Abstract
During HIV infection, it is unclear why different opportunistic pathogens cause disease at different CD4 T cell count thresholds. Early work showed that CD4 T cell depletion is influenced both by cellular activation status and expression of viral entry receptors. More recently, functional characteristics of the CD4 T cells, such as cytokine and chemokine production, have also been shown to influence cellular susceptibility to HIV. Here we examine how functional differences in pathogen-specific CD4 T cells could lead to their differential loss during HIV infection. This may have implications for when different opportunistic infections occur, and a better understanding of the mechanisms for functional imprinting of antigen-specific T cells may lead to improvements in design of vaccines against HIV and opportunistic pathogens.
HIV-induced CD4 T cell depletion increases susceptibility to opportunistic pathogens
Untreated Human Immunodeficiency Virus-1 (HIV) infection leads to Acquired Immunodeficiency Syndrome (AIDS), a disease characterized by immune suppression and a loss of immune-mediated control against diverse opportunistic pathogens. Immune suppression in AIDS results from the progressive loss of CD4 T cells brought on by persistent HIV replication. While the pathogenic mechanisms underlying CD4 T cell loss by HIV have been widely debated, a simple rule still applies: the lower the number of CD4 T cells, the higher the risk of opportunistic infections. However, the timing of opportunistic infection is not strictly related to the extent of CD4 depletion for a given pathogen. Some opportunistic pathogens, such as Mycobacterium tuberculosis, can reactivate at relatively high CD4 T cell counts, whereas other pathogens, such as cytomegalovirus (CMV) or toxoplasma gondii, typically reactivate and cause disease only after CD4 T cells have dropped substantially (eg <100/μl).
Whether the difference in the timing of infection between opportunistic pathogens relates to the requirements for total CD4 cell numbers to control a given pathogen, or if it is due to differences in the number and/or quality of opportunistic pathogen-specific CD4 T cells at different stages of total CD4 T cell depletion is unclear. However, arguing for the latter, CMV end-organ disease in AIDS patients closely correlates with depletion of CMV-specific CD4 T cell responses [1–3]. Initiation of antiretroviral therapy (ARV) stops HIV replication, restores both total and opportunistic pathogen-specific CD4 T cells and leads to clearance or control of opportunistic infections [4]. Thus, depletion and functional defects of pathogen-specific CD4 T cells most probably contribute to the co-pathogenesis of HIV and particular opportunistic infections. Here we examine recent data suggesting that HIV infection and depletion rates of pathogen-specific CD4 T cells might differ depending on the cellular maturation or functional status, at least for Mycobacterium tuberculosis (MTB)-specific and CMV-specific CD4 T cells [5–6]. These findings might provide insight into why certain opportunistic infections occur or reactivate at different total CD4 T cell counts.
Viral entry receptors and CD4 T cell depletion
HIV entry into T cells is dependent on expression of the primary HIV receptor CD4 and one of two chemokine receptors: CCR5 or CXCR4 [7–8]. HIV transmission occurs almost exclusively with CCR5-tropic HIV strains [9]. Historically, CCR5-tropic strains have been referred to as “M-tropic”, because of their potential to infect both macrophages and primary T cells. CXCR4-tropic strains typically appear late during the course of HIV disease progression, are more cytopathic and have historically been referred to as T-tropic because of their ability to infect transformed T cell lines [10]. CCR5 expression is common on memory CD4 T cells in mucosal lymphoid tissues, the mucosa of the reproductive tract and intestine, the lung, and inflamed tissues [11–13] and cells in these locations are frequent targets for HIV infection. CXCR4 is expressed on naïve CD4 T cells, a minor fraction of peripheral memory CD4 T cells [14] and on CD4 T follicular helper cells that reside in central lymphoid structures [15]. Antigen-specific stimulation induces CCR5, but reduces CXCR4 expression by memory CD4 T cells in vitro [16–19]. Thus antigen-specific stimulation would promote HIV infection of responding CD4 T cells via CCR5 during their subsequent expansion and differentiation in vivo [6, 16]. This is supported by the relatively high cell-associated viral load of circulating transitional CD4 T cells (CD28+ and CCR7-), which are on the pathway to full CD4 T cell differentiation [20].
After transmission of CCR5-tropic HIV there is a rapid and profound depletion of CCR5+ memory CD4 T cells from mucosal effector sites [12, 21]. This early decline in memory CD4 T cells is thought to occur due to cell death resulting from: 1) direct infection; 2) bystander effects from infected CD4 T cells; and 3) downstream immune activation [22–24]. Depletion of antigen-presenting cells, in particular dendritic cells (DCs), might also contribute to dysfunction or depletion of pathogen-specific T cell responses in HIV-infected subjects [25–27]. The expression of CCR5 on activated and dividing CD4 T cells certainly contributes to the early selection of CCR5-tropic strains [28–29] and to the depletion of pathogen-specific CD4 T cells during early and chronic HIV infection.
Integrin α4β7 in transmission and early dissemination of HIV
High integrin α4β7 expression defines a subset of memory CD4 T cells within genital mucosa, the rectum and the gut associated lymphoid tissue (GALT). The integrin α4β7 was recently described as a cellular receptor for HIV on CD4 T cells [30–31]. It contributes to efficient entry of HIV into the cell by capturing virions in close spatial proximity to the viral entry receptors CD4 and CCR5 [32–33]. Many cells in this population also express the viral coreceptor CCR5 and are in the active phase of the cell cycle, as defined by expression of Ki67 [33]. In vitro, α4β7high CD4 T cells are highly susceptible to HIV infection and therefore represent an ideal substrate for viral replication [33]. Whether the amount of α4β7 expressed contributes to the infection or depletion rate of pathogen-specific CD4 T cells has not been studied specifically, but preferential depletion of circulating gut-homing CD4 T cell subsets during acute HIV infection suggest that this might be the case [34]. The discovery of this additional cellular receptor may provide a further link between sexual transmission, early virus dissemination and the early depletion of memory CD4 T cells in the GALT.
T cell activation status and HIV infection
T cell activation and proliferation contribute to productive HIV infection of memory CD4 T cells [35–38]. Expression of HLA-DR and CD25 (IL-2 receptor alpha chain) on CD4 T cells within lymphoid tissue explants defines the main target CD4 T cell population for productive HIV infection [35]. Indeed, the IL-2 signalling pathway, which is essential for clonal expansion after antigen-specific T cell stimulation, promotes HIV replication [6, 36]. Other cytokines that promote T cell turnover, in particular IL-15, also increase viral replication [39–40]. Thus a substantial fraction of antigen-specific CD4 T cells might die as a result of either complete or abortive infection by HIV, thereby reducing the generation and maturation of pathogen-specific memory CD4 T cells. Abortive HIV infection of CD4 T cells is characterized by incomplete reverse transcription of the viral genome and leads to cell apoptosis through the induction of cellular host defence mechanisms [41]. Potentially, such a mechanism further enhances death of newly generated pathogen-specific CD4 T cells. This hypothesis is supported by the dramatic loss of short-lived effector memory CD4 T cells within effector sites, such as the lung, during experimental SIVmac239 infection. These cells have a high rate of turnover in lymphoid tissues in the absence of SIV infection, a characteristic that presumably contributes to their infection and depletion by SIV prior to their arrival at effector sites [11, 42]. Hence, cytokine signalling pathways and the downstream cellular events associated with activation and proliferation of antigen-specific memory CD4 T cells also promote productive HIV or SIV infection. Extensive cellular depletion is then thought to occur at two stages: first, during the initial stimulation and early expansion phase from naïve or memory T cell pools, and second during the phase of homeostatic maintenance of antigen-specific memory T cells.
CD4 T cell specificity and HIV-induced depletion
Both MTB and CMV typically cause latent or controlled infection in immunocompetent individuals but can reactivate and cause disease during periods of immunosuppression, and both infections appear to be controlled predominantly by pathogen-specific CD4 T cells [1, 3, 43–44]. Despite these similarities, the CD4 T cell thresholds at which these infections cause opportunistic disease are very different: active TB disease is often the first severe opportunistic infection affecting HIV+ patients in MTB endemic areas [45–46], whereas CMV end-organ disease typically occurs only after CD4 counts have declined to very low numbers. MTB-specific CD4 T cell responses are depleted relatively early after HIV infection (<12 months) and are significantly reduced in peripheral blood and bronchalveolar lavage during chronic HIV infection [6, 47–48] in subjects with latent infection. In contrast, CMV-specific CD4 T cells persist until late stage HIV infection [5–6, 49] but their eventual loss still precedes CMV end-organ disease [1–3]. These results imply that MTB-specific CD4 T cells are particularly vulnerable to HIV infection and depletion. Likewise, HIV reduces Streptococcus pneumonia-specific CD4 TH1 cell responses and invasive pneumococcal disease is often observed in HIV patients with relatively preserved T cell numbers[50]. The differential depletion of pathogen-specific CD4 T cells provides a potential explanation for the different CD4 T cell thresholds for active opportunistic disease caused by these pathogens during HIV infection.
Phenotypic differences might contribute to the early depletion of MTB-specific CD4 T cells and persistence of CMV-specific CD4 T cells. Most studies of immunodeficiency virus infection and depletion of CD4 T cells have stratified T cell subsets using markers of cellular activation and maturation such as CD45RO/RA, CD62L, HLA-DR, Ki67 or CCR5. Parameters associated with HIV-induced depletion of CD4 T cells of defined specificity are less clear. MTB- and CMV-specific CD4 T cells populations express similar amounts of CCR5[6]. However, during latency, most MTB-specific CD4 T cells are CD27+CD57−, which together with the frequent expression of CCR5 on these cells, is consistent with a transitional or early differentiated phenotype [47]. In contrast, CMV-specific CD4 T cells are almost exclusively CD27− and typically contain a large proportion of CD57+ cells consistent with an effector memory and terminally differentiated (more mature) phenotype. CD4 T cells that express CD57 have a reduced capacity to proliferate and a lower cellular HIV load in vivo [5, 51]. Thus as antigen-specific CD4 T cells become more differentiated (probably as a result of more vigorous antigenic stimulation by opportunistic pathogens) they tend to express more CD57, are less able to proliferate, and are less likely to become infected by HIV.
There are also functional differences in MTB- and CMV-specific CD4 T cells that could impact susceptibility to HIV infectivity. For example, it is well known that CCR5 ligands can protect CD4 T cells from infection with CCR5-tropic HIV strains in vitro, probably by blocking the interaction of HIV envelope protein gp120 with CCR5, which is necessary for viral entry into the target cell [52]. The CCR5 ligand MIP-1β is highly expressed in CMV-specific CD4 T cell populations, whereas during latency most MTB-specific cells do not produce MIP-1β [6]. Recent data suggest that autocrine production of the CCR5 ligand MIP-1β by CMV-specific CD4 T cells is operative in protecting these cells from infection in vivo [5]. The state of T cell maturation may determine the capacity of a cell to produce MIP-1β; terminally differentiated CD57+ CMV-specific CD4 T cell typically secrete MIP-1β upon restimulation (figure 1) [5-6]. These results link T cell maturation, the production of CCR5 ligands and cellular resistance to HIV infection, and support the hypothesis that autocrine production of CCR5 ligands and a reduction in proliferative potential can render certain antigen-specific CD4 T cells moderately resistant to HIV infection. IL-2 production also differs between these pathogen-specific T cells: IL-2 is produced by the majority of MTB-specific CD4 T cells but only a small minority CMV-specific CD4 T cells. IL-2 supports proliferation of CD4 T cells and might promote HIV infection of MTB-specific CD4 T cells [6].
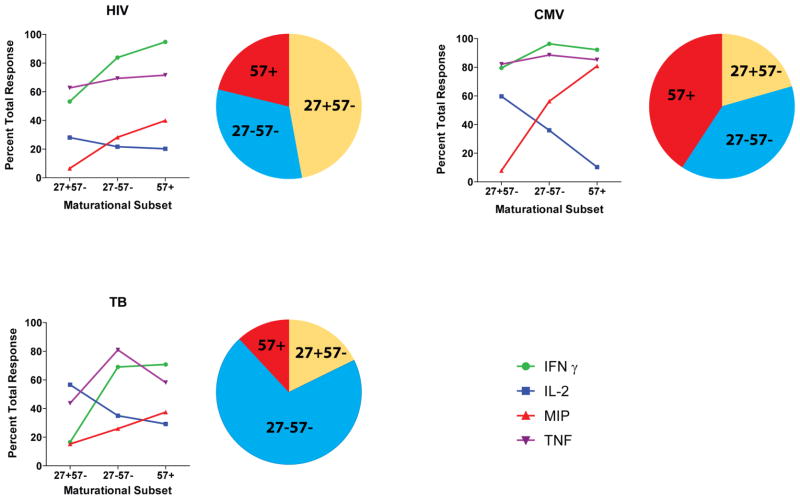
Figure 1. Maturation and function differences between pathogen-specific CD4 T cell populations.
Each pie chart shows the maturational subsets delineated by CD27 and CD57 expression for different, circulating pathogen-specific CD4 T cell populations. The pathogen-specificity is indicated. Less mature CD4 T cells express CD27, more mature CD4 T cells express the senescence marker CD57. The line charts depict the capacity of each of these 3 maturational subsets to secrete IFNγ, IL-2, TNFα and MIP-1β. There are trends toward more MIP-1β and less IL-2 production which potentially reduces cellular HIV susceptibility with increasing maturity of pathogen-specific CD4 T cells. The data illustrated in this figure are based on [5–6] .
Of note, during latent TB infection MTB- and HIV-specific CD4 T cells share several functional and phenotypic characteristics (Figure 1); a majority of cells are CD27+ and produce little or no MIP-1β. Furthermore, when compared to CMV-specific or the total memory population, HIV- and MTB-specific CD4 T cells are preferentially depleted and carry similar levels of HIV gag DNA. These results are consistent with the hypothesis that infection and depletion of different pathogen-specific CD4 T cell populations are influenced by their functional and phenotypic characteristics [5-6, 53], with potential implications for HIV vaccine design (text box 1).
Box 1. How functional and phenotypic analysis of vaccine induced CD4 T cells could support optimization of HIV vaccines.
Phenotype and function of vaccine-induced T cells can be modulated through use of different vaccine vectors and thus can potentially be optimized to induce HIV-specific CD4 T cells that are partially resistant to HIV infection. Preferential HIV infection of vaccine induced CD4 T cells is a major concern for HIV vaccine developers, because such a mechanism would lead to rapid depletion of vaccine-induced immunity upon encounter with HIV. In this context, vaccine-mediated induction of HIV- and vector-specific CD4 T cells of a mature phenotype with a high capacity to secrete CCR5 ligands, such as MIP-1β, could potentially protect these cells from being infected with HIV. SIV vaccine vectors based on replication competent CMV induce highly protective effector memory T cell responses that are associated with SIV-specific CD4 T cells with a high capacity to secrete MIP-1β upon stimulation [92]. In contrast, responses induced by BCG vectors could produce antigen-specific CD4 T cells that are highly susceptible to infection and depletion by HIV.
Antigen stimulation history affects CD4 T cell phenotype and function
Antigen exposure can influence CD4 T cell phenotype and function, and thus potentially affects susceptibility to HIV. In situations in which antigen is cleared, for example after tetanus toxoid vaccination, the antigen-specific CD4 T cells predominantly express a less differentiated, central memory phenotype, and produce IL-2 upon restimulation. Infections with low, but persistent antigen levels, such as latent/recurrent CMV or non-progressing HIV infections, are associated with more differentiated pathogen-specific CD4 T cells that produce less IL-2 [54-55]. Frequent reactivation of opportunistic infections also potentially contributes to the depletion of pathogen-specific CD4 T cell via the mechanism of T cell exhaustion. Latent MTB infection probably falls somewhere between true clearance (as seen for tetanus toxoid vaccination) and the more persistent antigen expression that is observed in CMV and HIV infections. Overall, different conditions of antigen exposure and persistence affect phenotypic and functional characteristics of pathogen-specific CD4 T cells [54]. Consistent with this is the observation that the higher antigen load in active, as compared to latent, tuberculosis drives maturation of MTB-specific CD4 T cells towards a more mature, CD27− phenotype [56–57], and these cells have an increased capacity to secrete MIP-1β and a decreased capacity to produce IL-2 [6]. Indeed, MTB-specific CD4 T cell responses can be detected in some HIV patients even with low CD4 T cell count in HIV+ subjects and are often associated with active symptomatic or subclinical, yet active TB [6, 58–61]. Thus, changes in the function and phenotype of MTB-specific and other pathogen-specific CD4 T cells during disease and changes in antigen load, potentially alters their susceptibility to HIV infection [6, 56, 60, 62]. Putative cellular parameters that are associated with an increased or decreased susceptibility to HIV infection of antigen-specific CD4 T cells are summarized in table 1.
Table 1.
Phenotypic and functional markers that influence HIV infection
Putative cellular parameters associated with increased or decreased HIV susceptibility of antigen-specific CD4 T cells
| Cellular marker | HIV infection | Comment |
|---|---|---|
| CD4 | ↑ | HIV receptor |
| CCR5 | ↑ | HIV receptor |
| CXCR4 | ↑ | HIV receptor |
| α4β7 | ↑ | HIV receptor |
| CD25 | ↑ | Activation marker |
| HLA-DR | ↑ | Activation marker |
| Ki67 | ↑ | Activation marker |
| IL2 | ↑ | T cell growth factor |
| IL15 | ↑ | T cell growth factor |
| CD57 | ↓ | Senescence marker |
| MIP-1β/α Rantes | ↓ | CCR5 ligands |
Antigen-presenting cells (APC) contribute to HIV infection of CD4 T cells
Conjugates of APCs (including DCs) and T cells are the predominant sites for HIV replication in vivo [63]. Infected DCs preferentially transmit HIV to responding antigen-specific CD4 T cells [64], a process that would contribute to efficient infection and subsequent depletion of newly emerging antigen-specific CD4 T cell populations during the expansion phase [53]. Not only are DCs crucial for the transfer of HIV to responding CD4 T cells, the co-stimulatory molecules and cytokines/chemokines they express can influence the function and phenotype of the responding CD4 T cells, which may further influence susceptibility to infection and subsequent HIV replication [65]. For example, DC production of proinflammatory cytokines (eg TNF) and stimulation of T cell surface molecules such as CD40 and B7-family members induce HIV replication and promote infection of T cells that interact with DCs [66]. Recognition of microbial products through pattern recognition receptors, such as the Toll-like receptors, modulate DC costimulatory properties during T cell priming [67]. Hence, innate microbial stimuli probably play a central role in the type of CD4 T cell response that is generated against various pathogens, and might therefore influence the likelihood that an antigen-specific CD4 T cell will become infected and depleted by HIV.
Depletion of CD4 T helper (Th) 17 cells contributes to impaired mucosal barrier function of the gut
Profound depletion of GALT memory CD4 T cells during HIV infection [12, 68] results in impaired mucosal immunity and systemic immune activation, which are hallmarks of pathogenic immunodeficiency virus infection [27]. Memory Th17 cells are found in high frequency in GALT [69] and their depletion plays a central role in the translocation of microbes and their products into the blood stream promoting systemic immune activation [27, 70]. The extent of immune activation is an important predictor of subsequent HIV disease progression [71]. The selective loss of mucosal Th17 cells may thus be important in the general pathogenesis of HIV.
In humans, Th17 cells appear to specifically target extracellular bacterial and fungal pathogens promoting their clearance [27, 72–73]. Thus Th17 cell depletion probably sets the stage for increased susceptibility to many opportunistic bacterial and fungal infections and certainly contributes to systemic dissemination of enteric infections in HIV patients [74]. Susceptibility of Th17 cells to HIV-infection is similar to that of Th1 cells. This probably relates to the fact that Th17 cells are highly activated cells in the gut which are under continuous exposure to bacterial antigens, and this promotes direct infection by HIV [69].
Depletion of pathogen-specific CD4 T cells might promote development of virus- associated malignancies in HIV infection
Loss of control of some viral pathogens can lead to cancer rather than disseminated infection. Cancers and lymphoproliferative disorders occurring in patients with HIV infection are often closely linked to oncogenic viruses, such as the gamma Herpes viruses Epstein-bar (EBV) and Kaposi Sarcoma Virus (KSV), or Human Papilloma Virus [75–78]. Perhaps most thoroughly studied are malignancies associated with HIV-induced immunosuppression that are caused by EBV co-infection, which typically occur only after CD4 T cell counts have dropped substantially. Development of EBV-related lymphomas in HIV-infected patients is associated with a loss of EBV-specific T cell function. In particular, EBV-specific CD4 T cells targeting the latency associated Epstein-Barr virus nuclear antigen (EBNA) 1 are preferentially lost in patients who progress to AIDS-related non-Hodgkin lymphoma [79]. Similarly, HIV+ patients that develop primary lymphoma of the central nervous system lack EBV-specific CD4 T cell responses [80], suggesting a role for the depletion of EBV-specific CD4 T cells in malignant B cell transformation by EBV. The mechanism for this cellular depletion is not known, but it has been hypothesized that EBNA1-specific CD4 T cell responses might be driven by proliferating EBV-infected B cells within secondary lymphoid organs. These areas support extensive HIV replication, which could potentially contribute to infection and depletion of EBNA1-specific CD4 T cells [79, 81-82]. Alternatively, frequent reactivation of EBV in HIV+ subjects [83] might drive cellular maturation of these cells, associated with an increased capacity for Mip-1β secretion, which is associated with partial resistance to HIV infection. Depletion might then be mediated through exhaustion rather than by direct infection of these cells.
HIV infection also significantly increases the incidence of Human Papilloma Virus (HPV) associated cervical cancer and other anogenital malignancies, even in the era of highly active antiretroviral treatment [75–76]. HPV infection in HIV+ subjects is characterized by a higher prevalence of persisting infections caused by multiple HPV types, more rapid disease progression (mean of 3 years vs. 15 years in HIV− subjects) and less frequent spontaneous regression [84–85]. Many HPV-associated lesions and cancers in HIV+ subjects are associated with non-16/18 HPV types, and would therefore not have been preventable by existing vaccines [86–87]. While vaccine-induced protection against HPV infection is mediated by antibodies, other evidence suggests that, once infection is established, T cells are responsible for controlling malignant outgrowth. Specifically, spontaneous regression of HPV-associated warts occurs in the presence of infiltrating memory CD4 and CD8 T cells [88] while a lack of HPV-specific CD4 T cell responses predisposes to HPV-associated cervical cancer [89–91]. While little is known about the influence of HIV infection and ARVs on the frequency and characteristics of the CD4 T cell response to HPV infection, the increase of HPV-associated cancers in HIV+ humans implies that defects in the HPV-specific CD4 T cell response play an important role.
Concluding remarks
Not all pathogen-specific CD4 T cells are created equal and this may have implications for opportunistic infection during HIV infection. Emerging data suggests that specific characteristics of CD4 T cells, including their phenotype, function, and location, can have direct influence on their likelihood of being infected and depleted by HIV. The susceptibility of MTB- and CMV-specific CD4 T cells to HIV infection has been linked to specific characteristics of these cells [5–6] and differential depletion rates of these cells could be a determinant in the timing of reactivation of these pathogens during HIV infection. For other pathogens (e.g. HPV, Cryptococcus, Salmonella, HHV8, and others), it is not known whether the characteristics of the pathogen-specific CD4 T cells impact susceptibility to HIV infection and depletion. However, themes arise in the study of MTB- and CMV-specific CD4 T cells that might be more general. For example, cellular activation and proliferation might promote HIV infection whereas expression of CCR5 ligands and replicative senescence (as observed in CMV-specific CD4 T cells) might inhibit infection (Table 1). Many of the T cell characteristics that influence infectivity are linked to maturational phenotype, such as the differential expression of MIP-1β and IL-2 during cellular differentiation (figure 1)[5–6]. It is likely that in addition to direct effects of antigen load and persistence on pathogen-specific responses, the cytokine and co-signalling milieu induced by antigen-presenting cells are also important. We therefore propose a model in which antigen, innate microbial stimuli, DCs, and T cells interact to establish pathogen-specific CD4 T cell characteristics that will ultimately determine their susceptibility to, and risk of depletion by, HIV (Figure 2).
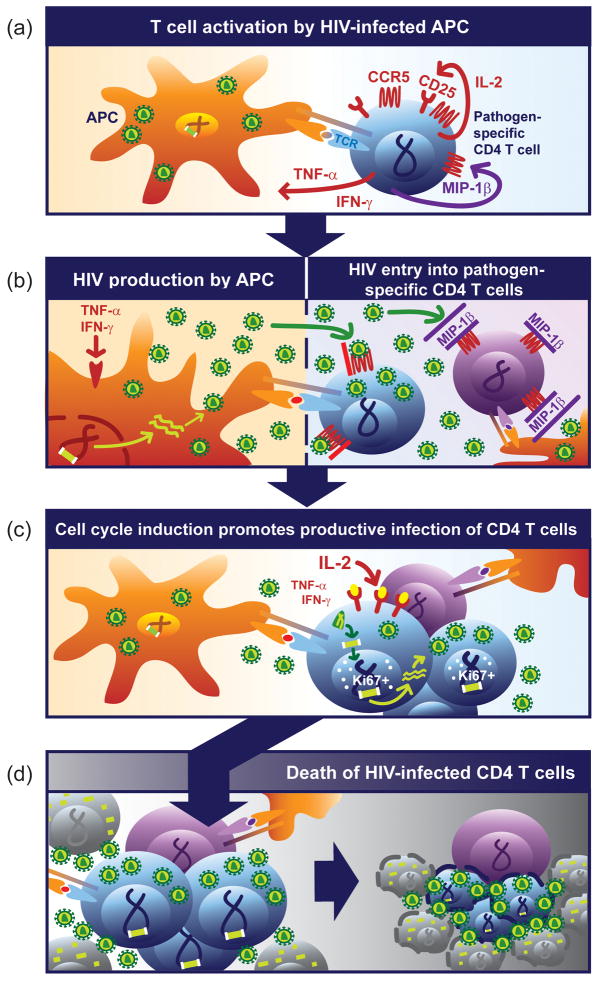
Figure 2. Proposed model for HIV-associated depletion of antigen-specific CD4 T cells.
(a) Within secondary lymphoid tissue, a pathogen-specific CD4 T cell (blue) is activated by a HIV-infected antigen-presenting cell (APC, orange) upon recognition of its cognate peptide presented on MHC class II. T cell activation induces the secretion of pro-inflammatory cytokines and up regulation of the HIV co-receptor CCR5 (red) and CD25. More mature CD57+ pathogen-specific CD4 T cells produce MIP-1β. (b) Pro-inflammatory cytokines, such as TNFα, promote HIV genomic transcription and production of virions by the APC, which are released into the interstitial space. HIV enters the T cell cytoplasm after binding its receptors CD4 and CCR5. Simultaneous production of MIP-1β (or other CCR5 ligands) by more mature pathogen-specific CD4 T cells can inhibit viral entry by blocking CCR5 entry. (c) IL2 signaling induces cell cycle induction within responding antigen-specific CD4 T cell expressing proliferation marker Ki67, and promotes complete reverse transcription of the viral RNA Subsequently, viral cDNA is integrated into host genome. CD57+ CD4 T cells are refractory to cell cycle induction and do not promote complete reverse transcription of the viral RNA genome. Continuous presence of proinflammatory cytokines, such as TNFα, promote proviral transcription. (d) HIV infection of expanding pathogen-specific CD4 T cells culminates in death of a significant fraction of these pathogen-specific CD4 T cells. More mature pathogen-specific CD4 T cells producing MIP-1β (or other CCR5 ligands) survive.
A better understanding of how pathogen-specific CD4 T cells with different characteristics are generated could also lead to better vaccines against HIV and opportunistic pathogens. Both CMV and mycobacteria (BCG) are being developed as potential HIV vaccine vectors [92–93]. Assuming the insert-specific responses will reflect the type of immunity induced to the vector, it can be expected that these vaccines will stimulate T cell responses of very different quality, that will impart very different the susceptibility of these T cells to HIV infection and depletion, thereby potentially affecting the efficacy of these vaccines.
Acknowledgments
The authors thank Joseph Casazza, Pratip Chattopadhyay, Joe Jarvis and Costas Petrovas for useful discussions. The illustrated data has kindly been provided by Joseph Casazza from the Vaccine Research Center, National Institutes of Health, Bethesda, USA We also like to thank Jennifer Ring for illustration of Figure 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bronke C, et al. Dynamics of cytomegalovirus (CMV)-specific T cells in HIV-1-infected individuals progressing to AIDS with CMV end-organ disease. J Infect Dis. 2005;191:873–880. doi: 10.1086/427828. [DOI] [PubMed] [Google Scholar]
- 2.Komanduri KV, et al. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J Infect Dis. 2001;183:1285–1289. doi: 10.1086/319683. [DOI] [PubMed] [Google Scholar]
- 3.Komanduri KV, et al. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4:953–956. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 4.Autran B, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 5.Casazza JP, et al. Autocrine production of beta-chemokines protects CMV-Specific CD4 T cells from HIV infection. PLoS Pathog. 2009;5:e1000646. doi: 10.1371/journal.ppat.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geldmacher C, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207:2869–2881. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dragic T, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 9.Moore JP, et al. The CCR5 and CXCR4 coreceptors--central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- 10.Levy JA. HIV pathogenesis: 25 years of progress and persistent challenges. AIDS. 2009;23:147–160. doi: 10.1097/QAD.0b013e3283217f9f. [DOI] [PubMed] [Google Scholar]
- 11.Picker LJ, et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin S, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura Y, et al. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc Natl Acad Sci U S A. 2004;101:12324–12329. doi: 10.1073/pnas.0404620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma CS, et al. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 16.Zaunders JJ, et al. Early proliferation of CCR5(+) CD38(+++) antigen-specific CD4(+) Th1 effector cells during primary HIV-1 infection. Blood. 2005;106:1660–1667. doi: 10.1182/blood-2005-01-0206. [DOI] [PubMed] [Google Scholar]
- 17.Maier R, et al. Kinetics of CXCR4 and CCR5 up-regulation and human immunodeficiency virus expansion after antigenic stimulation of primary CD4(+) T lymphocytes. Blood. 2000;96:1853–1856. [PubMed] [Google Scholar]
- 18.Bermejo M, et al. Activation of blood T lymphocytes down-regulates CXCR4 expression and interferes with propagation of X4 HIV strains. Eur J Immunol. 1998;28:3192–3204. doi: 10.1002/(SICI)1521-4141(199810)28:10<3192::AID-IMMU3192>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Jourdan P, et al. Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol. 2000;165:716–724. doi: 10.4049/jimmunol.165.2.716. [DOI] [PubMed] [Google Scholar]
- 20.Ganesan A, et al. Immunologic and virologic events in early HIV infection predict subsequent rate of progression. J Infect Dis. 2010;201:272–284. doi: 10.1086/649430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehandru S, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 23.Mattapallil JJ, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 24.Holm GH, Gabuzda D. Distinct mechanisms of CD4+ and CD8+ T-cell activation and bystander apoptosis induced by human immunodeficiency virus type 1 virions. J Virol. 2005;79:6299–6311. doi: 10.1128/JVI.79.10.6299-6311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soumelis V, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 26.Brown KN, et al. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5:e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenchley JM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutter G, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro RM, et al. Naive and memory cell turnover as drivers of CCR5-to-CXCR4 tropism switch in human immunodeficiency virus type 1: implications for therapy. J Virol. 2006;80:802–809. doi: 10.1128/JVI.80.2.802-809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkins RA, et al. Expression of mucosal homing receptor alpha4beta7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect Immun. 2000;68:5587–5594. doi: 10.1128/iai.68.10.5587-5594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner N, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 32.Arthos J, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 33.Cicala C, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A. 2009;106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krzysiek R, et al. Preferential and persistent depletion of CCR5+ T-helper lymphocytes with nonlymphoid homing potential despite early treatment of primary HIV infection. Blood. 2001;98:3169–3171. doi: 10.1182/blood.v98.10.3169. [DOI] [PubMed] [Google Scholar]
- 35.Biancotto A, et al. HIV-1 induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 2008;111:699–704. doi: 10.1182/blood-2007-05-088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou CS, et al. Highly purified CD25- resting T cells cannot be infected de novo with HIV-1. Proc Natl Acad Sci U S A. 1997;94:1361–1365. doi: 10.1073/pnas.94.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zack JA, et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 38.Maenetje P, et al. A steady state of CD4+ T cell memory maturation and activation is established during primary subtype C HIV-1 infection. J Immunol. 2010;184:4926–4935. doi: 10.4049/jimmunol.0903771. [DOI] [PubMed] [Google Scholar]
- 39.Eberly MD, et al. Increased IL-15 production is associated with higher susceptibility of memory CD4 T cells to simian immunodeficiency virus during acute infection. J Immunol. 2009;182:1439–1448. doi: 10.4049/jimmunol.182.3.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller YM, et al. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol. 2008;180:350–360. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doitsh G, et al. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okoye A, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caruso AM, et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 44.Gallegos AM, et al. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonnenberg P, et al. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191:150–158. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 46.WHO. Frequently asked questions about TB and HIV. 2008. [Google Scholar]
- 47.Geldmacher C, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198:1590–1598. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalsdorf B, et al. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am J Respir Crit Care Med. 2009;180:1262–1270. doi: 10.1164/rccm.200907-1011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldrop SL, et al. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glennie SJ, et al. Impaired CD4 T cell memory response to Streptococcus pneumoniae precedes CD4 T cell depletion in HIV-infected Malawian adults. PLoS One. 2011;6:e25610. doi: 10.1371/journal.pone.0025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenchley JM, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol. 2004;2:401–413. doi: 10.1038/nrmicro878. [DOI] [PubMed] [Google Scholar]
- 53.Douek DC, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 54.Harari A, et al. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 55.Harari A, et al. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol. 2004;34:3525–3533. doi: 10.1002/eji.200425324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Streitz M, et al. Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis. PLoS One. 2007;2:e735. doi: 10.1371/journal.pone.0000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuetz A, et al. Evaluating Mycobacterium tuberculosis activity in HIV-1 infected individuals in vivo. PLOSone accepted for publication Oct 2011. [Google Scholar]
- 58.Hammond AS, et al. Mycobacterial T cell responses in HIV-infected patients with advanced immunosuppression. J Infect Dis. 2008;197:295–299. doi: 10.1086/524685. [DOI] [PubMed] [Google Scholar]
- 59.Samandari T, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 60.Schuetz A, et al. Monitoring CD27 Expression to Evaluate Mycobacterium Tuberculosis Activity in HIV-1 Infected Individuals In Vivo. PLoS One. 2011;6:e27284. doi: 10.1371/journal.pone.0027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oni T, et al. High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening. Thorax. 2011;66:669–673. doi: 10.1136/thx.2011.160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harari A, et al. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pope M, et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 64.Lore K, et al. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujita H, et al. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci U S A. 2009;106:21795–21800. doi: 10.1073/pnas.0911472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoshino Y, et al. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med. 2002;195:495–505. doi: 10.1084/jem.20011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wille-Reece U, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehandru S, et al. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J Virol. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cecchinato V, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–288. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 71.Deeks SG, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 72.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 73.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raffatellu M, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adler DH. The impact of HAART on HPV-related cervical disease. Curr HIV Res. 2010;8:493–497. doi: 10.2174/157016210793499240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palefsky J. Human papillomavirus-related disease in people with HIV. Curr Opin HIV AIDS. 2009;4:52–56. doi: 10.1097/COH.0b013e32831a7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carbone A, et al. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113:1213–1224. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- 78.Chang Y, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 79.Piriou E, et al. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2005;106:3166–3174. doi: 10.1182/blood-2005-01-0432. [DOI] [PubMed] [Google Scholar]
- 80.Gasser O, et al. HIV patients developing primary CNS lymphoma lack EBV-specific CD4+ T cell function irrespective of absolute CD4+ T cell counts. PLoS Med. 2007;4:e96. doi: 10.1371/journal.pmed.0040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheynier R, et al. HIV and T cell expansion in splenic white pulps is accompanied by infiltration of HIV-specific cytotoxic T lymphocytes. Cell. 1994;78:373–387. doi: 10.1016/0092-8674(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 82.Klatt NR, et al. SIV infection of rhesus macaques results in dysfunctional T- and B-cell responses to neo and recall Leishmania major vaccination. Blood. 2011;118:5803–5812. doi: 10.1182/blood-2011-07-365874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Griffin E, et al. Oral mucosal reactivation rates of herpesviruses among HIV-1 seropositive persons. J Med Virol. 2008;80:1153–1159. doi: 10.1002/jmv.21214. [DOI] [PubMed] [Google Scholar]
- 84.McKenzie ND, et al. Women with HIV are more commonly infected with non-16 and -18 high-risk HPV types. Gynecol Oncol. 2010;116:572–577. doi: 10.1016/j.ygyno.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 85.Strickler HD, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 86.Luque AE, et al. Prevalence of human papillomavirus genotypes and related abnormalities of cervical cytological results among HIV-1-infected women in Rochester, New York. J Infect Dis. 2006;194:428–434. doi: 10.1086/505876. [DOI] [PubMed] [Google Scholar]
- 87.Sahasrabuddhe VV, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007;96:1480–1483. doi: 10.1038/sj.bjc.6603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coleman N, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102:768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 89.de Jong A, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64:5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 90.de Jong A, et al. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002;62:472–479. [PubMed] [Google Scholar]
- 91.Woo YL, et al. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int J Cancer. 2010;126:133–141. doi: 10.1002/ijc.24804. [DOI] [PubMed] [Google Scholar]
- 92.Hansen SG, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chapman R, et al. Recombinant Mycobacterium bovis BCG as an HIV vaccine vector. Curr HIV Res. 2010;8:282–298. doi: 10.2174/157016210791208686. [DOI] [PMC free article] [PubMed] [Google Scholar]