Abstract
Rationale
Affective disorders are twice as likely to occur in women as they are in men suggesting a critical role for gonadal hormones in their etiology. In particular, testosterone has been shown to have protective effects in men.
Objective
To investigate antidepressant effects and interactions between testosterone and imipramine in socially isolated male and female rats.
Methods
A chronic social isolation model was used to induce an anxiety and depressive-like state in adult gonadectomized (Gnx) male and ovariectomized (Ovx) female rats receiving chronic testosterone and imipramine treatments. Their anxiety and depression-like behaviors were examined using the light-dark box, elevated plus maze, open field, sucrose preference and novelty induced hypophagia tests.
Results
In socially isolated rats, the anxiolytic and antidepressant effects of testosterone and imipramine were limited to male rats. Additionally, testosterone enhanced the neurogenic effect of imipramine on hippocampal cell proliferation in male rats. Although female rats exhibited signs of anxiety and depressive-like behaviors following social isolation, testosterone and/or imipramine administration had no anxiolytic or antidepressant effects in Ovx females.
Conclusions
Testosterone and imipramine had anxiolytic and antidepressant effects in socially isolated male, but not female rats. Testosterone enhanced the effect of imipramine on cell proliferation in the hippocampus of male rats.
Keywords: testosterone, imipramine, social isolation, gonadectomy, depression, anxiety, neurogenesis
Introduction
There are well documented sex differences in the prevalence of anxiety and depressive disorders, where females are twice as likely as men to be affected (Angst et al., 2002; Bebbington et al., 2003; Earls, 1987; Kessler, 2003). Evidence from human and animal studies suggests that females react differently to stressors than males (Frye and Walf, 2009; Vermeulen and Kaufman, 2002). Although it is well established that women are more susceptible to the development of affective disorders, considerably less attention has been given to sex differences in the development, presentation, and features of depressive disorders as well as the response to antidepressant treatments.
Biological, psychological, and sociological theories attempt to explain the preponderance of women suffering from anxiety and depressive disorders, however, the prevailing hypothesis implicates a critical role for gonadal hormones in both the onset and regulation of mood disorders (Colangelo et al., 2008). In particular, testosterone had antidepressant effects in humans and rodents. Indeed, hypogonadal men with low circulating levels of testosterone have increased incidence of major depressive disorder and testosterone replacement effectively improved mood (Cunningham et al., 1989; McIntyre et al., 2006; McNicholas et al., 2003). Testosterone administration had protective effects against the development of anxiety and depressive-like symptoms and had antidepressant effects in aged male and female mice (Frye and Walf, 2009; Solomon et al., 2009). These studies suggested that testosterone is protective against the development and progression of affective disorders.
The onset of anxiety and depressive disorders is highly correlated with the occurrence of stressful life events (Agliati et al., 2006). In the laboratory, chronic social isolation constitutes an environmentally-induced stressful experience that may be effectively used to model a depressive-like state (Migues et al., 2005). In our recently published work (Carrier and Kabbaj, 2012), we have shown in stress-free conditions, that testosterone had antidepressant effects in male rats despite a lack of change in neurogenesis. In this work, Gnx male rats were subjected to a chronic social isolation protocol to examine if testosterone has protective effects and/or can affect neurogenesis under stressful conditions. We then extended our study to investigate potential anxiolytic and antidepressant effects of testosterone and imipramine administration in socially isolated Ovx female rats. We report prominent effects of testosterone and imipramine treatments in the response to social isolation stress in male, but not female rats.
Methods
Animals
Adult male (weighing 250–270g) and female (weighing 200–225g) Sprague-Dawley rats, were purchased from Charles River (Wilmington, MA, USA). All rats were initially pair-housed in 43x21.5x25.5cm Plexiglas cages and kept on a 12h: 12h light: dark cycle (lights on at 0700 hours). Food and water was available ad libitum except during testing. All behavioral experiments, except the sucrose preference test, were conducted during the first 4 h of the light phase of the light/dark cycle and all animal protocols were carried out in accordance with the NIH Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Florida State University.
Surgery
Immediately following gonadectomy/ovariectomy, male and female rats received placebo/testosterone pellet and saline/imipramine osmotic minipumps. Rats were anesthetized with a mixture of ketamine/xylazine (70 mg/kg of ketamine and 10 mg/kg xylazine; i.p.) and bupivicaine (0.25% solution; 0.4 mL/kg) was applied topically as analgesic. The non-steroidal anti-inflammatory drug (NSAID) meloxicam (1.0 mg/mL) was injected subcutaneously.
Gonadectomy/Ovariectomy
Following disinfection of the skin (with alcohol and betadine), a 1–2 cm ventral midline incision was made in the scrotum of adult male rats to expose the tunica. The tunica was pierced and both testes were extracted to expose the underlying blood vessels, which were ligated with silk suture. The testes were excised and all vessels and ducts were placed back into the tunica prior to suturing. A slightly larger 2–3 cm ventral midline incision was made in the lower abdominal region of adult female rats to expose the uterus. Visible blood vessels were ligated, the ovaries removed, and the muscle layers and skin were sutured.
Testosterone supplementation
Sixty-day slow release testosterone (25mg/pellet) or placebo pellets (Innovative Research of America, Sarasota FL) were inserted subcutaneously 7–10 cm from a small 1–2 cm incision below the shoulder blades. We have shown that these pellets reproduce the physiological levels of testosterone found in male rats (Carrier and Kabbaj, 2012).
Osmotic minipumps
Alzet Osmotic Minipumps (Alza, Mountain View, CA) for 28-day administration (Model 2ML4), containing imipramine HCl (Sigma-Aldrich, St. Louis, MO; 20mg/kg/day) or 0.9% saline were implanted subcutaneously in the dorsal rear flank region. Imipramine was prepared in 0.9% sterile saline.
Experimental Design
Experiment 1: Validation of chronic social isolation as an anxiety and depression model in male rats
Adult male rats (2–3 months of age at the start) were either pair-housed or socially isolated for 3 weeks prior to behavioral testing. During this time they were only handled twice a week for cage maintenance. Their anxiety and depression-like behaviors were examined using the light-dark box and sucrose preference tests respectively.
Experiment 2: Social isolation, testosterone, and imipramine exposure in males rats
Immediately following Gnx surgeries, all rats were socially isolated for two weeks to induce an anxiety and depressive-like state. Anxiety-like behaviors were assessed 13–16 days following isolation using the light dark box, open field, and elevated plus maze tests. Depressive-like behaviors were examined 3 weeks after social isolation using the sucrose preference and novelty-induced hypophagia tests. To label proliferating cells in the dentate gyrus, rats were injected with 5-bromo-2'-deoxyuridine (BrdU) under non-stress conditions, 3 d after all behavior testing was completed. Rats were transcardially perfused 24 h later, and their brains processed for BrdU immunohistochemistry.
Experiment 3: Validation of chronic social isolation as an anxiety and depression model in female rats
Adult female rats (2–3 months of age at the start) were either pair-housed or socially isolated for 3 weeks prior to behavioral testing. During this time they were only handled twice a week for cage maintenance. Their anxiety and depression-like behaviors were examined using the light-dark box and sucrose preference tests respectively.
Experiment 4: Social isolation, testosterone, and imipramine exposure in female rats
Immediately following Ovx surgeries, all rats were socially isolated for two weeks to induce an anxiety and depressive-like state. Anxiety-like behaviors were assessed 13–16 days following isolation using the light dark box, open field, and elevated plus maze tests. Depressive-like behaviors were examined 3 weeks after social isolation using the sucrose preference and novelty-induced hypophagia tests.
Behavioral Tests
Light Dark Box
Rats were placed into the dark compartment (200x310 mm) of the dual chamber apparatus (Model LE-812, EB Instruments, Pinellas Park, FL) and allowed to freely explore both compartments for 10 minutes. Total duration spent and frequency of entries in the light compartment (310x310 mm) were analyzed using PPCWIN software. The apparatus was cleaned with 70% ethanol between trials.
Open Field Test
Rats were placed in a large (1m×1m) open field under dim light and were allowed to freely explore the arena for 10 min. Rat behavior was recorded by a digital camcorder placed directly above the open field. Total duration spent in the center were analyzed in EthoVision XT version 6 (Noldus Information Technology, Leesburg, VA). The open field arena was cleaned with 70% ethanol between trials.
Elevated Plus Maze
Rats were placed into the elevated plus maze (MED Associates Inc., St. Albans, Vermont) facing a closed arm and were allowed to freely explore the maze for 10 minutes under dim light. Rats’ behavior was recorded by a digital camcorder placed directly above the elevated plus maze. Open arm duration and number of entries into open arms were analyzed in EthoVision XT version 6 (Noldus Information Technology, Leesburg, VA). The elevated plus maze was cleaned with 70% ethanol between trials.
Sucrose preference test
The sucrose preference test consisted of a two-bottle choice paradigm (Carrier and Kabbaj, 2012; Chen et al., 2012; Dagyte et al., 2011; Kentner et al., 2010). Rats were allowed to drink from two water bottles for five days prior to testing. The rats were given access to two preweighed bottles, one containing water and the other containing 0.5% sucrose for 48 hours. The bottles were weighed at 8am and 5pm and the preference for sucrose over water was used as a measure of anhedonia.
Novelty Induced Hypophagia
Rats were trained for 30 minutes/day for 3 days to drink a sweetened condensed milk solution (diluted 1:3 in distilled water) from plastic water bottles in their home cage. On the fourth day, the latency to drink the sweetened milk solution in the home cage was recorded. On the fifth day, rats were placed into a novel cage under bright light, which contained no bedding and had white sheets of paper underneath to enhance aversiveness. The latency to drink the sweetened milk solution in the novel environment was recorded.
Neurogenesis Studies
Hippocampal cell proliferation
The thymidine analog BrdU was administered intraperitoneally from a 40 mg/mL stock solution prepared in 0.9% NaCl. Rats received a single injection of BrdU (200 mg/kg, i.p.) 21 d following Gnx and pellet/minipump implant surgeries, and were sacrificed 24 h later. Rats were anesthetized with sodium pentobarbital (100 mg/kg) and were transcardially perfused with phosphate buffered saline (PBS, pH 7.4), followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The brains were extracted, postfixed at 4°C in paraformaldehyde fixative overnight, cryoprotected in increasing sucrose (12 hrs in 10%, 12 hrs in 20%, and 24 hrs in 30%) solutions at 4°C, and frozen at −80°C until further processing.
Immunohistochemistry for BrdU
Brains were sectioned on a cryostat at 30 µm, and a series of sections throughout the hippocampus were mounted on poly-L-lysine-coated slides and stored at −80°C until use. Sections were blocked with normal goat serum (NGS; Vector labs) in 0.1M PBS pH 7.4 for 1 h at room temperature and then incubated overnight at 4°C in 1:100 rat anti-BrdU antibody (Abcam, cat # ab6326). Slides were washed in 0.01% triton in 0.1M PBS pH 7.4 (3×5 min), and incubated at room temperature for 2 h in 1:1000 anti-rat IgG biotinylated antibody (Sigma, cat # B 7139), followed by biotin-peroxidase complex (Vector labs) for 1 h, and 3,3’-diaminobenzadine (DAB) solution (Vector labs).
Quantification of BrdU labeled cells
Stereological procedures were conducted by an investigator blind to treatment and were based on computerized stereology (StereoInvestigator, MicroBrightField, Inc., Colchester, VT) using a Leica fluorescence microscope (Ctr6000) at 400X magnification. Sampling of every 6th section began at −2.56 mm from bregma where the dentate gyrus first becomes clearly visible and ended at −4.16 mm from bregma where the hippocampus has dorsal and ventral components in coronal section (Paxinos G, 1998). Cell density of the dorsal hippocampus was calculated for each subject as the total number of BrdU positive cells within the granule cell layer and the hilus of the dentate gyrus divided by the volume (mm3) of the dentate gyrus estimated for each rat using Cavalieri’s method (Amstadter et al., 2009).
Statistical analysis
Unpaired t-test was used to compare pair-housed with isolated rats in the light-dark box and sucrose preference tests. Two-way (placebo/testosterone treatment and saline/imipramine treatment) analysis of variance (ANOVA) tests were conducted on all other measures. All ANOVAs were followed by post-hoc Fisher tests where appropriate to ensure significance. P values < 0.05 were considered statistically significant.
Results
Experiment 1: Validation of chronic social isolation as an anxiety and depression model in male rats
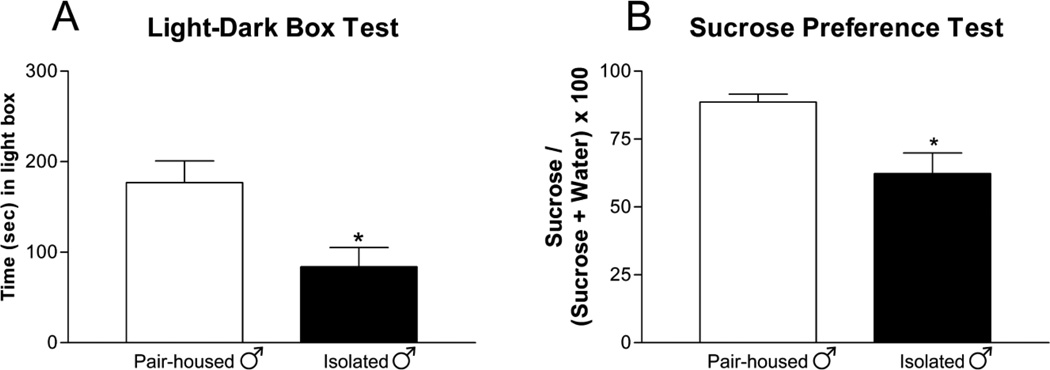
Two weeks of chronic social isolation induced anxiety-like behaviors in male rats. Isolated male rats spent less time in the light compartment of the light-dark box [Figure 1A; t=7.116; p<0.05] compared to pair-housed controls. Three weeks of chronic social isolation induced depressive-like symptoms in male rats where isolated male rats exhibited decreased sucrose preference [Figure 1B; t=5.743; p<0.05] compared to pair-housed male rats.
Figure 1. Chronic Social Isolation Induced Anxiety and Depressive-like Symptoms in Male Rats.
Socially isolated male rats (A) spent less time (mean ± SEM) in the light box and (B) have decreased sucrose consumption (mean ± SEM) compared to pair-housed controls. n=6–12 per group; t-test; *p<0.05 compared to same sex pair-housed.
Experiment 2: Social isolation, testosterone, and imipramine exposure in male rats
Anxiety-like Behaviors in Isolated Male Rats
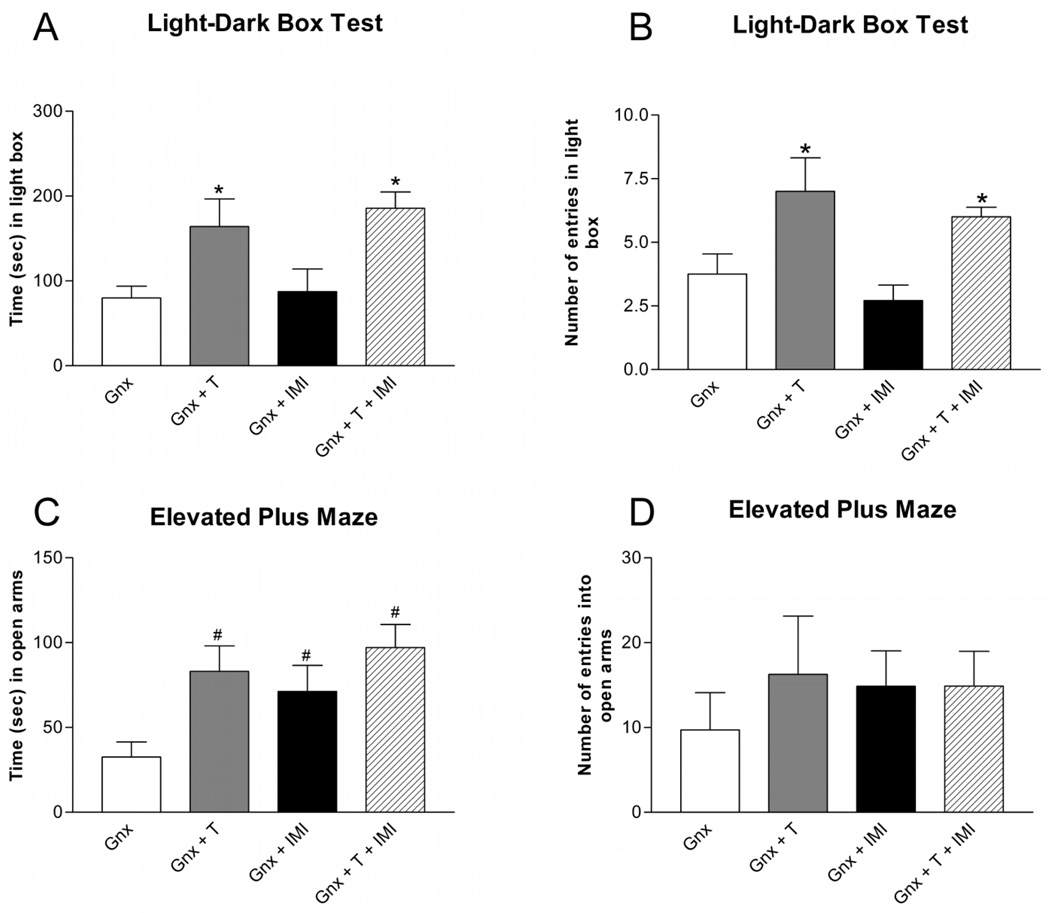
In the light-dark box test, testosterone replacements in Gnx male rats increased the time spent [Figure 2A; F(1,27) = 14.22; p < 0.001] and the number of entries [Figure 2B; F(1,27) = 13.84; p < 0.001] in the light box compared to placebo-treated Gnx male rats. Two weeks of chronic imipramine administration did not affect the time spent [Figure 2A; F(1,27) = 0.36; p > 0.05] or the number of entries [Figure 2B; F(1,27) = 1.34; p > 0.05] in the light box compared to saline-treated Gnx male rats. Co-administration of testosterone and imipramine increased the time spent and number of entries in the light box similar to testosterone treatment alone.
Figure 2. Anxiety-like Behaviors in Isolated Gnx Male Rats.
Testosterone supplementation increased (A) the time (mean ± SEM) spent and (B) the number (mean ± SEM) of entries in the light box in socially isolated Gnx male rats. Imipramine alone did not affect the time or entries in the light box. In the elevated plus maze, testosterone supplementation and chronic imipramine treatment increased (C) the time (mean ± SEM) spent but (D) had no effect on the number (mean ± SEM) of entries in the open arms. Co-administration of testosterone and imipramine was similar to testosterone or imipramine treatment alone. n=6–9 per group; T = Testosterone, IMI = Imipramine; *p<0.05 compared to Gnx and Gnx + IMI; #p<0.05 compared to Gnx.
In the elevated plus maze, testosterone supplementation in Gnx male rats significantly increased time spent [Figure 2C; F(1,26) = 6.07; p<0.05] and had no effect on the number of entries [Figure 2D; F(1,26) = 0.44; p > 0.05] in the open arms compared to placebo-treated Gnx male rats. Two weeks of chronic imipramine administration in Gnx male rats had no effect on the time spent [Figure 2C; F(1,26) = 0.64; p > 0.05] or number of entries [Figure 2D; F(1,26) = 0.53; p > 0.05] in the open arms compared to saline-treated Gnx male rats. Co-administration of testosterone and imipramine in Gnx male rats increased the time spent in the open arms similar to treatment with testosterone or imipramine alone in Gnx male rats and did not affect the number of entries into the open arms.
Time spent in the center of the open field arena was not affected by testosterone supplementation [F(1,27) = 0.66; p > 0.05] or two weeks of chronic imipramine administration [F(1,27) = 1.17; p > 0.05] in Gnx male rats compared to placebo and saline-treated Gnx male rats. Similarly, time spent in the center of the open field was not affected in Gnx rats receiving both testosterone and imipramine when compared to placebo and saline-treated Gnx male rats (Figure not shown).
Depression-like Behaviors in Isolated Male Rats
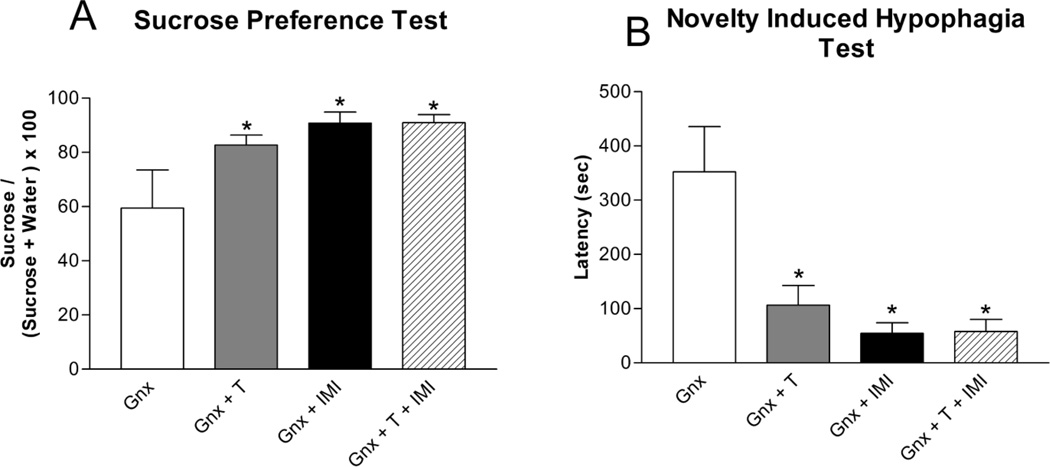
In the sucrose preference test, testosterone supplementation [Figure 3A; F(1,19) = 4.64; p<0.05] and three weeks of chronic imipramine administration [F(1,18) = 7.72; p<0.05] increased sucrose preference in Gnx male rats compared to placebo and saline-treated Gnx male rats. There was an interaction between testosterone and imipramine treatments [F(1,18) = 4.72; p<0.05]. In the novelty induced hypophagia test, testosterone supplementation [Figure 3B; F(1,22) = 5.30; p<0.05] and three weeks of chronic imipramine administration [F(1,22) = 10.80; p<0.05] decreased latency to drink in the novel cage in Gnx male rats compared to placebo and saline controls. There was an interaction between testosterone and imipramine treatments [F(1,22) = 5.57; p<0.05].
Figure 3. Depression-like Behaviors in Isolated Gnx Male Rats.
Testosterone supplementation and chronic imipramine treatment (A) increased sucrose preference (mean ± SEM) and (B) decreased latency (mean ± SEM) to drink in the novel cage in socially isolated Gnx male rats. Co-administration of testosterone and imipramine had similar effects of testosterone or imipramine treatment alone. n=6–9 per group; T = Testosterone, IMI = Imipramine; *p<0.05 compared to Gnx.
Cell Proliferation in Isolated Male Rats
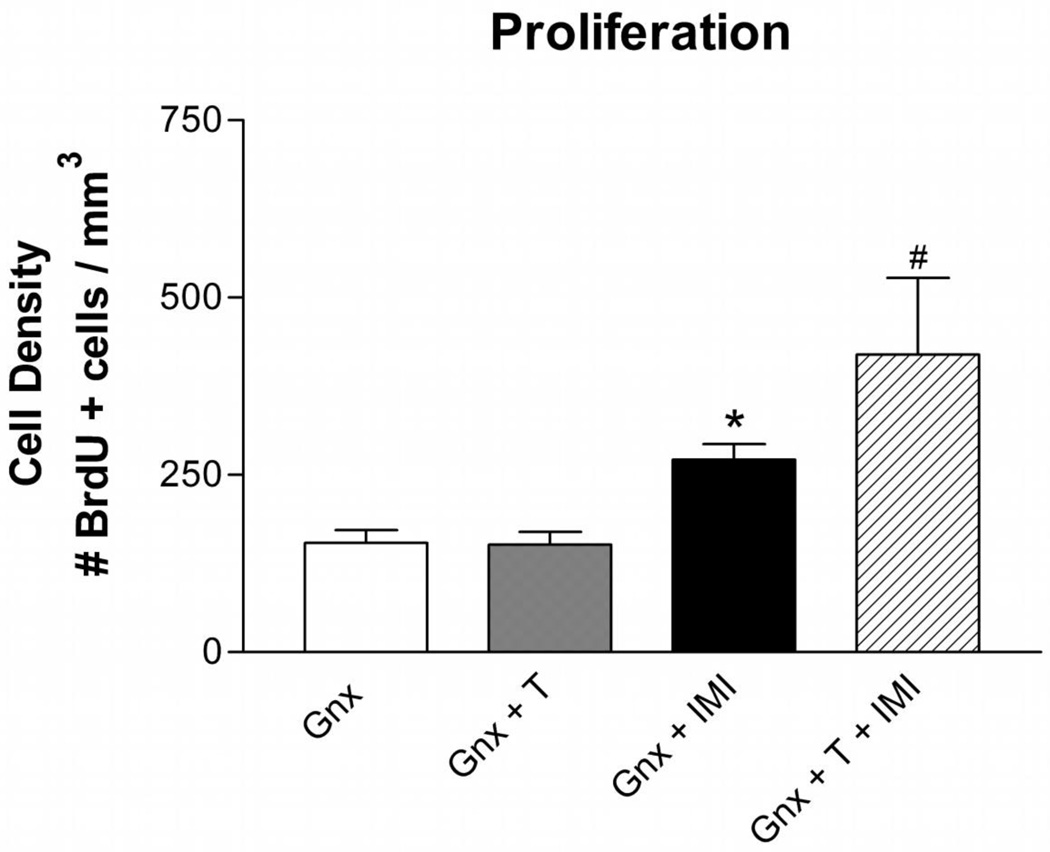
Three weeks of chronic imipramine treatment increased the density of BrdU+ cells [Figure 4; F(1,15) = 20.78; p< 0.0005] in Gnx male rats. While, testosterone supplementation alone did not increase the density of BrdU+ cells, there was an interaction between testosterone and imipramine treatment [F(1,15) = 4.61; p< 0.05]. Interestingly, imipramine treatment resulted in a greater number of BrdU+ cells in Gnx male rats with testosterone supplementation compared to placebo.
Figure 4. Testosterone Enhances the Neurogenic Effect of Imipramine in Isolated Gnx Male Rats.
Three weeks of chronic imipramine treatment increased the number (mean ± SEM) of BrdU+ cells in the subgranular zone and hilus of the dentate gyrus. Testosterone supplementation alone had no effects on the number of proliferating cells; however co-administration with imipramine resulted in an increased number of BrdU+ cells. n=6 per group; T = Testosterone, IMI = Imipramine; *p<0.05 compared to Gnx and Gnx+ T, #p<0.05 compared to Gnx, Gnx+ T, and Gnx + IMI.
Experiment 3: Validation of chronic social isolation as an anxiety and depression model in female rats
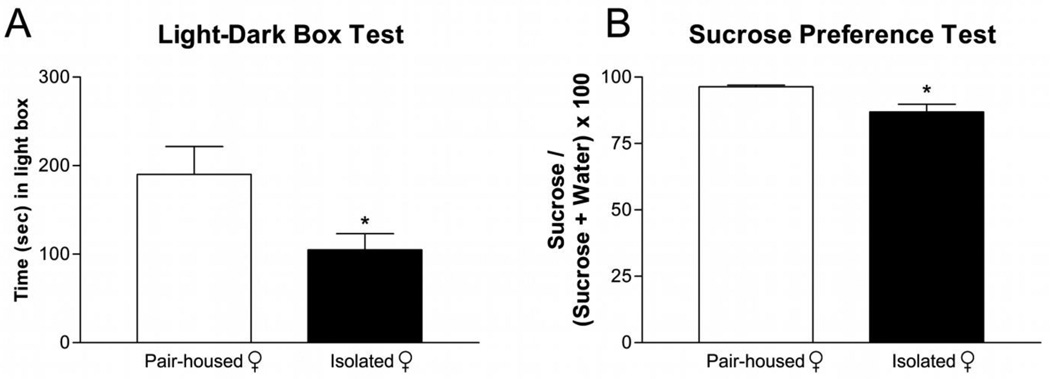
Two weeks of chronic social isolation induced anxiety-like behaviors in female rats. Isolated female rats spent less time in the light compartment of the light-dark box [Figure 5A; t= 6.381; p<0.05] compared to pair-housed controls. Similarly, three weeks of chronic social isolation induced depressive-like symptoms in female rats where isolated female rats exhibited decreased sucrose preference [Figure 5B; t= 5.253; p<0.05] compared to pair-housed female rats.
Figure 5. Chronic Social Isolation Induced Anxiety and Depressive-like Symptoms in Female Rats.
Socially isolated female rats (A) spent less time (mean ± SEM) in the light box and (B) had decreased sucrose consumption (mean ± SEM) compared to pair-housed controls. n=6–12 per group; *p<0.05 compared to same sex pair-housed.
Experiment 4: Social isolation, testosterone, and imipramine exposure in male rats
Anxiety-like Behaviors in Isolated Female Rats
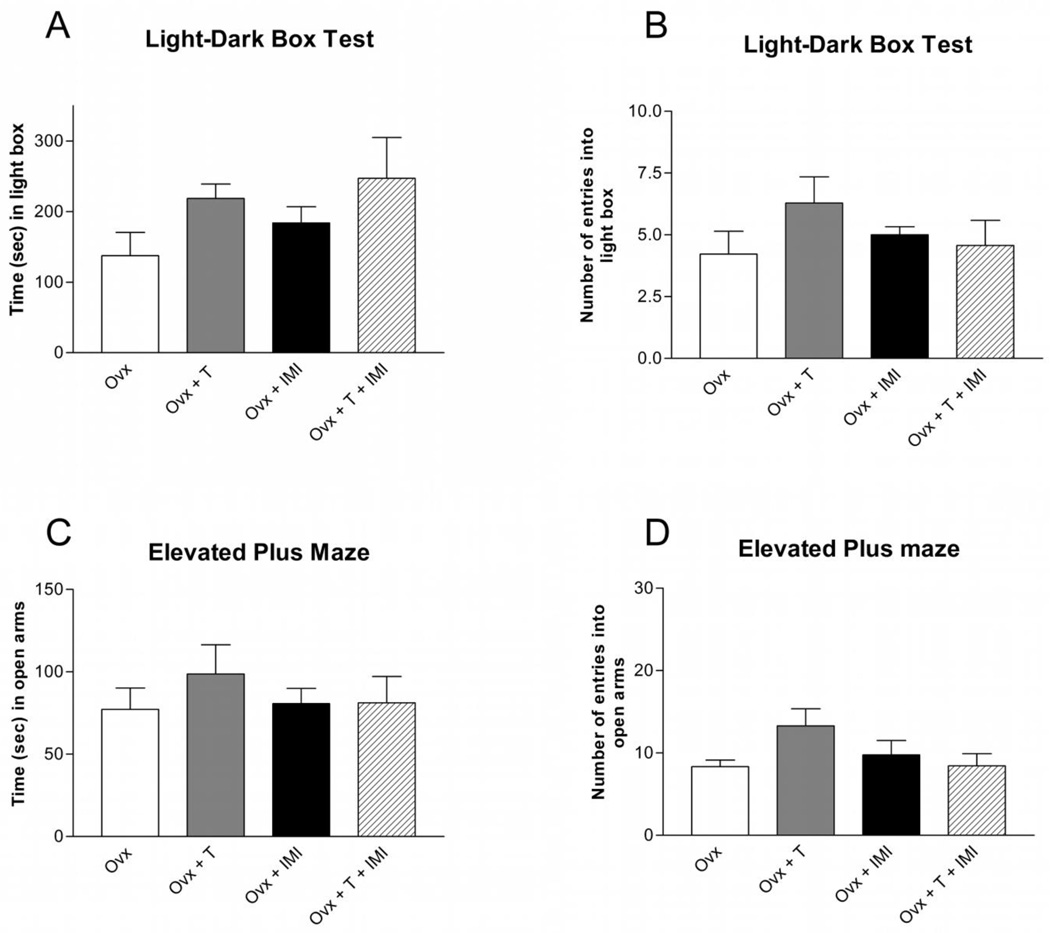
Testosterone supplementation in Ovx female rats did not affect the time spent [Figure 6A; F(1,24) = 3.50; p > 0.05] or the total number of entries [Figure 6B; F(1,24) = 0.695; p > 0.05] in the light box compared to placebo-treated Ovx female rats. Similarly, two weeks of chronic imipramine administration did not affect the time spent [Figure 6A; F(1.24) = 0.94; p > 0.05] or number of entries [Figure 6B; F(1,24) = 0.228; p>0.05] in the light box compared to saline-treated Ovx female rats. Co-administration of testosterone and imipramine in Ovx female rats did not affect time spent or number of entries in the light box compared to placebo and saline-treated rats.
Figure 6. Anxiety-like Behaviors in Isolated Ovx Female Rats.
Testosterone, imipramine, or co-administration of testosterone and imipramine had no effect on (A) the time (mean ± SEM) spent or (B) the number (mean ± SEM) of entries in the light box, or on the (C) time (mean ± SEM) spent, (D) or number (mean ± SEM) of entries in the open arms in socially isolated Ovx female rats. n=6–9 per group; T = Testosterone, IMI = Imipramine.
The time spent [Figure 6C; F(1,23) = 0.38; p > 0.05] and number of entries [Figure 6D; F(1,23) = 0.90; p > 0.05] in the open arms were not affected by testosterone supplementation in Ovx female rats. Two weeks of chronic imipramine treatment in Ovx female rats did not affect the time spent [Figure 6C; F(1,23) = 0.40; p > 0.05] or number of entries [Figure 6D; F(1,23) = 0.20; p > 0.05] in the open arms compared to saline-treated control Ovx female rats. Co-administration of testosterone and imipramine did not affect the time spent or number of entries into the open arms.
Time spent in the center of the open field arena was not affected by testosterone supplementation [F(1,24) = 0.24; p > 0.05] or two weeks of chronic imipramine administration [F(1,24) = 0.57; p > 0.05] in Ovx female rats compared to placebo and saline-treated Ovx female rats. Co-administration of testosterone and imipramine did not affect the time spent in the center of the open field compared to placebo and saline-treated controls (Figure not shown).
Depression-like Behaviors in Isolated Female Rats
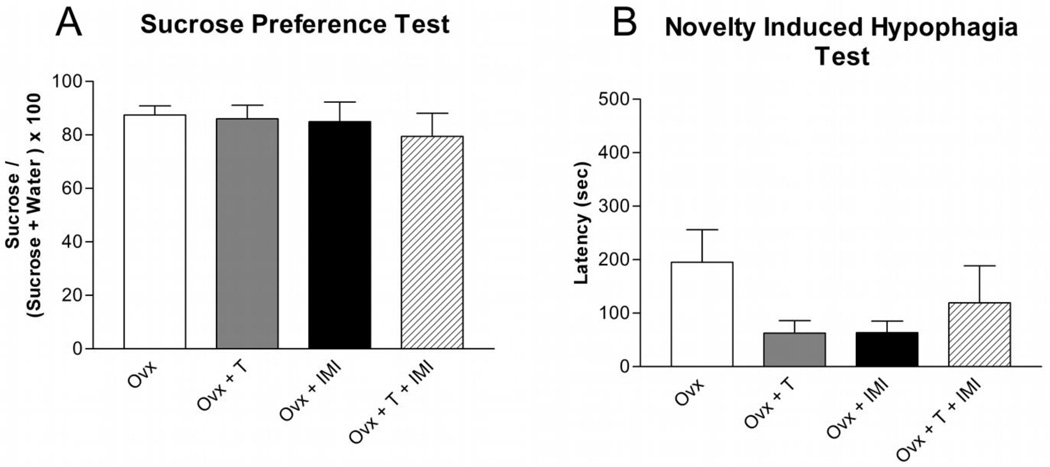
Testosterone supplementation [Figure 7A; F(1,24) = 1.23; p> 0.05] and three weeks of chronic imipramine administration [F(1,24) = 1.55; p> 0.05] had no effect on sucrose preference in Ovx female rats compared to placebo and saline-treated Ovx female rats. In the novelty induced hypophagia test, testosterone supplementation [Figure 7B; F(1,23) = 0.49; p> 0.05] and chronic imipramine administration [F(1,23) = 0.47; p> 0.05] had no effect on latency to drink in the novel cage in Ovx female rats compared to placebo and saline-treated controls. Co-administration of testosterone and imipramine did not affect sucrose preference or latency to drink compared to saline and placebo-treated Ovx female rats.
Figure 7. Depression-like Behaviors in Isolated Ovx Female Rats.
Testosterone, imipramine, or co-administration of testosterone and imipramine had no effect on (A) sucrose preference (mean ± SEM) or (B) latency (mean ± SEM) to drink in isolated Ovx female rats. n=6–9 per group; T = Testosterone, IMI = Imipramine.
Discussion
Social isolation stress revealed marked sex differences in the anxiolytic and antidepressant effects of testosterone and imipramine, as these treatments were beneficial in Gnx male rats, but had no effect in Ovx female rats. We observed a testosterone-imipramine interaction, where testosterone supplementation enhanced the neurogenic effect of imipramine in Gnx male rats, illustrated by an increase in the number of new proliferating cells in the dentate gyrus.
Chronic Social Isolation Stress
Neurobiological sex differences complicate our understanding of the mechanisms underlying the etiology of anxiety and depressive disorders. For instance, many gender disparities are attributable to organizational sex differences in brain development, while others are evident only after certain manipulations, including stress exposure or pharmacological drug treatments. We exposed adult male and female rats to chronic social isolation stress and examined their anxiety and depressive-like behavioral responses to testosterone and the tricyclic antidepressant imipramine. Social isolation stress followed by testosterone and/or imipramine treatments did not significantly impact the behavioral profile of Ovx female rats. Interestingly, early social isolation has been shown to increase emotional reactivity and alter HPA axis activity particularly in male rats (Ceravolo and del Vescovo, 1993; Mathews et al., 2008; Pelle-Ceravolo et al., 2004), and when coupled with additional stressors affects neurogenesis differently in male and female rats (Pelle-Ceravolo et al., 2004). Furthermore, social stressors during adolescence have long-lasting, sex-specific effects on behavioral and neuroendocrine responses (Bourke and Neigh, 2011; McCormick et al., 2004).
Sex Differences in the Antidepressant Response
Our data illustrate sexually dimorphic responses to antidepressant treatment, such that chronic imipramine administration is without effect in Ovx female rats. These results are in agreement with emerging evidence indicating sex-specific antidepressant responses (Li et al., 1999; Pitychoutis et al., 2011; Renoir et al., 2011). Gender specific differences in the response to tricyclic and selective serotonin reuptake inhibitor (SSRI) treatments have been reported, where women have a more favorable response to sertraline compared to imipramine and men respond better to imipramine compared to sertraline (Roth et al., 1999). Furthermore, a meta-analysis of thirty-five studies spanning over two decades, confirmed increased efficacy of imipramine treatment in men compared with women (Hagins et al., 1999). Evidence from several studies suggests that an effective antidepressant response in women requires estrogen. In two independent studies, depressed women over the age of sixty on estrogen replacement therapy had greater improvement with SSRI treatment compared to those without estrogen supplementations (Fischer et al., 1999; Kunze et al., 1999). The dramatic hormonal fluctuations experienced by females over the lifespan are associated with the incidence of depression, but may also be fundamental to sex-differences in the response to antidepressants. Whether or not these differences are a reflection of different subtypes of depression afflicting men and women, or involve the influence of gonadal hormones on the response to particular antidepressants requires further investigation.
A particularly interesting observation in the treatment of affective disorders, is that androgen administration to hypogonadal men alleviates depression nearly as effectively as antidepressants (Adler et al., 1999; Gentry and Wade, 1976), suggesting a major role for testosterone in mediating antidepressive effects. Our data support a significant antidepressant effect of testosterone. However, this effect appears to be limited to males, as we found no antidepressive effects of testosterone in Ovx female rats. Although testosterone treatment in male rodents has similar antidepressant activity to traditional drug treatments, few studies have investigated the potential interaction between androgens and antidepressants.
The physiological mechanism of these interactions remains largely unknown. However, imipramine - a tricyclic antidepressant that inhibits both 5-hydroxytryptamine and norepinephrine reuptake (Racagni and Popoli, 2010) - likely interacts with testosterone through the noradrenergic system. Indeed, gonadectomized rats do not respond to the noradrenaline reuptake inhibitor desipramine, and testosterone supplementation restores its antidepressant activity. Furthermore, in this same study, testosterone did not influence the antidepressant efficacy of serotonin reuptake inhibitors such as fluoxetine and clomipramine (Martinez-Mota and Fernandez-Guasti, 2004).
The antidepressant activity of testosterone observed in male, but not female rats is likely due to the sex-specific organization of the brain established during development which generates permanent dimorphisms in brain regions important in cognition and affect such as the hippocampus, amygdala, and prefrontal cortex. These organizational effects result in circuitries within male and female brains that respond differently to endogenous and exogenous challenges (Arnold and Breedlove, 1985). Sex differences in intracellular signaling pathways likely contribute to sex-specific behaviors and responses to environmental challenges, including social isolation stress and antidepressant treatments.
In the present study, testosterone supplementation had anxiolytic and antidepressant effects in Gnx male rats regardless of imipramine administration. Three weeks of chronic imipramine administration had anxiolytic effects in the elevated plus maze and antidepressant effects in Gnx male rats regardless of testosterone supplementation, suggesting prominent antidepressant activity of each treatment. We did not observe anxiolytic effects in the light-dark box following two weeks of imipramine administration, suggesting that prolonged treatment may be required for the manifestation of anti-anxiety behaviors using this test. Furthermore, we did not observe cumulative behavioral effects of combined testosterone and imipramine treatment; however these results are likely due to the potent antidepressant activity of each independent treatment resulting in ceiling and flooring effects in the depression tests (Page et al., 2007).
Imipramine, testosterone, and neurogenesis
A hallmark of depression and antidepressant activity is altered hippocampal neurogenesis in the subgranular zone of the dentate gyrus (Boldrini et al., 2009). Stem cell proliferation and survival components of adult hippocampal neurogenesis are influenced by multiple factors, including antidepressant treatment and sex hormones (Galea et al., 2006). As expected, three weeks of chronic imipramine treatment induced a significant increase in the number of newly proliferated cells. In line with our recent findings (Carrier and Kabbaj, 2012), we observed no significant effect of testosterone supplementation in Gnx male rats on cell proliferation independent of imipramine administration. Interestingly, in a recent study, testosterone supplementation was unable to reverse reduced cell proliferation observed in isolated gonadectomized males (Spritzer et al., 2011). Accumulating evidence suggests prolonged exposure to testosterone may enhance the cell survival component of neurogenesis without affecting the number of newly proliferating cells (Buwalda et al., 2010; Spritzer and Galea, 2007). Interestingly, we observed an enhanced neurogenic effect with testosterone and imipramine co-administration, suggesting a potential influence of testosterone on imipramine antidepressant activity in male rats.
Conclusions
Sex differences in the incidence, manifestation, and treatment responses of mood disorders suggest a critical role for gonadal hormones. In a social isolation model for depression, we found that testosterone supplementation had anxiolytic and antidepressant effects in Gnx male rats and was without effect in Ovx female rats. Similarly, three weeks of chronic imipramine administration had antidepressant effects in male, but not female rats. Co-administration of testosterone and imipramine resulted in increased neurogenesis compared to imipramine alone in Gnx male rats. These critical interactions between stress, gonadal hormones, and antidepressant efficacy have implications for sex specific treatment of mood disorders.
Highlights.
Social isolation induces anxiety and depressive-like behaviors in rats.
Testosterone and imipramine have antidepressant effects in male but not female rats.
Testosterone enhances the neurogenic effect of imipramine in male rats.
Acknowledgements
This work was supported by funds from the National Institute of Mental Health (R01MH087583), the FSU College of Medicine and an FSU Developing Scholar Award to Mohamed Kabbaj. We thank Hussam Jourdi for proof reading the final version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: There are no conflicts of interest
References
- Adler A, et al. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- Agliati A, et al. A new methodological approach to nonverbal behavior analysis in cultural perspective. Behav Res Methods. 2006;38:364–371. doi: 10.3758/bf03192789. [DOI] [PubMed] [Google Scholar]
- Amstadter AB, et al. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. J Anxiety Disord. 2009;23:369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst J, et al. Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. Eur Arch Psychiatry Clin Neurosci. 2002;252:201–209. doi: 10.1007/s00406-002-0381-6. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Bebbington P, et al. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Int Rev Psychiatry. 2003;15:74–83. doi: 10.1080/0954026021000045976. [DOI] [PubMed] [Google Scholar]
- Boldrini M, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, et al. Testosterone decrease does not play a major role in the suppression of hippocampal cell proliferation following social defeat stress in rats. Physiol Behav. 2010;101:719–725. doi: 10.1016/j.physbeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Erk2 signaling in the hippocampus mediates the antidepressant effects of testosterone. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceravolo MP, del Vescovo A. Another look at steroids: intraluminal methylprednisolone in retropectoral augmentation mammoplasty. Aesthetic Plast Surg. 1993;17:229–232. doi: 10.1007/BF00636266. [DOI] [PubMed] [Google Scholar]
- Chen YW, et al. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Colangelo N, et al. A cardioplegia circuit with versatility: the 'ReVerse' system. How to do it. Perfusion. 2008;23:205–207. doi: 10.1177/0267659108101498. [DOI] [PubMed] [Google Scholar]
- Cunningham GR, et al. Testosterone replacement with transdermal therapeutic systems. Physiological serum testosterone and elevated dihydrotestosterone levels. Jama. 1989;261:2525–2530. [PubMed] [Google Scholar]
- Dagyte G, et al. Agomelatine reverses the decrease in hippocampal cell survival induced by chronic mild stress. Behav Brain Res. 2011;218:121–128. doi: 10.1016/j.bbr.2010.11.045. [DOI] [PubMed] [Google Scholar]
- Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr Dev. 1987;5:1–23. [PubMed] [Google Scholar]
- Fischer BM, et al. Tumor necrosis factor-alpha stimulates mucin secretion and cyclic GMP production by guinea pig tracheal epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999;20:413–422. doi: 10.1165/ajrcmb.20.3.3393. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Depression-like behavior of aged male and female mice is ameliorated with administration of testosterone or its metabolites. Physiol Behav. 2009;97:266–269. doi: 10.1016/j.physbeh.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, et al. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol. 1976;90:18–25. doi: 10.1037/h0077264. [DOI] [PubMed] [Google Scholar]
- Hagins M, et al. Effects of practice on the ability to perform lumbar stabilization exercises. J Orthop Sports Phys Ther. 1999;29:546–555. doi: 10.2519/jospt.1999.29.9.546. [DOI] [PubMed] [Google Scholar]
- Kentner AC, et al. Sex-dependent effects of neonatal inflammation on adult inflammatory markers and behavior. Endocrinology. 2010;151:2689–2699. doi: 10.1210/en.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kunze II, et al. The green fluorescent protein targets secretory proteins to the yeast vacuole. Biochim Biophys Acta. 1999;1410:287–298. doi: 10.1016/s0005-2728(99)00006-7. [DOI] [PubMed] [Google Scholar]
- Li CM, et al. Development of monoclonal antibodies against bovine mucin core 2 beta6 N-acetylglucosaminyltransferase. Glycoconj J. 1999;16:555–562. doi: 10.1023/a:1007030223118. [DOI] [PubMed] [Google Scholar]
- Martinez-Mota L, Fernandez-Guasti A. Testosterone-dependent antidepressant-like effect of noradrenergic but not of serotonergic drugs. Pharmacol Biochem Behav. 2004;78:711–718. doi: 10.1016/j.pbb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, et al. Increased depressive behaviour in females and heightened corticosterone release in males to swim stress after adolescent social stress in rats. Behavioural Brain Research. 2008;190:33–40. doi: 10.1016/j.bbr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- McCormick CM, et al. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Hormones and Behavior. 2004;46:458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, et al. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology. 2006;31:1029–1035. doi: 10.1016/j.psyneuen.2006.06.005. [DOI] [PubMed] [Google Scholar]
- McNicholas TA, et al. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU Int. 2003;91:69–74. doi: 10.1046/j.1464-410x.2003.04016.x. [DOI] [PubMed] [Google Scholar]
- Migues A, et al. Peripheral foot blockade versus popliteal fossa nerve block: a prospective randomized trial in 51 patients. J Foot Ankle Surg. 2005;44:354–357. doi: 10.1053/j.jfas.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Page AC, et al. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in depressed clinical samples. Br J Clin Psychol. 2007;46:283–297. doi: 10.1348/014466506X158996. [DOI] [PubMed] [Google Scholar]
- Paxinos G WC. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pelle-Ceravolo M, et al. A technique to decrease breast shape deformity during muscle contraction in submuscular augmentation mammaplasty. Aesthetic Plast Surg. 2004;28:288–294. doi: 10.1007/s00266-003-3023-0. [DOI] [PubMed] [Google Scholar]
- Pitychoutis PM, et al. Individual differences in novelty-seeking predict differential responses to chronic antidepressant treatment through sex- and phenotype-dependent neurochemical signatures. Behav Brain Res. 2011;223:154–168. doi: 10.1016/j.bbr.2011.04.036. [DOI] [PubMed] [Google Scholar]
- Racagni G, Popoli M. The pharmacological properties of antidepressants. Int Clin Psychopharmacol. 2010;25:117–131. doi: 10.1097/YIC.0b013e3283311acd. [DOI] [PubMed] [Google Scholar]
- Renoir T, et al. Sexually dimorphic serotonergic dysfunction in a mouse model of Huntington's disease and depression. PLoS One. 2011;6:e22133. doi: 10.1371/journal.pone.0022133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth KA, et al. 9 enhanced tyramide signal amplification immunohistochemical detection. J Histochem Cytochem. 1999;47:1644D–1645D. [PubMed] [Google Scholar]
- Solomon MB, et al. Gonadal hormones modulate the display of conditioned defeat in male Syrian hamsters. Horm Behav. 2009;56:423–428. doi: 10.1016/j.yhbeh.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, et al. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011;195:180–190. doi: 10.1016/j.neuroscience.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM. Diagnosis of hypogonadism in the aging male. Aging Male. 2002;5:170–176. [PubMed] [Google Scholar]