Abstract
Aims
We conducted a systematic review of studies reporting seroincidence of Hepatitis C infection (HCV) in relation to shared syringes and drug preparation equipment among injection drug users (IDUs). We identified 21 published and unpublished studies that met inclusion criteria.
Design
We estimated the relative contributions of shared syringes and drug preparation equipment to HCV transmission using random-effects meta-analysis, and analyzed potential sources of heterogeneity of effects among studies.
Findings
Syringe sharing was associated with HCV seroconversion (Pooled Risk Ratio (PRR) = 1.97, 95% confidence interval (95% CI) = 1.57, 2.49), as was sharing drug preparation containers (PRR = 2.42, 95% CI = 1.89, 3.10), filters (PRR = 2.61, 95% CI = 1.91, 3.56), rinse water (PRR = 1.98, 95% CI = 1.54, 2.56), combinations of this equipment (PRR = 2.24, 95% CI = 1.28, 3.93), and “backloading,” a syringe-mediated form of sharing prepared drugs (PRR = 1.86, 95% CI = 1.41, 2.44). Meta-regression results showed that the association between syringe sharing and seroconversion was modified by HCV seroprevalence in the IDU populations.
Conclusions
The risk of Hepatitis C infection through shared syringes is dependent upon Hepatitis C infection seroprevalence in the population. The risk of Hepatitis C infection through shared drug preparation equipment is similar to that of shared syringes. Since the infection status of sharing partners is often unknown, it is important for injection drug users to consistently avoid sharing unsterile equipment used to prepare, divide, or inject drugs, and avoid backloading with an unsterile syringe.
Hepatitis C virus (HCV) infection is endemic among injection drug users (IDUs) [1], and is transmitted through shared injection syringes [2]. In addition, seroincidence studies have demonstrated transmission of HCV through the shared use of drug preparation equipment [3–6]. Estimation of infection risk is complicated by the frequent co-occurrence of sharing behaviors [7, 8]. A recent multi-site incidence study found that co-occurrence of sharing drug cookers, filtration cottons, rinse water, or of “backloading” (a syringe-mediated form of sharing prepared drugs) ranged from 86% to 96% [3]. In many IDU populations, syringe sharing has declined since HIV was first recognized, but shared use of drug preparation equipment has persisted, with 50–70% of IDUs reporting recent sharing of cookers or cotton, or backloading [3–6, 9–11]. Synthesizing knowledge on the relative risk of HCV infection associated with sharing behaviors, and estimating the relative importance of these behaviors is important, as it may be used to guide the development of more effective HCV prevention programs and information.
Previous research syntheses of risk behaviors in regard to HCV acquisition have included an insufficient number of studies for quantitative analysis of distinct risk behaviors. One such synthesis grouped data from 10 studies regarding shared drug preparation equipment into a single meta-analysis [12]. Their analysis showed that risks associated with sharing drug preparation equipment generally ranged from 2.0 to 5.9, but that substantial heterogeneity precluded calculation of an overall effect. Heterogeneity of effects could have arisen for a number of reasons, including variability in the concentration of virus and amount of contaminated blood by equipment type. Such heterogeneity could be reduced by stratifying analyses by the specific type of equipment that was shared, but there were few studies included that reported effects with such detail.
In this study, we examined the associations between shared syringes and drug preparation equipment with HCV seroconversion using data from the HCV Synthesis Project, a systematic review and meta-analysis of published and unpublished research studies of HCV epidemiology and prevention in drug users throughout the world [13]. Using pooled estimates, and data on the proportions of IDUs engaging in sharing behaviors, we calculated population attributable risk percentages reflecting the percentage of HCV seroconversions that could be prevented by eliminating specific sharing behaviors.
METHODS
As part of the HCV Synthesis Project, we conducted a systematic review on potential risk behaviors related to shared drug preparation and injection equipment in HCV incidence studies. Data collection and abstraction methods have been described in detail elsewhere [13]. Briefly, staff retrieved and coded published and unpublished studies from throughout the world on the prevalence and incidence of HCV infection, the molecular epidemiology of HCV genotypes, and HCV co-infection with HIV, HBV and HAV in drug users. To be eligible for inclusion in the HCV Synthesis Project, studies must have reported HCV prevalence or incidence rates, measures of association, HIV/HCV co-infection rates or HCV genotype distributions in eligible samples of drug users. In addition, HCV status must have been determined by serologic testing of either sera or saliva. Although the scope of the research includes all individuals at risk for HCV via drug-related practices including via inhalation, this current paper only draws on the sample of studies of HCV seroincidence among drug injectors in relation to shared syringes and/or injection preparation equipment. Reports published or released between January, 1989 and December, 2006 were included in an initial search which identified a total of 2,375 reports that were screened for eligibility, of which 628 were determined to be eligible and included in the HCV Synthesis sample, of which 71 reported seroincidence. To update the systematic review of literature related to infection risk behaviors, the automated searches and searching of proceedings from scientific conferences were carried out again in April, 2010 to encompass the period after December, 2006, resulting in the addition of 10 studies, including 2 incidence studies. Bibliographies of qualitative reviews of HCV prevention were also examined to identify any additional studies. Overlapping reports, i.e., duplicate data from a single study, were identified based on matching study names, setting and authors; this was followed by comparing sample sizes, years of data collection and other study characteristics to select the most complete and informative report in terms of our research question of interest. Although the HCV Synthesis database includes studies written in other languages, all reports in this analysis were in English.
For the purposes of this analysis syringe sharing is defined as the self-reported use of at least one syringe that was previously used by another IDU (receptive sharing) or the use of a sterile syringe by the participant followed by the use of the same syringe by another IDU (distributive sharing). Although HCV acquisition by the participant is only possible by receptively sharing a syringe, some studies did not differentiate receptive and distributive sharing. We analyzed data on sharing and seroincidence if questionnaire items specified receptive sharing, or did not specify between receptive and distributive sharing. Sharing drug cookers, filtration cottons and rinse water are defined as the self-reported use of this equipment that was previously used by another IDU, although as with syringe sharing, not all studies explicitly indicated that another IDU had used the equipment before the participant. Some studies reported risk of HCV seroconversion in relation to a shared drug preparation equipment category (e.g., “other drug-preparation equipment”, “other injecting equipment”) that excluded syringes, and in some cases referred specifically to shared drug preparation containers, filters or rinse water. Backloading is defined as drawing up drug into the syringe of an IDU (the primary syringe) and then transferring a portion of the solution into the barrel of a second syringe (the secondary syringe) belonging to another IDU. Pathogens that are present on the primary syringe may be transmitted to users of the secondary syringe through this process. Across studies included in the analysis, survey questions varied in the duration of the observation time period when participants were susceptible to infection and during which sharing could have occurred.
Table 1 describes the studies analyzed. We identified and removed overlapping reports (i.e., duplicate data from a single study). We extracted measures of association from the incidence studies. Reported effects were extracted in their original units and variance estimates, when available. When these measures were unavailable for the comparisons of interest, we used published numbers of cases and controls, or total person time to calculate effects and variances. Data were combined from available studies and reported as pooled risk ratios (PRRs).
Table 1.
Study Characteristics.
| First Author and publication year |
Location | Data Collection Period |
Number of Sero- conversions |
Hepatitis C prevalence in the population |
Risk Behaviors, Effect Calculations Used in the Analyses, Duration of Susceptibility Per Participant (months), Quality Score |
|---|---|---|---|---|---|
| Brunton 2000 [34] | New Zealand | 1994–1996 | 9 | 64%[[14] | Syringes, equipment* Cumulative Incidence, 24, 6 |
| Champion 2004 [35] | Scotland, UK | 1999–2000 | 5 | 16% | Syringe Cumulative Incidence, 6, 5 |
| Craine 2009 [36] | South Wales, UK | 2004–2006 | 17 | 26.3% | Syringea Incidence Density**, 12, 7 |
| Crofts 1997 [37] | Victoria, Canada | 1990–1995 | 19 | 62.4% | Syringe Incidence Density, 8.5, 6 |
| Des Jarlais unpublished [7] | New York City | 1997–1999 | 25 | 43.8%[7] | Syringes, container, filter, water, equipment Incidence Density, 6, 6 |
| Edlin unpublished [28] | New York City | 1987–2001 | 62 | 73% | Syringe, equipment Cumulative Incidence, 6, 1 |
| Garfein 1998 [8] | Baltimore, MD | 1994–1996 | 13 | 37.6% | Syringe Incidence Density, 6, 8 |
| Hagan 2001 [4] | Seattle, WA | 1994–1997 | 53 | 82.4% | Syringes, containera, filtera, watera, backloadinga Cumulative Incidence, 12, 6 |
| Hagan 2010 [3] | Multiple US cities | 2002–2004 | 55 | 34.4%[15] | Syringea,s, containera, filtera, watera, backloadinga, equipmenta Survival Analysis**, 3, 6 |
| Hahn 2002 [5] | San Francisco, CA | 2000–2001 | 48 | 39% | Syringes, equipmenta, backloading Survival Analysis, 3, 7 |
| Kapadia 2002 [9] | Multiple US cities | 1997–1999 | 78 | 36% | Syringe, container, water, backloading Conditional Logistic Regression**, 6, 5 |
| Lamothe unpublished [38] | Montreal, Canada | 1992–1996 | 28 | 70.1%[29] | Syringe Survival Analysis, 12, 5 |
| Lucidarme 2004 [10] | Northern and Eastern France | 1999–2000 | 16 | 21.4% | Syringea, containera, filtera, watera, equipmenta Survival Analysis, 3, 6 |
| Maher 2006 [11] | New South Wales, Australia | 1999–2002 | 68 | 31.2% | Syringea,s, container, filtera, water, backloading, equipment Incidence Density/Survival Analysis, 6, 6 |
| Miller 2002 [39] | Vancouver, Canada | 1996-unknown (study published in 2002) | 37 | 46% | Syringea Survival Analysis, 6, 7 |
| Rezza 1996 [40] | Naples, Italy | 1991–1993 | 21 | 63.1% | Syringea, equipmenta Cumulative Incidence, 6, 5 |
| Roy 2007 [1] | Eastern Central Canada | 1997–2003 | 199 | 60.4% | Syringea,s, equipment Survival Analysis, 6, 5 |
| Thorpe 2002 [6] | Chicago, IL | 1997–1999 | 29 | 27% | Syringea,s, containera, filtera, watera, backloadinga Survival Analysis, 6, 9 |
| Van Beek 1998 [41] | Sydney, Australia | 1992–1995 | 31 | 45% | Syringea Cumulative Incidence, 12, 5 |
| Van den Berg 2007 [19] | Amsterdam, the Netherlands | 1985–2005 | 59 | 82.2% | Syringe Incidence Density, 12, 3.4 |
| Vilano 1997 [20] | Baltimore, MD | 1988–1989 | 47 | 91.1% | Syringe Cumulative Incidence, 12, 5 |
Equipment refers to sharing any drug preparation equipment other than syringes.
adjusted,
receptive syringe sharing specified.
We investigated 7 study characteristics as potential modifiers of the association between syringe sharing and seroconversion: (1) HCV seroprevalence in the study IDU population, (2) the calendar year at the end of data collection, (3) the duration of the period of susceptibility covered by survey questions that assessed the sharing behaviors, (4) study quality rating, (5) whether data specified receptive sharing or did not specify if sharing was receptive or distributive, (6) effect estimation calculation category (odds ratio from cumulative incidence or matched case-control designs, rate ratio from cumulative incidence designs, or hazard ratio from survival analysis), and (7) whether or not effect measures were adjusted for covariates. Potential effects of HCV seroprevalence, calendar year, duration of susceptibility and study quality were assessed with mixed-effects meta-regressions (using unrestricted maximum likelihood estimation). For 2 studies that did not report HCV prevalence in the sample, we used prevalence estimates in published reports from the same study population [14, 15]. To test potential differences by effect estimation calculation category, receptive sharing specificity, and effect adjustment, we compared effects of meta-analyses calculated separately for each category, and tested heterogeneity of effects by category using Q statistics. There were too few studies to test potential effect modification for shared drug preparation equipment or backloading.
Adjusted effect measures were used in the analysis where they were included in the source studies, under the assumption that adjustment was carried out to remove bias in the estimate of the association between the risk behavior and risk of HCV seroconversion. Several cohort studies used odds ratios (ORs) as their estimator. It has been shown that when the disease is common (incidence above 10/100PY), the OR will no longer approximate the risk ratio [16]. To correct for this, the ORs (crude or adjusted) and their 95% confidence intervals (CIs) were transformed into relative risks and their respective CIs [16]. In studies that did not report effect estimates, an unadjusted relative risk was calculated using the incidence data provided. For one study [17] that reported only hazard ratios and p-values, confidence intervals were calculated using standard methods [18]. For three studies [10, 19, 20] published effects were reported in relation to non-injector reference groups. For these studies, risk ratios were re-calculated by comparing the risk ratios among the sharing behavior group to the group of injectors who did not share [21].
We developed a quality rating scale based on published recommendations, [22] and applied them to each study. Some examples of quality items included: 1) Were dates of data collection given?, 2) Were details of recruitment methods given? More complete descriptions of our methods for rating study quality are available elsewhere [23]. Nine items were used to rate quality of incidence studies. Cronbach's alpha analysis of individual items reflected adequate internal consistency (alpha = 0.71), and the items were summed to form a scale.
PRR values of HCV seroconversion for each sharing behavior were calculated and meta-regressions were performed using Comprehensive Meta-Analysis software [24]. Random effects models were used throughout to account for potential unmeasured sources of variation among studies [25]. Heterogeneity of effects was evaluated using Q and I2 statistics. Q values were tested for significance using the chi-square distribution; I2 values above 50% are considered to reflect meaningful heterogeneity [26]. We examined asymmetry in funnel plots of syringe sharing effects against standard errors, and Egger’s tests of significance to assess potential publication bias [27].
For each sharing behavior shown to be associated with HCV seroconversion, a population attributable risk percent (PAR%) measure was calculated using the formula:
| (1) |
where pe is the proportion exposed in the cohort, [21] and PRR values from the meta-analyses are used as the RR (risk ratio). This represents the percentage of seroconversions that could have been avoided if sharing was eliminated among IDUs. A range of estimates of the prevalence of the exposure in the population (minimum, maximum, median) was taken from the studies included in the meta-analysis.
RESULTS
Twenty-one studies were eligible to be included in the analysis (Table 1). All contributed data on syringe sharing, 8 contributed data on equipment sharing, 7, 6, and 7 contributed data on sharing containers, filters, and water, respectively, and 6 contributed data on backloading. Three studies were unpublished [28–30]. Data collection periods ranged from 1985 to 2005. The duration of the susceptibility period ranged from the previous 1 month to the previous 24 months. Nine studies took place in the US, 5 in Europe, 4 in Canada, and 3 in Australia or New Zealand.
Meta-analysis results are shown in Table 2. There was an association between HCV seroconversion and syringe sharing (PRR = 1.97, 95% CI) = 1.56, 2.49). However, there was evidence of moderate heterogeneity (Q = 38.9, p < 0.05, I2 = 46%). HCV seroconversion was also associated with shared drug preparation containers (PRR = 2.42, 95% CI = 1.89, 3.10); filters (PRR = 2.61, 95% CI = 1.91, 3.56); and rinse water (PRR = 1.98, 95% CI = 1.54). Additionally, HCV seroconversion was associated with “shared drug preparation equipment, combined” (PRR = 2.24, 95% CI = 1.28, 3.93) and backloading (PRR = 1.86, 95% CI = 1.41, 2.44). In analyses of shared drug preparation equipment, heterogeneity was significant only for “shared drug preparation equipment, combined” (Q = 33.0, p < 0.01, I2 = 79%). We found in subsequent analyses that this heterogeneity was significant due to the small effect found in one study [17]. After removing that study the pooled risk ratio was 2.66 (95 % CI = 1.82, 3.91), and heterogeneity was no longer significant (Q = 7.4, p = 0.28, I2 = 19%).
Table 2.
Meta-analyses of sharing syringes, drug preparation equipment and backloading on HCV seroconversion.
| First author, publication year; summary statistic |
Relative Risk (95% CI) | Forest plot |
|---|---|---|
| 2a. Shared syringes (injected with a syringe previously used by another injector) |
||
| Brunton 2000 | 28.12 (1.50 528.18) | 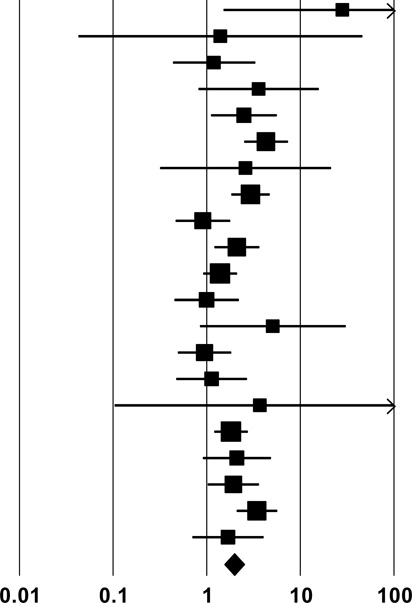 |
| Champion 2004 | 1.40 (0.04, 43.79) | |
| Craine 2009 | 1.20 (0.44, 3.40) | |
| Crofts 1997 | 3.59 (0.81, 15.89) | |
| Des Jarlais unpublished | 2.50 (1.10, 5.65) | |
| Edlin unpublished | 4.30 (2.50, 7.50) | |
| Garfein 1998 | 2.60 (0.30, 20.40) | |
| Hagan 2001 | 2.94 (1.73, 4.53) | |
| Hagan 2010 | 0.91 (0.46, 1.80) | |
| Hahn 2002 | 2.10 (1.20, 3.70) | |
| Kapadia 2002 | 1.39 (0.89, 2.09) | |
| Lamothe unpublished | 1.00 (0.45, 2.23) | |
| Lucidarme 2004 | 5.09 (0.84, 30.81) | |
| Maher 2006 | 0.95 (0.49, 1.85) | |
| Miller 2002 | 1.13 (0.47, 2.73) | |
| Rezza 1996 | 3.70 (0.10, 129.10) | |
| Roy 2007 | 1.82 (1.19, 2.78) | |
| Thorpe 2002 | 2.10 (0.90, 4.90) | |
| Vanbeek 1998 | 1.93 (0.96, 3.44) | |
| Vandenberg 2007 | 3.44 (2.07, 5.70) | |
| Vilano 1997 | 1.69 (0.70, 4.12) | |
| Pooled Estimate | 1.94 (1.53, 2.46) | |
| Q, I2 | 38.1**, 48% | |
| 2b. Shared Combinations of Drug Preparation Equipment other than Syringes | ||
| Brunton 2000 | 49.08 (2.67, 901.90) | 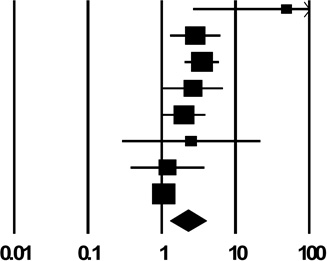 |
| Des Jarlais unpublished | 2.84 (1.30, 6.22) | |
| Edlin unpublished | 3.50 (2.05, 5.97) | |
| Hagan 2010 | 2.66 (1.05, 6.74) | |
| Hahn 2002 | 2.00 (1.03, 3.90) | |
| Lucidarme 2004 | 2.50 (0.29, 21.59) | |
| Rezza 1996 | 1.20 (0.38, 3.79) | |
| Roy 2007 | 1.07 (0.91, 1.26) | |
| Pooled Estimate | 2.24 (1.28, 3.93) | |
| Q, I2 | 33.0**, 79% | |
| 2c. Shared Drug Preparation Container (“Cooker”) | ||
| Des Jarlais unpublished | 2.88 (1.31, 6.32) | 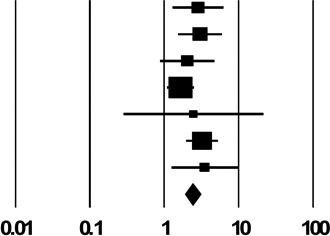 |
| Hagan 2001 | 3.06 (1.56, 6.00) | |
| Hagan 2010 | 2.06 (0.89, 4.77) | |
| Kapadia 2002 | 1.67 (1.10, 2.53) | |
| Lucidarme 2004 | 2.50 (0.29, 21.59) | |
| Maher 2006 | 3.25 (1.98, 5.34) | |
| Thorpe 2002 | 3.54 (1.26, 9.94) | |
| Pooled Estimate | 2.42 (1.89, 3.10) | |
| Q, I2 | 5.7, 0% | |
| 2d. Shared Drug Preparation Filter (Cotton) | ||
| Des Jarlais unpublished | 2.92 (1.33, 6.41) | 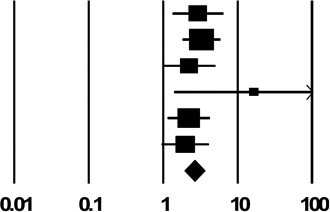 |
| Hagan 2001 | 3.28 (1.82, 5.90) | |
| Hagan 2010 | 2.22 (0.98, 5.02) | |
| Lucidarme 2004 | 16.41 (1.41, 190.79) | |
| Maher 2006 | 2.21 (1.15, 4.24) | |
| Thorpe 2002 | 1.98 (0.95, 4.11) | |
| Pooled Estimate | 2.61 (1.91, 3.56) | |
| Q, I2 | 3.8, 0% | |
| 2b. Shared Drug Preparation Rinse Water | ||
| Des Jarlais unpublished | 2.83 (1.29, 6.22) | 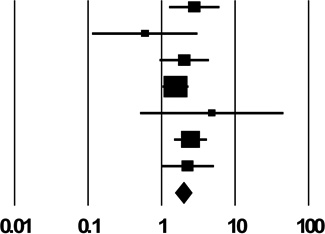 |
| Hagan 2001 | 0.60 (0.12, 3.12) | |
| Hagan 2010 | 2.07 (0.96, 4.46) | |
| Kapadia 2002 | 1.56 (1.04, 2.33) | |
| Lucidarme 2004 | 4.88 (0.52, 45.84) | |
| Maher 2006 | 2.50 (1.50, 4.16) | |
| Thorpe 2002 | 2.29 (1.01, 5.20) | |
| Pooled Estimate | 1.98 (1.54, 2.56) | |
| Q, I2 | 5.7, 0% | |
| 2b. Backloading | ||
| Hagan 2001 | 1.10 (0.23, 5.33) | 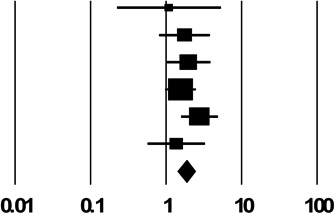 |
| Hagan 2010 | 1.77 (0.82, 3.82) | |
| Hahn 2002 | 2.00 (1.03, 3.90) | |
| Kapadia 2002 | 1.57 (0.99, 2.49) | |
| Maher 2006 | 2.80 (1.59, 4.92) | |
| Thorpe 2002 | 1.38 (0.58, 3.30) | |
| Pooled Estimate | 1.86 (1.14, 2.44) | |
| Q, I2 | 3.5, 0% | |
p < 0.05,
p < 0.01
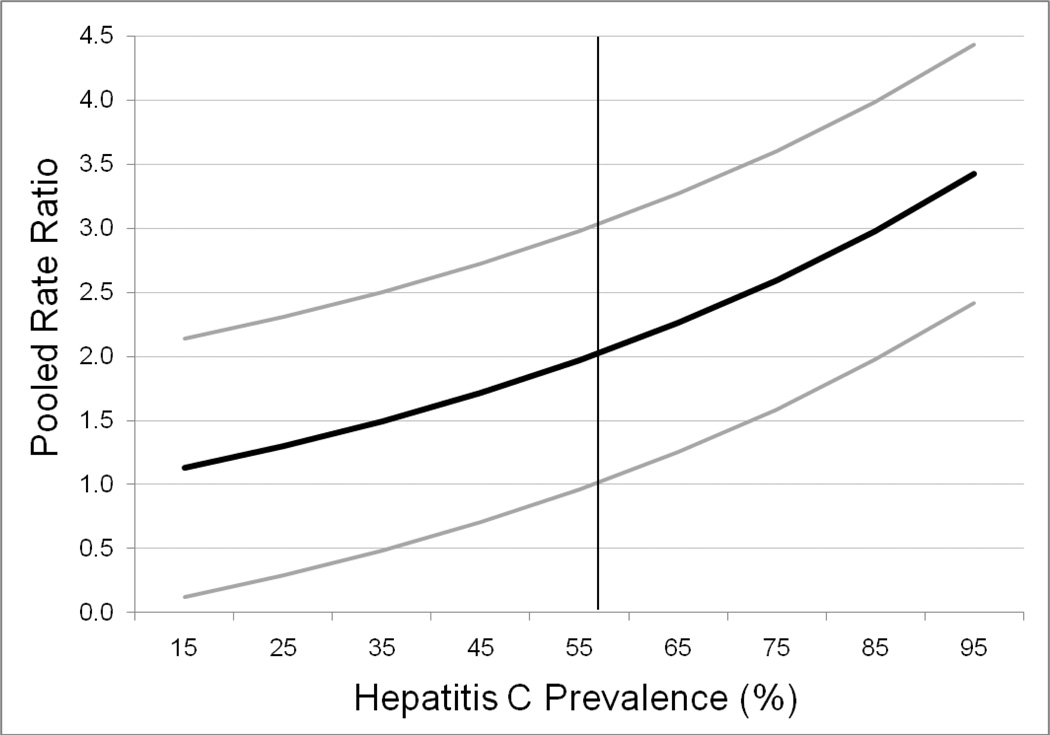
In meta-regression analysis, studies with higher HCV seroprevalence in the study IDU population had larger effects of syringe sharing on seroconversion (Estimate (Est.) = 0.014, standard error (SE) = 0.004, p < 0.001). PRR values and 95% CI bounds predicted from the regression equation are plotted in Figure 1. The lower bound of the PRR 95% CI exceeds 1 at 56% HCV prevalence and higher. The duration of susceptibility and calendar time were not significant moderators of the effect of syringe sharing on HCV seroconversion (Est. = 0.48, SE = 0.39; Est. = −0.02, SE = 0.03, respectively). However, higher study quality was associated with a smaller effect (Est. = −0.12, SE = 0.06). After removing the 2 studies of syringe sharing with the lowest quality scores, heterogeneity was reduced (Q = 23, p = ns, I2 = 22%), and the PRR was 1.71 (95 % CI) = 1.45, 2.03). Differences by estimation method (Hazard Ratio = 1.6, 95% CI = 1.2, 2.3; Odds Ratio = 2.1, 95% CI = 1.3, 3.5; Rate Ratio = 2.5, 95% CI = 1.5, 4.0), by effect adjustment (Unadjusted PRR = 2.3, 95% CI =1.6, 3.3; Adjusted PRR = 1.7, 95% CI = 1.2, 2.4) and by whether syringe sharing specifically referred to receptive sharing (Specified PRR = 1.85, 95% CI = 1.29, 2.65; Unspecified PRR = 2.02, 95 % CI = 1.45, 2.83) were relatively small and not significant by Q statistic test.
Figure 1.
Pooled risk ratios (black line) and 95% confidence bounds (gray lines) predicted from a meta-regression on the association of syringe sharing with hepatitis C seroconversion by hepatitis C seroprevalence in the studies’ populations of injection drug users.
Note. The vertical line represents the point in hepatitis C prevalence (56%) at which the 95% confidence interval lower bound begins to exceed 1.
A funnel plot of logged risk ratios with their respective standard error values showed little evidence of potential publication bias; an Egger’s test was not significant (t = 0.35, p = 0.73).
Table 3 shows the PAR% estimates in relation to the proportion exposed in each injection equipment category, as calculated with formula 1. Values of the proportion exposed were obtained from the included studies. In a population of IDUs where the prevalence of syringe sharing is 36% among those susceptible to HCV infection (the median value), we estimate that 25% of HCV seroconversions could be prevented if syringe sharing were eliminated. Median exposure rates for drug preparation containers, filters, rinse water and combinations of these equipment types were higher than for syringe sharing, and the corresponding PAR%s were 43%, 42% and 37%, respectively. The maximum exposure rates for these variables were quite high (60–85%), which increased our upper limit estimates of the PAR% to approximately 50%. In populations where syringe sharing is reported by the majority of HCV-susceptible IDUs, the PAR% rises to 41%. Our median-exposure rate estimates of the PAR% for sharing rinse water and backloading were somewhat lower (31% and 27%, respectively).
Table 3.
Population attributable risk percentages based on values of the proportion exposed by equipment type.
| Exposure values | |||
|---|---|---|---|
| Shared Injection Equipment Category | Minimum | Median | Maximum |
| Population Attributable Risk Percentage (Proportion Exposed) | |||
| Syringes | 2% (0.02) | 25% (0.36) | 41% (0.73) |
| Combinations of drug preparation equipment other than syringes | 19% (0.19) | 37% (0.47) | 51% (0.85) |
| Drug preparation container | 35% (0.38) | 43% (0.54) | 50% (0.70) |
| Drug preparation filter | 41% (0.43) | 42% (0.44) | 49% (0.60) |
| Drug preparation rinse water | 23% (0.30) | 31% (0.46) | 35% (0.54) |
| Backloading | 13% (0.17) | 27% (0.43) | 33% (0.57) |
Note. Minimum, median and maximum values of the proportion exposed were obtained from the studies included in the meta-analysis.
DISCUSSION
This analysis demonstrated great consistency across studies in the association between HCV seroconversion and a range of equipment sharing behaviors. Indeed, differences in pooled risk ratios by shared equipment category were relatively small, varying within 30% of 2.0. Syringe sharing is an important risk factor for HCV acquisition, but shared drug preparation equipment may account for a substantial proportion of risk. This was clearly demonstrated in the calculation of the PAR% values, which showed that a large proportion of HCV seroconversions could be prevented by eliminating the shared use of syringes or drug preparation equipment or eliminating backloading.
Our meta-analyses also showed that the risk of acquiring HCV from sharing syringes was dependent upon HCV seroprevalence in the studies’ IDU populations, which we presume to reflect HCV prevalence among sharing partners. While there were not enough studies to test effect modification by HCV prevalence for all sharing behaviors, it is reasonable to assume that risk of infection from shared drug preparation equipment or backloading is also dependent upon HCV prevalence among sharing partners. HCV seroprevalence in the included studies averaged 50% (data not shown). Thus, risks of infection from shared syringes, equipment or backloading are likely to be higher than our results suggest in IDU populations where prevalence is higher than 50%, and lower in IDU populations where prevalence is lower than 50%. This relationship is reflected in Figure 1. Effects of other study characteristics on the observed associations were relatively small. Effects of potential publication bias in the syringe sharing effect were minimal.
The potential for preventing HCV transmission by reducing sharing of drug preparation equipment is significant, in part, because sharing drug preparation equipment is common. For example, recent cohort data showed that sharing drug preparation equipment was more common than sharing syringes [3, 31]. Since the data in the meta-analyses did not specify the number of injections in which equipment was shared, the similar effect sizes should not be interpreted as similar likelihood of HCV transmission on a per-act basis. Although the studies did not include data on the proportion of injections in which syringes or drug preparation equipment was shared, the proportion was likely higher for drug preparation equipment than for syringes. IDUs can divide drugs without risking HCV (or HIV) transmission by using a single sterile container in which to heat drugs mixed with sterile water, a sterile filter, sterile syringes for each IDU, and a sterile syringe for distributing specified amounts to the syringes of each IDU, if one is used. Since a small amount of blood, which may not be visible, can remain in a syringe after injecting, even after rinsing, it is important to protect the sterile drug solution, container, filter, and any syringe used to distribute the drug solution, from potential contamination by used syringes.
There are several limitations to this study. Since risk behaviors are self-reported, participants in the individual studies may have underreported risk behaviors, potentially biasing the results. However, a recent study of the association between injection risk behavior and HCV seroconversion used A-CASI to collect stigmatized behaviors, [3] and showed that the estimates were similar to other studies where face to face interviewing may have encouraged socially-desirable responses. Perhaps more importantly, it can be difficult for IDUs to keep track of drug preparation and injection equipment during injection events, particularly when multiple people are present. Self-reported risk behavior data may contain inaccuracies based on imperfect recall and lack of perfect attention to who may have used which piece of equipment in which order. HCV antibody tests used to determine HCV infection may have missed infections before antibodies were detectable, potentially leading to underestimation of the associations between seroincidence and sharing behaviors. While we believe we are justified in combining RR, HR and adjusted OR effect estimates, the underlying study designs are not identical, and combining their effects introduces some error. Even though we investigated potential sources of effect modification, differences in study designs and harm reduction efforts likely introduced unmeasured factors that could have led to variation in effects. While we attempted to extract adjusted effects, by necessity we included some unadjusted effects, and adjusted effects did not necessarily adjust for confounding. In some studies attrition was high, potentially biasing those results. The sample size for analyses of effect modifiers of syringe sharing was relatively small. The relatively small number of studies included in analyses of drug preparation equipment also limited our ability to assess publication bias and heterogeneity, and evaluate potential effect modification.
The consistency in findings among the studies included in this meta-analysis provides strong support for current harm reduction recommendations that include access to sterile syringes and drug preparation equipment, and safe injection education programs [32]. Safe injection programs should train users on how to avoid exposure to potentially contaminated drug preparation equipment, as well as potentially contaminated syringes, when sharing drugs. Our previous synthesis showed that multi-component prevention strategies that seek to reduce injection frequency and increase injection safety through education and access have the most substantial impact on HCV transmission [33]. Given that we possess substantial knowledge on how to prevent HCV in this population, future research should emphasize questions of implementation and scale up of effective programs.
Acknowledgments
Supported by US National Institute on Drug Abuse (R01 DA018609). The authors would like to thank Emily Winkelstein for her comments on an earlier draft.
REFERENCES
- 1.Roy E, Haley N, Leclerc P, Cedras L, Boivin JF. Drug Injection Among Street Youth: the First Time. Addiction. 2002;97:1003–1009. doi: 10.1046/j.1360-0443.2002.00161.x. [DOI] [PubMed] [Google Scholar]
- 2.van den Hoek JA, van Haastrecht HJ, Goudsmit J, de Wolf F, Coutinho RA. Prevalence, incidence, and risk factors of hepatitis C virus infection among drug users in Amsterdam. Journal of Infectious Diseases. 1990;162:823–826. doi: 10.1093/infdis/162.4.823. [DOI] [PubMed] [Google Scholar]
- 3.Hagan H, Pouget ER, Williams IT, et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201:378–385. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 4.Hagan H, Thiede H, Weiss NS, et al. Sharing of drug preparation equipment as a risk factor for hepatitis C. American Journal of Public Health. 2001;91:42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: Relationships and risks. Journal of Infectious Diseases. 2002;186:1558–1564. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 6.Thorpe LE, Ouellet LJ, Hershow R, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645–653. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 7.Des Jarlais DC, Diaz T, Perlis T, et al. Variability in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among young injecting drug users in New York City. American Journal of Epidemiology. 2003;157:467–471. doi: 10.1093/aje/kwf222. [DOI] [PubMed] [Google Scholar]
- 8.Garfein RS, Doherty MC, Monterroso ER, et al. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(Suppl 1):S11–S19. doi: 10.1097/00042560-199802001-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kapadia F, Vlahov D, Des Jarlais DC, et al. Does bleach disinfection of syringes protect against hepatitis C infection among young adult injection drug users? Epidemiology. 2002;13:738–741. doi: 10.1097/00001648-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Lucidarme D, Bruandet A, Ilef D, et al. Incidence and risk factors of HCV and HIV infections in a cohort of intravenous drug users in the North and East of France. Epidemiol Infect. 2004;132:699–708. doi: 10.1017/s095026880400247x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher L, Jalaludin B, Chant KG, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101:1499–1508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 12.De P, Roy E, Boivin JF, Cox J, Morissette C. Risk of hepatitis C virus transmission through drug preparation equipment: a systematic and methodological review. J Viral Hepat. 2008;15:279–292. doi: 10.1111/j.1365-2893.2007.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern RK, Hagan H, Lelutiu-Weinberger C, et al. The HCV Synthesis Project: scope, methodology, and preliminary results. BMC Med Res Methodol. 2008;8:62. doi: 10.1186/1471-2288-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemp R, Miller J, Lungley S, Baker M. Injecting behaviours and prevalence of hepatitis B, C and D markers in New Zealand injecting drug user populations. N Z Med J. 1998;111:50–53. [PubMed] [Google Scholar]
- 15.Garfein RS, Golub ET, Greenberg AE, et al. A peer-education intervention to reduce injection risk behaviors for HIV and hepatitis C virus infection in young injection drug users. AIDS. 2007;21:1923–1932. doi: 10.1097/QAD.0b013e32823f9066. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 17.Roy E, A M, Morissette C, Leclerc P, Boudreau JF, Parent R, Rochefort J, Claessens C, Group SW. High hepatitis C virus prevalence and incidence among Canadian intravenous drug users. Int J STD AIDS. 2007;18:23–27. doi: 10.1258/095646207779949880. [DOI] [PubMed] [Google Scholar]
- 18.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 19.van den Berg CH, Smit C, Bakker M, et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. Eur J Epidemiol. 2007;22:183–193. doi: 10.1007/s10654-006-9089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villano SA, Vlahov D, Nelson KE, et al. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35:3274–3277. doi: 10.1128/jcm.35.12.3274-3277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Lelutiu-Weinberger C, Pouget ER, Des Jarlais DD, et al. A meta-analysis of the hepatitis C virus distribution in diverse racial/ethnic drug injector groups. Soc Sci Med. 2009;68:579–590. doi: 10.1016/j.socscimed.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 28.Edlin BR. Hepatitis C: the new prevalence; Hepatitis C: Breaking the Silence on the Epidemic, New York State Department of Health 2007 Statewide Hepatitis C Conference; 20–21 March 2007; New York. (opening plenary address) [Google Scholar]
- 29.Lamothe F, Bruneau J, Franco EL, Vincelette J, Lachance N. A report prepared under contract(5498) with the Laboratory Centre for Disease Control, Health Canada. Health Canada: Laboratory Centre for Disease Control; 1997. Prevalence, incidence, and risk factors for hepatitis C infection among injection drug users participating in the Saint-Luc Cohort. [Google Scholar]
- 30.Des Jarlais DC. personal communication.
- 31.Wand H, Spiegelman D, Law M, et al. Estimating population attributable risk for hepatitis C seroconversion in injecting drug users in Australia: implications for prevention policy and planning. Addiction. 2009;104:2049–2056. doi: 10.1111/j.1360-0443.2009.02704.x. [DOI] [PubMed] [Google Scholar]
- 32.Beyrer C, Malinowska-Sempruch K, Kamarulzaman A, et al. Time to act: a call for comprehensive responses to HIV in people who use drugs. Lancet. 2010;376:551–563. doi: 10.1016/S0140-6736(10)60928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204:74–83. doi: 10.1093/infdis/jir196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunton C, Kemp R, Raynel P, Harte D, Baker M. Cumulative incidence of hepatitis C seroconversion in a cohort of seronegative injecting drug users. N Z Med J. 2000;113:98–101. [PubMed] [Google Scholar]
- 35.Champion JK, Taylor A, Hutchinson S, et al. Incidence of hepatitis C virus infection and associated risk factors among Scottish prison inmates: a cohort study. Am J Epidemiol. 2004;159:514–519. doi: 10.1093/aje/kwh061. [DOI] [PubMed] [Google Scholar]
- 36.Craine N, Hickman M, Parry JV, et al. Incidence of hepatitis C in drug injectors: the role of homelessness, opiate substitution treatment, equipment sharing, and community size. Epidemiol Infect. 2009;137:1255–1265. doi: 10.1017/S095026880900212X. [DOI] [PubMed] [Google Scholar]
- 37.Crofts N, Aitken CK. Incidence of bloodborne virus infection and risk behaviours in a cohort of injecting drug users in Victoria, 1990–1995. Med J Aust. 1997;167:17–20. doi: 10.5694/j.1326-5377.1997.tb138757.x. [DOI] [PubMed] [Google Scholar]
- 38.Lamothe F, Bruneau J, Franco E, Vincelette J, Lachance N. A report prepared under contract(5498) with the Laboratory Centre for Disease Control, Health Canada. Montreal, Canada: McGill University; 1997. Prevalence, incidence and risk factors for hepatitis C infection among injection drug users participating in the Saint-Luc cohort. [Google Scholar]
- 39.Miller CL, Johnston C, Spittal PM, et al. Opportunities for Prevention: Hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36:737–742. doi: 10.1053/jhep.2002.35065. [DOI] [PubMed] [Google Scholar]
- 40.Rezza G, Sagliocca L, Zaccarelli M, et al. Incidence rate and risk factors for HCV seroconversion among injecting drug users in an area with low HIV seroprevalence. Scand J Infect Dis. 1996;28:27–29. doi: 10.3109/00365549609027145. [DOI] [PubMed] [Google Scholar]
- 41.van Beek I, Dwyer R, Dore GJ, Luo K, Kaldor JM. Infection with HIV and hepatitis C virus among injecting drug users in a prevention setting: retrospective cohort study. BMJ. 1998;317:433–437. doi: 10.1136/bmj.317.7156.433. [DOI] [PMC free article] [PubMed] [Google Scholar]