and amygdala and is used as a model of temporal lobe-dependent memories. This training-dependent induction in the hippocampus was dependent on the binding of C/EBPβ to IGF-2 promoters 26, in agreement with previous findings indicating that the IGF-2 promoters bears C/EBPβ consensus sequences 81. Furthermore, either antisense mediated knockdown of IGF-2 in the dorsal hippocampus or injections of a function-neutralizing anti-IGF-2 receptor (anti-IGF-2R) antibody significantly impaired long-term inhibitory avoidance memory, suggesting that the learning-dependent increase in IGF-2 (via IGF-2R) is required for inhibitory avoidance memory consolidation 26. Conversely, injections of recombinant IGF-2 into the dorsal hippocampus immediately after training, but not 24 hours later, significantly and dose-dependently enhanced long-term inhibitory avoidance and contextual fear memory retention (Figure 3a and 3b), as well as preventing memory loss (Figure 3a) 26. In fact, rats given a single hippocampal injection of IGF-2 immediately after training still had significant memory retention 3 weeks after training, while vehicle-injected control rats had largely forgotten (Figure 3a) 26. Injections of IGF-2 into the amygdala had no effect on memory retention, suggesting that the mechanisms regulated by IGF-2 in memory enhancement target selective regions like the hippocampus but not the amygdala 26. Furthermore, hippocampal injections of IGF-2 have also been found to facilitate fear extinction in mice (Figure 3d) 82, confirming and extending the conclusion that IGF-2 promotes memory enhancement. It will be important to investigate other brain regions as targets of IGF-2-mediated memory enhancement, such as the cortex. Such future studies will help to address questions of region-specific mechanisms and/or selective effects on specific memory systems in memory enhancement.
Figure 3.
Insulin-like growth factor 2 (IGF-2) enhances fear as well as fear extinction memories. a) Rats underwent inhibitory avoidance training. Immediately after training, they received bilateral intra-hippocampal injections of IGF-1, IGF-2, or vehicle solution (indicated by ↓). Memory retention was tested at 24 hours (Test 1) and 7 days (Test 2) after training. Rats that received IGF-2, compared to IGF-1 and vehicle solution, had significantly higher memory retention at both tests 26. Similar intra-hippocampal injections of IGF-2 immediately after inhibitory avoidance training, compared to vehicle solution, significantly prevented memory forgetting. Rats injected with IGF-2 immediately after inhibitory avoidance training showed significantly higher memory retention at 3 weeks after training (Test) while the rats that received vehicle solution showed a significant memory retention decay 26. b). Rats underwent contextual/auditory fear conditioning, in which the rats associate a context and a tone to a footshock and freeze in subsequent exposure to the training context or to the tone in a different context. Immediately after conditioning, the rats received bilateral intra-hippocampal injections of IGF-2 or vehicle (indicated by ↓). Rats that were injected with IGF-2, compared to vehicle solution, had significantly higher freezing score when they were tested in the training context. In contrast, no effect was seen on the auditory fear-conditioning test, as rats injected with IGF-2 had freezing scores similar to rats injected with vehicle26. c) Rats underwent inhibitory avoidance training and were divided into two groups. Twenty-four hours later, one group received a bilateral intra-hippocampal injection of either IGF-2 or IGF-1 whereas the other group underwent inhibitory avoidance memory retrieval (Test 1) and immediately thereafter received similar bilateral intra-hippocampal injections (indicated by ↓). All groups were tested for memory retention 48 hours after training (Final Test). IGF-2 had no effect when given 24 hours after training (NoR Final test), but, if given in concert with memory retrieval, compared to IGF-1, significantly enhanced memory retention 26. d) Mice underwent contextual fear conditioning. Context-dependent freezing was assessed 24 hour later. Extinction of contextual fear was performed on consecutive days at 24-hour intervals consisting of re-exposure to the training context without footshock. Fear extinction was significantly enhanced in mice that received injections of IGF-2 into the dorsal hippocampus immediately after each extinction trial (indicated by ↓) compared to the vehicle-injected group 82. Data are shown as mean latency or % freezing ± s.e.m.; *P < 0.05, **P< 0.01, ***P< 0.001. Adapted, with permission, from [26] (A–C) and [82] (D).
IGF-2 is an interesting candidate for potential clinical applications because it is a naturally produced growth factor that readily crosses the blood-brain barrier. In the adult rat, IGF-2 mRNA can be found in the brain, heart, kidney, uterus, and liver 83, and peripheral IGF-2 can be transported into the brain via IGF-2 receptors localized in brain capillaries 84. The temporally limited effect of hippocampally-injected IGF-2 that occurs from the time of training (ie. less than 24 hours) as well as that of the post-retrieval effect in young but not old memories (discussed below) indicates that both consolidation or reconsolidation mechanisms can be targeted to produce memory enhancement. Mechanistically, IGF-2-dependent memory enhancement requires the function of IGF-2R, but not IGF-1R 26. IGF-2R, unlike insulin- and IGF-1R, is not a tyrosine kinase receptor 85. While insulin and IGF-1R mediate growth and activate the ras/raf/mitogen activated protein kinase (MAPK) and phosphatidylinositol 3’-kinase (PI3K)/Akt pathways (Figure 2), IGF-2R/mannose-6-phosphate receptor either binds IGF-2 extracellularly and/or transports lysosomal acid hydrolase precursors from the Golgi apparatus to the lysosome 79 (Figure 4). IGF-2Rs from both the cell surface and Golgi traffic to the early endosome, where the relatively low pH environment causes the release of the IGF-2Rs cargo. The IGF-2Rs are recycled back to the Golgi by the retromer complex. The cargo proteins are then trafficked to the lysosome via the late endosome independently of the IGF-2Rs 79 (Figure 4). How these and potentially other functions of IGF-2Rs - lead to memory enhancement is currently unknown.
Figure 4.
Insulin-like growth factor 2 receptor (IGF-2R)-dependent lysosomal enzyme trafficking, signaling transduction and endocytosis. Intracellular or extracellular lysosomal enzymes such as acid hydrolase are transported to late endosomes by IGF-2R (also known as mannose 6-phosphate receptor) 80. Lysosomal enzymes dissociate from IGF-2R within the low-pH environment of late endosomes and are subsequently delivered to lysosomes. IGF-2R is recycled to the Golgi or to the cell surface 79. At the cell surface, IGF-2 binding to IGF-2R is followed by endocytosis to form early endosomes. From the early endosomes, IGF-2R dissociates from IGF-2: IGF-2R is recycled to cell surface while IGF-2 is targeted to late endosome for lysosomal degradation 79. A recent study 26 suggests that, in the hippocampus, learning leads to IGF-2 binding to cell surface IGF-2R which results in glycogen synthase kinase 3β (GSK3β) activation and activity-regulated cytoskeletal-associated protein (Arc) synthesis, both of which have been implicated in regulating AMPA receptor (AMPAR) endocytosis 88, 93. Internalized AMPARs can recycle to cell surface or be targeted for degradation 112. IGF-2/IGF-2Rs could also directly regulate AMPAR endocytosis independently of GSK3β and Arc.
IGF-2-mediated memory enhancement also requires de novo protein synthesis. Although IGF-2 is a target gene of C/EBPβ, IGF-2-mediated memory enhancement does not depend on de novo C/EBPβ synthesis and does not correlate with increased levels of pCREB and C/EBPβ26. This suggests that IGF-2 does not exert its effects by amplifying these transcriptional mechanisms, but rather by acting on translational and synaptic mechanisms that enhance memory. Interestingly, one of the newly synthesized proteins required for IGF-2-mediated memory enhancement is activity-regulated cytoskeletal-associated protein (Arc); its functions are needed for long-term plasticity and memory formation. For example, Arc is required in the hippocampus and the amygdala, respectively, for the consolidation of spatial memory and cued-fear conditioning 86, 87. Studies have suggested that Arc is critical for long-term plasticity and memory because, by interacting with the endocytic machinery proteins endophilin and dynamin, it regulates membrane trafficking of AMPA receptors 88, 89. AMPA receptor trafficking is critically involved in LTP, long-term depression (LTD), and memory. It is thought that AMPA receptor subunits are rapidly transported in and out of synapses to strengthen or weaken synaptic activity during plasticity and learning 90, 91. In agreement with this hypothesis of a positive correlation between plasticity and memory formation and increased trafficking of synaptic AMPA receptors, IGF-2-mediated memory enhancement was found to correlate with increased levels of synaptic GluA1 AMPA receptor subunits 26.
Endocytosis is also regulated by glycogen synthase kinase 3β (GSK3β), a serine/threonine kinase that was originally identified as a regulator of cell metabolism but has since been implicated in various functions, including proliferation, cell survival, neural development, and shaping nerve terminal development and function 92. In fact, endocytosis was found to be dependent on the phosphorylation of dynamin I (at the Ser 774 residue) by GSK3β93. IGF-2-mediated memory enhancement correlates with the activation of GSK3β and requires the activity of GSK3 26, supporting our hypothesis that the memory-enhancing effect critically targets functions of the endocytic pathway. Previous findings suggested a correlation between increased endocytosis and increased GSK3 activation and Arc expression 88, 93. Such findings appear to contrast with the finding that memory enhancement is accompanied by increased synaptic expression of certain receptor subunits (e.g. GluA1) 26. However, it is possible that endocytosis of other receptors and related membrane synapse restructuring are also critical for enhancing synaptic strength. Moreover, increased endocytosis may play an important role in homeostatic synaptic scaling, which may enhance the system capacity. Future studies will hopefully extend our knowledge regarding the role of endocytosis in memory enhancement.
An intriguing property of IGF-2 and IGF-2R is that they are both imprinted genes. Imprinted genes are expressed only by one allele, either maternal or paternal, and this pattern of expression is maintained epigenetically in almost all tissues. How imprinted genes participate in behavior is a topic of great interest and recent investigations 94–96.
Interestingly, insulin and other insulin regulatory proteins have been shown to promote memory enhancement in rodents as well as in humans 97–99. However the mechanisms by which this effect occurs seems to be different from that of IGF-2. Further studies are needed to better dissect the mechanisms of these effects.
Targeting reconsolidation to enhance memory
Why does memory become labile after retrieval and undergo reconsolidation? The biological purpose of reconsolidation has been the topic of debates and investigations since its rediscovery more than 10 years ago 13. One debated issue in the reconsolidation field is its relationship with the role of the passage of time 100. In inhibitory avoidance conditioning, retrieval leads to protein synthesis-dependent reconsolidation only if the memory is less than 2 weeks old. In contrast, 2- or 4-week old memories do not become fragile after retrieval and, in fact, are resilient to protein synthesis inhibitor treatment administered either systemically or intra-amygdala 18, 101. Similarly, contextual fear conditioning becomes increasingly resistant to post-retrieval administration of protein synthesis inhibitors with time 19. In contrast, retrieval of auditory fear conditioning is still susceptible to disruption by inhibition of new protein synthesis in the amygdala after 45 or 60 days, and no temporal gradient of resilience has been described 102, 103. In inhibitory avoidance conditioning, while hippocampal protein synthesis is required for the initial consolidation, it is dispensable during reconsolidation, indicating that mechanisms in the amygdala, but not the hippocampus, are critical for memory reconsolidation 104. We propose that in inhibitory avoidance and, perhaps, other temporal-lobe-dependent memories, hippocampal resistance to post-retrieval amnesic treatments may reflect the more cortically distributed representation of the memory trace. In contrast, in Pavlovian fear conditioning, where the amygdala is necessarily involved in the acquisition, storage, and expression of conditioned fear memory, reactivation of the very localized trace renders the memory labile. Whether this reflects differences in molecular and/or circuit-based mechanisms remains to be understood.
One of the hypotheses proposed to explain the function of reconsolidation is that memory reconsolidates to become stronger and longer lasting 14. Supporting this hypothesis, studies have demonstrated that a second training trial strengthens a contextual fear memory 105. This strengthening requires the function of transcription factor EGR-1 (early growth response protein 1) also known as Zif268, which is also essential for the reconsolidation, but not consolidation, of contextual fear conditioning 105. A recent study reported, using inhibitory avoidance conditioning in rats, that unreinforced contextual reminders given when the memory is young, but not when it is older, a enhance memory retention and prevent forgetting after significant consolidation training 22. Such findings indicate that reconsolidation does indeed promote memory enhancement. Targeting reconsolidation mechanisms should therefore also provide opportunities to enhance memories.
Several studies have explored the possibility of enhancing memory by targeting mechanisms activated during reconsolidation. For example, activating PKA in the basolateral amygdala (BLA) with sp-cAMP during reconsolidation leads to an enhancement of rat auditory fear memory retention; conversely, inhibiting PKA by administering 6-BNZ-cAMP to the same brain region immediately after retrieval impairs reconsolidation and leads to amnesia 106. Similarly, in the crab Chasmagnathus, blocking angiotensin II impairs reconsolidation, whereas exogenous administration of recombinant angiotensin II enhances it 107.
IGF-2, which enhances inhibitory avoidance memory when administered to the hippocampus following training (Figure 3a), also significantly strengthens inhibitory avoidance memory when administered in the same brain region after memory retrieval (Figure 3c) 26. The effect is retrieval-dependent and limited to the same exact temporal window during which inhibitory avoidance memory undergoes reconsolidation; that is, less than 2 weeks after training. Hence, although the hippocampus is not a substrate for post-retrieval memory disruption after inhibitory avoidance training, it is a substrate for post-retrieval memory enhancement that occurs during the reconsolidation-sensitive temporal window. We conclude that in medial temporal lobe-dependent fear memories, retrieval activates the amygdala to control consolidation that is mediated by hippocampal mechanisms, leading to subsequent strengthening and cortical redistribution of the trace. These mechanisms can be targeted to promote memory enhancement. Thus, to effectively target mechanisms of memory consolidation or reconsolidation to enhance temporal-lobe-dependent memories, it is important to keep in mind that there are temporal constraints. Hence, an enhancer that targets the hippocampal CREB-C/EBP-dependent cascade will likely be most effective within the first day after training or after reactivation when reactivation leads to reconsolidation. However, as the effect of IGF-2 on cortical regions still needs to be determined, it is possible that any memory enhancing effects it has in the cortex may have different temporal evolutions.
Future Directions and perspectives
Important questions arise concerning memory enhancement that targets consolidation and reconsolidation mechanisms (Box 1). First, are the enhancing effects selective for the active trace and therefore only affect an active memory? This would restrict their effect, eliminating them as treatments that might globally enhance all stored memories. However, these types of pharmacological treatments could perhaps be paired with brain stimulation, which would activate the memory traces, allowing them to respond to the treatment. Second, from the results obtained, we can infer that if consolidation or reconsolidation is impaired by, for example, neurodegenerative disease, memory enhancers may be ineffective. Thus, it is important that memory enhancers are tested and investigated in disease models at multiple stages of disease progression. Memory enhancers targeting consolidation and reconsolidation may hopefully be able to compensate for or rescue defects that may very well be the cause of the disease, thus significantly slowing the progression of the disorder. In other words, prevention would be the most, or perhaps the only, successful strategy. Third, as memory-enhancing treatments would be needed for non-aversive memories, the consolidation and reconsolidation mechanisms of appetitive or problem-solving memories will have to be better investigated and understood. Finally, a safe and clinically applicable memory enhancer would also need to be highly specific and have minimal side-effects. Importantly, studies on memory enhancement should more carefully examine the effect of treatment on multiple tasks and brain functions to assess whether memory enhancement compromises the flexibility of memory or brain plasticity. Given that memory formation is such a dynamic process, gaining a more complete understanding of the anatomical and temporal dynamics of the molecular and systems changes required after learning and retrieval to consolidate memories will enable us to develop the most specific and efficacious memory enhancers.
Box 1. Outstanding questions.
What are the mechanisms of action of memory enhancers? Memory enhancers may provide substrates that cooperate with encoding, synaptic/system consolidation and/or memory retrieval mechanisms and either prolong and/or potentiate these processes, or oppose negative constraints. An example is hippocampally-administered IGF-2 that acts only if present during active phases of the memory, either post-training or post-retrieval 26. Other examples likely include changing the neurotransmitter or glutamate receptor-mediate responses 113 and the general state of chromatin to promote the expression of genes 114 or cell excitability 23, 115 that mediate synaptic plasticity to promote memory formation or persistence. It would also be interesting to determine whether/which mechanisms have universal or selective effects in different brain regions, memory systems, memory tasks and/or memory trace. Alternatively, memory enhancers may target the basal state of the system and prolong memory storage, like in the case of enhanced expression of PKM 116, 117. With this approach, memories could be, in principle, enhanced at any time after their formation and the effect should be unselective, therefore targeting all stored memories.
Memory enhancers as potential treatments in diseases of memory decay: will they help? How? Little is known about whether or how memory enhancers can be used as effective treatments in memory decay disorders, including aging-related memory loss and Alzheimer’s disease (AD). Although animal model studies suggest that some compounds that produce memory enhancement also prevent memory loss in models of AD 73, it needs to be determined whether the effect on the memory is long lasting. Importantly, it also needs to be determined whether the compounds enhance the system’s abilities (and whatever remains of it) or actually rescues or bypass defective mechanisms. If the compound reverses the deficits, an early intervention may significantly prevent the progression of the disease and may be the best or only successful intervention.
Memory enhancers: are they producing enhanced memory but inflexible behavior? Overlapping memories and memory systems exist, and little, if anything, is known about whether enhancement of certain memories come at the expense of the retention of other memories already formed, or of modifying/updating memories, or, finally, at the potentials of encoding new memories. In other words, like in LTP 118, 119, does a memory potentiation occlude the system or make the system less flexible?
What aspect of memory is enhanced? Behavioral studies need to dissect whether memory enhancers affect the general representation of memory, thereby enhancing it as a whole, or whether selected components of the memory are affected (e.g. emotion, spatial, perception, execution etc.).
Enhancing memory in normal subjects: ethical problems It is important to briefly mention that ethical issues should be discussed and Ethics Committees should provide guidelines about the use of memory enhancers, according to the effects found 120. For example, enhancing memories may change emotional aspects of the memory that can cause affect disregulations (e.g. enhancing painful, stressful and/or traumatic memories). Thus, studies are required to carefully understand the mechanisms and both the positive and negative consequences of enhancing memories.
Acknowledgments
We dedicate this review to Rusiko Bourtchouladze who passed away August 6th 2011. She was a pioneer of the work on the role of CREB in memory, an outstanding scientist and a wonderful friend. The writing of this review, and part of the work described within, were supported by the National Institute of Mental Health (R01-MH065635 and R01-MH074736 to C.M.A. and F31-MH816213 and T32-GM007280 to D.Y.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Meek PD, et al. Economic considerations in Alzheimer's disease. Pharmacotherapy. 1998;18:68–73. discussion 79–82. [PubMed] [Google Scholar]
- 2.Lynch G, et al. The likelihood of cognitive enhancement. Pharmacol. Biochem. Behav. 2011;99:116–129. doi: 10.1016/j.pbb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roesler R, Schroder N. Cognitive enhancers: focus on modulatory signaling influencing memory consolidation. Pharmacol. Biochem. Behav. 2011;99:155–163. doi: 10.1016/j.pbb.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Wallace TL, et al. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol. Biochem. Behav. 2011;99:130–145. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 6.McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- 7.Korol DL. Enhancing cognitive function across the life span. Ann. N. Y. Acad. Sci. 2002;959:167–179. doi: 10.1111/j.1749-6632.2002.tb02091.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith MA, et al. Glucose enhancement of human memory: a comprehensive research review of the glucose memory facilitation effect. Neurosci. Biobehav. Rev. 2011;35:770–783. doi: 10.1016/j.neubiorev.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 9.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 10.Alberini CM. The role of protein synthesis during the labile phases of memory: revisiting the skepticism. Neurobiol. Learn. Mem. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberini CM, et al. Memory consolidation and its underlying mechanisms. In: Giese KP, editor. Memory mechanisms in health and disease: Mechanistic basis of memory. World Scientific Publishing; 2012. [Google Scholar]
- 12.Dudai Y. Reconsolidation: the advantage of being refocused. Curr. Opin. Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Nader K, et al. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 14.Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn. Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 15.Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front. Behav. Neurosci. 2011;5:12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberg M, Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in Medaka: old fears don't die. Eur. J. Neurosci. 2004;20:3397–3403. doi: 10.1111/j.1460-9568.2004.03818.x. [DOI] [PubMed] [Google Scholar]
- 17.Frankland PW, et al. Stability of recent and remote contextual fear memory. Learn. Mem. 2006;13:451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litvin OO, Anokhin KV. Mechanisms of memory reorganization during retrieval of acquired behavioral experience in chicks: the effects of protein synthesis inhibition in the brain. Neurosci. Behav. Physiol. 2000;30:671–678. doi: 10.1023/a:1026698700139. [DOI] [PubMed] [Google Scholar]
- 21.Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Inda MC, et al. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J. Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat. Rev. Neurosci. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsien JZ. Building a brainier mouse. Sci. Am. 2000;282:62–68. doi: 10.1038/scientificamerican0400-62. [DOI] [PubMed] [Google Scholar]
- 25.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen DY, et al. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberini CM, et al. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 28.Bartsch D, et al. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- 29.Tully T, et al. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 30.Dudai Y. Molecular bases of long-term memories: a question of persistence. Curr. Opin. Neurobiol. 2002;12:211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- 31.Squire LR, et al. Retrograde amnesia. Hippocampus. 2001;11:50–55. doi: 10.1002/1098-1063(2001)11:1<50::AID-HIPO1019>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 33.Silva AJ, et al. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 34.Dubnau J, Tully T. Gene discovery in Drosophila: new insights for learning and memory. Annu. Rev. Neurosci. 1998;21:407–444. doi: 10.1146/annurev.neuro.21.1.407. [DOI] [PubMed] [Google Scholar]
- 35.Balschun D, et al. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J. Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin JC, et al. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 37.Perazzona B, et al. The role of cAMP response element-binding protein in Drosophila long-term memory. J. Neurosci. 2004;24:8823–8828. doi: 10.1523/JNEUROSCI.4542-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JA, et al. Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses. Learn. Mem. 2001;8:220–226. doi: 10.1101/lm.40201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, et al. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat. Neurosci. 2004;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- 40.Jentsch JD, et al. Stimulation of protein kinase a activity in the rat amygdala enhances reward-related learning. Biol. Psychiatry. 2002;52:111–118. doi: 10.1016/s0006-3223(02)01358-6. [DOI] [PubMed] [Google Scholar]
- 41.Barad M, et al. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutten K, et al. Rolipram reverses scopolamine-induced and time-dependent memory deficits in object recognition by different mechanisms of action. Neurobiol. Learn. Mem. 2006;85:132–138. doi: 10.1016/j.nlm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Reneerkens OA, et al. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–443. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li YF, et al. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J. Neurosci. 2011;31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prickaerts J, et al. Dissociable effects of acetylcholinesterase inhibitors and phosphodiesterase type 5 inhibitors on object recognition memory: acquisition versus consolidation. Psychopharmacology (Berl) 2005;177:381–390. doi: 10.1007/s00213-004-1967-7. [DOI] [PubMed] [Google Scholar]
- 46.Rutten K, et al. The selective PDE5 inhibitor, sildenafil, improves object memory in Swiss mice and increases cGMP levels in hippocampal slices. Behav. Brain. Res. 2005;164:11–16. doi: 10.1016/j.bbr.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 47.Shafiei M, et al. Effect of sildenafil (Viagra) on memory retention of a passive avoidance response in rats. Acta. Physiol. Hung. 2006;93:53–59. doi: 10.1556/APhysiol.93.2006.1.6. [DOI] [PubMed] [Google Scholar]
- 48.Josselyn SA, et al. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Restivo L, et al. Viral-mediated expression of a constitutively active form of CREB in hippocampal neurons increases memory. Hippocampus. 2009;19:228–234. doi: 10.1002/hipo.20527. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki A, et al. Upregulation of CREB-Mediated Transcription Enhances Both Short- and Long-Term Memory. J. Neurosci. 2011;31:8786–8802. doi: 10.1523/JNEUROSCI.3257-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han JH, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 52.Han JH, et al. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Y, et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez de Armentia M, et al. cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J. Neurosci. 2007;27:13909–13918. doi: 10.1523/JNEUROSCI.3850-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abel T, et al. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- 56.Bartsch D, et al. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 57.Chen A, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 58.Yin JC, et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 59.Costa-Mattioli M, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Costa-Mattioli M, et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Barrett RM, Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn. Mem. 2008;15:460–467. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrij F, et al. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- 64.Oike Y, et al. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum. Mol. Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- 65.Korzus E, et al. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alarcon JM, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 67.Bourtchouladze R, et al. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levenson JM, et al. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 69.Fischer A, et al. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 70.Yeh SH, et al. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol. Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- 71.Bredy TW, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn. Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stefanko DP, et al. Modulation of long-term memory for object recognition via HDAC inhibition. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chuang DM, et al. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr. Opin. Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer A, et al. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol. Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Russo VC, et al. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr. Rev. 2005;26:916–943. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- 77.Rotwein P, et al. Differential expression of insulin-like growth factor genes in rat central nervous system. Proc. Natl. Acad. Sci. U. S. A. 1988;85:265–269. doi: 10.1073/pnas.85.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown J, et al. Keeping IGF-II under control: lessons from the IGF-II-IGF2R crystal structure. Trends Biochem. Sci. 2009;34:612–619. doi: 10.1016/j.tibs.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 79.Ghosh P, et al. Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell. Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 80.Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochim. Biophys. Acta. 2009;1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 81.Shamblott MJ, et al. Characterization of a teleost insulin-like growth factor II (IGF-II) gene: evidence for promoter CCAAT/enhancer-binding protein (C/EBP) sites, and the presence of hepatic C/EBP. Mol. Mar. Biol. Biotechnol. 1998;7:181–190. [PubMed] [Google Scholar]
- 82.Agis-Balboa RC, et al. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J. 2011;30:4071–4083. doi: 10.1038/emboj.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy LJ, et al. Tissue distribution of insulin-like growth factor I and II messenger ribonucleic acid in the adult rat. Endocrinology. 1987;120:1279–1282. doi: 10.1210/endo-120-4-1279. [DOI] [PubMed] [Google Scholar]
- 84.Pardridge WM. Transport of insulin-related peptides and glucose across the blood-brain barrier. Ann. N. Y. Acad. Sci. 1993;692:126–137. doi: 10.1111/j.1749-6632.1993.tb26211.x. [DOI] [PubMed] [Google Scholar]
- 85.Hawkes C, Kar S. The insulin-like growth factor-II/mannose-6-phosphate receptor: structure, distribution and function in the central nervous system. Brain Res. Brain Res. Rev. 2004;44:117–140. doi: 10.1016/j.brainresrev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Guzowski JF, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ploski JE, et al. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J. Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bramham CR, et al. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J. Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chowdhury S, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choquet D. Fast AMPAR trafficking for a high-frequency synaptic transmission. Eur. J. Neurosci. 2010;32:250–260. doi: 10.1111/j.1460-9568.2010.07350.x. [DOI] [PubMed] [Google Scholar]
- 91.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 92.Smillie KJ, Cousin MA. The role of GSK3 in presynaptic function. Int. J. Alzheimers Dis. 2011 doi: 10.4061/2011/263673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clayton EL, et al. Dynamin I phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nat. Neurosci. 2010;13:845–851. doi: 10.1038/nn.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gregg C, et al. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gregg C, et al. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilkinson LS, et al. Genomic imprinting effects on brain development and function. Nat. Rev. Neurosci. 2007;8:832–843. doi: 10.1038/nrn2235. [DOI] [PubMed] [Google Scholar]
- 97.Babri S, et al. Intrahippocampal insulin improves memory in a passive-avoidance task in male wistar rats. Brain Cogn. 2007;64:86–91. doi: 10.1016/j.bandc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Craft S, et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch. Gen. Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 99.Irvine EE, et al. Insulin receptor substrate 2 is a negative regulator of memory formation. Learn. Mem. 2011;18:375–383. doi: 10.1101/lm.2111311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 101.Milekic MH, et al. Temporal requirement of C/EBPbeta in the amygdala following reactivation but not acquisition of inhibitory avoidance. Learn. Mem. 2007;14:504–511. doi: 10.1101/lm.598307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Debiec J, et al. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 103.Wang SH, et al. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat. Neurosci. 2009;12:905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- 104.Taubenfeld SM, et al. The consolidation of new but not reactivated memory requires hippocampal C/EBPbeta. Nat. Neurosci. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- 105.Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat. Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- 106.Tronson NC, et al. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat. Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- 107.Frenkel L, et al. Memory strengthening by a real-life episode during reconsolidation: an outcome of water deprivation via brain angiotensin II. Eur. J. Neurosci. 2005;22:1757–1766. doi: 10.1111/j.1460-9568.2005.04373.x. [DOI] [PubMed] [Google Scholar]
- 108.Eckel-Mahan KL, Storm DR. Rookie snail protein LAPS veteran C/EBP: net transcriptional proceeds for long-term facilitation. Neuron. 2006;49:645–646. doi: 10.1016/j.neuron.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 109.Belfiore A, et al. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 110.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol. Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 111.Morcavallo A, et al. Research resource: New and diverse substrates for the insulin receptor isoform A revealed by quantitative proteomics after stimulation with IGF-II or insulin. Mol. Endocrinol. 2011;25:1456–1468. doi: 10.1210/me.2010-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell. Dev. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 113.Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology. 2011;36:227–250. doi: 10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guan JS, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murphy GG, et al. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr. Biol. 2004;14:1907–1915. doi: 10.1016/j.cub.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 116.Drier EA, et al. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat. Neurosci. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- 117.Shema R, et al. Boundary conditions for the maintenance of memory by PKMzeta in neocortex. Learn. Mem. 2009;16:122–128. doi: 10.1101/lm.1183309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sacchetti B, et al. Time-dependent inhibition of hippocampal LTP in vitro following contextual fear conditioning in the rat. Eur. J. Neurosci. 2002;15:143–150. doi: 10.1046/j.0953-816x.2001.01844.x. [DOI] [PubMed] [Google Scholar]
- 119.Schroeder BW, Shinnick-Gallagher P. Fear learning induces persistent facilitation of amygdala synaptic transmission. Eur. J. Neurosci. 2005;22:1775–1783. doi: 10.1111/j.1460-9568.2005.04343.x. [DOI] [PubMed] [Google Scholar]
- 120.Erler A. Does Memory Modification Threaten Our Authenticity? Neuroethics. 2011;4:235–249. doi: 10.1007/s12152-010-9090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]