Abstract
Activation of rat adenosine 2A receptors (A2A R) dilates preglomerular microvessels, an effect mediated by epoxyeicosatrienoic acids (EETs). High salt (HS) intake increases epoxygenase activity and adenosine levels and greater vasodilator response to a stable adenosine analog, 2-chloroadenosine (2-CA), was seen in kidneys obtained from HS-fed rats which was mediated by increased EET release. Because this pathway is antipressor, we examined the role of the A2A R-EET pathway in a genetic model of salt-sensitive hypertension, the Dahl salt-sensitive (SS) rats. Dahl S resistant (R) rats fed a HS diet demonstrated a greater renal vasodilator response to 2-CA. In contrast, Dahl SS rats did not exhibit a difference in the vasodilator response to 2-CA whether fed normal salt (NS) or HS diet. In Dahl SR but not Dahl SS rats, HS intake significantly increased purine flux, augmented the protein expression of A2A R and cytochrome P450 2C23 and 2C11 epoxygenases, and elevated the renal efflux of EETs. Thus the Dahl SR rat is able to respond to HS intake by recruiting EET formation, whereas the Dahl SS rat appears to have exhausted its ability to increase EET synthesis above the levels observed on NS intake. In vivo inhibition of the A2A R-EET pathway in Dahl SR rats fed a HS diet results in reduced renal EETs levels, diminished natriuretic capacity and hypertension, thus supporting a role for the A2A R-EET pathway in the adaptive natriuretic response to modulate blood pressure during salt loading. An inability of Dahl SS rats to upregulate the A2A R-EET pathway in response to salt loading may contribute to the development of salt-sensitive hypertension.
Keywords: Cytochrome P450, Epoxyeicosatrienoic acids, Adenosine, Kidney, Salt-sensitive hypertension
1. Introduction
Salt-sensitivity, as defined by blood pressure elevation in response to a dietary salt load, is not only a causative factor for a subgroup of humans with essential hypertension, but has also been reported to be an independent cardiovascular risk factor in patients with hypertension (1). Therefore, understanding the mechanisms that contribute to the development of salt-sensitivity and identifying potential therapeutic targets for the management of salt-sensitive hypertension should provide novel approaches to treat elevated blood pressure. It has been recognized for many years that sodium chloride intake is one of the main environmental factors responsible for the development of hypertension (2). Increased sodium chloride intake results in increased renal sodium chloride excretion. This adaptive process prevents progressive salt retention and volume expansion, with elevation of blood pressure; thus, an impaired ability of the kidney to excrete sodium requires an increase in blood pressure to increase natriuresis and correct the sodium balance, resulting in hypertension (3).
2. Renal Adenosine Receptors
The physiological effects of adenosine, a metabolite of ATP, are observed in nearly every tissue and modulates cellular and organ function by binding to specific cell-surface P1 purinergic receptors (R), of which there are four known subtypes (A1, A2A, A2B and A3) (4). These receptors are members of the large family of seven transmembrane spanning heterotrimeric G protein-coupled receptors (5).
The renal localization of the adenosine receptor subtypes is well documented, but the reported distribution throughout the kidney vasculature and tubular segments varies depending on the technique employed. A1 R have a high affinity for adenosine and are expressed in preglomerular microvessels (PGMV), including the afferent arterioles, in glomeruli including mesangial cells, juxtaglomerular cells and vasa recta (6;7). The A1 R mRNA is expressed throughout every nephron segment (8–11). The A2A R and the A2B R, which possess a high and a low adenosine affinity, respectively, have been demonstrated in whole kidney preparations (6) and in glomeruli of rat and mouse kidney, in the outer medullary descending vasa recta and in the papilla (9;12;13). Although expression of A2A R was not detected in rat PGMV (7), using primer-specific polymerase chain reaction A2A R were found to be expressed in microdissected mouse efferent arterioles (14). Expression of A2B R was reported in rat PGMV (7) and have been implicated in the efferent arteriole tubular glomerular feedback (TGF) response (14–16). In addition, A2B R mRNA has been detected in the cortical thick ascending loop of Henle (TALH) and in the distal convoluted tubule as well as in the outer medullary descending vasa recta (9;12;13). There is scant information on the distinct intrarenal localization of A3 R, although both the mRNA and protein are present in whole kidney preparations of various species (6). The A3 R protein and mRNA was undetectable in PGMV (7), but was found in rat cortical and medullary tissue (17).
The kidney plays an integral role in the maintenance of extracellular fluid volume and electrolyte balance and thus, contributes to the long-term control of arterial pressure (18). In the kidney, adenosine plays a critical role in the regulation of renal vascular tone and reactivity, and additionally affects tubular transport (19;20). Adenosine also inhibits renin release, sympathetic neurotransmission, platelet aggregation, and lipolysis (20). Stimulation of A1 R constricts the renal vasculature and inhibits renin release and enhances proximal tubular NaCl reabsorption (21;22). The vasoconstrictor effects of A1 R activation in the afferent arteriole is a major focus as adenosine is a primary mediator of TGF (22–25). Stimulation of A2 R results in endothelium-dependent relaxation via stimulation of adenylyl cyclase (26–28), thus increasing renal blood flow (29) and decreasing blood pressure (30). Stimulation of A2A R promotes natriuresis by reducing NaCl reabsorption in the TALH and collecting duct (31–33) and A2 R activation attenuated TGF responses by stimulation of endothelial NOS (34). In afferent arterioles, A2R activation has been shown to counteract the A1 R-induced constriction leading to dilation and decreased autoregulation (35;36). Activation of A2B R dilates renal arteries in a NO-dependent manner (37) and in juxtamedullary afferent arterioles, functional expression of both A2A R and A2B R was observed, but the opposing vasodilator effect during adenosine-mediated afferent arteriolar vasoconstriction was predominantly via activation of A2B R (38). The renal function of the A3 R is poorly characterized. It may play a role in sodium and fluid balance by regulating the Na+/H+ exchanger (39) and may exacerbate renal ischemia-reperfusion injury (40). Interestingly A3 R expression increase with age and expression upregulated by high salt (HS) intake (6;17).
As production of adenosine is increased under stressful conditions such as hypoxia, ischemia, and inflammation, adenosine has traditionally been implicated in the renal functional responses to pathological events (41;42). However, there is now evidence supporting the contribution of adenosine to renal mechanisms that respond to nonpathological challenges to renal function. Adenosine levels have been shown to correlate with salt intake; switching rats from a normal salt (NS) to a HS or low-salt diet results in parallel changes in renal interstitial and urinary adenosine levels (43). The increase in adenosine concentration during HS intake may contribute to a reduction of macula densa-mediated renin secretion and enhance sodium excretion (44).
3. Cytochrome P450-derived arachidonic acid metabolites
The cytochrome P450 (CYP)-derived arachidonic acid (AA) metabolites, 20-hydroxyeicosatetraenoic acids (20-HETE) and four regioisomeric cis-epoxyeicosatrienoic acids (EETs), 5,6-, 8,9-, 11,12- and 14,15-EETs, generated by hydroxylases and epoxygenases, respectively, occupy a key position in the regulation of renal vascular tone (45;46). The constrictor effect of 20-HETE on PGMV established its importance as a modulator of TGF and renal autoregulation (47). In contrast, EETs are important modulators of cardiovascular function and have been recognized for their vasodilator, antiinflammatory, antiproliferative, and profibrinolytic properties (48–53). These lipid derived metabolites mediate/modulate the vascular responses of many vasoactive peptides, for example, angiotensin II (54;55), endothelin-1 (56) and bradykinin (57;58).
Furthermore, the contribution of EETs to blood pressure regulation has been established in several different animal models (59–61). It is well documented that the activity of renal epoxygenase is increased with dietary salt loading (62;63). EETs are thought to be natriuretic by virtue of their ability to dilate the renal vasculature (54;64;65), as well as regulating Na+ transport in proximal and distal tubules (66–68). Thus, an increase in the production of natriuretic EETs is one of the significant components of the kidney's adaptive response to prevent elevation of blood pressure in response to HS intake (60;69).
Although there is much evidence showing an increase in epoxygenase activity and subsequent increased production of antihypertensive EETs in response to salt loading, the stimulus for this increased epoxygenase activity has not been identified. We have linked A2A R activation to EET production in rat arcuate arteries (70) and have reported that activation of A2A R is coupled to EET release upstream of adenylyl cyclase activation and that EETs stimulate mono-ADP ribosyltransferase resulting in Gsα protein activation (28).
4. The renal A2A R – EET pathway
As adenosine levels are increased by dietary salt intake, we proposed that adenosine is the stimulus for increased renal epoxygenase activity in response to salt loading. More specifically, we hypothesized that HS intake increases the renal response to adenosine, resulting in increased epoxygenase activity and EET levels (71).
We examined the renal response to adenosine in isolated, perfused kidneys obtained from Spraque-Dawley (SD) rats fed a 4% HS diet or NS diet for 7 days (71). To define the vascular responses to adenosine, we used a stable adenosine analog, 2-chloroadenosine (2-CA) that is not subject to inactivation by either adenosine deaminase/kinase or rapid removal by nucleoside carriers. Bolus injections of 2-CA elicited biphasic responses: a transient vasoconstriction followed by a prolonged dilation (Fig. 1). The dilator responses to 2-CA were dose-dependent and were exaggerated in kidneys from rats fed a HS diet compared with those receiving a NS diet for 7 days. Under both conditions of NS and HS, 2-CA resulted in dilation, but the magnitude of the response was enhanced in kidneys obtained from rats fed HS for 7 days. As seen in Fig. 1, the inhibitory effect of a selective epoxygenase inhibitor, N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH), was greater under HS conditions. However, MS-PPOH did not completely abolish the response; thus EETs may not be the sole mediators of adenosine-induced dilation in isolated, perfused rat kidneys. Our study was performed in the presence of nitric oxide synthetase (NOS) and cyclooxygenase (COX) inhibition, which eliminated the contribution of NO and prostaglandins (i.e., PGI2 and PGE2) to the 2-CA response. In a previous study, we showed that the renal responses to 2-CA in the perfused kidney were not dependent on NO or PG synthesis (70). A possible candidate mediator of the epoxygenase-independent dilation in response to adenosine is carbon monoxide (CO), a product of heme degradation by heme oxygenase. An interaction between CO and adenosine in the nucleus of the solitary tract of rats has been reported: adenosine receptor antagonism attenuated the vasodepressor effect of hemin, while heme oxygenase inhibition attenuated the vasodepressor effect of adenosine (72).
Figure 1. Responsiveness of isolated, perfused rat kidneys to 10 µg 2-chloroadenosine (2-CA).
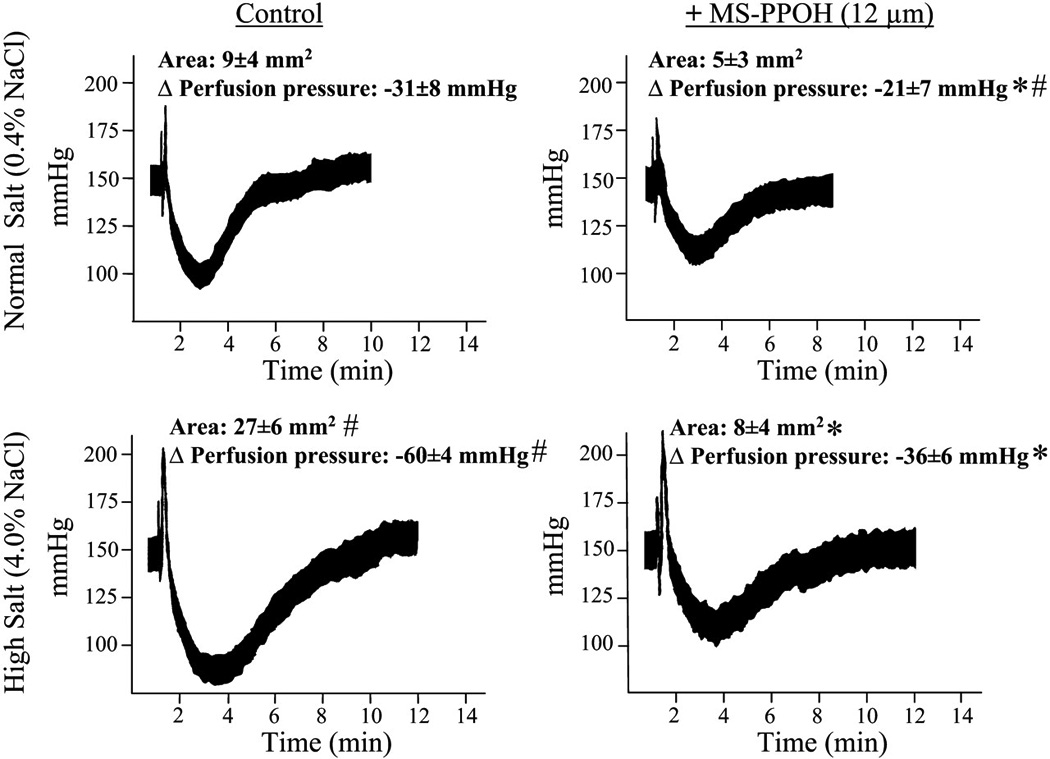
Changes in perfusion pressure and area of response to a bolus injection of 10 µg 2-CA were compared in kidneys obtained from normal salt (NS)-fed rats vs. high salt (HS)-fed rats for 7 days in the absence and presence of MS-PPOH (12 µM). Data expressed as means ± SE; n = 4. *P < 0.05 vs. control (i.e., NS vs. NS + MS-PPOH or HS vs. HS + MS-PPOH). #P < 0.05 NS vs. HS. *#P < 0.05 HS vs. NS + MS-PPOH.
As salt loading increases renal epoxygenase activity (62;63), we assessed the role of EETs in the enhanced dilator response to 2-CA seen in kidneys from HS-fed rats (Fig. 2). Although EETs can be metabolized via a number of different pathways (73;74), the rapid hydrolysis to their corresponding dihydroxyeicosatrienoic acids (DHTs) by soluble epoxide hydrolase (sEH) seems to be the dominant pathway in the kidney (75;76). Thus total EET and DHT release was measured, to account for total epoxygenase activity. Compared with NS intake, salt loading significantly augmented epoxygenase activity, as reflected by an increase in EET and DHT release, without affecting 20-HETE levels, in response to 10 µg of 2-CA. Epoxygenase inhibition abolished this increase, suggesting that de novo synthesis of EETs, rather than release of preformed EETs from storage in phospholipids (54), is responsible for this enhanced renal response.
Figure 2. Renal release of CYP epoxygenase metabolites in response to 10 µg 2-CA.
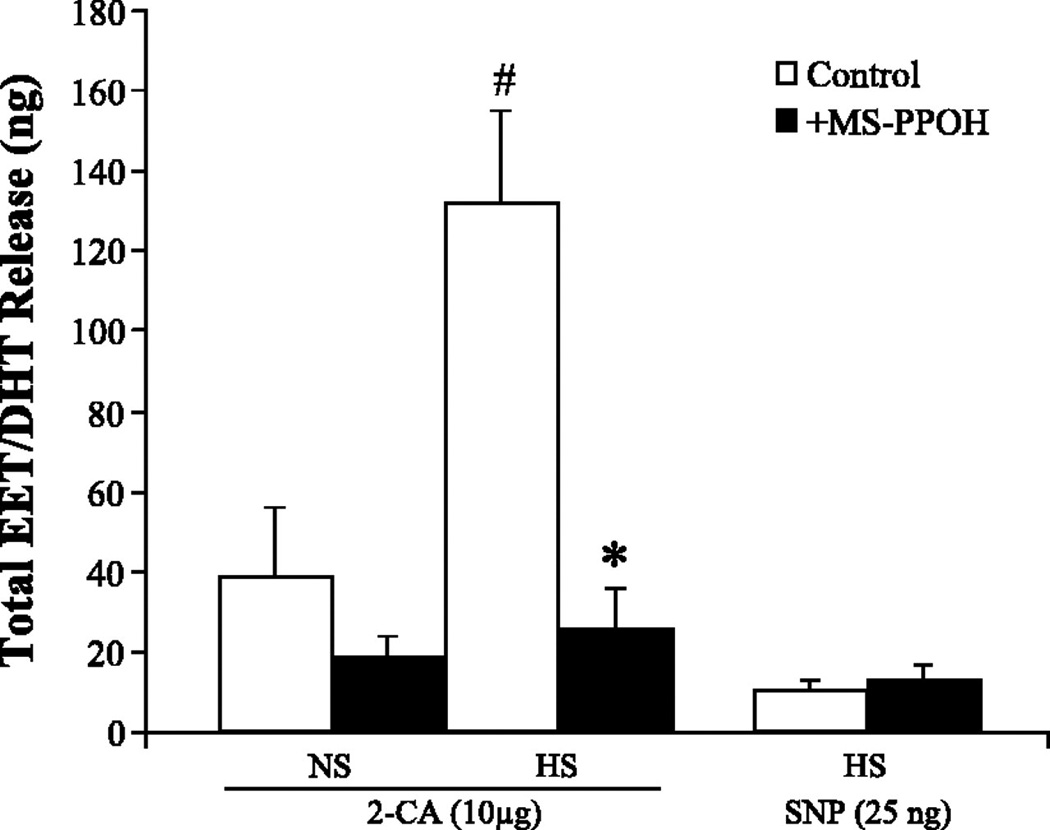
Release of total EETs + DHTs in response to 10 µg 2-CA was compared in NS- and HS-fed rats in the absence and presence of MS-PPOH. Total EET + DHT release in response to sodium nitroprusside (SNP; 25 ng) in HS-fed rats was also measured. Data are expressed as means ± SE; n = 4–5. *P < 0.05 vs. control (i.e., HS vs. HS + MS-PPOH). #P < 0.05 NS vs. HS.
Because all four adenosine receptor subtypes are expressed within the kidney (6) and 2-CA is a nonselective agonist of the adenosine receptors, it was necessary to elucidate which receptor isoform mediated the 2-CA-induced vasodilation and EET stimulation in the isolated, perfused rat kidney. Previously, we showed that the A2A R-selective agonist, CGS-21680, induced dilation of rat renal PGMV was mediated by stimulation of EET synthesis (70). Adenosine-dependent vasodilation is mediated by increased cAMP levels via stimulation of A2 R subtypes (A2A R and/or A2B R) (28). Our data revealed that as with the pressurized arcuate artery preparation (70), 2-CA-induced vasodilation of the isolated, perfused rat kidney was also mediated through activation of A2A R, a finding confirmed by experiments with ZM-241385, a selective A2A R antagonist. As seen in Fig. 3A in the presence of ZM-241385, the dilator response to 2-CA was abolished; in fact, only a transient vasoconstriction was observed. This suggests that 2-CA-induced dilation in the isolated, perfused rat kidney is solely mediated by A2A R activation. The abrogated dilator response was accompanied by a significant reduction in total EET and DHT release after ZM-241385 (Fig. 3B). As activation of A2B R dilates renal arteries in a NO-dependent manner (37), we eliminated this pathway as a mediator of 2-CA-induced dilation by inhibition of NOS. The association between A2A R activation and EET release in arcuate arteries and isolated, perfused kidney experiments was based on the pharmacological studies using an A2A R-selective agonist, CGS-21680 and A2A R antagonism with ZM-241385. It is feasible that ZM-241385 may antagonize both A2A R and A2B R, under our experimental conditions. The expression of A2 receptors in arcuate arteries has not has been addressed and further studies exploring the contribution of the A2A R and A2B R are required to reconcile the differences in the functional responses to adenosine in the arcuate artery versus the afferent arteriole (38;70). The vasodilator link between A2A R and EETs was confirmed with A2A R knockout mice. Relaxation responses to A2A R agonists of aorta obtained from wild type mice were abrogated in aorta of A2A R knockout mice and epoxygenase expression and activity was also reduced in A2A R knockout mice (77). Further, greater dilation to A2A R agonists was observed with HS intake and A1 R was downregulated, whereas A2A R was upregulated with HS compared with NS intake (78).
Figure 3. Effect of A2A R inhibition on the responsiveness of isolated, perfused rat kidneys to 10 µg 2-CA under NS intake.
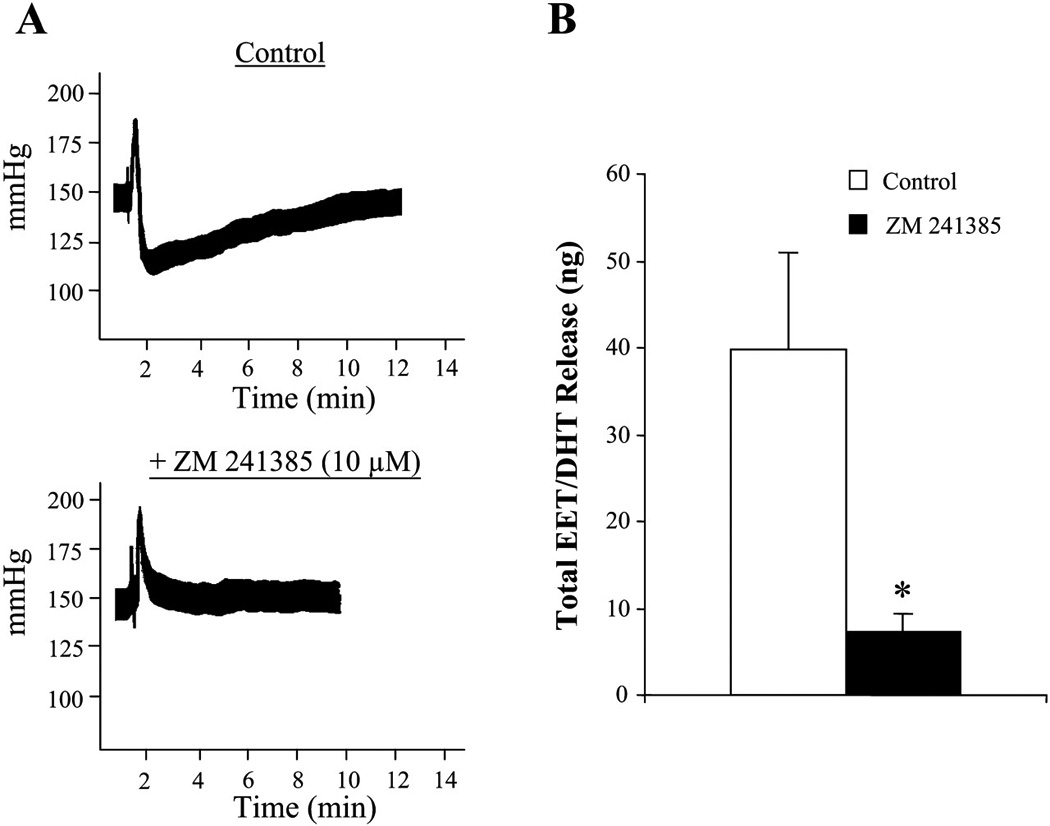
A: vascular response to 10 µg 2-CA was assessed before and after A2AR blockade by ZM-241385 (10 µM). B: total release of EET + DHT in response to 10 µg 2-CA was compared in the absence and presence of ZM-241385. Data are expressed as means ± SE; n = 3. *P < 0.05 vs. control.
5. Upregulation of CYP2C23 and A2A R under conditions of salt loading
Most renal EET biosynthesis has been attributed to the CYP2C and 2J epoxygenase families (79–81). In the rat kidney, CYP2C23 has been identified as the major 2C arachidonate epoxygenase and the isoform of the 2C family that is subject to regulation by dietary salt (79). In agreement with other studies (79;82), renal CYP2C23 expression was upregulated with increased dietary salt intake. We showed that HS increased medullary CYP2C23 protein expression, without affecting levels of cortical CYP2C23. In contrast to Zhao et al. (82), who reported that an 8% NaCl diet for 14 days increased CYP2C11 cortical protein expression, we did not detect changes in either cortical or medullary homogenates under our experimental conditions of 4% NaCl intake for 7 days. Changes in adenosine receptor protein expression in response to dietary salt intake have also been observed (17). Under conditions of low salt, the renal expression of A1 R was increased, whereas HS diet downregulated A1 R expression (17;73). Similarly, we showed that the expression of cortical A1 R protein was decreased by ~20%, whereas in medullary homogenates, A2A R expression was increased from HS-fed rats compared with NS-fed rats (71). Thus the enhanced dilator response to 2-CA seen in kidneys obtained from HS-fed rats is mediated by increased EET and DHT release and is associated with a downregulation of cortical A1 R protein expression and increased medullary A2A R and CYP2C23 protein expression.
In summary in normotensive SD rats, HS intake augmented renovascular responses to a non-selective adenosine receptor agonist, 2-CA, associated with increased renal protein expression of the A2A R and CYP2C23, a salt-inducible epoxygenase, that promotes increased renal efflux of EETs and the hydrolysis products of EETs and DHTs.
6. Inability of Dahl salt-sensitive (SS) rats to upregulate the A2A R-EET pathway may contribute to the development of salt-sensitive hypertension
We have reported that adenosine-activated renovascular dilatation is mediated by stimulating the A2A R that is linked to increasing EET synthesis, the latter mediating adenosine actions on the renal vasculature and that A2A R-EET pathway is upregulated by HS intake in normotensive rats (71). As this pathway is antipressor, we examined the role of the adenosine-epoxygenase pathway in Dahl salt-sensitive (SS) hypertensive rats. The Dahl SS rat is a genetic model of salt-dependent hypertension (83), that exhibits a rightward shift in the pressure-natriuresis curve (84), the hallmark of salt-sensitive hypertension. A common underlying adaptation in hypertension is an increase in vascular resistance, although the mechanisms of the changes of vascular reactivity induced by a HS intake are not fully known. Many mechanisms have been proposed to contribute to the development of hypertension; enhanced reactivity to constrictors such as norepinephrine, endothelin and angiotensin II as well as increased tissue oxygen delivery have been reported, whereas, endothelium-dependent relaxations to hypoxia, acetylcholine, and sodium nitroprusside are depressed (85). In addition, in Dahl SS rats, a deficiency of 20-HETE production by TALH (59), an inability to increase renal epoxygenase activity in response to dietary salt intake (60;86) has been documented, as well as an inability to produce or release eicosanoid precursors from phospholipid stores in response to dietary salt (87). Interactions between NO and CYP metabolites have been described; both NO and CYP metabolites contribute to the regulation of kidney function and blood pressure control, however, the role of 20-HETE and EETs increases with HS intake or after NOS inhibition (88). 20-HETE has been shown to result in endothelium dysfunction by reducing NO release and increasing superoxide production (89), whereas, CYP epoxygenases are directly inhibited by hydrogen peroxide, and this interaction may modulate vascular EET bioavailability (90). Increased NO production may suppress 20-HETE synthesis yet permit EETs-dependent vasodilation (91).
We studied Dahl salt-resistant (SR) and SS rats with the expectation of demonstrating abnormalities in the adenosine A2A R-epoxygenase pathway in the Dahl SS rat (92). Dahl SS rats challenged with HS intake, in contrast to Dahl SR rats, failed to respond to 2-CA elicited renal vasodilation above the levels produced by 2-CA in Dahl SS rats on NS intake. Thus, the renal vasodilator dose-response curves to 2-CA and the efflux of EETs in response to 2-CA did not differ in Dahl SS rats irrespective of salt intake, whether fed NS or HS. Moreover, differences in the renal vasodilator effect of 2-CA demonstrated by Dahl SS and SR rats on either NS or HS intake were entirely accounted for by their ability to release EETs because inhibition of epoxygenase activity with MS-PPOH eliminated differences in renal vasodilator responses to 2-CA observed in Dahl SR rats on NS vs. HS intake, as well as differences in the renal vasodilator responses to 2-CA occurring between Dahl SR rats and Dahl SS rats. Thus, inhibiting EET synthesis obliterated all differences in renal vasodilator responses between NS and HS-fed Dahl SS rats produced by 2-CA as well as those between Dahl SR and SS rats, demonstrating the crucial role of EETs as mediators of renovascular responses on activating the renal adenosine system. The dilator response to 2-CA was abolished in both Dahl SR and SS rats in the presence of A2A R antagonism with ZM 241385.
The role of adenosine in cardiovascular regulation of blood pressure has been evaluated in the Dahl SS model of hypertension (93). Plasma adenosine concentrations were significantly higher in Dahl SS than in Dahl SR rats, irrespective of the NaCl content of the diet and adenosine levels positively correlated with blood pressure, data indicating that an abnormality in adenosine signaling may play a role in the pathophysiology of hypertension of Dahl SS rats. In our study, total purines, which reflect increased flux through the adenosine pathway, were also higher in Dahl SS rats than in Dahl SR rats on NS intake (Fig. 4) and Dahl SS rats exhibited an inability to upregulate the A2A R, suggesting that an abnormality in adenosine signaling contributes to the development of hypertension in Dahl SS rats. In Dahl SR rats, HS intake did increase urinary purine excretion in contrast to the unresponsiveness to additional increases of urinary purine excretion that occurred in Dahl SS rats on HS intake (92). As renal purine excretion increased in Dahl SR rats on HS intake, the upregulation of A2A R was unexpected. Adenosine receptors are G-protein coupled receptors, which typically exhibit desensitization. However, an increased A2A R expression in the face of increased purine levels in Dahl SR rats does not conform to typical G-protein coupled receptor pharmacology, and instead displays a feed-forward mechanism. Expression A2B R was not significantly affected by salt intake in Dahl SR rats or in Dahl SS rats. In agreement with other studies (17), the renal expression of A1 R decreased with HS intake in both cortex and medulla of Dahl SS rats but not in Dahl SR rats. Spontaneously hypertensive rats (SHR) develop tolerance to chronic administration of a selective A1 R agonist, but not a selective A2A R agonist, over the course of 21 days (94). Although the functional relevance remains unknown, HS intake has also been shown to increase the expression of A3 R (17).
Figure 4. Urinary purine levels after 7 days of HS diet in Dahl SR and SS rats.
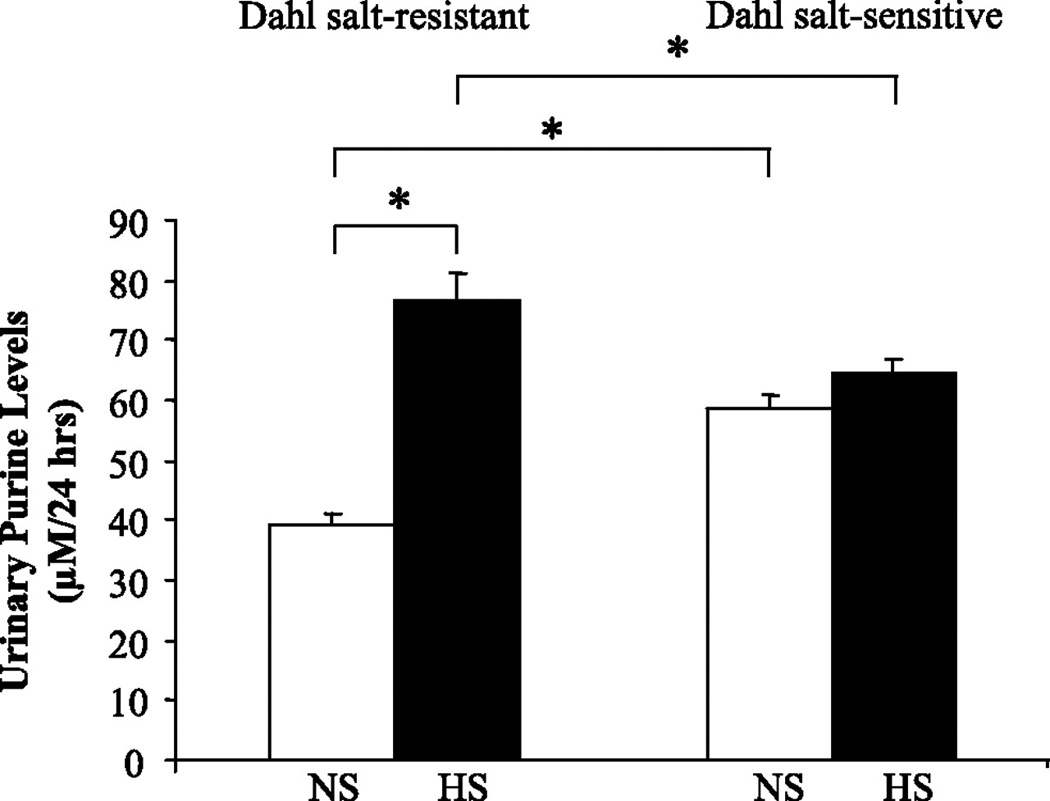
Levels of urinary purines (i.e., adenosine, hypoxanthine, xanthine, and inosine) were measured in Dahl SR and SS rats on NS diet and after 7 days of HS diet. Data are means ± SE; n = 5–7. *P < 0.05.
Renal efflux of EETs and DHTs in response to 2-CA was greater in Dahl SR rats fed a HS diet than a NS diet (Fig. 5). Epoxygenase inhibition abolished the HS-induced increase in EET/DHT release in Dahl SR rats, indicating that de novo synthesis of EETs rather than release of preformed EETs from storage in phospholipids (54), was responsible for the enhanced renovascular response. In contrast to Dahl SR rats, salt-loading of Dahl SS rats failed to increase the renal protein expression of either CYP2C23 or CYP2C11. Rather, levels of medullary CYP2C23 and CYP2C11 tended to decrease with HS intake in Dahl SS rats. The functional significance of this reduction in CYP2C protein is unknown, as the renovascular responsiveness of Dahl SS kidneys to 2-CA mediated by EETs was not decreased with HS feeding compared to NS intake.
Figure 5. Renal release of cytochrome P-450 (CYP) epoxygenase metabolites in response to 2-CA in Dahl SR and SS rats.
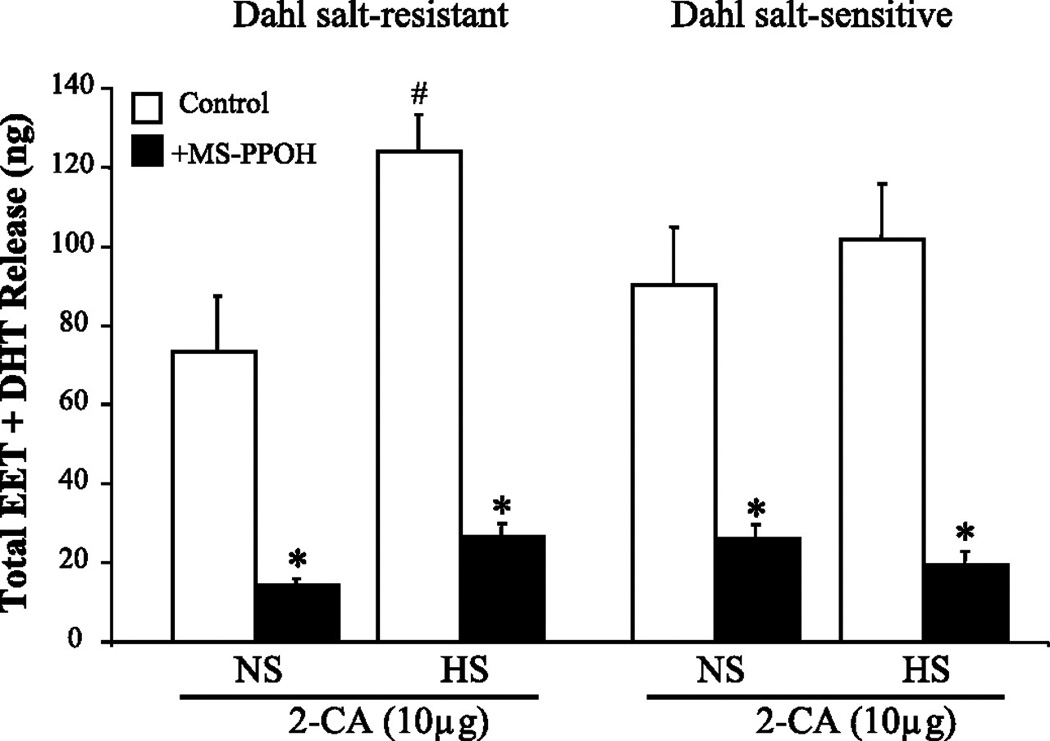
Data are means ± SE; n = 4–5. *P < 0.05 vs. control (i.e., HS vs. HS + MS-PPOH). #P < 0.05 NS vs. HS.
As we did not observe any significant change in the EET to DHT ratios with salt-loading, it is unlikely that the increased EET levels seen with HS intake in Dahl SR rats can be accounted for by decreased sEH activity. In view of the antagonistic effects of 20-HETE on EET-induced renal vasodilation (64), we also examined the effects of HS diet on 2-CA elicited renal release of 20-HETE. Salt-loading did not affect 20-HETE renal release in response to 2-CA thereby eliminating the potential contribution of changes in 20-HETE production to the altered renal vasodilator effects of 2-CA in Dahl SS and SR rats. Deficient medullary TALH 20-HETE production associated with deficient cortical EETs have been proposed to contribute to hypertension in Dahl SS rats (86). A 20-HETE deficiency in the outer medulla of Dahl SS rats may promote increased TALH sodium and chloride reabsorption with elevation of blood pressure (59). In contrast to our findings, the production of 20-HETE and EETs by renal cortical microsomes actually fell in Dahl SS rats fed a HS diet (86). Further, we did not find differences in renal 20-HETE production by Dahl SR and SS rats, which reflect critical differences in the assays between our study and that of Ma et al., (59). Namely, we measured renal efflux of CYP-AA metabolites from the isolated, perfused kidney in response to an adenosine analog, whereas they measured the synthesis of EETs and 20-HETE by renal microsomes in the presence of AA, which is an index of CYP450 enzyme synthetic capability rather than an index of the response of endogenous CYP products to experimental conditions, such as changes in salt intake.
Our findings suggest a ceiling imposed on both Dahl SR and SS rats that limited the ability of Dahl SS rats to recruit epoxygenase activity as indicated by renal EET release/efflux. Several factors that relate to the adenosine-epoxygenase system have been identified in our study that may explain the inability of HS intake to mobilize increased EET production in the Dahl SS rat, in response to HS intake. Namely: 1) purine levels reflecting activity of the adenosine pathway were fixed at levels found in NS-fed Dahl SS rats; 2) protein expression of the preeminent CYP2C isozyme, CYP2C23, responsible for increased EET synthesis in HS-fed rats was unresponsive to HS feeding in Dahl SS rats; 3) expression of the A2A R linked to epoxygenase activity was also unresponsive to HS intake in the Dahl SS rat.
7. Inhibition of the A2A R-EET pathway renders Dahl salt-resistant rats (SR) hypertensive
Single nucleotide polymorphisms in the CYP2J2 epoxygenase gene have been associated with a hypertensive phenotype in humans (95). The inability to upregulate CYP2C epoxygenases in response to salt-loading has been associated with the development of salt-sensitive hypertension (60;82) and inhibition of the epoxygenase pathway with MS-PPOH, has been reported to increase blood pressure in pregnant rats (96). Although adenosine has traditionally been implicated in the renal functional responses to pathological events such as ischemia and inflammation (41;42), its role in the adaptation of the kidney to enhance salt excretion has only recently become appreciated (44;71). It has been reported that treatment of Wistar rats with 1,3-dipropyl-8-sulphophenylxanthine (DPSPX), a non-selective adenosine receptor antagonist, results in hypertension (97). Furthermore, blood pressure was elevated in transgenic A2A R knockout mice on a NS diet (30), suggesting that A2A R activation can serve in mechanisms that contribute to the basal regulation of blood pressure.
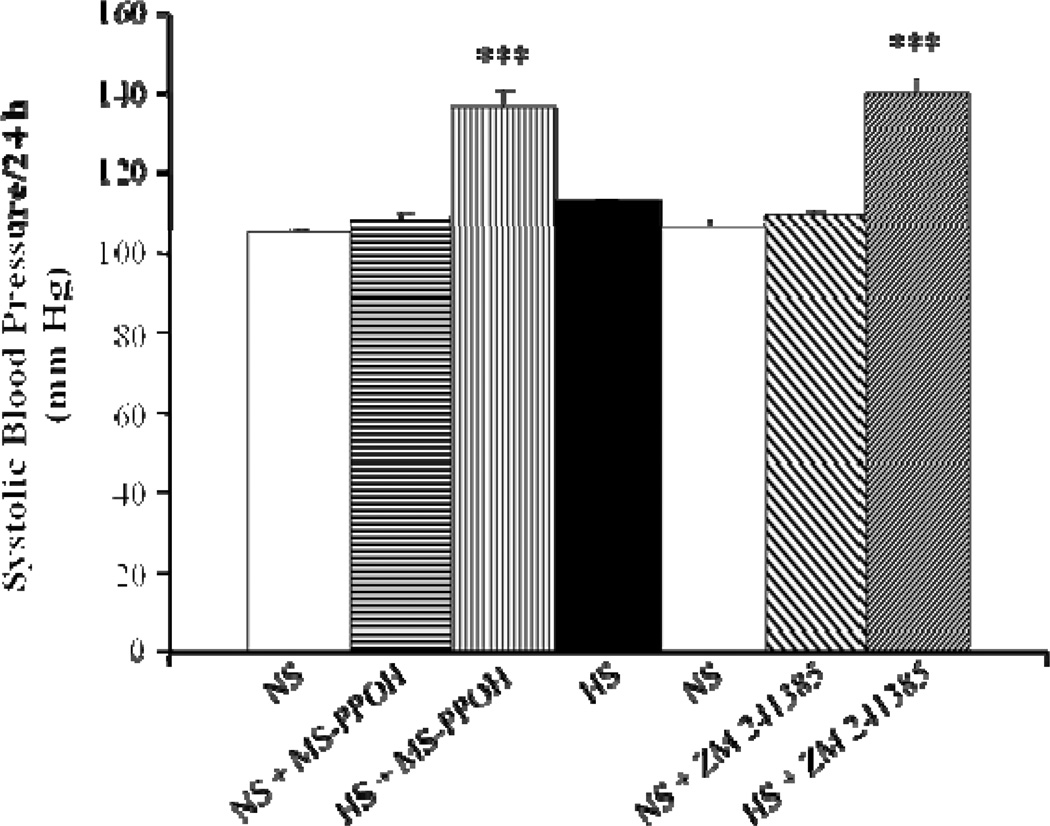
In Dahl SR rats, salt-loading augmented renovascular responses to an adenosine analog, an effect associated with upregulation of the protein expression of the CYP2C23 and CYP2C11, as well as A2A R, changes that were not observed in Dahl SS rats (92). Therefore, we examined the effect of in vivo inhibition of the A2A R-epoxygenase pathway on the adaptive natriuretic response to salt-loading in Dahl SR rats, both at the level of epoxygenase inhibition and A2A R antagonism. In agreement with Makita et al. (60), we saw an increase in blood pressure in salt-loaded Dahl SR rats treated with an epoxygenase inhibitor. In the study by Makita et al., clotrimazole, a non-selective inhibitor of CYP epoxygenases was used, whereas we used MS-PPOH, an inhibitor selective for CYP epoxygenases. In addition, in our study we examined the first 3 days of HS intake (i.e. the early, adaptive natriuretic response to salt-loading) after epoxygenase inhibition, whereas Makita et al. examined the effect of epoxygenase inhibition after rats were maintained for 6 weeks on a HS diet. We observed a similar increase in blood pressure in salt-loaded Dahl SR rats treated with a selective A2A R antagonist, ZM 241385. In vivo administration of ZM 241385 to SHR attenuated the hypotensive response produced by exogenous adenosine (98). Moreover, oral administration of a selective A2A R adenosine agonist elicited a sustained hypotensive response in SHR (99).
The increase in blood pressure in response to salt-loading seen in both MS-PPOH- and ZM 241385-treated Dahl SR rats was associated with a more positive sodium balance (as assessed by the daily difference between sodium intake and urinary sodium excretion), compared with vehicle-treated Dahl SR rats, during the first two days of salt-loading. By the third day of HS intake however, no differences in sodium balance were detected among the three groups, indicating that either in vivo epoxygenase inhibition or antagonism of A2A R results in a delay in the natriuretic response to salt-loading. On Day 3 of HS intake, MS-PPOH- and ZM-241385-treated rats, plasma Na+ levels were significantly increased (163.3 ± 1.2 and 158.1 ± 4.5 mEq/L, respectively) compared with vehicle-treated rats (142.1 ± 1 mEq/L), reflecting a diminished natriuretic capacity. Such increases in plasma Na+ concentration are not without precedent, as previous studies have shown similar changes in rats rendered salt-sensitive by uninephrectomy and DOCA (100). To our knowledge, our observations provide the first evidence for a role of the A2A R-EET pathway in the early, adaptive natriuretic response to salt-loading, as we have been able to render Dahl SR rats salt-sensitive, by either in vivo epoxygenase inhibition or A2A R antagonism.
Compared with vehicle-treated Dahl SR rats, in Dahl SR rats treated with MS-PPOH showed a tendency for urinary K+ excretion to be reduced, as reflected by the positive difference between K+ intake and urinary K+ excretion, irrespective of salt diet. These results are in agreement with those of Sun et. al. (101), who showed that EETs activate large-conductance calcium-activated K+ (BKCa) channels and flow-stimulated K+ secretion in the cortical collecting duct, thereby regulating K+ secretion. Moreover, in that study, epoxygenase inhibition abolished K+ secretion mediated by BKCa and renal outer medullary K+ channels.
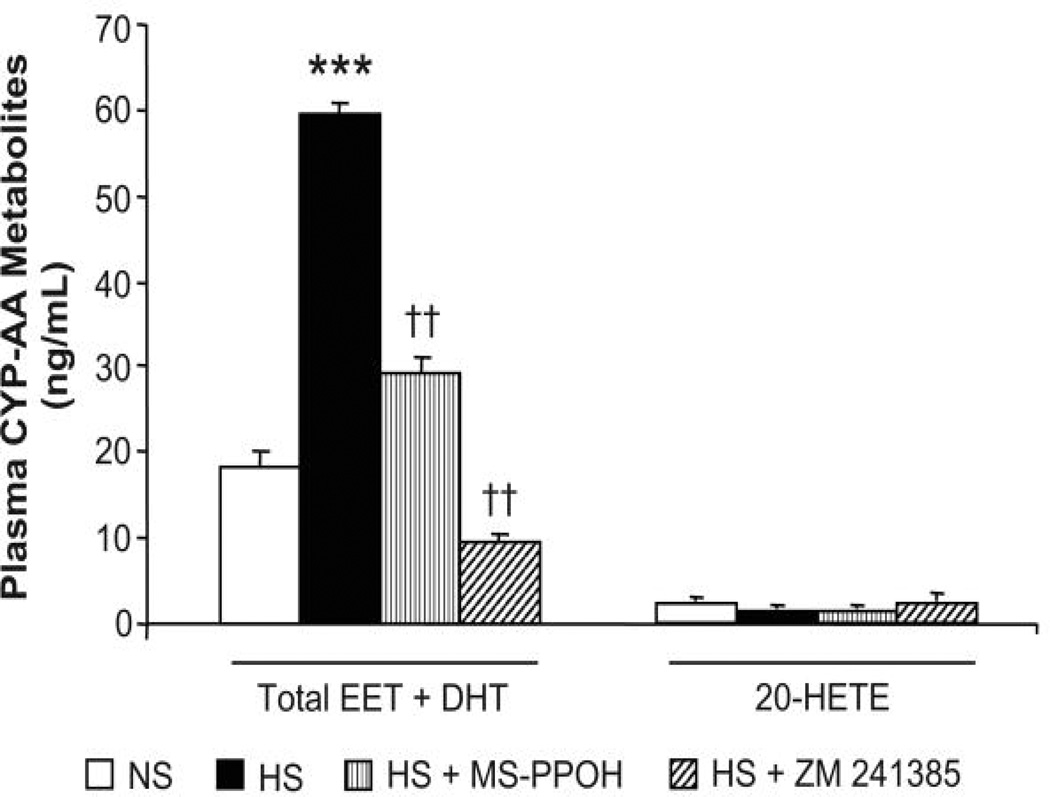
Dahl SR rats treated with either a CYP epoxygenase inhibitor or antagonist of A2A R were unable to increase plasma levels of EETs and DHTs to the same extent as vehicle-treated Dahl SR rats, in response to salt-loading (Fig. 7). In fact, in Dahl SR rats treated with an A2A R antagonist, the concentration of plasma EETs and DHTs declined to levels below the basal levels on NS diet. Renal levels of EETs and DHTs were also significantly lower with either CYP epoxygenase inhibition or A2AR antagonism, thus further supporting a role for the A2A R-EET pathway in the adaptive natriuretic response to salt-loading in normotensive, Dahl SR rats.
Figure 7. Effect of epoxygenase inhibition or A2A R antagonism on plasma levels of CYP-AA metabolites in Dahl SR rats.
Plasma levels of CYP-AA metabolites were measured rats during NS (0.4% NaCl) diet, and after 3 days of HS (2% saline drinking water) intake or 3 days of HS intake with either epoxygenase inhibition (HS + MS-PPOH; 20 mg/Kg/day) or A2A R antagonism (HS + ZM 241385; 5 mg/Kg/day). Data are expressed as means ± SEM; n = 4–6; *** p<0.001 vs. NS, †† p<0.001 vs. HS.
8. Concluding Remarks
The stimulation of adenosine levels with salt loading and downregulation of A1 R, with increased adenylyl cyclase activity via A2A R stimulation, may play an important role in the adaptation of the kidney to enhance salt excretion. Indeed, there is evidence linking changes in A2A R-coupled activity to blood pressure regulation: 1) blood pressure is elevated in transgenic A2A R knockout mice and 2) A2A R wild-type mice exhibit a decrease in blood pressure in response to an A2A R agonist. The central finding of this study is that the renal response to adenosine is exaggerated in rats fed a HS diet, presumably via A2AR activation and subsequent increased production of EETs. Unlike Dahl SR rats, Dahl SS rats exhibit an inability to upregulate the A2A R-EET pathway with salt loading. With inhibition of the A2A R or epoxygenase pathway, Dahl SR rats exhibited a positive sodium balance, reflecting a diminished natriuretic capacity and became hypertensive. These studies support a role for the A2A R -EET pathway in the adaptive natriuretic response to modulate blood pressure during salt loading. As salt-sensitivity is an important characteristic of a subgroup of humans with essential hypertension and other forms of salt-dependent hypertension that occurs in African-Americans, diabetics, and the aged (102), identification of potential targets for the management of salt-sensitive hypertension may be of therapeutic benefit. The adenosine-A2A R-EET pathway may be an important therapeutic target for managing salt-sensitive hypertension.
Highlights.
Epoxyeicosatrienoic acids (EETs) are renal vasodilator and natriuretic eicosanoids.
Adenosine-activated renovascular dilatation is mediated via A2A receptor (R) which is linked to increasing EET synthesis.
The A2AR-EET pathway is upregulated in normotensive rats and contributes to the adaptive response to high salt intake.
Failure to upregulate the A2AR-EET pathway may contribute to the development of salt-sensitive hypertension
Figure 6. Effect of epoxygenase inhibition or A2A R antagonism on systolic BP of Dahl SR rats after 3 days treatment.
Dahl SR rats on NS diet were pretreated with the epoxygenase inhibitor, MS-PPOH (20 mg/Kg/day), for 3 days prior to switching rats to a HS (2% saline drinking water) intake. After 3 days of HS intake with MS-PPOH treatment, MS-PPOH was withdrawn while rats remained on HS intake for 3 more days. Rats were then switched to NS diet for 7 days. The selective A2A R antagonist, ZM 241385 (5 mg/Kg/day), was then given for 3 days prior to switching rats back to HS intake. ZM 241385 treatment continued during 3 days of HS intake. Data are expressed as means ± SEM; n = 6–7; *** p<0.001 vs. NS.
Acknowledgements
This review is dedicated to my dear brother, John Carroll, who died on July 4th, 2011. I wish to thank Monica K. Cheng, Anabel B. Doumad, Elvira L. Liclican and Jing Li for their significant contribution to these studies. In addition, I wish to thank J.R. (Camille) Falck for providing MS-PPOH and John C. McGiff for his constant advice. This work was supported by grants from the National Institutes of Health DK69687, HL-25394, and GM31278 and from the Pharmaceutical Research and Manufacturers of America Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How NaCl raises blood pressure: A new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He FJ, Jenner KH, Macgregor GA. WASH-world action on salt and health. Kidney Int. 2010;78(8):745–753. doi: 10.1038/ki.2010.280. [DOI] [PubMed] [Google Scholar]
- 3.Cowley AW., Jr Genetic and nongenetic determinants of salt sensitivity and blood pressure. Am J Clin Nutr. 1997;65(2 Suppl):587S–593S. doi: 10.1093/ajcn/65.2.587S. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic receptors in the heart. Circ Res. 1980;46(6 Pt 2):I175–I182. [PubMed] [Google Scholar]
- 5.Olah ME, Stiles GL. The role of receptor structure in determining adenosine receptor activity. Pharmacol Ther. 2000;85(2):55–75. doi: 10.1016/s0163-7258(99)00051-0. [DOI] [PubMed] [Google Scholar]
- 6.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86(3):901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 7.Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol. 2002;283(1):F41–F51. doi: 10.1152/ajprenal.00232.2001. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi S, Umemura S, Tamura K, Iwamoto T, Nyui N, Ishigami T, Ishii M. Adenosine A1 receptor mRNA in microdissected rat nephron segments. Hypertension. 1995;26(6 Pt 2):1181–1185. doi: 10.1161/01.hyp.26.6.1181. [DOI] [PubMed] [Google Scholar]
- 9.Vitzthum H, Weiss B, Bachleitner W, Kramer BK, Kurtz A. Gene expression of adenosine receptors along the nephron. Kidney Int. 2004;65(4):1180–1190. doi: 10.1111/j.1523-1755.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith JA, Sivaprasadarao A, Munsey TS, Bowmer CJ, Yates MS. Immunolocalisation of adenosine A(1) receptors in the rat kidney. Biochem Pharmacol. 2001;61(2):237–244. doi: 10.1016/s0006-2952(00)00532-3. [DOI] [PubMed] [Google Scholar]
- 11.Pawelczyk T, Grden M, Rzepko R, Sakowicz M, Szutowicz A. Region-specific alterations of adenosine receptors expression level in kidney of diabetic rat. Am J Pathol. 2005;167(2):315–325. doi: 10.1016/S0002-9440(10)62977-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silldorff EP, Pallone TL. Adenosine signaling in outer medullary descending vasa recta. Am J Physiol Regul Integr Comp Physiol. 2001;280(3):R854–R861. doi: 10.1152/ajpregu.2001.280.3.R854. [DOI] [PubMed] [Google Scholar]
- 13.Weaver DR, Reppert SM. Adenosine receptor gene expression in rat kidney. Am J Physiol. 1992;263(6 Pt 2):F991–F995. doi: 10.1152/ajprenal.1992.263.6.F991. [DOI] [PubMed] [Google Scholar]
- 14.Al-Mashhadi RH, Skott O, Vanhoutte PM, Hansen PB. Activation of A(2) adenosine receptors dilates cortical efferent arterioles in mouse. Kidney Int. 2009;75(8):793–799. doi: 10.1038/ki.2008.684. [DOI] [PubMed] [Google Scholar]
- 15.Ren Y, Garvin JL, Liu R, Carretero OA. Possible mechanism of efferent arteriole (Ef-Art) tubuloglomerular feedback. Kidney Int. 2007;71(9):861–866. doi: 10.1038/sj.ki.5002161. [DOI] [PubMed] [Google Scholar]
- 16.Singh P, Thomson SC. Renal homeostasis and tubuloglomerular feedback. Curr Opin Nephrol Hypertens. 2010;19(1):59–64. doi: 10.1097/MNH.0b013e3283331ffd. [DOI] [PubMed] [Google Scholar]
- 17.Zou AP, Wu F, Li PL, Cowley AW., Jr Effect of chronic salt loading on adenosine metabolism and receptor expression in renal cortex and medulla in rats. Hypertension. 1999;33(1 Pt 2):511–516. doi: 10.1161/01.hyp.33.1.511. [DOI] [PubMed] [Google Scholar]
- 18.Guyton AC, Coleman TG. Quantitative analysis of the pathophysiology of hypertension. Circ Res. 1969;24(5 Suppl):1–19. [PubMed] [Google Scholar]
- 19.Nishiyama A, Navar LG. ATP mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R273–R275. doi: 10.1152/ajpregu.00071.2002. [DOI] [PubMed] [Google Scholar]
- 20.Spielman WS, Arend LJ. Adenosine receptors and signaling in the kidney. Hypertension. 1991;17(2):117–130. doi: 10.1161/01.hyp.17.2.117. [DOI] [PubMed] [Google Scholar]
- 21.Holz FG, Steinhausen M. Renovascular effects of adenosine receptor agonists. Ren Physiol. 1987;10(5):272–282. doi: 10.1159/000173135. [DOI] [PubMed] [Google Scholar]
- 22.Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 23.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. Epub;%2002 May 1.:501–529. [DOI] [PubMed] [Google Scholar]
- 24.Hansen PB, Hashimoto S, Oppermann M, Huang Y, Briggs JP, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the mouse kidney due to preferential activation of A1 or A2 adenosine receptors. J Pharmacol Exp Ther. 2005;315(3):1150–1157. doi: 10.1124/jpet.105.091017. [DOI] [PubMed] [Google Scholar]
- 25.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98(17):9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 27.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76(2):425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 28.Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol. 2006;291(1):F155–F161. doi: 10.1152/ajprenal.00231.2005. [DOI] [PubMed] [Google Scholar]
- 29.Levens N, Beil M, Jarvis M. Renal actions of a new adenosine agonist, CGS 21680A selective for the A2 receptor. J Pharmacol Exp Ther. 1991;257(3):1005–1012. [PubMed] [Google Scholar]
- 30.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388(6643):674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 31.Zou AP, Nithipatikom K, Li PL, Cowley AW., Jr Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol. 1999;276(3 Pt 2):R790–R798. doi: 10.1152/ajpregu.1999.276.3.R790. [DOI] [PubMed] [Google Scholar]
- 32.Beach RE, Good DW. Effects of adenosine on ion transport in rat medullary thick ascending limb. Am J Physiol. 1992;263(3 Pt 2):F482–F487. doi: 10.1152/ajprenal.1992.263.3.F482. [DOI] [PubMed] [Google Scholar]
- 33.Wei Y, Sun P, Wang Z, Yang B, Carroll MA, Wang WH. Adenosine inhibits ENaC via cytochrome P-450 epoxygenase-dependent metabolites of arachidonic acid. Am J Physiol Renal Physiol. 2006;290(5):F1163–F1168. doi: 10.1152/ajprenal.00301.2005. [DOI] [PubMed] [Google Scholar]
- 34.Carlstrom M, Wilcox CS, Welch WJ. Adenosine A2A receptor activation attenuates tubuloglomerular feedback responses by stimulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2011;300(2):F457–F464. doi: 10.1152/ajprenal.00567.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng MG, Navar LG. Adenosine A2 receptor activation attenuates afferent arteriolar autoregulation during adenosine receptor saturation in rats. Hypertension. 2007;50(4):744–749. doi: 10.1161/HYPERTENSIONAHA.107.094961. [DOI] [PubMed] [Google Scholar]
- 36.Carlstrom M, Wilcox CS, Welch WJ. Adenosine A(2) receptors modulate tubuloglomerular feedback. Am J Physiol Renal Physiol. 2010;299(2):F412–F417. doi: 10.1152/ajprenal.00211.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin PL, Potts AA. The endothelium of the rat renal artery plays an obligatory role in A2 adenosine receptor-mediated relaxation induced by 5'-N-ethylcarboxamidoadenosine and N6-cyclopentyladenosine. J Pharmacol Exp Ther. 1994;270(3):893–899. [PubMed] [Google Scholar]
- 38.Feng MG, Navar LG. Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Renal Physiol. 2010;299(2):F310–F315. doi: 10.1152/ajprenal.00149.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di SF, Cerull R, Babich V, Casavola V, Helmle-Roth C, Burckhardt G. Short- and long-term A3 adenosine receptor activation inhibits the Na+/H+ exchanger NHE3 activity and expression in opossum kidney cells. J Cell Physiol. 2008;216(1):221–233. doi: 10.1002/jcp.21399. [DOI] [PubMed] [Google Scholar]
- 40.Lee HT, Ota-Setlik A, Xu H, D'Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol. 2003;284(2):F267–F273. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- 41.Miller WL, Thomas RA, Berne RM, Rubio R. Adenosine production in the ischemic kidney. Circ Res. 1978;43(3):390–397. doi: 10.1161/01.res.43.3.390. [DOI] [PubMed] [Google Scholar]
- 42.Okusa MD. A(2A) adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol. 2002;282(1):F10–F18. doi: 10.1152/ajprenal.2002.282.1.F10. [DOI] [PubMed] [Google Scholar]
- 43.Siragy HM, Linden J. Sodium intake markedly alters renal interstitial fluid adenosine. Hypertension. 1996;27(3 Pt 1):404–407. doi: 10.1161/01.hyp.27.3.404. [DOI] [PubMed] [Google Scholar]
- 44.Lorenz JN, Weihprecht H, He XR, Skott O, Briggs JP, Schnermann J. Effects of adenosine and angiotensin on macula densa-stimulated renin secretion. Am J Physiol. 1993;265(2 Pt 2):F187–F194. doi: 10.1152/ajprenal.1993.265.2.F187. [DOI] [PubMed] [Google Scholar]
- 45.Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56(4):336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGiff JC, Quilley J. 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids and blood pressure. Curr Opin Nephrol Hypertens. 2001;10(2):231–237. doi: 10.1097/00041552-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459(6):881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfister SL, Gauthier KM, Campbell WB. Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol. 2010;60:27–59. doi: 10.1016/B978-0-12-385061-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sudhahar V, Shaw S, Imig JD. Epoxyeicosatrienoic acid analogs and vascular function. Curr Med Chem. 2010;17(12):1181–1190. doi: 10.2174/092986710790827843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellien J, Joannides R, Richard V, Thuillez C. Modulation of cytochrome-derived epoxyeicosatrienoic acids pathway: a promising pharmacological approach to prevent endothelial dysfunction in cardiovascular diseases? Pharmacol Ther. 2011;131(1):1–17. doi: 10.1016/j.pharmthera.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 52.Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276(19):15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- 53.Potente M, Michaelis UR, Fisslthaler B, Busse R, Fleming I. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of cyclin D1. J Biol Chem. 2002;277(18):15671–15676. doi: 10.1074/jbc.M110806200. [DOI] [PubMed] [Google Scholar]
- 54.Carroll MA, Balazy M, Margiotta P, Huang DD, Falck JR, McGiff JC. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Am J Physiol. 1996;271(4 Pt 2):R863–R869. doi: 10.1152/ajpregu.1996.271.4.R863. [DOI] [PubMed] [Google Scholar]
- 55.Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol. 2000;279(3):F544–F551. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- 56.Hercule HC, Oyekan AO. Cytochrome P450 omega/omega-1 hydroxylase-derived eicosanoids contribute to endothelin(A) and endothelin(B) receptor-mediated vasoconstriction to endothelin-1 in the rat preglomerular arteriole. J Pharmacol Exp Ther. 2000;292(3):1153–1160. [PubMed] [Google Scholar]
- 57.Fulton D, McGiff JC, Quilley J. Role of phospholipase C and phospholipase A2 in the nitric oxide-independent vasodilator effect of bradykinin in the rat perfused heart. J Pharmacol Exp Ther. 1996;278(2):518–526. [PubMed] [Google Scholar]
- 58.Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res. 2001;38(3):247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- 59.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension. 1999;33(1 Pt 2):419–423. doi: 10.1161/01.hyp.33.1.419. [DOI] [PubMed] [Google Scholar]
- 60.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest. 1994;94(6):2414–2420. doi: 10.1172/JCI117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87(11):992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 62.Capdevila JH, Wei S, Yan J, Karara A, Jacobson HR, Falck JR, Guengerich FP, DuBois RN. Cytochrome P-450 arachidonic acid epoxygenase. Regulatory control of the renal epoxygenase by dietary salt loading. J Biol Chem. 1992;267(30):21720–21726. [PubMed] [Google Scholar]
- 63.Oyekan AO, Youseff T, Fulton D, Quilley J, McGiff JC. Renal cytochrome P450 omega-hydroxylase and epoxygenase activity are differentially modified by nitric oxide and sodium chloride. J Clin Invest. 1999;104(8):1131–1137. doi: 10.1172/JCI6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br J Pharmacol. 1999;127(6):1399–1405. doi: 10.1038/sj.bjp.0702662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol. 1996;7(11):2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 66.McGiff JC, Carroll MA. Cytochrome P450-dependent arachidonate metabolites, renal function and blood pressure regulation. Adv Prostaglandin Thromboxane Leukot Res. 1991;21B:675–682. [PubMed] [Google Scholar]
- 67.Romero MF, Madhun ZT, Hopfer U, Douglas JG. An epoxygenase metabolite of arachidonic acid 5,6 epoxy- eicosatrienoic acid mediates angiotensin-induced natriuresis in proximal tubular epithelium. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:205–208. [PubMed] [Google Scholar]
- 68.Staudinger R, Escalante B, Schwartzman ML, Abraham NG. Effects of epoxyeicosatrienoic acids on 86Rb uptake in renal epithelial cells. J Cell Physiol. 1994;160(1):69–74. doi: 10.1002/jcp.1041600109. [DOI] [PubMed] [Google Scholar]
- 69.Liclican EL, Doumad AB, Wang J, Li J, Falck JR, Stier CT, Jr, Carroll MA. Inhibition of the adenosine2A receptor-epoxyeicosatrienoic acid pathway renders Dahl salt-resistant rats hypertensive. Hypertension. 2009;54(6):1284–1290. doi: 10.1161/HYPERTENSIONAHA.108.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol. 2004;141(3):441–448. doi: 10.1038/sj.bjp.0705640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liclican EL, McGiff JC, Pedraza PL, Ferreri NR, Falck JR, Carroll MA. Exaggerated response to adenosine in kidneys from high salt-fed rats: role of epoxyeicosatrienoic acids. Am J Physiol Renal Physiol. 2005;289(2):F386–F392. doi: 10.1152/ajprenal.00421.2004. [DOI] [PubMed] [Google Scholar]
- 72.Lin CH, Lo WC, Hsiao M, Tseng CJ. Interaction of carbon monoxide and adenosine in the nucleus tractus solitarii of rats. Hypertension. 2003;42(3):380–385. doi: 10.1161/01.HYP.0000088561.17252.42. [DOI] [PubMed] [Google Scholar]
- 73.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43(1):55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 74.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276(39):36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 75.Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem. 1993;268(9):6402–6407. [PubMed] [Google Scholar]
- 76.Zeldin DC, Wei S, Falck JR, Hammock BD, Snapper JR, Capdevila JH. Metabolism of epoxyeicosatrienoic acids by cytosolic epoxide hydrolase: substrate structural determinants of asymmetric catalysis. Arch Biochem Biophys. 1995;316(1):443–451. doi: 10.1006/abbi.1995.1059. [DOI] [PubMed] [Google Scholar]
- 77.Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol. 2008;295(5):H2068–H2078. doi: 10.1152/ajpheart.01333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nayeem MA, Ponnoth DS, Boegehold MA, Zeldin DC, Falck JR, Mustafa SJ. High-salt diet enhances mouse aortic relaxation through adenosine A2A receptor via CYP epoxygenases. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R567–R574. doi: 10.1152/ajpregu.90798.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holla VR, Makita K, Zaphiropoulos PG, Capdevila JH. The kidney cytochrome P-450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest. 1999;104(6):751–760. doi: 10.1172/JCI7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma J, Qu W, Scarborough PE, Tomer KB, Moomaw CR, Maronpot R, Davis LS, Breyer MD, Zeldin DC. Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney. J Biol Chem. 1999;274(25):17777–17788. doi: 10.1074/jbc.274.25.17777. [DOI] [PubMed] [Google Scholar]
- 81.Zeldin DC, DuBois RN, Falck JR, Capdevila JH. Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys. 1995;322(1):76–86. doi: 10.1006/abbi.1995.1438. [DOI] [PubMed] [Google Scholar]
- 82.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension. 2003;41(3 Pt 2):709–714. doi: 10.1161/01.HYP.0000047877.36743.FA. [DOI] [PubMed] [Google Scholar]
- 83.Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension. 1985;7(3 Pt 1):340–349. [PubMed] [Google Scholar]
- 84.Roman RJ, Kaldunski M. Pressure natriuresis and cortical and papillary blood flow in inbred Dahl rats. Am J Physiol. 1991;261(3 Pt 2):R595–R602. doi: 10.1152/ajpregu.1991.261.3.R595. [DOI] [PubMed] [Google Scholar]
- 85.Kunert MP, Drenjancevic-Peric I, Dwinell MR, Lombard JH, Cowley AW, Jr, Greene AS, Kwitek AE, Jacob HJ. Consomic strategies to localize genomic regions related to vascular reactivity in the Dahl salt-sensitive rat. Physiol Genomics. 2006;26(3):218–225. doi: 10.1152/physiolgenomics.00004.2006. [DOI] [PubMed] [Google Scholar]
- 86.Ma YH, Schwartzman ML, Roman RJ. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol. 1994;267(2 Pt 2):R579–R589. doi: 10.1152/ajpregu.1994.267.2.R579. [DOI] [PubMed] [Google Scholar]
- 87.Kelly RA, Pfeffer JM, Mitch WE, Smith TW. Plasma nonesterified fatty acids in the Dahl rat. Response to salt loading. Hypertension. 1987;10(2):198–203. doi: 10.1161/01.hyp.10.2.198. [DOI] [PubMed] [Google Scholar]
- 88.Kuczeriszka M, Olszynski KH, Gasiorowska A, Sadowski J, Kompanowska-Jezierska E. Interaction of nitric oxide and the cytochrome P-450 system on blood pressure and renal function in the rat: dependence on sodium intake. Acta Physiol (Oxf) 2011;201(4):493–502. doi: 10.1111/j.1748-1716.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 89.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294(2):H1018–H1026. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 90.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102(1):59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu X, Li C, Falck JR, Roman RJ, Harder DR, Koehler RC. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am J Physiol Heart Circ Physiol. 2008;295(2):H619–H631. doi: 10.1152/ajpheart.01211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liclican EL, McGiff JC, Falck JR, Carroll MA. Failure to upregulate the adenosine2A receptor-epoxyeicosatrienoic acid pathway contributes to the development of hypertension in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2008;295(6):F1696–F1704. doi: 10.1152/ajprenal.90502.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamada K, Goto A, Ishii M, Yoshioka M, Sugimoto T. Plasma adenosine concentrations are elevated in Dahl salt-sensitive rats. Experientia. 1995;51(3):227–229. doi: 10.1007/BF01931102. [DOI] [PubMed] [Google Scholar]
- 94.Casati C, Monopoli A, Dionisotti S, Zocchi C, Bonizzoni E, Ongini E. Repeated administration of selective adenosine A1 and A2 receptor agonists in the spontaneously hypertensive rat: tolerance develops to A1-mediated hemodynamic effects. J Pharmacol Exp Ther. 1994;268(3):1506–1511. [PubMed] [Google Scholar]
- 95.King LM, Gainer JV, David GL, Dai D, Goldstein JA, Brown NJ, Zeldin DC. Single nucleotide polymorphisms in the CYP2J2 and CYP2C8 genes and the risk of hypertension. Pharmacogenet Genomics. 2005;15(1):7–13. doi: 10.1097/01213011-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Huang H, Chang HH, Xu Y, Reddy DS, Du J, Zhou Y, Dong Z, Falck JR, Wang MH. Epoxyeicosatrienoic Acid inhibition alters renal hemodynamics during pregnancy. Exp Biol Med (Maywood) 2006;231(11):1744–1752. doi: 10.1177/153537020623101112. [DOI] [PubMed] [Google Scholar]
- 97.Guimaraes S, Albino-Teixeira A. Hypertension due to chronic blockade of P1-purinoceptors. J Auton Pharmacol. 1996;16(6):367–370. doi: 10.1111/j.1474-8673.1996.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 98.Poucher SM, Keddie JR, Brooks R, Shaw GR, McKillop D. Pharmacodynamics of ZM 241385, a potent A2a adenosine receptor antagonist, after enteric administration in rat, cat and dog. J Pharm Pharmacol. 1996;48(6):601–606. doi: 10.1111/j.2042-7158.1996.tb05981.x. [DOI] [PubMed] [Google Scholar]
- 99.Yagil Y, Miyamoto M. The hypotensive effect of an oral adenosine analog with selectivity for the A2 receptor in the spontaneously hypertensive rat. Am J Hypertens. 1995;8(5 Pt 1):509–515. doi: 10.1016/0895-7061(95)00020-p. [DOI] [PubMed] [Google Scholar]
- 100.Kunes J, Zicha J, Jelinek J. The role of chloride in deoxycorticosterone hypertension: selective sodium loading by diet or drinking fluid. Physiol Res. 2004;53(2):149–154. [PubMed] [Google Scholar]
- 101.Sun P, Liu W, Lin DH, Yue P, Kemp R, Satlin LM, Wang WH. Epoxyeicosatrienoic Acid Activates BK Channels in the Cortical Collecting Duct. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2008040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(2 Part 2):429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]