Abstract
Objective
Our aim was to determine if silent myocardial infarction (MI) is more common in women with type 2 diabetes than in men. Our secondary aim was to examine the relationships between silent MI and risk factors for cardiovascular disease.
Research Design and Methods
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) database was used to determine if women had more silent MI on baseline electrocardiograms (ECGs) than did men with a similar unremarkable cardiovascular history. MI was diagnosed using ECG analysis according to the Minnesota code. Multivariable logistic regression analysis was used to compare demographic and clinical associations. Interactive effects of risk factors by gender were tested using a forward selection algorithm.
Results
Men were found to have a higher prevalence of silent MI on baseline ECGs than women (6% vs 4%, p=0.001). Women had lower odds of silent MI than men after adjusting for other risk factors (OR=0.80, p=0.04). Race and ethnicity were significantly associated with silent MI (p=0.02), with Asians having the highest and African Americans and Hispanics having lower odds relative to whites.
Conclusions
Our main findings provide no evidence that silent MI, as detected by the Minnesota code, was more common in women than in men in the ACCORD cohort. If, as in the general population, the women in ACCORD are found to have a higher heart disease mortality rate than the men, it seems unlikely that failure to recognize clinically silent heart disease in the years before study enrollment could be a major cause.
Keywords: silent myocardial infarction, type 2 diabetes, Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial, cardiovascular disease in women
Cardiovascular disease (CVD) is a major cause of death in the diabetic population (Gu, Cowie, Harris 1998; Moss, Klein, Klein 1991). Patients with diabetes have an estimated two- to four- fold increased risk of developing cardiovascular disease. Adjusting for other risk factors, a 1% increase in A1c is associated with an 18% increased in risk of cardiovascular events (Selvin, Marinopolous, Berkenbilt, Rami, Brancati, Powe, Golden 2004) and a 12 to 14 % increased risk of death (Gerstein, Pogue, Mann, Lonn, Dagenais, McQueen, Yusuf 2005). The prevalence of silent myocardial infarction in patients with diabetes has been recognized for decades. Kannel reported in the 1980s that up to 25% of all myocardial infarctions in patients with diabetes are clinically silent and found on screening ECG or at autopsy (Kannel 1986). Recent observations from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study found that 36.8% of MIs identified by EKG during the study were clinically silent (Burgess, Hunt, Li, Zannino, Williamson, Davis, Laakso, Kesaniemi, Zhang, Sy, Lehto, Mann, Keech 2010). Silent myocardial ischemia occurs in one of five asymptomatic patients with type 2 diabetes (Wackers, Young, Inzucchi, Chyun, Davey, Barrett, Taillefer, Wittlin, Heller, Filipchuk, Engel, Ratner, Iskandrian 2004. Based on these results it has been suggested that all patients with diabetes should be treated as though they have a history of cardiovascular disease.
Over the last 30 years, mortality from coronary artery disease has declined in men but not in women with diabetes. In fact, age adjusted cardiovascular disease mortality in females with diabetes has increased over this period (Gu, Cowie, Harris 1999). In this study we sought to determine if women without a self-reported history of clinical cardiovascular disease have more myocardial infarction (definite or possible) changes on ECG than do men with a similar unremarkable history. We used data collected at the baseline visit of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial to examine the relationship between gender, diabetes, risk factors for cardiovascular disease, and the presence of cardiovascular heart disease as defined by ECG criteria. This large database gave us the opportunity to examine this question in a well-characterized population of patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
The ACCORD trial is a double 2×2 factorial trial designed to determine if intensive versus standard glucose control, intensive versus standard blood pressure control; and a lipid treatment strategy that targets both LDL cholesterol and triglyceride levels versus LDL levels only, will reduce cardiovascular events in participants with type 2 diabetes. The primary outcome is myocardial infarction, stroke or cardiovascular death (Buse 2007). The results of intensive glycemia treatment, as well as a full description of the methodology and rationale for the trial have previously been reported (Gerstein, Miller, Byington, Goff, Bigger, Buse, Cushman, Genuth, Ismail-Beigi, Grimm, Probstfield, Simons-Morton, Friedewald 2008).
At baseline, 10,251 patients between the ages of 55–79 years were enrolled in the ACCORD trial. Of these patients, 38% were women. Eligibility criteria included a glycated hemoglobin A1c between 7.5% and 9.0% or 11.0%, depending on current treatment. To be included in the primary prevention cohort, subjects must be free of a clinical history of cardiovascular disease or stroke at enrollment, but have markers of cardiovascular risk including albuminuria, left ventricular hypertrophy, hypertension, smoking, obesity, or dyslipidemia. The primary prevention cohort included 65% of the enrolled participants totaling 6642. Participants were assigned to the secondary prevention group at baseline if they ever had any of the following: MI, stroke, angina, ischemic changes (ECG) on Graded Exercise Tolerance Test or positive imaging, or any revascularization procedure.
Identical electrocardiograph equipment (MAC 1200, Marquette Electronics, Inc., Milwaukee, Wisconsin) was used at all clinic sites. Resting standard simultaneous twelve-lead ECGs were recorded in all participants using strict standardized procedure, including precise placement of chest electrodes. All ECGs were processed in a central ECG laboratory, EPICARE (Epidemiological Cardiology Research Center at Wake Forest University, Winston-Salem, NC), and were visually inspected for technical errors and inadequate quality. The ECGs were then processed by the 2001 version of the GE Marquette 12-SL program, which automatically read and coded all tracings on the basis of the Minnesota criteria (Prineas, Crow, Blackburn 1982) (Marquette 12SL ECG Physician Guide, available at www.ge-healthcare.com).
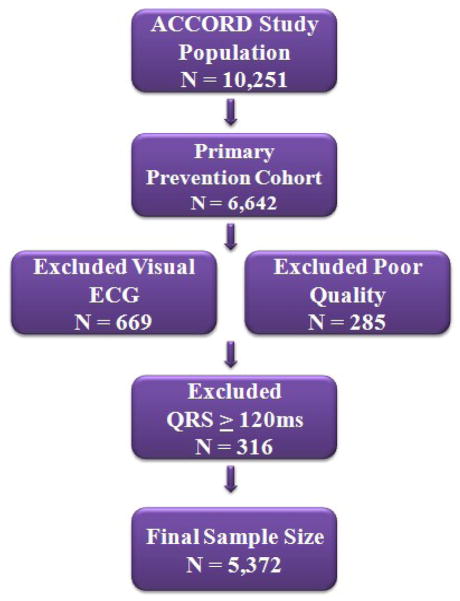
The ACCORD coordinating center identified participants in the primary prevention cohort with Q/STT abnormalities (denoting myocardial infarction/ischemia) on baseline ECG by gender. Myocardial infarction (possible or definite) was defined using the Minnesota code (MC) where an old myocardial infarction is defined by a major Q wave abnormality [MC 1.1.x or 1.2.x] or minor Q/QS waves with major ST-T abnormalities [MC 1.3.x with 4.1.x, or 4.2, or 5.1, or 5.2]. The following additional ischemic abnormalities were also considered: major isolated ST-T abnormalities, defined as MC 4.1.x, or 4.2, or 5.1, or 5.2,; minor isolated Q/QS waves, defined as MC 1.3.x; and minor isolated ST-T abnormalities, defined as MC 4.3, 4.4, 5.3, or 5.4. Participants with poor-quality baseline ECG recordings, or baseline ECG conditions affecting the interpretation of Q/STT abnormalities (such as complete right and left bundle branch block or pacemakers) were excluded from this analysis. Of the 6,642 participants in the primary prevention cohort, 669 subjects were excluded from this analysis because their ECGs required visual reading and thus were not assigned classifications made by electronic reading that are used as inclusion criteria, 285 were excluded for having poor quality ECGs (defined as “quality=5”), and another 316 were excluded for having QRS duration ≥ 120 ms, resulting in a final sample size of 5,372 (Figure 1).
Figure 1.
Primary prevention cohort participants final sample size and ECG exclusion criteria.
Risk factors of heart disease were examined. Age was calculated as the difference in years between the participant’s date of birth and date at the time of randomization. Self-reported race/ethnicity was categorized in the following order: Hispanic, Spanish, or Latino (regardless of race); Black, African American/Canadian; Asian; other; and White/Caucasian. Participants who selected more than one race were placed in the first relevant category in the list (for example, someone who selected both Asian and White would be categorized as Asian). Duration of diabetes was calculated as the difference between the year of randomization and the year of diagnosis of diabetes. Left ventricular hypertrophy (LVH) on the baseline ECG was defined by MC 3.1 or 3.4 with MC 4.1 x, 4.2, 5.1 or 5.2. Systolic and diastolic blood pressure, lipid profile, HbA1c, weight, waist circumference, and BMI were obtained from the baseline physical exam. Participants with albumin/creatinine ≥300 mg/g were defined as having macroalbuminuria, and those albumin/creatinine ≥30 mg/g and <300mg/g were defined as having microalbuminuria. Education level, family history of heart disease, patient history of lower limb amputation, and medication use were assessed at baseline. Participants were classified as current smokers if they had smoked within the past 30 days at the baseline visit and as current drinkers if they consumed one or more alcoholic drinks in a typical week. Depression was defined by whether the participant had ever been told by a physician that he/she had depression.
Statistical Analysis
Among primary prevention patients, ECG abnormalities denoting myocardial ischemia were compared in males versus females. A five degree of freedom chi-square test was performed to determine if the proportion of participants with these five types of ischemic abnormalities differed by gender. A one degree of freedom chi square test was used to determine if the proportion of participants with a silent MI differed by gender, unadjusted for other risk factors (Table 2).
Table 2.
Primary prevention participants with types of ischemic abnormalities based upon gender.
| Males | Females | |||
|---|---|---|---|---|
| Minnesota Code for Ischemic ECG findings | N | % | N | % |
| Major Q wave abnormalities | 157 | 5.3% | 80 | 3.3% |
| Minor Q/QS waves with major ST-T abnormalities | 20 | 0.7% | 18 | 0.7% |
| Major isolated ST-T abnormalities | 156 | 5.3% | 161 | 6.6% |
| Minor isolated Q/QS waves | 243 | 8.3% | 191 | 7.9% |
| Minor isolated ST-T abnormalities | 336 | 11.4% | 450 | 18.5% |
| None of the above | 2030 | 69.0% | 1530 | 63.0% |
| Total | 2942 | 2430 | ||
p< 0.001 for the 5 degree of freedom chi-square test of the six categories above by gender
p=0.001 for the 1 degree of freedom chi-square test of silent MI (Major Q wave abnormalities or Minor Q/QS wave with major ST-T abnormalities) vs. no silent MI (other abnormalities or none of the above) by gender
Baseline characteristics are presented for primary prevention participants overall and by gender (Table 2). Logistic regression analysis was used to examine and compare the demographic and clinical associations of silent MI. To further characterize the differences, risk factors for cardiovascular disease using logistic regression analysis were examined. To determine whether any of the risk factors had a differential effect by gender on the odds of a silent MI, interaction terms were computed for risk factors by gender. A forward selection process was used to determine if any interaction terms were significant when added to the model presented in Table 3. For any interactions which were significant, the predicted odds of a silent MI were computed separately for males and females at various levels of the risk factor, setting all other risk factors in the model to the population mean.
Table 3.
Logistic regression examining previous Myocardial Infarction* vs. no MI among primary prevention participants.
| All primary prevention participants | ||
|---|---|---|
| Parameters | OR | P value |
| Female | 0.80 | 0.04 |
| Age (years) | 1.01 | 0.38 |
| Race/Ethnicity | ||
| -White | -- | 0.02 |
| -Spanish, Hispanic, Latino | 0.94 | |
| -Black (American/Canadian) | 0.80 | |
| -Asian | 1.41 | |
| -other | 0.66 | |
| Education | ||
| -Less than high school | -- | 0.45 |
| -High school graduate | 1.13 | |
| -Some college | 1.07 | |
| -College degree or higher | 0.85 | |
| Duration of DM (years) | 0.99 | 0.61 |
| Left Ventricular Hypertrophy on baseline ECG | 1.53 | 0.06 |
| Hypertension | ||
| -Systolic (mmHg) | 1.01 | 0.07 |
| -Diastolic (mmHg) | 1.00 | 0.72 |
| Hyperlipidemia | ||
| -Total Cholesterol (mg/dL) | 1.00 | 0.84 |
| -Low-density lipoprotein (mg/dL) | 1.00 | 0.85 |
| -High-density lipoprotein (mg/dL) | 1.00 | 0.91 |
| -Triglyceride (mg/dL) | 1.00 | 0.70 |
| A1c | 1.09 | 0.18 |
| Albumin/Creatinine ratio (mg/g) | ||
| -macroalbuminuria (≥300) | 1.27 | 0.22 |
| -microalbuminuria (>=30 and <300) | 0.96 | |
| - <30 | -- | |
| Weight (kg) | 1.00 | 0.78 |
| Waist circumference (cm) | 1.00 | 0.97 |
| Body Mass Index | 1.01 | 0.70 |
| Family h/o heart disease** | 1.07 | 0.30 |
| Current Smoker | 0.99 | 0.83 |
| Alcohol use | 1.02 | 0.81 |
| History of Amputation | 0.91 | 0.75 |
| Depression | 0.96 | 0.60 |
| Insulin | 0.96 | 0.61 |
| Number of oral glycemia medications | 0.97 | 0.72 |
| Angiotensin-converting-enzyme inhibitor | 1.03 | 0.69 |
| Aspirin | 0.97 | 0.62 |
| Beta-Blocker | 1.14 | 0.11 |
| Statin | 0.96 | 0.55 |
| Angiotensin II Receptor Blocker | 0.81 | 0.32 |
Myocardial Infarction (Major Q wave abnormalities or Minor Q, QS waves with major ST-T abnormalities)
Heart disease, heart attack, stoke (before 55 for father/brother, 65 for mother/sister)
RESULTS
The ACCORD trial recruited and randomized 10,251 patients (6299 males and 3952 females). As shown in Figure 1, 5372 subjects were eligible for participation in this analysis. Table 1 describes the baseline characteristics of participants included in this analysis.
Table 1.
Baseline characteristics of participants enrolled in the primary prevention cohort in the ACCORD trial included in this analysis: mean (standard deviation) or N (%).
| Parameters | Males (N=2942) | Females (N=2430) | Total (N= 5372) |
|---|---|---|---|
| Age (years) | 62.3 (5.9) | 62.1 (5.8) | 62.2 (5.9) |
| Race/Ethnicity (%) | |||
| -White | 1919 (65 %) | 1357 (56 %) | 3276 (61 %) |
| -Spanish, Hispanic, Latino | 189 (6 %) | 215 (9 %) | 404 (8 %) |
| -Black (American, Canadian) | 510 (17 %) | 573 (24 %) | 1083 (20 %) |
| -Asian | 177 (6 %) | 156 (6 %) | 333 (6 %) |
| -other | 147 (5 %) | 129 (5 %) | 276 (5 %) |
| Education | |||
| -Less than high school | 358 (12 %) | 392 (16 %) | 750 (14 %) |
| -High school graduate | 708 (24 %) | 700 (29 %) | 1408 (26 %) |
| -Some college | 947 (32 %) | 809 (33 %) | 1756 (33 %) |
| -College degree or higher | 926 (32 %) | 529 (22 %) | 1455 (27 %) |
| Duration of DM (years) | 10.3 (7.2) | 10.2 (7.4) | 10.2 (7.3) |
| Left Ventricular Hypertrophy on baseline ECG (%) | 25 (1 %) | 29 (1 %) | 54 (1 %) |
| Hypertension | |||
| -Systolic (mmHg) | 136.1 (16.3) | 136.9 (17.5) | 136.5 (16.9) |
| -Diastolic (mmHg) | 76.1 (10.4) | 75.7 (10.2) | 75.9 (10.3) |
| Hyperlipidemia | |||
| -Total Cholesterol (mg/dL) | 180.0 (39.7) | 195.9 (42.3) | 187.2 (41.7) |
| -Low-density lipoprotein (mg/dL) | 103.8 (32.4) | 112.6 (35.4) | 107.8 (34.0) |
| -High-density lipoprotein (mg/dL) | 39.4 (9.8) | 47.2 (12.5) | 42.9 (11.7) |
| -Triglyceride (mg/dL) | 192.1 (159.5) | 184.0 (122.2) | 188.5 (143.9) |
| A1c (%) | 8.3 (1.0) | 8.3 (1.1) | 8.3 (1.1) |
| Albumin/Creatinine ratio (mg/g) | |||
| -macroalbuminuria (≥300) | 164 (6 %) | 120 (5 %) | 284 (5 %) |
| -microalbuminuria (>=30 and <300) | 721 (25 %) | 456 (19 %) | 1177 (22 %) |
| - <30 | 2057 (70 %) | 1854 (76 %) | 3911 (73 %) |
| Weight (kg) | 98.2 (18.4) | 86.7 (17.2) | 93.0 (18.7) |
| Waist circumference (cm) | 108.4 (13.6) | 103.8 (14.1) | 106.3 (14.0) |
| Body Mass Index | 31.6 (5.2) | 33.2 (5.8) | 32.3 (5.5) |
| Family h/o heart disease*(%) | 995 (35 %) | 1099 (47 %) | 2094 (41 %) |
| Current Smoker (%) | 1465 (50 %) | 683 (28 %) | 2148 (40 %) |
| Alcohol use (%) | 983 (33 %) | 292 (12 %) | 1275 (24 %) |
| History of Amputation (%) | 55 (2 %) | 18 (1 %) | 73 (1 %) |
| Depression (%) | 571 (20 %) | 681 (28 %) | 1252 (23 %) |
| Insulin (%) | 861 (29 %) | 820 (34 %) | 1681 (31 %) |
| Number of oral glycemia medications | 1.4 (0.9) | 1.3 (0.9) | 1.4 (0.9) |
| Angiotensin-converting-enzyme inhibitor (%) | 1580 (54 %) | 1134 (47 %) | 2714 (51 %) |
| Angiotensin II receptor antagonists (%) | 397 (13 %) | 488 (20 %) | 885 (16 %) |
| Aspirin (%) | 1473 (50 %) | 1098 (45 %) | 2571 (48 %) |
| Beta-Blocker (%) | 462 (16 %) | 447 (18 %) | 909 (17 %) |
| Statin (%) | 1646 (56 %) | 1290 (53 %) | 2936 (55 %) |
| Estrogen or Progestin (%) | n/a | 248 (10 %) | n/a |
Heart disease, heart attack, stoke (before 55 for father/brother, 65 for mother/sister)
The proportion of primary prevention participants with Q/STT abnormalities in their ECGs (Table 2) differed significantly by gender (p<0.001 for the five degree of freedom chi-square test). Males had more major Q-wave abnormalities and minor isolated Q/QS waves. Females were more likely to have major and minor isolated ST-T abnormalities. Abnormalities were identified in the anterior leads in 36%, in the lateral leads in 8%, and the inferior leads in 66% of participants. Overall, males were found to have higher prevalence of silent old MI or possible silent old MI (vs. isolated ST-T abnormalities, isolated minor Q/QS waves, or no ischemic abnormalities) on baseline ECG than were females among primary prevention participants (6% vs 4%, p=0.001 for the unadjusted one degree of freedom chi square test), and the higher odds among males remained significant once adjusted for baseline covariates (p=0.04, Table 3).
Table 3 provides the results of logistic regression analyses performed to detect any correlation between cardiovascular risk factors and baseline evidence of silent MI (Major Q wave abnormalities or Minor Q/QS waves with major ST-T abnormalities) vs. no MI. Factors other than gender that influenced the odds of having a silent MI on baseline ECG in this cohort included race/ethnicity (p=0.02), with Asians having the highest odds, and African Americans, Hispanics and Other races having lower odds than Whites. While not statistically significant, a trend towards association with lower odds was noted for participants with left ventricular hypertrophy on baseline ECG (p=0.06) and higher baseline systolic blood pressure (p=0.07). A significant interaction between gender and history of depression (p=0.009) was identified by the forward selection algorithm. The predicted odds of a silent MI were higher for males with a history of depression than males without a history of depression (0.071 vs. 0.055). The predicted odds of a silent MI were lower for females with a history of depression than females without a history of depression (0.023 vs. 0.044). No other gender by risk factor interactions were found to be significant.
DISCUSSION
In this study, our main finding was that more men than women in the primary prevention cohort of ACCORD were found to have evidence of silent MI changes on baseline ECG. These observations do not support the theory that failure to recognize silent cardiovascular disease in diabetic women contributes to a higher age-adjusted heart disease mortality in women as compared to men.
Cardiovascular disease (CVD) is a major cause of death in the diabetic population (Gu, Cowie, Harris 1998; Moss, Klein, Klein 1991). Silent myocardial ischemia is known to occur more frequently in patients with diabetes than in people without this disorder (Selvin, Marinopolous, Berkenbilt, Rami, Brancati, Powe, Golden 2004), but whether more women than men experience silent infarction is uncertain. Our observations suggest that women may not have more silent infarction than men.
Mortality from coronary artery disease has declined in men but not in women with diabetes; in fact it has increased in women (Gu, Howie, Harris 1999). Women with diabetes and a history of CVD tend to have poorer control of modifiable risk factors than do men, (systolic blood pressure and LDL cholesterol) which may contribute to the overall sex disparity in CVD mortality trends (Ferrara, Mangione, Kim, Marrero, Curb, Stevens, Selby 2008). Due to different symptoms of coronary artery disease, women may have disease that is less likely to be detected in a pre-clinical state (Hochman, Tamis, Thompson, Weaver, White, Van de Werf, Aylward, Topol, Califf 1999).
Aggressive strategies for early detection of subclinical CVD could lead to more effective prevention and reduce morbidity and mortality in both women and men with diabetes. The American Diabetes Association consensus statement recommends that patients with diabetes should be screened for the presence of CVD based upon a risk factor–based approach (American Diabetes Association, 2009), although recent studies have concluded that this approach does not identify patients who have silent ischemia on screening tests (Wackers, Young, Inzucchi, Chyun, Davey, Barrett, Taillefer, Wittlin, Heller, Filipchuk, Engel, Ratner, Iskandrian 2004). The routine application of cardiac stress testing may identify some individuals with diabetes and silent ischemia, but, as demonstrated by The Detection of Ischemia in Asymptomatic Diabetics (DIAD) trial, such screening has no effect on subsequent rates of cardiac death or nonfatal MI (Young, Wackers, Chyun, Davey, Barrett, Taillefer, Heller, Iskandrian, Wittlin, Filipchuk, Ratner, Inzucchi 2009). In our analysis, depression increased the risk of having a silent MI in men but decreased the risk of silent MI in women, which was surprising since depression has been shown to increase the risk of cardiac disease in both genders (Frasure-Smith, Lesperance). Until more aggressive strategies have been proven to improve outcomes, efforts to reduce CVD morbidity and mortality in patients with type 2 diabetes should focus risk factor modification, early diagnosis of depression, and early attention to any symptoms suggestive of cardiac ischemia.
Strengths of our study include the a large sample size, centrally-interpreted ECGs, and the fact that the Minnesota Code for MI is part of the American Heart Association recommendation for the diagnosis of MI (Luepker, Apple, Christenson, Crow, Fortmann, Goff, Goldberg, Hand, Jaffe, Julian, Levy, Mamolio, Mendis, Mensah, Parak, Prineas, Redd, Roger, Rosamond, Shahar, Sharrett, Sorlie, Tunstall-Pedoe 2003), but the available data is limited by use of self-reported history of previous heart disease. It is possible there was less silent ischemia in women as they had been more effectively screened than men and thus were placed in the secondary prevention cohort; however, the utilization of such screening tests in our population prior to enrollment is unknown
In conclusion, the analysis of men and women enrolled in the ACCORD trial with type 2 diabetes and no previously diagnosed cardiovascular disease, provide no evidence that silent myocardial infarction is more common in women than in men. These observations do not support the hypothesis that failure to recognize silent heart disease contributes to the greater age adjusted heart disease mortality rate noted in diabetic women. The possible relationship between gender and silent infarction should be explored in more detail in subsequent ACCORD trial analyses.
Acknowledgments
Funding: This study was supported by grants from the National Heart, Lung, and Blood Institute (NO1-HC-95178, NO1-HC-95179, NO1-HC-95180, NO1-HC-95181, NO1-HC-95182, NO1-HC-95183, NO1-HC-95184, IAA-Y1-9035, and IAA-Y-HC-1010), by other components of the National Institutes of Health, including the National Institute of Diabetes and Digestive and Kidney Disease, the National Institute of Aging, and the National Eye Institute, by the Centers for Disease Control and Prevention; and by the General Clinical Research Centers. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amlyn Pharmaceuticals, AstraZeneca, Bayer HealthCare, Closer HealthCare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, Omron Healthcare, Sanofi-Aventis, and Schering-Plough
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Diabetes Association. Executive summary: standards of medical care in diabetes—2009. Diabetes Care. 2009;32:S6–S12. doi: 10.2337/dc09-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess DC, Hunt D, Li LP, Zannino D, Williamson E, Davis TME, Laakso M, Kesaniemi A, Zhang J, Su RW, Lehto S, Mann S, Keech AC. Incidence and predictors of silent myocardial infarction in type 2 diabetes and the effect of fenofibrate: an analysis from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Eur Heart J. 2010;31 (1):92–99. doi: 10.1093/eurheartj/ehp377. [DOI] [PubMed] [Google Scholar]
- Buse JB. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12A):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- De Bacquer D, De Baker G, Blackburn H. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart. 1998;80:570–577. doi: 10.1136/hrt.80.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara A, Mangione CM, Kim C, Marrero DG, Curb D, Stevens M, Selby JV. Sex disparities in control and treatment of modifiable cardiovascular disease risk factors among patients with diabetes. Diabetes Care. 2008;31:69–74. doi: 10.2337/dc07-1244. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F. Reflections on depression as a cardiac risk factor. Psychosomatic Medicine. 2005;67:S19–25. doi: 10.1097/01.psy.0000162253.07959.db. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Pogue J, Mann JF, Lonn E, Degenais GR, McQueen M, Yusuf S. The relationship between dysglycemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia. 2005;48:1749–55. doi: 10.1007/s00125-005-1858-4. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the US population, 1971–1993. Diabetes Care. 1998;21:1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US Adults. JAMA. 1999;281:1291–1297. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Toppol EF, Califf RM. Sex, clinical Presentation, and outcome in patients with Acute Coronary Syndrome. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Silent myocardial ischemia and infarction: insights from the Framingham Study. Cardiol Clin. 1986;4:583–91. [PubMed] [Google Scholar]
- Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Prevention; National Heart, Lung, and Blood Institute. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003 Nov 18;108(20):2543–9. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- Moss SE, Klein R, Klein BEK. Cause-specific mortality in a population-based study of diabetes. Am J Public Health. 1991;81:1158–1162. doi: 10.2105/ajph.81.9.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Boston, MA: John Wright PSB; 1982. [Google Scholar]
- Selvin E, Marinopoulous S, Berkenbilt G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Heller GV, Filipchuk N, Engel S, Ratner RE, Iskandrian AE. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- Young LH, Wackers FJTh, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE for the DIAD Investigators. Cardiac Outcomes After Screening for Asymptomatic Coronary Artery Disease in Patients With Type 2 Diabetes: The DIAD Study: A Randomized Controlled Trial. JAMA. 2009;301:1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]