Abstract
Post-ingestive factors are known to strongly modulate feeding behavior by providing feedback signals to the central nervous system on the current physiological state of the organism. Of particular interest is the identification of the physiological pathways that permit the brain to sense post-ingestive signals. We will review recent evidence supporting the concept that direct stimulation of the gastrointestinal tract with nutrients induces release of the catecholamine neurotransmitter dopamine. In addition, changes in dopamine efflux produced by direct stimulation of the gastrointestinal tract were found to reflect the caloric load of the infusates, suggesting that dopamine signaling may function as a central caloric sensor that mediates adjustments in intake according to the caloric density of a meal. Consistent with the above, blockade of dopamine signaling disrupts flavor-nutrient associations and impair the regulatory capacity to maintain constant caloric intake during intra-gastric feeding. Future research must determine the exact pathways linking gut nutrient administration to dopamine efflux. Current evidence points to parallel contributions by pre- and post-absorptive pathways, indicating that dopamine systems constitute a site of convergence through which distinct physiological signals can exert control over ingestive behaviors.
Keywords: Calorie intake, Gastrointestinal tract, Dopamine, Flavor
The Role of Brain Dopamine in Post-Ingestive Reinforcement
Extensive evidence demonstrates that nutrients activate physiological pathways that do not depend on oral sensation to stimulate food intake. On one hand, gut nutrient infusions performed concurrently to oral ingestion of a distinct flavor produce long-lasting preferences for that particular flavor, in so-called “flavor-nutrient conditioning” paradigms [1, 2]. The physiological relevance associated with flavor preference learning is demonstrated by the ability of post-ingestive signals to influence food preferences in humans [2, 3]. Even mutant ageusic mice lacking the taste ion channel TRPM5 are capable of acquiring preferences for sipper positions associated with nutrient intake [4] to the point where sugar intake levels become, within hours, comparable to those observed in sweet-sensitive wild-type mice [5].
Such flavor-independent stimulation of intake must ultimately be regulated by brain circuits involved in controlling ingestive behavior, and research effort has been placed on determining the identity of these circuits [6]. The central catecholamine transmitter dopamine is one primer candidate given its critical role in eliciting intake, as demonstrated both by the deeply aphagia displayed by dopamine-deficient mice [7] and by the robust effluxes of dopamine observed during active feeding [8, 9]. In fact, while the orosensory properties of palatable foods are sufficient to stimulate brain dopamine release [10, 11], significant efflux is also observed during sugar intake in the abovementioned ageusic animals [4]. Consistent with a central role for dopamine signaling in post-ingestive reward, dopamine receptor (D1) antagonists injected into the nucleus accumbens, amygdala, medial prefrontal cortex or lateral hypothalamus either block or attenuate flavor-nutrient conditioning by gut glucose infusions [6]. Overall, current evidence points therefore to a predominant role for dopamine signaling in mediating the rewarding effects of nutrient-derived post-ingestive signals.
Gut Infusions of Nutrients Regulate Dopamine Release
The abovementioned results obtained with using ageusic mice suggest that orosensation and post-ingestive signals are each capable, via dedicated pathways, of increasing dopamine levels in brain reward circuits. Direct evidence that dopamine release is stimulated by nutrient delivery to the gut was given by experiments demonstrating that intragastric infusions (i.e., completely bypassing of the oral cavity) of glucose produce different effects on dopamine release when compared to similar infusions of the free amino acid L-serine [5]. Specifically, intragastric infusions of glucose stimulated significantly higher levels of dopamine release in nucleus accumbens of the ventral striatum compared to isocaloric infusions of L-serine. In fact, and rather interestingly, L-serine infusions did actually result in equivalent decreases in accumbal dopamine levels. Furthermore, similar measurements were performed on the dorsal aspect of the striatum. Whereas no significant decreases in dopamine levels were detected during L-serine infusions, significant dopamine efflux resulted from glucose infusions. These microdialysis measurements provided the first direct evidence that nutrient-specific dopamine efflux is produced upon direct stimulation of the gastrointestinal tract [5].
Extracellular Dopamine Release Reflects the Caloric Density of Gut Infusates
More recently, we have further explored the sensitivity of dopamine circuits to intra-gastric infusions of nutrients [12]. We first found that, within certain limits, mice are capable of regulating calorie intake even if denied the perception of flavor cues – specifically, by activating intra-gastric infusions upon licking a dry sipper. This regulatory capacity was however limited to caloric loads ranging above certain threshold values, with low-calorie infusions exerting relatively weak influences on the animal’s behavior. In any event, and most relevant for the present discussion, microdialysis measurements taken concomitantly to the dry licking behaviors revealed that extracellular dorsal striatum dopamine levels increased in proportion to the infusates’ caloric density. In addition, and rather remarkably, while dopamine efflux in dorsal striatum was linearly associated with the amounts of calories self-infused, this did not hold for the numbers of motor responses (i.e. dry licks) produced to obtain the caloric intra-gastric infusions. In other words, extracellular levels of dopamine were more closely associated with the amounts of calories consumed via the gastric route than with the motor behaviors associated with initiating the infusions.
We note that it is unlikely that the observed calorie-driven dopamine effluxes was accounted for by alternative functions associated with dorsal striatal activity, such response vigor, the actual cost of responding, or shifts in motivational state. First, as was mentioned above, striatal extracellular dopamine levels did not bear associations with the number of dry licks produced. This suggests that response cost (in our case represented by the number of dry licks required to achieve a certain amount of calories infused into the gastro intestinal tract) were not the primary drivers of dopaminergic stimulation. In fact, additional tests performed on animals passively receiving intra-gastric infusions of fat emulsions revealed similar calorie-dependent dopamine efflux in dorsal striatum, as had been the case for the previous experiments employing glucose infusions [5]. Note that in these passive infusions experiments the caloric value of a given infusion could not be predicted by the animals, which rules out the possibility that dopamine release was driven by external predictive stimuli [which in other settings are known to induce dopamine release, 13]. Finally, while motivational shifts – such as those associated with transitions from hunger to satiety – may also contribute to changes in dopamine levels, we note that such efflux levels correlated with caloric density even when motivational state was fixed. At this point it must be noted that it has not yet been tested whether gastrointestinal-stimulated changes in dopamine levels could be recapitulated in non-deprived animals. However, previous behavioral studies revealed that animals will self-infuse via the gastric route significant amounts of the fat emulsions even when not food- or water-deprived [although they do increase the number of self-infusions in response to increases in food deprivation, 12]. Given that animals were visibly motivated to sustain intra-gastric feeding in the absence of deprivation, one must expect to observe significant increases in striatal dopamine efflux under the same conditions.
Now, if extracellular dopamine levels do actually encode caloric density, one would expect that inhibiting dopamine receptor signaling should increase the intake of a highly caloric emulsion as if the emulsion’s caloric density had been diluted. In fact, pretreatment with the dopamine receptor blocker haloperidol led to a significant increase in the numbers of dry licks required to infuse highly caloric emulsions – a response analogous to those observed when the less-caloric emulsions are employed [12]. These effects are entirely consistent with calorie-dependent increases in dopamine efflux, since the disruption of normal dopamine receptor signaling led the animal to treat the infusions as being less caloric than they actually were. On a more general level of analysis, our results lead us to speculate that dopaminergic sensitivity to caloric load may be one important factor contributing to the critical role of striatal dopamine signaling in food reinforcement [14].
Regarding the above, it is important to note that there is no definitive evidence to rule out the possibility that calorie-dependent dopamine release is involved in mediating satiation effects produced by the intra-gastric infusions, i.e. independently of their putative reinforcing value [see 15]. However, the current body of evidence favors a role for calorie-dependent dopamine release in food reinforcement independently of satiety per se. First, our own previous behavioral studies show that mice have the ability to develop conditioned preferences for dry sipper positions associated with the more caloric infusions, with preferences being tested during extinction (sham infusion) sessions [12]. This suggests that calorie-dependent dopamine release produces conditioning effects independently of calorie-dependent suppression of intake. Second, inhibiting dopamine receptor signaling in different dopaminergic targets [6] abolishes the expression of nutrient-conditioned flavor preferences, further indicating that gut-stimulated dopamine release is involved in reward-related behaviors independently of satiation.
The Role of Dorsal Striatum Dopaminergic Signaling in Feeding Behavior
Our decision to assess dopamine efflux in the dorsal aspect of the striatum builds on previous findings showing that dopamine signaling in this brain region is required for the expression of ingestive motivated behaviors [16]. More specifically, the profound aphagia observed in genetically engineered dopamine-deficient mice has been shown to be reversed by induction of local dopamine production within the dorsal striatum of these mutant mice [7]. The animal literature is corroborated by reports of experiments performed on humans revealing marked dopaminergic and metabolic activity in dorsal striatum in response to food-associated stimuli, including whole meals [9] or anticipatory cues [17]. While it is true that brainstem circuits are sufficient to produce feeding to satiation in decerebrate rats [18, 19], such autonomy of may rely on taste relays arising from the nucleus of the solitary tract [20, 21] that in principle are not under direct dopaminergic control. This is consistent with the finding that dopamine-deficient mice retain normal preferences for sucrose over water while nevertheless displaying aphagia [22]. In other words, if on one hand taste-elicited ingestion may not require forebrain dopamine tone, behaviors leading to appropriate nutrition do. It also intriguing to note that dopamine receptor downregulation in striatum induces a dramatic increase in caloric intake in trained animals [23], further corroborating the notion that disrupted dopaminergic signaling in striatum leads to a loss of control over caloric regulation. These observations are also consistent with the more general notion that dopamine acts to regulate feeding as a flavor-independent calorie sensor [4, 5]. Finally, it is interesting to note that obesity has been associated with impaired dopamine release in rodents [24, 25] and with altered striatal responses in humans [26]. From our point of view, such diminished evoked striatal dopamine in obese organisms is expected to be 1. Recapitulated when food is delivered directly to the gut, and 2. Associated with decreased intra-gastric feeding (to the extent that striatal dopamine levels represent a reinforcement signal arising from the gastrointestinal tract). In fact, we have verified that high-fat fed mice not only fail to show the expected dopamine efflux following intra-gastric infusions of fat, but also display much lower motivation to consume fat calories via the gastric route (L. Tellez & I.E. de Araujo, unpublished observations). Such deficits are therefore consistent with the observed diminishing in motivation to eat upon disruption of striatal dopamine signaling [7]. Such putative alteration in dopamine efflux associated with excessive caloric intake is entirely consistent with the proposed role for striatal dopamine signaling as one central calorie sensor.
The Role of Pre-Absorptive Signals in Gut-Stimulated Dopamine Release
The above discussion will remain restricted to little more than speculation unless it is determined how exactly dopamine cells may sense fluctuations in physiological state (i.e. without the assistance of oral sensory cues). Both pre- and post-absorptive pathways may equally be involved in stimulating dopamine efflux during intra-gastric feeding. While the list of potential candidate signals is rather extensive, we may start at the level of gastric stimulation itself since the stomach is the first organ to be exposed to nutrients upon intra-gastric infusions. At this level, one putative regulatory cue relates to the precise control on gastric emptying exerted by caloric density. A number of pioneering studies [27–30] have demonstrated that gastric emptying rates decrease in proportion to the caloric density of the gut infusates. In fact, in our own studies [12] we verified that the differences in gastric emptying rate associated with high vs. low caloric lipid emulsions were comparable to the equivalent differences in dopamine efflux associated with the same emulsions. In addition, further pressures on stomach distention associated with infusions of highly caloric compounds may arise from potent effects on gastric secretion. Overall, these observations would place calorie-regulated gastric distention as one candidate signal mediating calorie-regulated release of striatal dopamine. Alternatively, we may also consider the possibility that the stomach-released orexigenic peptide ghrelin [31] may influence dopamine signaling via direct effects on the brain. In this regard, it is of note that Andrews et al. [32] demonstrated that ghrelin promotes tyrosine hydroxylase gene expression in dopaminergic neurons of Substantia Nigra concomitantly to increasing dopamine concentration in striatum. While these findings are in striking contrast with our own observations that incoming nutrients into the gut should increase – rather than reduce – dopamine release in dorsal striatum, they raise the intriguing possibility that ghrelin signaling may act to modulate dopaminergic efflux in ventral striatum upon nutrient intake. In fact, an inhibitory influence of ghrelin signaling on mesolimbic – but not on nigrostriatal – dopamine [33–35] is consistent with our own findings of decreased accumbal dopamine upon injections of L-serine [5]or lipids [12]. It is interesting to note that such decreases are not observed if isocaloric glucose solutions are infused instead [5], indicating that post-gastric properties associated with sugar metabolism counteracts the effects associated with ghrelin inhibition (see considerations on post-absorptive signals below).
Finally, a gastric-related mechanism would also be consistent with the established role of the intestinal peptide cholecystokinin in slowing gastric emptying [36], specifically given that this hormone is released in direct proportion to the amounts of lipids ingested [37]. Conversely, deficient cholecystokinin signaling entails impaired gastric mechanodetection [38]. Because calorie-mediated slowing in gastric emptying is generally dependent on vagal transmission, it will be important to determine in the near future whether the significant effluxes in dopamine release are abolished/attenuated by subdiaphragmatic vagotomies. In any event, the possibility therefore exists that duodenum-derived signals such as cholecystokinin [36, 38] or fatty acid amides [39] regulate dopamine release via their effects on gastric emptying/distention. The above further stresses the fact calorie-dependent dopamine efflux may be under the simultaneous control of several different regions of the gastrointestinal tract.
Consistent with the above are previous propositions that cholecystokinin per se may alter extracellular dopamine levels [40], a concept that may well be extended to other intestinally released factors such as GLP-1 or PPY. Alternatively, dopamine cells may also be modulated by activation of lipid-sensing molecules expressed in the small intestine [41]. More generally, two lines of evidence suggest an important role for intestinal-mediated signaling in transducing the nutritive properties of lipids into signals to brain. First, it is noticeable that intestinal vagal afferents are critical mediators of fat-induced satiation in rats [42]. Second, and perhaps more critically, experiments employing flavor-nutrient conditioning paradigms – where animals are allowed to associate a distinct flavor with the physiological consequences of administering nutrients to post-oral sites – suggest that the small intestine stimulation with nutrients may be required for animals to develop robust flavor preferences [43]. Specifically, it has been shown that male rats infused with glucose into the duodenum or mid-jejunum as they drank a saccharin-sweetened flavor did develop robust preferences for that flavor, an effect that was not observed in animals infused with glucose into the distal ileum [43]. Furthermore, duodenal and mid-jejunal infusions of glucose reduced the intake of palatable solutions while ileal infusions did not. Now, when the researchers performed similar tests using post-intestinal hepatic portal vein infusions instead, no flavor preferences developed. Overall, the comparable preferences displayed by animals infused in the mid-jejunum and duodenum (but not in distal ileum or hepatic portal vein) implicate the former as a critical site for glucose-conditioned flavor preferences [43]. Therefore, pre-absorptive sites seem to play a regulatory role in mediating preferences for flavors paired with post-oral glucose infusions. It remains to be addressed whether similar findings would result when lipids are substituted for glucose. In any event, these findings strongly suggest that direct infusions into the proximal small intestine would produce dopamine release to levels comparable to those observed after intra-gastric infusions. This is one relevant hypothesis that must be tested in the near future.
Finally, it is noticeable that taste receptor expression has been detected at extra-oral sites including the gastrointestinal tract [44–46], pancreas [47] and brain [48]. Specifically, gut expression of taste proteins could constitute one possible pre-absorptive signal conveying information on the chemical composition of luminal contents to brain dopamine circuits. In fact, intestinal taste receptor levels have been shown to be responsive to changes in gut microbiota composition [49] and to regulate intestinal peptide release [44]. However, two previous findings indicate that gut taste receptor signaling may not be involved in detecting the rewarding post-ingestive effects of sugars or in inducing gastrointestinal-stimulated dopamine release. Thus, mice lacking TRPM5, a taste transient receptor potential ion channel required for sweet sensation [50], not only acquire sugar preferences but also show robust dopamine release upon sucrose, but not artificial sweetener, intake [4]. In addition, mice lacking T1R3, an obligatory subunit for the T1R2/T1R3 sweet taste receptor heterodimer [51], do show normal preferences for arbitrary flavors associated with intra-gastric glucose infusions [52].
The Role of Post-Absorptive Signals in Gut-Stimulated Dopamine Release
However, current evidence does not allow us to limit the list of potential post-oral dopamine modulators to pre-absorptive cues. Favoring a role for post-absorptive signals in modulating dopamine release are our own previous findings that dopamine efflux is disrupted upon glucose oxidation inhibition with intra-venous (jugular) injections of 2-deoxy-D-glucose [henceforth “2DG”, 5]. The hypothesis that dopamine neurons of the midbrain are sensitive to glucose utilization rates had been brought about by our finding that higher intake levels of glucose compared to the nongluconeogenic amino acid L-serine were strongly correlated with glucose oxidation levels, as assessed by indirect calorimetry performed concomitantly to ingestive behavior [5]. To test the hypothesis of whether glucose metabolism influences dopamine efflux via post-absorptive pathways, we designed an experiment where extracellular dopamine levels in dorsal striatum were measured previous to, during and following jugular infusions of 2-DG or glucose [thereby bypassing not only the oral but also the gastrointestinal tract, 5]. Accordingly, upon infusion of a bolus of 2-DG via the jugular catheter, dopamine levels were monitored for 1h. This treatment was followed by a jugular infusion of glucose. We anticipated that, if glucose metabolism is indeed relevant for increasing striatal dopamine levels, then significant decreases in extracellular dopamine levels should follow the 2-DG injection. In fact, we observed that jugular infusions of 2-DG produced robust suppressions in striatal extracellular dopamine levels, with approximately 35% suppression in detected dopamine compared to baseline (pre-infusion) levels.
Now, one should expect that the inhibitory effects of 2-DG on dopamine release must be at least partially reversed when glucose – now acting as metabolism-promoting 2-DG competitor – is administered following 2-DG injection. In fact, intravenous infusions of glucose subsequent to 2-DG infusions resulted in partial reversal of the suppressive effects of 2-DG on dopamine release, to the extent that overall dopamine concentration approximately reaches baseline levels within 30min of glucose infusions. When the comparison is made directly against the period following 2-DG infusions, glucose infusions were in fact found to produce robust increases in striatal dopamine levels [5]. We should therefore conclude that cellular glucose metabolism – presumably within brain cells but certainly independently of the oral-gastrointestinal tract – is required for normal dopamine tone in dorsal striatum [53].
With respect to the experiments described above, we should note that the modulatory influence of 2-DG on dopamine release may have originated at post-intestinal peripheral sites such as the pancreas, the liver or the adrenal glands, where inhibition of cellular glucose utilization may trigger counter-regulatory pathways in an attempt to defend the organism against glucoprivation [54]. Alternatively, glucoprivation may be directly sensed by dopamine neurons themselves, which in this case would function as glucosensors, i.e., capable of modulating membrane potentials in direct response to the availability of intracellular glucose. While the actual mechanisms linking glucose utilization rates to dopamine release remain to be identified, these findings strongly indicate that post-absorptive cues act to modulate dopamine activity in nigrostriatal pathways [53].
Different lines of evidence support the notion that post-absorptive signals may directly affect the activity of dopaminergic cells. First, earlier work by Figlewicz and colleagues revealed the expression of the functional forms of both the insulin and leptin receptors – and of their downstream substrates – in dopaminergic neurons located in both Substantia Nigra (pars compacta), and ventral tegmental area [55, 56]. In addition, leptin receptors expressed in dopaminergic neurons of the midbrain were shown to be functional and to influence dopamine release [57]. It would therefore appear that both insulin and leptin signaling pathways act as critical mediators of the influence of physiological (post-absorptive) signals on dopamine release.
However, evidence exists to challenge a dominant role for either of these hormones in regulating dopamine release. First, a preponderant role for insulin as a post-oral reinforcement signal – thereby acting as dopamine release stimulator – was ruled out by the finding that hyperglycemic, hypoinsulinemic rats display normal preferences for flavors associated with intra-gastric infusions of nutrients [58]. This would suggest that lower insulin levels do not preclude the ability of intra-gastric nutrients to stimulate dopamine release. Second, conditional knockout mice selectively lacking functional leptin receptor expression in dopamine neurons have been recently shown to exhibit normal body weight and unimpaired feeding patterns [59]. Intriguingly, these conditional knockout mice displayed anxiogenic-like phenotypes that were partially reversed by antagonizing D1-receptor dopamine transmission in central amygdala. In other words, while leptin receptors expressed in dopaminergic neurons seem to regulate amygdala-dependent anxiogenic responses, they do not appear as important mediators of food intake and body weight gain.
An alternative hypothesis states that dopaminergic cells of the midbrain may be under the direct influence of the intracellular availability of glucose for metabolism, that is, intracellular molecules sense nutrient availability to regulate synthesis and release of neurotransmitters. Several different intracellular nutrient sensors arise as relevant candidates, including the 5′-adenosine monophosphate-activated protein kinase [AMPK, 60], a molecule that displays the ability to detect and initiate signaling pathways that react to intracellular nutrient depletion as indicated by rises in AMP:ATP ratios [as has been shown to be the case in hindbrain, 61]. In fact, intracellular nutrient sensing in dopamine neurons is a hypothesis consistent with previous findings revealing the sensitivity of dopaminergic neurons of the Substantia Nigra to direct contact with glucose inflow via locally applied reverse microdialysis [62].
Non-dopaminergic brain circuits via their projections to the midbrain may also act to link cellular nutrient sensing to dopamine efflux. In fact, hindbrain circuits containing catecholaminergic cell groups are known to detect glucose deficits [63–65] and are required for the expression of both the consummatory and appetitive phases of glucoprivic feeding [66]. It is therefore of great interest to inquiry whether midbrain-projecting hindbrain neurons mediate the suppressive effects produced by 2-DG on dopamine release. The possibility that dopamine cells act on behavior downstream to hindbrain catecholaminergic glucosensensing is a promising topic for future research. Finally, it is also relevant to mention a similarly important role for nutrient-sensing hypothalamic neurons [67] that send efferent fibers into midbrain dopamine cells [68].
Pre- and Post-Absorptive Signals may interact to Stimulate Dopamine Release
The rather straightforward picture of pre- and post-absorptive mechanisms working in parallel to influence dopamine efflux may become considerably more complicated once one takes into account the fact that these two pathways may convey physiological signals to one another. First, it is possible that pre- and post-absorptive pathways are selective to certain nutrient types. In fact, and as mentioned above, our previous findings using intra-gastric infusions of glucose and amino acids revealed that intra-gastric infusions of L-amino acids significantly decreased accumbal dopamine release, while glucose infusions did not [5]. In addition, after intra-gastric infusions of fats, accumbal dopamine levels did also fluctuate in response to the caloric densities of the infusates; however, whereas accumbal dopamine was actually suppressed by the more caloric emulsions, it remained stable after infusions of less caloric emulsions [12]. Note that the suppression in dopamine levels following inhibition of glucose utilization is selectively attenuated by intravenous glucose infusions [5], suggesting that post-absorptive mechanisms may be important in modulating dopamine release to glucose infusions but not to other nutrients. While future research must determine the circuit mechanisms associated with this singular dissociation between dorsal and ventral striatum in response to calorie intake, they suggest nutrient-specific calorie coding in mesolimbic pathways.
Second, it is also important to stress that pre- and post-absorptive influences on dopaminergic activity are not necessarily mutually exclusive. In fact, one could consider rather complex arrangements where pre-absorptive signals would eventually influence the activity of post-absorptive physiological pathways in such a way that signaling disruption at any of these levels is sufficient to impair the expression of dopamine-dependent feeding behaviors. For example, it has been established that lipid sensing in the upper intestine activates a brain-liver axis which ultimately regulates glucose homeostasis [69, 70]. Specifically, the ability of duodenal sensing of lipids to inhibit liver glucose production can be disrupted by either subdiaphragmatic or hepatic vagotomies, suggesting that the gut, the central nervous system and post-intestinal peripheral organs constitute an interconnected integral pathway that continuously monitor the physiological state of the organism. Conversely, it is conceivable that post-absorptive central nutrient sensors may act via descending vagal efferents to control gastric distension and motility, which in turn may be sufficient to affect dopamine efflux. Physiological interactions between pre- and post-absorptive signals represent another avenue for future research on gut-controlled neurotransmitter release.
Conclusion
Post-oral signals exert strong influence on feeding behaviors by providing feedback signals to the central nervous system on the ongoing physiological state of the organism. Current evidence indicates that the central catecholamine dopamine is a critical mediator of the ability displayed by animals to detect the physiological consequences of ingesting caloric nutrients and thereby acquire food preferences. Accordingly, recent evidence supports the notion that direct stimulation of the gastrointestinal tract with nutrients is sufficient to stimulate the release of dopamine in brain circuits controlling food intake, including the dorsal aspect of the striatum. Interestingly, gut infusions of nutrients are such that the subsequent changes in extracellular dopamine levels reflect the caloric load of the infusates, indicating that dopamine acts analogously to a central caloric sensor that mediates adjustments in intake according to the caloric density of a meal. It is relevant to note that, in fact, inhibiting dopamine receptor signaling disrupts flavor-nutrient associations and impair the regulatory capacity to maintain constant caloric intake during intra-gastric feeding. The actual physiological pathways allowing for dopamine cells to respond to caloric intake remain to be determined and constitute an important topic for future investigations. The current picture suggests that both pre- and post-absorptive post-oral cues converge onto dopaminergic circuits, depicting dopamine circuits as a major site of convergence where metabolic/hormonal and visceral sensory cues interact to regulate ingestive behavior (see Figure 1).
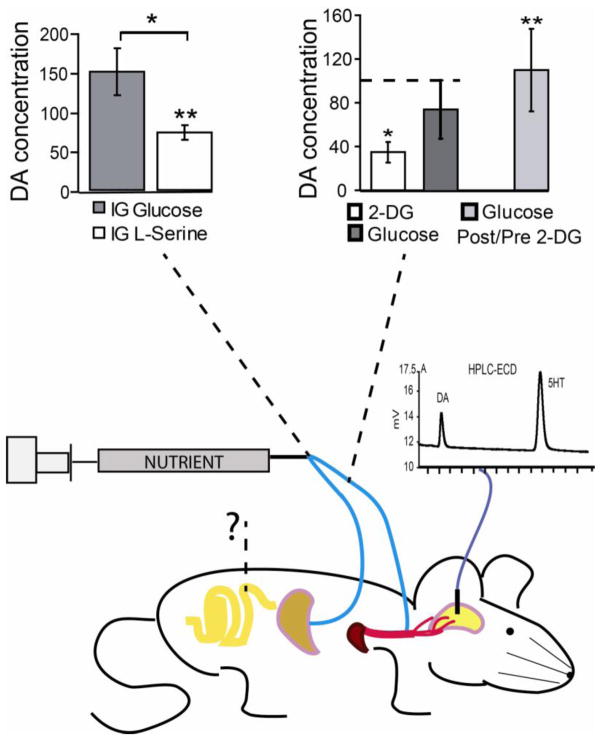
Figure 1. Post-oral pathways modulating dopamine release.
Direct infusions of nutrients into post-oral peripheral sites regulate dopamine release, as measured from microdialysates collected form either the ventral or dorsal striatum. The figure depicts peripheral sites where nutrient infusions were shown to modulate dopamine release. Gastric injections of the non-gluconeogenic amino acid L-serine produced marked reductions of extracellular dopamine levels in ventral striatum, while isocaloric injections of glucose did not (upper left, data shown as percentage dopamine change with respect to pre-infusion baseline periods). This finding demonstrates that stimulating the oral cavity is not required for differential nutrient sensing by dopamine cells, although the relative contributions of gastric, intestinal and post-absorptive sites remain to be dissected. Furthermore, jugular infusions of the glucose antimetabolite 2-deoxy-D-glucose (2-DG) strongly suppressed dopamine release in dorsal striatum, an effect that was shown to be reversed by subsequent jugular infusions of glucose (upper right, data shown as percentage dopamine change with respect to pre-infusion baseline periods, as well with respect to 2-DG post-infusion period for glucose effects, “Glucose Post/Pre 2-DG”). The diagram illustrates the concept that a network of pre- and post-absorptive physiological signals converge onto dopamine circuits to regulate ingestive behaviors. Interrogation mark represents the requirement of future experiments to assess the effects produced by direct nutrient stimulation of proximal intestine on dopamine release. Chromatogram represents the use of liquid chromatography coupled to electrochemical detection (HPLC-ECD) methods to separate and quantify dopamine (DA) and serotonin (5HT) content in brain dialysates. [Adapted from 5]. IG = Intra-Gastric infusion; 2-DG = 2-deoxy-D-glucose.
Highlights.
We will review recent evidence supporting the notion that direct stimulation of the gastrointestinal tract with nutrients induces release of the neurotransmitter dopamine in brain circuits controlling food intake;
Elevations in extracellular dopamine levels produced by direct stimulation of the gastrointestinal tract reflect the caloric load of the infusates, suggesting that dopamine signaling may function as a central caloric sensor;
Current evidence points to parallel contributions by pre- and post-absorptive pathways to stimulate dopamine release upon gastrointestinal stimulation with nutrients, indicating that dopamine systems constitute a site of convergence through which distinct physiological signals can exert control over ingestive behaviors.
Acknowledgments
Supported by NIH grant DC009997 to IEA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36(1):79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- 2.Mobini S, Chambers LC, Yeomans MR. Effects of hunger state on flavour pleasantness conditioning at home: flavour-nutrient learning vs. flavour-flavour learning. Appetite. 2007;48(1):20–8. doi: 10.1016/j.appet.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Yeomans MR, et al. Effects of energy density and portion size on development of acquired flavour liking and learned satiety. Appetite. 2009;52(2):469–78. doi: 10.1016/j.appet.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 4.de Araujo IE, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57(6):930–41. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Ren X, et al. Nutrient Selection in the Absence of Taste Receptor Signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol Behav. 2011;104(1):64–8. doi: 10.1016/j.physbeh.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sotak BN, et al. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005;1061(2):88–96. doi: 10.1016/j.brainres.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42(18):1705–12. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- 9.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19(4):1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 10.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. AJP - Regulatory, Integrative and Comparative Physiology. 2004;286(1):R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 11.Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1236–9. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira JG, et al. Regulation of fat intake in the absence of flavor signaling. J Physiol. 2012;590:953–972. doi: 10.1113/jphysiol.2011.218289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 14.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1149–58. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackroff K, Sclafani A. Energy density and macronutrient composition determine flavor preference conditioned by intragastric infusions of mixed diets. Physiol & Behav. 2006;89:250–60. doi: 10.1016/j.physbeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30(8):375–81. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Malik S, et al. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7(5):400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201(4352):267–9. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan JM, Seeley RJ, Grill HJ. Daily caloric intake in intact and chronic decerebrate rats. Behav Neurosci. 1993;107(5):876–81. [PubMed] [Google Scholar]
- 20.Norgren R. Gustatory afferents to ventral forebrain. Brain Res. 1974;81:285–295. doi: 10.1016/0006-8993(74)90942-1. [DOI] [PubMed] [Google Scholar]
- 21.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neurosci. 1978;3:207–218. doi: 10.1016/0306-4522(78)90102-1. [DOI] [PubMed] [Google Scholar]
- 22.Cannon CM, Palmiter RD. Reward without Dopamine. J Neurosci. 2003;23(34):10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger BM, et al. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159(4):1193–9. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rada P, et al. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav. 2010;101(3):394–400. doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stice E, et al. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHugh PR. The control of gastric emptying. J Auton Nerv Syst. 1983;9(1):221–31. doi: 10.1016/0165-1838(83)90143-1. [DOI] [PubMed] [Google Scholar]
- 28.McHugh PR, Moran TH. Calories and gastric emptying: a regulatory capacity with implications for feeding. Am J Physiol. 1979;236(5):R254–60. doi: 10.1152/ajpregu.1979.236.5.R254. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan JM, Spector AC, Grill HJ. Dynamics of gastric emptying during and after stomach fill. Am J Physiol. 1992;263(4 Pt 2):R813–9. doi: 10.1152/ajpregu.1992.263.4.R813. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan JM, et al. Gastric branch vagotomy and gastric emptying during and after intragastric infusion of glucose. Am J Physiol. 1997;273(5 Pt 2):R1786–92. doi: 10.1152/ajpregu.1997.273.5.R1786. [DOI] [PubMed] [Google Scholar]
- 31.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 32.Andrews ZB, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29(45):14057–65. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jerlhag E, Engel JA. Ghrelin receptor antagonism attenuates nicotine-induced locomotor stimulation, accumbal dopamine release and conditioned place preference in mice. Drug Alcohol Depend. 2011;117(2–3):126–31. doi: 10.1016/j.drugalcdep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Skibicka KP, et al. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jerlhag E, et al. Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl) 2010;211(4):415–22. doi: 10.1007/s00213-010-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz GJ, et al. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol. 1993;264(3 Pt 2):R630–7. doi: 10.1152/ajpregu.1993.264.3.R630. [DOI] [PubMed] [Google Scholar]
- 37.Beglinger C, Degen L. Fat in the intestine as a regulator of appetite--role of CCK. Physiol & Behav. 2004;83:617–21. doi: 10.1016/j.physbeh.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 38.De Jonghe BC, Hajnal A, Covasa M. Decreased gastric mechanodetection, but preserved gastric emptying, in CCK-1 receptor-deficient OLETF rats. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G640–9. doi: 10.1152/ajpgi.00109.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aviello G, et al. Inhibitory effect of the anorexic compound oleoylethanolamide on gastric emptying in control and overweight mice. J Mol Med (Berl) 2008;86(4):413–22. doi: 10.1007/s00109-008-0305-7. [DOI] [PubMed] [Google Scholar]
- 40.Feifel D, et al. Altered extracellular dopamine concentration in the brains of cholecystokinin-A receptor deficient rats. Neurosci Lett. 2003;348:147–50. doi: 10.1016/s0304-3940(03)00767-5. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz GJ. Gut fat sensing in the negative feedback control of energy balance - Recent advances. Physiol & Behav. 2011 doi: 10.1016/j.physbeh.2011.05.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav. 2003;78(2):285–94. doi: 10.1016/s0031-9384(02)00968-x. [DOI] [PubMed] [Google Scholar]
- 43.Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol & Behav. 2010;99:402–11. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margolskee RF, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 2010;339(3):493–504. doi: 10.1007/s00441-009-0907-6. [DOI] [PubMed] [Google Scholar]
- 46.Janssen S, et al. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci U S A. 2011;108(5):2094–9. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawa Y, et al. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4(4):e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren X, et al. Sweet taste signaling functions as a hypothalamic glucose sensor. Frontiers in Integrative Neuroscience. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swartz TD, et al. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br J Nutr. 2012;107(5):621–30. doi: 10.1017/S0007114511003412. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 51.Zhao GQ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 52.Sclafani A, et al. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1643–50. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Araujo IE, Ren X, Ferreira JG. Metabolic sensing in brain dopamine systems. Results Probl Cell Differ. 2010;52:69–86. doi: 10.1007/978-3-642-14426-4_7. [DOI] [PubMed] [Google Scholar]
- 54.McCrimmon R. The mechanisms that underlie glucose sensing during hypoglycaemia in diabetes. Diab Med. 2008;5:513–522. doi: 10.1111/j.1464-5491.2008.02376.x. [DOI] [PubMed] [Google Scholar]
- 55.Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2003;284(4):R882–92. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- 56.Pardini AW, et al. Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain Res. 2006;1112(1):169–78. doi: 10.1016/j.brainres.2006.06.109. [DOI] [PubMed] [Google Scholar]
- 57.Hommel JD, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 58.Ackroff K, Sclafani A, Axen KV. Diabetic rats prefer glucose-paired flavors over fructose-paired flavors. Appetite. 1997;28:73–83. doi: 10.1006/appe.1996.0058. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, et al. Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic-like behavior and increases dopaminergic activity in amygdala. Mol Psychiatry. 2011;16(10):1024–38. doi: 10.1038/mp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horvath TL, Andrews ZB, Diano S. Fuel utilization by hypothalamic neurons: roles for ROS. Trends Endocrinol Metab. 2009;20(2):78–87. doi: 10.1016/j.tem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Li AJ, Wang Q, Ritter S. Participation of hindbrain AMP-activated protein kinase in glucoprivic feeding. Diabetes. 2011;60(2):436–42. doi: 10.2337/db10-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levin BE. Glucose-regulated dopamine release from substantia nigra neurons. Brain Res. 2000;874:158–64. doi: 10.1016/s0006-8993(00)02573-7. [DOI] [PubMed] [Google Scholar]
- 63.Hudson B, Ritter S. Hindbrain catecholamine neurons mediate consummatory responses to glucoprivation. Physiol Behav. 2004;82(2–3):241–50. doi: 10.1016/j.physbeh.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 64.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213(4506):451–2. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 65.Watts AG, Donovan CM. Sweet talk in the brain: Glucosensing, neural networks, and hypoglycemic counterregulation. Frontiers in Neuroendrocrinology. 2009 doi: 10.1016/j.yfrne.2009.10.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ritter S, Dinh TT, Li AJ. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav. 2006;89(4):490–500. doi: 10.1016/j.physbeh.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 67.Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res. 2010;209(1):1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 68.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–82. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat Med. 2010;16(4):392–5. doi: 10.1038/nm0410-392. [DOI] [PubMed] [Google Scholar]
- 70.Wang PY, et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452(7190):1012–6. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]