Abstract
Objective
Patients with Lewy body spectrum disorders (LBSD) such as Parkinson’s disease (PD), Parkinson’s disease with dementia (PDD), and dementia with Lewy bodies (DLB) exhibit deficits in both narrative comprehension and narrative expression. The present research examines the hypothesis that these impairments are due to a material-neutral deficit in organizational executive resources rather than to impairments of language per se. We predicted that comprehension and expression of narrative would be similarly affected and that deficits in both expression and comprehension of narrative would be related to the same anatomic distribution of prefrontal disease.
Method
We examined 29 LBSD patients and 26 healthy seniors on their comprehension and expression of narrative discourse. For comprehension, we measured accuracy and latency in judging events with high and low associativity from familiar scripts such as “going fishing.” The expression task involved maintaining the connectedness of events while narrating a story from a wordless picture book.
Results
LBSD patients were impaired on measures of narrative organization during both comprehension and expression relative to healthy seniors. Measures of organization during narrative expression and comprehension were significantly correlated with each other. These measures both correlated with executive measures but not with neuropsychological measures of lexical semantics or grammar. Voxel-based morphometry revealed overlapping regressions relating frontal atrophy to narrative comprehension, narrative expression, and measures of executive control.
Conclusions
Difficulty with narrative discourse in LBSD stems in part from a deficit of organization common to comprehension and expression. This deficit is related to prefrontal cortical atrophy in LBSD.
Keywords: Parkinson’s disease, speech, language, dementia with Lewy bodies
INTRODUCTION
Telling a story of personal experience or giving directions on how to perform an everyday task are communicative acts that healthy people take for granted. Comprehension of such speech acts is likewise a part of daily life. Effective interaction with one’s family and community depend on such routine communications. The present report focuses on difficulties with this form of communication in patients with Lewy body spectrum disorder, including Parkinson’s disease (PD), Parkinson’s disease in which a progressive reduction in cognitive functioning has reached the status of dementia (PDD), and dementia with Lewy bodies (DLB).
Though it is primarily considered to be a motor disorder, PD is also known to affect cognition. Cognitive deficits in mild PD may include executive dysfunction and visuospatial deficits, and some patients may also have impaired memory (Bosboom, Stoffers, & Wolters, 2004; Brown & Marsden, 1990; Levin, Tomer, & Rey, 1992). Hypothesized mechanisms of a cognitive deficit implicate dopamine depletion in the substantia nigra. This causes impaired functioning of the basal ganglia, an area that may mediate cognitive functioning through its rich connections with frontal cortex. It may also lead to impaired frontal lobe functioning more directly through compromised projections from the ventral tegmental portion of the substantia nigra to regions of the frontal lobe.
A progressive reduction in cognitive functioning in a proportion of PD patients eventually reaches the status of dementia (PDD). This is estimated to occur in about 20% of PD patients when first seen (Brown & Marsden, 1984; Ebmeier et al., 1991; Grossman, 1999; Mayeux et al., 1988), with estimates ranging from 11% to 36% (Giladi et al., 2000; Girotti et al., 1988; Lees, 1985; Parashos, Johnson, Erickson-Davis, & Wielinski, 2009). Up to 80% of patients with PD may eventually develop PDD as the disease progresses (Aarsland, Andersen, Larsen, & Lolk, 2003; Buter et al., 2008; Hely, Reid, Adena, Halliday, & Morris, 2008). Dementia in PD is associated with a proliferation of Lewy bodies in the cerebral cortex. This histopathologic picture is identical to that seen in dementia with Lewy bodies (DLB), a condition that is said to differ clinically from PDD in that there is a relatively later onset of a motor disorder in DLB compared to PDD (McKeith et al., 2005). Thus there exists a spectrum of cognitive disorders associated with extrapyramidal features, unified by the presence of Lewy bodies, varying in the relative onset of motor and cognitive features, and including PD patients potentially converting to clear dementia. We refer to this family of conditions as Lewy body spectrum disorder (LBSD). It includes nondemented patients (PD), cognitively impaired patients with a relatively early onset motor disorder (PDD), and demented patients with minimal or late onset motor disorder (DLB). We acknowledge that this view of PD, PDD, and DLB as a spectrum of cognitive and movement disorders is not universally accepted. Other researchers have identified both similarities and differences in the cognitive consequences of these diseases (Aarsland et al., 2003; Downes et al., 1998). In general, however, both the cognitive and brain atrophy differences that have been found among the groups are interpretable as quantitative differences in degree of change, rather than qualitative differences in the nature of these conditions (Double et al., 1996; Harrington et al., 1994). The shared features of proliferation of Lewy bodies in cerebral cortex and a qualitatively similar range of cognitive deficits are the grounds for our regarding these conditions as a spectrum of disorders.
There is evidence that cognitive deficits in PD can affect language as well (Bastiaanse & Leenders, 2009; Colman et al., 2009; Grossman, 1999; Hochstadt, 2009; Pereira et al., 2009). For example, some authors have reported reduced syntactic complexity in speech production (Ash et al., 2011; Cummings, Darkins, Mendez, Hill, & Benson, 1988; Murray & Lenz, 2001). Recent work examining grammatical comprehension has focused on the role of limited executive resources such as poor working memory in the comprehension deficits of these patients (Colman et al., 2009; Gross, Camp et al., submitted). Most studies of language in LBSD are limited to nondemented patients, although there are exceptions (Ash et al., 2011; Gross, Camp et al., submitted; Gross, McMillan et al., in press; Parashos et al., 2009; Piatt, Fields, Paolo, Koller, & Troster, 1999).
In the present study, we focus on narrative language. Narrative is a verbal technique of recapitulating past experience by matching a sequence of narrative units to the temporal sequence of events that occurred (Labov & Waletzky, 1967). It is a term usually applied to language production. In the present report, we also consider the receptive aspect of narrative, that is, we consider the comprehension of scripts describing familiar routines as narrative comprehension. While lexical selection and syntactic structure clearly play a role in a narrative, a feature of narrative that is not present at the levels of the single word or sentence is the top- down organization of events contributing to the narrative (Farag et al., 2010; Mar, 2004). We examine the hypothesis that both the comprehension of a narrative consisting of events from a brief script and the production of a narrative in a semi-structured speech sample are impaired in LBSD. We test non-aphasic patients with LBSD in order to determine whether there are linguistic or cognitive factors that are common to difficulty with both the comprehension and expression of connected speech and contribute to these patients’ narrative impairments.
We consider first narrative comprehension. Previous studies have examined the association between events contributing to a script in several conditions, including focal brain lesions (Joanette, Goulet, Joanette, & Brownell, 1990), traumatic brain injury (Coelho, Grela, Corso, Gamble, & Feinn, 2005), and neurodegenerative diseases (Cosentino, Chute, Libon, Moore, & Grossman, 2006; Farag et al., 2010). Cosentino and colleagues conducted a study examining the processing of four-event scripts describing familiar activities (e.g., “making coffee”). Non-aphasic patients with behavioral variant frontotemporal degeneration (bvFTD) showed greater difficulty detecting sequencing errors relative to semantic errors. This finding supports the notion that there is an organizational component to script processing, separable from knowledge of script content, that is compromised in patients with frontal/executive dysfunction (Cosentino et al., 2006). More recently, a deficit of top-down organization has been demonstrated in the impaired comprehension of narratives in bvFTD ((Farag et al., 2010). Further, patients with left and bilateral frontal injuries have exhibited difficulty recognizing the pragmatic connection between pairs of successively presented sentences (Ferstl, Guthke, & von Cramon, 2002). We are aware of only one previous study of narrative comprehension in non-aphasic patients with LBSD (Gross, Camp et al., submitted). The authors described poor comprehension of the organization of events from a script in patients with PDD and DLB compared to non-demented PD patients and healthy seniors. The patients’ impairment correlated with impaired performance on measures of executive control that are important for organization and planning. In the present study, we also assessed narrative comprehension by examining LBSD patients’ ability to judge the association of temporally ordered events in a narrative script.
We next consider narrative expression. In our prior work, we asked non-aphasic patients with bvFTD to narrate a wordless children’s picture story. A detailed analysis of performance revealed a limited grasp of the story’s overall theme and poor connectedness between successive events in the patients’ stories, even though lexical and grammatical aspects of word and sentence use were relatively preserved (Ash et al., 2006). We recently demonstrated a similar deficit in LBSD (Ash et al., 2011). This impairment was most evident in patients with PDD and DLB relative to non-demented patients with PD and healthy seniors.
Executive deficits on tasks requiring working memory, planning, and organizational skills have been described extensively in LBSD (Calderon et al., 2001; Kraybill et al., 2005; Lambon Ralph et al., 2001; Libon et al., 2001). Such deficits in executive resources may interfere with the capacity for effective communication in LBSD at the level of narrative, due to the demands placed on organization and planning. These higher-level organizational functions provide for the relating of narrative events to each other and support communicative coherence by maintaining the theme of the narrative, even if associated events are not adjacent to each other. Consistent with a model of narrative processing that involves executive functioning, previous work with non-aphasic bvFTD patients has related deficits of narrative comprehension and narrative expression to impaired performance on measures of executive functioning (Ash et al., 2006; Farag et al., 2010). Similarly, narrative deficits in LBSD have been associated with limited executive resources during expression (Ash et al., 2011) and during comprehension (Gross, Camp et al., submitted). We also examine linguistic deficits that may interfere with comprehension and production, such as difficulty with syntactic or semantic aspects of language comprehension or expression. In the present study of LBSD, we predict that difficulties with narrative comprehension and narrative expression will be correlated with each other. Furthermore, we predict that impairments on executive measures involving organization and planning will be correlated with deficits in both comprehension and expression of narrative.
We related impairments of discourse comprehension and expression in LBSD to their neuroanatomic underpinnings using volumetric MRI. Consistent with the hypothesized basis for cognitive difficulty in LBSD, volumetric MRI studies have shown frontal gray matter loss in PD, although there may also be extension to temporal, occipital, and parietal cortical areas in DLB/PDD (Ash et al., 2011; Burton, McKeith, Burn, Williams, & O’Brien, 2004; Gross, Camp et al., submitted; Gross, McMillan et al., in press; Whitwell et al., 2007). Voxel-based diffusion tensor imaging has shown white matter abnormalities in multiple brain regions including the frontal lobes (Lee et al., 2010). Functional imaging has shown disturbance of frontostriatal metabolism in these disorders (Eckert et al., 2005; Eidelberg, 1992; Eidelberg et al., 1990; Eidelberg et al., 1994; Huang et al., 2007; Lewis, Dove, Robbins, Barker, & Owen, 2003; Lozza et al., 2004; Sawamoto et al., 2008). Previous assessments of narrative have underlined the crucial role of prefrontal regions following focal brain lesions (Joanette et al., 1990) and traumatic brain injury (Coelho et al., 2005). In bvFTD, prefrontal disease has been associated with narrative disorders in both comprehension (Farag et al., 2010) and expression (Ash et al., 2006; Chapman et al., 2002). Our prior investigations of LBSD have related narrative deficits in comprehension (Gross, Camp et al., submitted) and expression (Ash et al., 2011) to prefrontal disease. In the present study, we seek to determine whether deficits in narrative comprehension and expression are associated with the same brain regions in LBSD. Further, we investigated whether a prefrontal basis for a deficit common to both comprehension and expression overlaps with a prefrontal region also shown to be crucial for organizational resources in LBSD.
METHODS
Subjects
We studied 29 non-aphasic patients with LBSD, diagnosed in the Cognitive Neurology or Movement Disorders clinics of the Department of Neurology at the University of Pennsylvania by experienced neurologists (RGG, AS, MG) according to published criteria (Hughes, Daniel, Kilford, & Lees, 1992; McKeith et al., 2005). The non-demented group consisted of 20 patients with PD. A group of nine patients exhibited evidence of dementia, including four patients with DLB and five with a diagnosis of PDD. Some of these patients had participated in both Ash et al. (2011) and Gross, Camp et al. (submitted). In determining the diagnosis, the convention recommended by the Third Report of the DLB Consortium (McKeith et al., 2005) was followed: a diagnosis of PDD was made when motor symptoms preceded the onset of dementia by at least one year, and a diagnosis of DLB was made when dementia preceded the development of motor symptoms by at least one year. Features of DLB recognized in the Third Report of the DLB Consortium (McKeith et al., 2005), such as fluctuating cognition, variations in attention and alertness, and visual hallucinations, were relatively mild and did not interfere with performance at the time of testing.
Patients were assigned to PD, PDD, or DLB subgroups using a consensus evaluation based on published criteria that entailed two independent raters reviewing a semi-structured neurologic history, a complete neurologic exam, and a detailed mental status exam. In addition to clinical criteria, patients were classified as having dementia if (1) the Mini-Mental State Exam (MMSE) score was less than or equal to 24, or (2) if the MMSE was greater than 24 but the patient performed in the demented range on the Mattis Dementia Rating Scale (DRS-2; age-adjusted score less than or equal to 5) (Folstein, Folstein, & McHugh, 1975; Lucas et al., 1998; Mattis, Jurica, & Leitten, 2001). This latter criterion was implemented for patients judged clinically to be demented who had a predominantly dysexecutive syndrome that was not detected by the MMSE, an instrument that is relatively insensitive to executive deficits.
Demographic and clinical characteristics are summarized in Table 1. Because LBSD is a spectrum disorder, means are presented for the combined subgroups and also for the demented and non-demented patient subgroups separately. Clinical features include dopaminergic medication use, Unified Parkinson’s Disease Rating Scale (UPDRS) motor assessment (Fahn, Elton, & UPDRS Program Members, 1987), and Hoehn & Yahr stage (Hoehn & Yahr, 1967). Dopaminergic medication use is expressed as levodopa equivalents. In accordance with Hobson, et al. (2002), the following dosages of medication are taken as equivalent: 100 mg levodopa; 130 mg controlled-release levodopa; 70 mg levodopa in conjunction with catechol-O-methyl transferase (COMT) inhibitor; 1 mg pergolide; 1 mg pramipexole; 5 mg ropinirole. Other PD medications (e.g., anticholinergics and monoamine oxidase inhibitors) were not included in the determination of levodopa equivalent dose. Exclusionary criteria included other causes of dementia, such as metabolic, endocrine, vascular, structural, nutritional, and infectious etiologies, and primary psychiatric disorders. The DLB/PDD patients were mildly impaired according to the Mini Mental State Exam (MMSE) (Folstein et al., 1975). One-way analyses of variance (ANOVAs) indicated that control, PD, and DLB/PDD subject groups were matched for age and education. Disease duration and UPDRS motor disorder did not differ significantly across LBSD subgroups. Control subjects consisted of 14 age- and education-matched healthy seniors evaluated on the expression task, 10 evaluated on the comprehension task, and 2 subjects who completed both tasks. Although the proportions of male and female subjects differed significantly in the LBSD and control groups (χ2= 9.70, p<.005), there was no significant difference between males and females on any of the comprehension, expression, or neuropsychological variables that were studied. This finding held true for both LBSD patients and control subjects. All subjects completed an informed consent procedure in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Pennsylvania.
Table 1.
Mean ± standard deviation of demographic, clinical and neuropsychological characteristics of patients and controls1,2
| Lewy Body Spectrum Disorder |
DLB/PDD subgroup |
PD subgroup |
Controls | |
|---|---|---|---|---|
| N (male/female) | 20/ 9 | 7 / 2 | 13 / 7 | 7/19 |
| Age (yrs) | 71.1 ±7.9 | 75.1 ±6.2 | 69.2 ±8.1 | 69.7 ±7.5 |
| Education (yrs) | 15.2 ±2.8 | 14.8 ±2.4 | 15.4 ±3.1 | 15.4 ±2.8 |
| MMSE (max=30) | 26.3 ±3.3** | 22.2 ±2.7** | 28.1 ±1.3* | 29.1 ±1.2 (13) |
| Disease duration (yrs) | 6.4 ±2.8 | 6.8 ±2.3 | 6.2 ±3.1 | -- |
| Levadopa equivalent dose |
570 ±407 (26) | 439 ±407 | 639 ±4023 (17) | -- |
| UPDRS total motor score |
24.8 ±9.1 (26) | 26.3 ±9.0 | 23.9 ±9.3 (17) | -- |
| Hoehn & Yahr stage | 2.4 ±0.6 (26) | 2.8 ±0.4 | 2.3 ±0.6(17) | -- |
|
Executive function Executive composite3 |
-1.33 ±1.35 | -2.70 ±0.30 | -.72 ±1.17 | -- |
| Category fluency (animals) |
14.6 ±7.0** | 7.8 ±2.9** | 17.6 ±6.0* | 22.3 ±5.1 (12) |
| Trails B time | 134 ±45 | 178 ±7** | 114 ±41 | 108 ±43 (10) |
| Reverse digit span | 4.48±1.55 | 2.89±.78** | 5.20±1.24 | 5.44±1.59 (9) |
| Letter-guided fluency (FAS) |
34.2 ±15.1* | 22.4 ±9.1** | 39.4 ±14.3 | 43.8 ±9.8 (10) |
|
Semantics Comprehension: Boston Naming Test (% correct) |
89.3 ±9.8 | 81.0 ±11.6 | 93.0 ±6.2 | 91.90 ±11.8 (10) |
| Expression: Open class words (%) |
41.0 ±3.6 | 40.1 ±5.0 | 41.4 ±2.9 | 42.8 ±3.1 |
|
Grammaticality Comprehension: Complex sentences |
36.8 ±8.3** (16) | 28.7 ±6.4** (6) | 41.6 ±4.9*(9) | 46.0 ±1.2 (5) |
| Expression: Composite grammar score (max=2)4 |
1.24 ±.21* | 1.16 ±.27 | 1.27 ±.17 | 1.38 ±.22 |
NOTES
Pairwise statistical differences between groups: * differs from controls, p<.05; ** differs from controls, p<.01. Since not all participants were available for testing on all neuropsychological measures, and because of technical limitations in recovering some demographic and clinical features, we provide in parentheses the numbers of participants for which each characteristic was ascertained if fewer than the total number in the group.
For all statistically significant comparisons of DLB/PDD patients to Controls, the effect size is “medium” or “large” (Cohen’s d > 0.7). For all statistically significant comparisons of LBSD and PD patients to Controls, the effect size is “small” or “medium” (Cohen’s d ranges from 0.3 to 0.7).
The composite score of executive function was constructed by averaging the Z-scores of category naming fluency and time to complete Trails B.
The composite score of grammatical expression was derived by summing the proportions of well-formed sentences and utterances containing complex structures.
Narrative comprehension materials
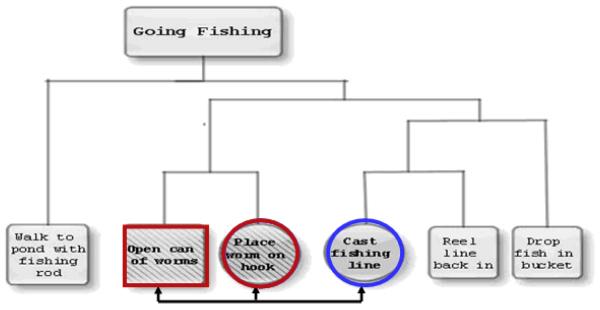
We created 22 scripts, each consisting of six events, describing familiar activities such as going fishing or making a sandwich. The development of this task has been described in detail elsewhere (Farag et al., 2010). The associativity of the events in each script was established by judgments from a group of ten young, healthy subjects. These pilot subjects were asked to arrange the events first in chronological order and then into clusters showing the degree of association of the events. Figure 1 displays an example of the clustered structure of the events of a script. We then established the following types of event pairs, also shown in Figure 1: adjacent events contained within the same cluster (WC); adjacent events from different clusters (DC), where one event was the same as an event from the WC pair; and nonadjacent event pairs from different clusters where one event was the same as an event from the WC pair (NA). Half of all event pairs were shown in the correct order and half were shown in the incorrect order. Subjects were shown a total of 198 pairs of events and were asked to judge whether the order of presentation was the correct order for performance of the actions. The stimuli were randomized with respect to correctness of order of presentation and were distributed over six runs, resulting in 33 items per run. Each type of event pair was presented from two to five times per run.
Figure 1.
Structure of the events in the script “Going Fishing,” showing clustering by associativity. Events outlined in red are Adjacent, within-cluster (WC); events outlined in circles are Adjacent, different cluster (DC); events outlined in a red square and a blue circle are Non-adjacent (NA).
Narrative comprehension procedure
The stimuli were presented using a Dell Inspiron 1100 laptop computer. E-Prime 1.4.1 presentation software was used to record response accuracy and latency. For each trial, a story title and pair of events were displayed on the monitor in black 18-point Arial type. First, the story title was presented at the top of the screen for 3000 msec, followed by a blank screen for 200 msec. Then the previously displayed story title was shown with one story event beneath the title for 4000 msec, again followed by a blank screen for 200 msec. Finally, the story title and the first event were shown with a second event presented below the first event for 4000 msec. Subjects were asked to judge whether the two events were shown in the correct order for the performance of the complete action named in the title. Response time was not limited. “Yes” or “no” responses were recorded by a button press on the computer keyboard. Subjects were given a practice run of six items before administration of the experiment. During the practice trials, incorrect answers were corrected and explained by the experimenter to ensure comprehension of task instructions.
Only responses to event pairs that had been presented in the correct order were analyzed. Outliers of less than 500 msec or more than 14,000 msec were eliminated. An individualized filter for latencies of 2.5 standard deviations was employed to normalize responses based on each subject’s own distribution of reaction times. On average, 7.5% of responses were excluded across subjects.
Narrative expression materials
In this task, the subjects were directed to tell the story of the wordless children’s picture book, Frog, Where Are You (Mayer, 1969). An outline of the story is given elsewhere (Ash et al., 2006). Briefly, the story begins with a boy and his dog admiring a frog that they keep in a large jar as they prepare to go to bed for the night. The frog escapes, and the following morning, the boy and his dog find that the window is open and the frog is gone. The story illustrates the adventures of the boy and his dog as they search for the frog in the forest outside their house. Ultimately, they find their frog with a lady frog and a brood of baby frogs. The book’s sequence of 24 drawings elicited an extended speech sample with a known target that was comparable in content across subjects and gave patients an opportunity to demonstrate the breadth of their language production capability. We elected to study speech production in this manner to elicit a narrative without taxing the memory resources of the speakers and to eliminate the interruptions of turn-taking that occur in free conversation. We used a longer story rather than the description of a single picture in order to elicit a reasonably lengthy speech sample that was representative of the patient’s speech and language abilities. We used a relatively unknown story rather than a fairy tale to avoid the intrusion of previously learned material.
Narrative expression procedure
Each subject was asked to look through the book for as long as s/he wished to become familiar with the story. When ready, the subject was asked to start at the beginning and narrate the story as if telling it to a child. Due to the nature of the protocol, there was no influence of the examiner on the time taken by the subjects to tell the story. Ten narrations were recorded on a Macintosh Powerbook G3 laptop computer using the Macintosh external microphone (part #590-0670) and the computer program SoundEdit 16, v. 2 at a sampling frequency of 44.1 kHz. Twenty-eight were recorded on a Dell Inspiron 2200 PC using the signal processing software Praat (Boersma & Weenink, 1992-2009) at a sampling rate of 22.05 kHz, using a Radio Shack omnidirectional lavaliere electret condenser microphone. Seven were recorded on a Marantz PMD 670 digital recorder at a sampling frequency of 32 kHz, using a Sennheiser MKE2 omnidirectional lavaliere condenser microphone.
The recordings of the narratives were transcribed in detail by trained transcribers using the signal processing software Praat. The transcription conventions used to capture the irregularities in patients’ speech are defined elsewhere (Ash et al., 2006). The narratives were coded from the transcripts by trained judges, referring to the original speech files as needed. All coding was checked by a linguist (SA) with expertise in grammatical, phonetic, and phonological analysis.
Analysis of narrative organization in expression
To assess the hypothesis that LBSD patients have a disorder of organization in narrative production, we evaluated two aspects of coherence in the patients’ narratives:
1. Local connectedness
An event was scored as locally connected if the narrative gave a relationship between a mentioned event and the preceding material. This was accomplished by rhetorical devices such as sequencing adverbials, pronominal reference to preceding events, reference by definite as opposed to indefinite determiners (Given vs. New information), and statements of cause and effect. Alternatively, the requirements of local connectedness were violated if a new element in the story was referred to in terms that are only appropriate for an element that has already been mentioned, as with definite determiners or pronominal reference when there is no immediately preceding noun antecedent. An event was scored as not connected if appropriate connecting devices were not present and the reported event did not follow logically from the preceding utterances.
2. Search theme
The essence of the story is that a boy and his dog have a pet frog which escapes from the boy’s room while he and the dog are asleep. They search for the frog, first in the boy’s room and then outdoors in the woods near his house. Ultimately, they find the frog. Thus, the search for the frog is the central theme of the story, and keeping the theme in mind unifies the narrative and maintains its coherence. Search theme maintenance was scored from 0 to 4 by counting points accrued according to these criteria: one point for noting that the frog is missing, one point for noting that the boy is searching for the frog, one point for one or two further mentions of the search theme, and one point for any additional mentions of the search theme (Reilly, Losh, Bellugi, & Wulfeck, 2004).
An example of a story narration produced by an LBD patient is given in Appendix A.
Language performance at the level of semantics was assessed by calculating the percentage of words spoken by the subject that were open class (content) words. Grammaticality was judged by a composite measure. First, the proportion of sentences spoken that were well-formed (complete and free of grammatical errors) was calculated. Second, the proportion of utterances that contained complex structures, either dependent clauses or phrasal adjuncts, was assessed (Ash et al., 2006). These two measures were added together, yielding a score that ranged from 0 to 2.
Results of the assessment of language production of semantics and grammar are summarized in Table 1. Patients did not differ from controls in their semantic performance as represented by the proportion of open class words produced. LBSD patients as a group were impaired compared to controls on the composite measure of grammatical expression.
Neuropsychological evaluation
The patients underwent neuropsychological testing within an average of four months of the dates of testing. For the comprehension task, the mean ± SD number of days of separation of the tests was 113 ± 118. For the expression task, the corresponding number of days was 107 ± 187. As Parkinson’s disease progresses slowly, with survival often decades long, an average discrepancy of four months between neuropsychological testing and the experimental task is acceptable. Nevertheless, a larger study would be needed to help establish the maximum acceptable difference that allows reasonable inferences about relationships among behavioral measures. We assessed subjects on tests of executive functioning, semantics, and grammaticality. Executive functioning was assessed by category naming fluency for animals (total number of non-repeated animal names in 1 minute), a test of the mental planning needed to search a semantic field; time taken to complete Trails B (up to 180 sec), a test of planning and mental flexibility requiring alternation between letter and number sequences; reverse digit span (total number of digits correctly repeated in reverse order), a test of working memory; and letter-guided word-naming fluency (FAS − averaged total number of non-repeated words in 1 minute for each letter), a test of mental search capability. Semantics was tested by an abbreviated form of the Boston Naming Test (% correct). Comprehension of syntax was tested by probing the subject’s ability to identify the agent or patient in sentences with active and passive voice, subject- and object-relative clauses, and right-branched vs. center-embedded clauses (maximum score = 48).
Results of the neuropsychological testing are summarized in Table 1. The LBSD patients differed significantly from controls on neuropsychological measures of executive functioning. Examination of the demented and non-demented subgroups revealed that the greatest differences between patients and controls were found in the DLB/PDD subgroup, in which patients were impaired on all measures. In addition, PD patients were impaired relative to controls on a measure of mental search.
On measures of receptive language performance, patients did not differ from controls on semantics as assessed by the Boston Naming Test. LBSD patients, including both the PD and DLB/PDD subgroups, were impaired in the comprehension of complex sentences.
Statistical considerations
Levene’s test of homogeneity of variance indicated that some measures of language and neuropsychological test scores did not meet the requirement of homogeneity of variance for parametric statistical tests. Both sample sizes and variances differed substantially between groups that were being compared; therefore we used the Welch statistic to test the equality of group means. The equality of paired samples was tested using Wilcoxon’s signed ranks test, and correlations were calculated using Spearman’s rho. All statistical analyses were conducted using SPSS 12.0.
Imaging methods
Fourteen LBSD patients, including 8 patients with PD and 6 patients with DLB/PDD, had a volumetric brain MRI scan within one year of the narrative task. These 14 patients did not differ statistically from the larger set of 29 LBSD patients on any neuropsychological or language measures (see Appendix B, Table B1).
Eleven patients had MRI scans acquired using a GE 1.5T scanner with 1.2-mm slice thickness and a 144 × 256 matrix. For three patients and 40 age-matched controls, images were collected using a SIEMENS Trio 3.0T scanner with 1-mm slice thickness and a 195 × 256 matrix. Images from both scanners were deformed into a standard local template space with a 1-mm3 resolution using PipeDream (https://sourceforge.net/projects/neuropipedream/) and Advanced Normalization Tools (ANTS, http://www.picsl.upenn.edu/ANTS/). These tools have been validated as stable and reliable for performing multivariate normalization (Avants, Epstein, Grossman, & Gee, 2008; Klein et al., 2009). Both PipeDream and ANTS mapped T1 structural MRI images to an optimal template space, using diffeomorphic and symmetric registration methods (Avants & Gee, 2004; Avants et al., 2010). The registered images were segmented into gray matter probability maps using template-based priors and then registered to MNI-template space for statistical comparisons. Gray matter probability images were smoothed in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/sortware/spm5) using a 4-mm full-width half-maximum Gaussian kernel to minimize individual gyral variations.
In SPM5, a two-sample t-test covarying for scanner contrasted gray matter probability between patients with LBSD and healthy controls to identify regions of significant cortical atrophy. For this atrophy analysis, an explicit mask was defined by generating a mean gray matter image from the healthy controls in order to limit the analysis to voxel-wise comparisons within gray matter. We used a p<.05 (uncorrected) height threshold, 400-voxel extent, and accepted clusters with a peak voxel Z-score >3.09 (p<0.001).
The regression module in SPM5 was used to relate gray matter atrophy to expression as measured by search theme. We also related gray matter atrophy to comprehension as measured by latency for adjacent WC judgments. Finally, we related gray matter atrophy to the composite measure of executive functioning. We performed a whole-brain analysis for each of these regressions using an explicit mask based on the anatomic distribution of gray matter atrophy. This enabled us to examine the relationship between the narrative and executive measures and brain areas known to be significantly diseased, based on the prior analysis of whole-brain gray matter atrophy. We interpreted only regions where measures of language and executive performance were related to atrophied gray matter areas because these diseased areas were likely to be implicated in the patients’ deficits, and it would be difficult to explain with confidence significant associations between patients’ performance and non-atrophied regions. For the regression analyses, we used a height threshold of p<.05 (uncorrected), 200-voxel extent, and we accepted clusters with a peak voxel Z-score >3.09 (p<.001). Coordinates for all accepted clusters were converted to Talairach space (Talairach & Tournaux, 1988).
RESULTS
Narrative comprehension results
Performance on the measures of narrative comprehension and expression are summarized in Table 2. To assess narrative comprehension, we first measured overall response latency. We found that LBSD patients as a whole and the subgroup of PD patients did not differ significantly from controls. In contrast, DLB/PDD patients were slower on average to respond in judging the order of events in the scripts. This slowing of response time was also seen in judgments of adjacent WC events and adjacent DC events in both LBSD patients as a group and in the DLB/PDD subgroup. Non-demented PD patients did not exhibit this slowing of response time. A within-group analysis was conducted to compare subjects’ response times to WC and DC events. Healthy seniors were significantly faster at making ordering judgments for WC event pairs compared to DC event pairs [Z= −2.82; p<0.01]. LBSD patients as a group also judged WC event pairs faster than DC pairs [Z=-2.20; p<0.05]. Of the LBSD subgroups, PD patients exhibited an advantage for judging WC event pairs compared to DC pairs [Z= 2.46; p<0.05], resembling controls. However, DLB/PDD patients differed from controls by not showing this advantage; their response time did not differ for WC and DC event pairs [Z= 0.42, NS]. These results replicate the findings of Gross, Camp et al. (submitted). They suggest that the faster response time for controls and non-demented PD patients indicates sensitivity to the closer association of WC events compared to events that are adjacent but belong to different clusters. DLB/PDD patients, in contrast, are relatively insensitive to the closer association of WC events compared to events belonging to different clusters, suggesting that these patients have difficulty appreciating the hierarchically organized structure of narratives during comprehension.
Table 2.
| Lewy Body Spectrum Disorder |
DLB/PDD subgroup |
PD subgroup |
Controls | |
|---|---|---|---|---|
| Narrative comprehension: Latencies (msec) for accurate ordering judgments | ||||
| Overall latency | 4545 ±2063 | 6101 ±2368* | 3845 ±1500 | 3553 ±1081 |
| Within-cluster judgments | 4458 ±2050* | 5948 ±2374* | 3788 ±1514 | 3331 ±1048 |
| Different-cluster judgments | 4678 ±2221* | 6169 ±2773* | 4007 ±1581 | 3572 ±1108 |
| Narrative expression | ||||
| Search theme maintenance (max=4) |
2.5 ±1.7** | 1.2 ±1.5** | 3.1 ±1.5* | 4.0 ±0.0 |
| Local connectedness (max=30) |
22.4 ±6.6** | 16.7 ±6.0** | 25.0 ±5.2* | 27.9 ±2.5 |
NOTE
Pairwise statistical differences between groups: * differs from controls, p<.05; ** differs from controls, p<.01
For all statistically significant comparisons of DLB/PDD patients to Controls, the effect size is “medium” (Cohen’s d ranges from 0.5 to less than 0.8). For all statistically significant comparisons of LBSD and PD patients to Controls, the effect size is “small” or “medium” (Cohen’s d ranges from 0.3 to 0.6).
Narrative expression results
Organization of a narrative during expression is quantifiable as maintenance of the theme--in this case, the theme of the search for the frog--and maintenance of connectedness ofsuccessive events to each other. These results are summarized in Table 2. LBSD patients as a group are impaired on both of these measures of narrative organization during expression. The non-demented subgroup of PD patients and the demented subgroup of DLB/PDD patients both exhibit impairments on these measures of narrative organization. These results replicate the previous findings of Ash et al. (2011) and support the conclusion that organizational deficits in LBSD patients affect their production of narrative discourse.
Correlations of narrative comprehension, narrative expression, and executive measures
We examined correlations between measures of narrative comprehension, narrative production, neuropsychological factors, and non-narrative aspects of language competence to investigate the relationship between narrative comprehension and expression and to study the basis of the impairments observed in the group of 29 LBSD patients. Narrative comprehension is quantified by latency for ordering judgments of adjacent WC events. Narrative expression is quantified by the score on maintenance of the search theme in the narration of the story. The correlations are summarized in Table 3.
Table 3.
Significant correlations of narrative discourse with neuropsychological and language measures in LBSD (N=29)1
| Narrative comprehension | Narrative expression | |
|---|---|---|
| Within-cluster latency | Search theme maintenance | |
| Comprehension | ||
| Within-cluster latency | −.62** | |
| Executive functioning | ||
| Category fluency (Animals) | −.72** | .67** |
| Trails B time | .70** | −.70** |
| Reverse digit span | −.45* | .46* |
| Letter-guided fluency (FAS) | −.48** | .60** |
| Average Z-score of Trails time and Category fluency |
−.73** | .68** |
| Semantics | ||
| Comprehension: Boston Naming Test |
−.15 | .18 |
| Expression: % open class words | −.23 | −.10 |
| Grammaticality | ||
| Comprehension: Complex sentences |
−.75** (16) | .70**(16) |
| Expression: Composite grammar score |
−.01 | −.03 |
NOTE
Significant correlations: * p<0.05; ** p<0.01. Since not all participants were available for testing on all neuropsychological measures, and because of technical limitations in recovering some demographic and clinical features, we provide in parentheses the numbers of participants for which each characteristic was ascertained if fewer than the total number in the group.
We found that narrative comprehension and narrative expression are highly correlated with each other. In addition, comprehension and expression are both correlated with measures of executive functioning, providing evidence that resources supporting organization and planning are related to both the comprehension and production of narrative discourse. There is no correlation of the measures of narrative comprehension and expression with features of semantic competence. Neither narrative comprehension nor narrative expression is correlated with grammaticality in the production of the spoken narratives, but both comprehension and expression are correlated with the comprehension of complex syntax. In addition, performance on the test of comprehension of complex sentences, a test of receptive grammatical competence, is correlated with measures of executive function. For the LBSD group, these correlations are as follows: comprehension of complex sentences is correlated with category fluency (animals), s=.92, p<0.01; with Trails B time, s=-.79, p<0.01; with reverse digit span, s=.69, p<0.01; with letter-guided fluency (FAS), s=.72, p<0.01. For PD patients, there was a significant correlation of performance on comprehension of complex sentences with category fluency (animals): s=.78, p<0.01. For DLB/PDD patients, there were no significant correlations of performance on comprehension of complex sentences with executive measures. Since comprehension of complex sentences, measures of narrative comprehension, and measures of narrative expression were all highly correlated with executive measures in LBSD, we calculated partial correlations of the measures of narrative comprehension and production with complex sentence comprehension while controlling for performance on executive measures. The partial correlations showed that there was no significant correlation of complex sentence comprehension with narrative comprehension or expression when we controlled for executive functioning. Thus, performance on measures of narrative comprehension and expression correlate significantly with each other, and both correlate with measures of executive functioning. However, narrative comprehension and expression do not correlate with linguistic measures of syntax or lexical semantics.
Imaging results
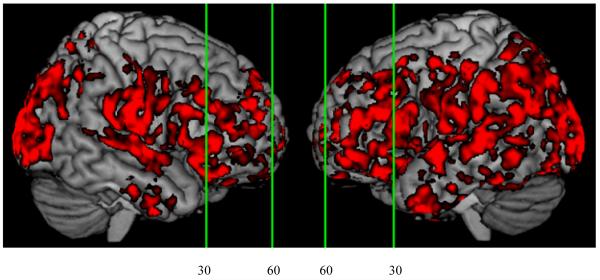
The structural images for 14 LBSD patients exhibited extensive gray matter atrophy compared to healthy seniors (Figure 2). The coordinates of atrophy peaks are given in Table 4. Extensive atrophy was observed in the frontal lobe, seen bilaterally in medial, ventromedial, and ventrolateral frontal regions, left dorsolateral frontal regions, and in right anterior, middle, and cingulate frontal regions. Atrophy was also observed bilaterally in middle temporal, inferior temporal, and hippocampal regions. In addition, atrophy extended to right inferior parietal and left postcentral regions.
Figure 2.
Cortical atrophy in Lewy body spectrum disorder patients.1
NOTE
1. Vertical lines show locations of coronal slices displayed in Figure 3: y = 60 and y = 30.
Table 4.
Regional distribution of significant atrophy in Lewy body spectrum disorder patients
| Anatomic locus (Brodmann area) |
Coordinates | Z-score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left ventral medial prefrontal (11) | −40 | 49 | −15 | 3.23 | 647 |
| Left medial frontal (9) | −10 | 49 | 13 | 4.36 | 2148 |
| Left dorsolateral prefrontal (46) | −40 | 37 | 17 | 4.05 | 2654 |
| Left dorsolateral prefrontal (8) | −32 | 36 | 41 | 3.1 | 898 |
| Left ventral lateral prefrontal (47) | −30 | 27 | −21 | 3.54 | 441 |
| Left middle temporal (21) | −58 | −2 | −6 | 3.71 | 3059 |
| Left postcentral (1) | −54 | −19 | 49 | 3.38 | 1697 |
| Left hippocampus | −32 | −20 | −14 | 5.42 | 9225 |
| Left paracentral lobule | −16 | −27 | 46 | 3.4 | 694 |
| Left inferior temporal (20) | −45 | −30 | −20 | 3.91 | 666 |
| Left lingual (18) | −11 | −84 | −8 | 4.03 | 1191 |
| Right anterior frontal (10) | 12 | 64 | 8 | 3.36 | 1409 |
| Right middle frontal (10) | 40 | 56 | 6 | 3.32 | 1553 |
| Right ventral medial prefrontal (11/47) |
20 |
36 |
−20 |
3.38 |
553 |
| Right medial frontal (32) | 11 | 20 | 35 | 3.27 | 406 |
| Right inferior temporal (20) | 41 | −12 | −33 | 3.6 | 832 |
| Right inferior temporal (20) | 59 | −14 | −21 | 4.6 | 1859 |
| Right cingulate (31) | 10 | −21 | 41 | 3.29 | 1029 |
| Right inferior parietal (40) | 65 | −27 | 27 | 3.71 | 3141 |
| Right middle temporal (21) | 65 | −28 | −10 | 5.26 | 101195 |
| Right hippocampal | 23 | −52 | 10 | 3.27 | 802 |
| Right fusiform (19) | 25 | −61 | −6 | 3.75 | 450 |
| Right lingual (18) | 4 | −82 | −6 | 3.44 | 422 |
We performed a series of whole brain regression analyses to relate cortical atrophy to narrative comprehension, quantified by latency of correct WC ordering judgments; to narrative expression, quantified by search theme score; and to executive functioning, quantified as the average Z-score for category fluency and Trails time. The coordinates of peaks of the correlations for the three regression variables are given in Tables B2-B4 of Appendix B.
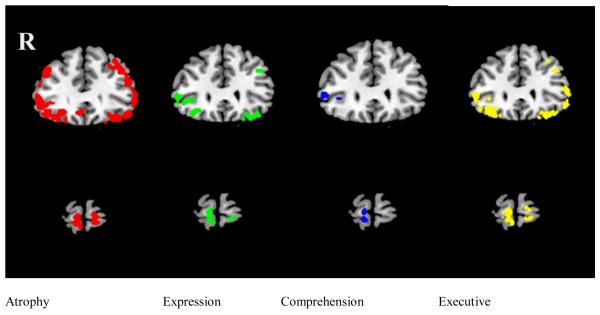
We identified significant cortical areas where there was overlap of atrophy related to narrative comprehension, narrative expression, and executive functioning. These areas are shown in Figure 3 in coronal slices at y=30 (top row) and y=60 (bottom row). At y=30, overlap with atrophy is seen bilaterally in an inferior portion of ventrolateral prefrontal cortex (BA 47) and a middle portion of ventrolateral prefrontal cortex (BA 47) for regressions involving both narrative expression and executive functioning. These areas overlap the area of regression relating narrative comprehension to atrophy in right middle ventrolateral prefrontal cortex (BA 47). At y=60, overlap of the regressions relating all three measures to atrophy is seen in the right anterior frontal region (BA 10).
Figure 3.
Coronal slices showing correlations of measures of language production and neuropsychological test performance with cortical atrophy. Red shows region of cortical atrophy; green shows correlation of cortical atrophy with expression (search theme maintenance); blue shows correlation of cortical atrophy with comprehension (latency of within-cluster judgments); yellow shows correlation of cortical atrophy with composite executive test Z-score (average of category fluency for animals and Trails B time). Slices in the top row are at y=30; slices in the bottom row are at y=60.
DISCUSSION
In this study, we investigated the relationship between the comprehension and expression of narrative discourse in LBSD. Previous work has shown that executive functioning plays a major role in organizing discourse during both comprehension and expression (Ash et al., 2006; Farag et al., 2010; Troiani et al., 2008). Since LBSD patients have limited executive resources, we predicted that executive deficits would interfere with their organization of narrative discourse in both comprehension and expression. Given the prefrontal anatomic distribution of disease in these patients, we predicted that their narrative impairment would be related to prefrontal disease. We found highly correlated deficits in the comprehension and expression of narratives in LBSD, and this was related to limitations in executive resources such as organization and planning. Furthermore, the narrative deficits in these patients were related to overlapping areas of prefrontal disease. We discuss each of these issues in turn below.
In the comprehension task, we confirmed earlier results (Gross, Camp et al., submitted) showing that LBSD patients are impaired in their processing of brief scripts. The observed deficits in script comprehension were most pronounced in the DLB/PDD subgroup. Most importantly, DLB/PDD patients apparently have lost the sensitivity to the close association of WC events that is exhibited by PD patients and healthy seniors. The high associativity of events within a cluster is important in the hierarchical organization of a narrative (Farag et al., 2010; Mar, 2004). In the expression task, we found that LBSD patients are impaired at maintaining the search theme and local connectedness, replicating previous findings (Ash et al., 2011). Theme maintenance and local connectedness are critical in the organization of a narrative during expression (Ash et al., 2006). Both comprehension and expression tasks thus place demands on the speaker’s organizational competence in narrative discourse.
Previous studies have demonstrated the contribution of executive resources to the organization of narrative discourse in both comprehension and production. In comprehension, one study showed that non-aphasic patients with bvFTD exhibited deficits in judging the ordering of events in a brief script, although their ability to identify semantically anomalous single events was relatively spared (Cosentino et al., 2006). A follow-up study of comprehension, using the same materials as the present report, showed that bvFTD patients, like the DLB/PDD patients in the present study, were impaired in appreciating the closer association of events within a cluster compared to adjacent events in different clusters (Farag et al., 2010). An earlier study of narrative production in bvFTD also showed deficits in the organization of narratives produced in the same task as that reported here (Ash et al., 2006). In all of these studies, deficits in the comprehension and expression of narrative organization were related to deficits on neuropsychological measures of executive control. In the present study, we found that impairments of narrative organization in comprehension and expression were related to executive functioning.
In other areas of language, performance on production and comprehension tasks may not always be governed by the same processing mechanism. Sentence-level studies of language in stroke aphasics, for example, have shown a dissociation of grammatical processing in comprehension and production (Miceli, Mazzucchi, Menn, & Goodglass, 1983). By comparison, the language impairments of non-aphasic LBSD patients appear to be due in large part to a cognitive impairment associated with deficits in executive functioning (Bastiaanse & Leenders, 2009; Colman et al., 2009; Gross, Camp et al., submitted; Grossman, 1999; Grossman et al., 2003). The evidence presented here suggests that the organization of narrative discourse in comprehension and production is not significantly dependent on language-specific capabilities but rather appears to rely at least in part on executive resources. Evidence from earlier studies support this view (Farag et al., 2010; Mar, 2004).
Despite deficits in narrative comprehension and expression, neither bvFTD patients (Ash et al., 2006; Cosentino et al., 2006; Farag et al., 2010) nor LBSD patients (Ash et al., 2011; Gross, Camp et al., submitted) examined in previous studies were aphasic. In the present study, we found no significant impairment of LBSD patients on measures of semantics in either comprehension or expression. This is consistent with previous reports of relatively intact lexical semantic processing in PD (Bayles, 1990; Piatt et al., 1999). Resource-demanding lexical processing such as that involved in resolving the meaning of homophones with multiple meanings has, however, been found to be compromised in LBSD (Chenery, Angwin, & Copland, 2008; Copland, 2006; Copland, McMahon, Silburn, & de Zubicaray, 2009). In further support of the prediction that simple lexical semantic processing does not play a significant role in the organization of narratives in these patients, we found no correlation between the measures of semantics taken during the present study and narrative comprehension or expression. In contrast, LBSD patients were impaired on both the receptive and the productive measures of grammaticality. Yet difficulty in producing grammatical utterances seems to contribute minimally to the difficulty exhibited by LBSD patients in narrative organization, since there was no correlation between narrative comprehension or expression and the composite score of grammatical production. We did find that grammatical comprehension correlated with narrative comprehension and expression. However, partial correlation analyses suggest that this may have been observed because all three of these measures correlate with executive functioning. When we controlled for executive functioning, there was no significant correlation of performance on complex sentence comprehension with the measures of either narrative comprehension or narrative expression. The association of comprehension of complex sentences in LBSD with executive functioning may be a reflection of the importance of working memory in understanding these sentences. Comprehension of complex sentences has been found previously to correlate with executive functioning (Colman et al., 2009; Gross, Camp et al., submitted). Production of grammar may be less likely to correlate with executive functioning since production is under the control of the speaker, so speakers may limit themselves to producing grammatical constructions that are within the competence provided by their level of executive functioning. These findings reinforce the conclusion that narrative organization depends minimally on language-specific processing resources.
In sum, the organization of temporally ordered discourse in LBSD patients exhibits a strong parallelism between comprehension and expression. Both of these facets of communication reveal a reliance on executive functioning. As has been reported many times, language at the level of words and sentences in LBSD is relatively spared. However, everyday communication takes place at the higher level of discourse, where executive resources are required. At this level, LBSD patients may experience difficulty.
Further evidence of the correspondence of narrative comprehension, narrative expression, and their combined dependence on executive functioning comes from the imaging study. We observed extensive atrophy bilaterally in frontal, temporal, and parietal/occipital regions, consistent with previous reports (Burton et al., 2009; Burton et al., 2004; Sauer, ffytche, Ballard, Brown, & Howard, 2006; Tam, Burton, McKeith, Burn, & O’Brien, 2005; Whitwell et al., 2007). Within the areas of atrophy, we found a pattern of gray matter thinning related to narrative organization. In the top row of Figure 3, at y=30, it can be seen that narrative comprehension, narrative expression, and executive functioning all were related to atrophy in a right ventral lateral region of the frontal lobe (BA 47). This suggests that ventral lateral frontal cortex is involved in both the comprehension and expression of narrative discourse coherence and that comprehension and expression are subserved, at least in part, by the same anatomic structures. These regions have been shown to be implicated in narrative comprehension and expression in a series of studies of healthy subjects and patients. Farag et al. (2010) found bilateral activation in judgments of more closely related compared to less closely related events in short scripts. In the same study, patients with progressive nonfluent aphasia and with bvFTD did not distinguish between more and less closely related events, and these patients exhibited significantly more atrophy in a left ventral frontal region. In a study of narrative expression, we used arterial spin labeling perfusion fMRI in healthy young adults during the narration of a continuous story relative to describing single, unconnected pictures and found bilateral activation in ventral prefrontal regions (Troiani et al., 2008). Using the same materials, Ash et al. (2006) found that poor local connectedness during narrative production correlated significantly with cortical atrophy in right ventral prefrontal regions in bvFTD. A study of LBSD patients revealed a correlation of local connectedness during narrative expression with atrophy, also in a left ventral prefrontal region (Ash et al., 2011). In a study of narrative comprehension, Gross, Camp et al. (submitted) found a correlation of significant cortical atrophy with a difference in response latencies for highly associated and less closely associated events in a script. Independent evidence that these frontal brain regions contribute to executive functioning comes from numerous imaging studies of healthy adults, showing activation of these areas during performance of planning, working memory, and decision-making tasks (Ramnani & Owen, 2004).
The regression analyses also identified an area of overlap of cortical atrophy, narrative expression, narrative comprehension, and executive function in a right anterior prefrontal region (BA 10). This is displayed in the bottom row of Figure 3. Imaging studies have demonstrated a role for this area bilaterally in attention, initiation, and higher-level planning (Badre & D’Esposito, 2007; Gilbert et al., 2006; Ramnani & Owen, 2004). This region has also been found to be associated with impaired speech fluency in LBSD patients (Ash et al., in press). Thus impairments on the present tasks of narrative discourse organization, including both comprehension and expression, appear to depend at least in part on the same prefrontal regions, and these regions overlap with areas of prefrontal cortical atrophy that contribute to executive deficits. In sum, our findings are consistent with the claim that a deficit in narrative comprehension and narrative expression in LBSD is due to a single source, namely, limited executive functioning. We find this deficit to be related to atrophy in prefrontal brain regions that are compromised in LBSD.
ACKNOWLEDGMENTS
This work was supported by the Morris K. Udall Parkinson’s Disease Research Center of Excellence and the National Institutes of Health (AG17586, AG15116, NS44266, and NS53488).
APPENDIX A
Transcript of a narration of Frog, Where Are You? by a 69-year-old male patient with Lewy body disease. Disease duration = 7 years; education = 12 years; MMSE = 25; total count of complete words = 281; local connectedness score = 17 (max = 30); search theme score = 1 (max = 4). Phonetic detail has been somewhat simplified. The notation of silences of 2.0 sec or longer has been deleted from the transcript.
He catches a frog and puts him in a jar, takes it home
and he’s in his bedroom.
While he’s asleep, the frog g- gets out of the .. jar.
Eh the boy get … is putting on his clothes
and him a- and the dog are calling apparently to the do- eh the .. frog.
The dog get … th- his head, caught in a b- in the jar.
and apparently it falls and breaks.
Now the boy’s calling.
A flock of birds .. fly over.
and the boy s- finds a hole in the ground
and then bu- u … and he finds a beehive
and the bee can start coming out,
the dog’s j- j- barking at the- at the beehive,
and the- the groundhog sticks his- comes out of the little, his hole
The dog disturbs the bees
They start to … swa- swa- eh swar- eh swarm.
They begin to swarm.
And theys, to chase the- they’re chasing the dog
and the dog is running from them
Boy he hi- is trying to hide behind a, a ro- o- a boulder
And the boulder .. apparently is the back of a elk
and the elk, is running with the dog barking at it.
And the elk chases the boy
and the boy .. falls off of the- the top, fell out of a, falls off from the- falls ff- ff- … from the- the- eh eh top
He falls from a tree, out of a tree.
The boy he falls into a … whirlpool
Boy’s sibitting {sitting} up, in the- in the water
and he has the dot- -og sitting on his shoulders.
Eh .. the boy seems to be stalking
They’re hiding behind a log where boy and the do- ug find the, frog .. with its, mate.
The boy is waving goodbye to the frogs.
That’s about it.
APPENDIX B
Table B1.
Mean ± standard deviation of demographic, clinical, neuropsychological, and language characteristics of 29 LBSD patients and the subset of 14 LBSD patients for whom MRI scans were available.1
| Lewy Body Spectrum Disorder |
LBSD patients in imaging analysis |
|
|---|---|---|
| N (male/female) | 20/9 | 12/2 |
| Age (yrs) | 71.1 ±7.9 | 73.3 ±6.4 |
| Education (yrs) | 15.2 ±2.8 | 15.9 ±2.6 |
| MMSE (max=30) | 26.3 ±3.3 | 25.6 ±3.8 |
| Disease duration (yrs) | 6.4 ± 2.8 | 6.9 ± 2.6 |
| Levadopa equivalent dose | 570 ±407 (26) | 353 ±290(11) |
| UPDRS total motor score | 24.8 ±9.1 (26) | 24.4 ±7.6 (11) |
| Hoehn & Yahr stage | 2.4 ±0.6 (26) | 2.4 ±0.6 (11) |
|
Executive function Executive composite2 |
−1.31 ±1.36 |
−1.46 ±1.37 |
| Category fluency (animals) | 14.5 ±6.9 | 13.9 ±6.0 |
| Trails B time | 132 ±46 | 137 ±51 |
| Reverse digit span | 4.48±1.55 | 4.57±1.60 |
| Letter-guided fluency (FAS) | 34.3 ±15.2 | 36.9 ±16.9 |
|
Semantics Comprehension: Boston Naming Test (% correct) |
a89.4 ±9.8 |
89.4 ±7.6 |
| Expression: Open class words (%) | 41.0 ±3.6 | 41.0 ±2.5 |
|
Grammaticality Comprehension: Complex sentences |
36.4 ±8.4 (15) |
38.1 ±8.4 (12) |
| Expression: Composite grammar score (max=2)3 | 1.24 ±.21 | 1.33 ±.19 |
| Narrative comprehension: Latencies (msec) for accurate ordering judgments | ||
| Overall latency | 4545 ±2063 | 4849 ±2192 |
| Within-cluster judgments | 4458 ±2050 | 4837 ±2154 |
| Different-cluster judgments | 4678 ±2221 | 4907 ±2378 |
| Narrative expression | ||
| Search theme maintenance (max=4) | 2.5 ±1.7 | 2.4 ±1.8 |
| Local connectedness (max=30) | 22.4 ±6.6 | 21.8 ±6.7 |
NOTES
There are no statistically significant differences between the two groups. Since not all participants were available for testing on all neuropsychological measures, and because of technical limitations in recovering some demographic and clinical features, we provide in parentheses the numbers of participants for which each characteristic was ascertained if fewer than the total number in the group.
The composite score of executive function was constructed by averaging the Z-scores of category naming fluency and time to complete Trails B.
The composite score of grammatical expression was derived by summing the proportions of well-formed sentences and utterances containing complex structures.
Table B2.
Correlation of regional distribution of significant atrophy in Lewy body spectrum patients with comprehension: Latency of judgments of adjacent within-cluster events
| Anatomic locus (Brodmann area) | Coordinates | Z-score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left inferior temporal (20) | −43 | 5 | −36 | 3.62 | 317 |
| Left putamen | −15 | 1 | −9 | 3.18 | 380 |
| Left uncus (28) | −25 | −12 | −27 | 3.19 | 1091 |
| Left hippocampus | −34 | −22 | −6 | 3.39 | 1737 |
| Right anterior frontal (10) | 12 | 67 | −8 | 3.28 | 207 |
| Right middle temporal (21) | 54 | 0 | −10 | 3.61 | 1485 |
| Right hippocampal | 14 | −2 | −12 | 4.14 | 412 |
| Right caudate nucleus | 33 | −28 | −5 | 3.25 | 1157 |
Table B3.
Correlation of regional distribution of significant atrophy in Lewy body spectrum patients with expression: Search theme maintenance
| Anatomic locus (Brodmann area) | Coordinates | Z-score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left anterior frontal (10) | −13 | 64 | −9 | 3.64 | 643 |
| Left dorsolateral prefrontal (8) | −12 | 51 | 38 | 3.46 | 207 |
| Left ventral lateral prefrontal (47) | −47 | 39 | −4 | 3.09 | 861 |
| Left ventral medial prefrontal (11/47) |
−29 |
30 |
−19 |
3.26 |
1573 |
| Left insula | −37 | 16 | −1 | 3.24 | 380 |
| Left inferior temporal (20) | −44 | 6 | −35 | 3.54 | 1074 |
| Left superior temporal (22) | −61 | −6 | 5 | 3.75 | 695 |
| Left hippocampus | −34 | −10 | −13 | 4.46 | 5449 |
| Left insula | −37 | −16 | 1 | 3.09 | 1278 |
| Left superior temporal (42) | −62 | −23 | 13 | 3.18 | 462 |
| Left inferior parietal (40) | −52 | −43 | 25 | 3.64 | 1335 |
| Left temporal/occipital (19) | −43 | −76 | 21 | 3.11 | 955 |
| Right anterior frontal (10) | 10 | 66 | −9 | 3.12 | 758 |
| Right anterior frontal (10) | 26 | 55 | 19 | 4 | 2170 |
| Right ventral lateral prefrontal (47) | 31 | 30 | −12 | 3.73 | 407 |
| Right ventral lateral prefrontal (47) | 49 | 21 | −4 | 3.18 | 2011 |
| Right inferior frontal (45) | 57 | 15 | 12 | 3.24 | 377 |
| Right frontal operculum | 38 | −2 | 16 | 4.03 | 1724 |
| Right superior temporal (22) | 58 | −2 | 2 | 3.58 | 2888 |
| Right hippocampus | 36 | −21 | −11 | 3.72 | 2499 |
| Right superior temporal (41) | 52 | −24 | 12 | 4.4 | 464 |
| Right inferior parietal (39) | 49 | −57 | 25 | 3.15 | 276 |
| Right middle occipital (19) | 41 | −66 | −6 | 3.11 | 602 |
| Right middle occipital (19) | 30 | −82 | 15 | 3.28 | 2318 |
| Right middle occipital (18) | 23 | −95 | 13 | 3.24 | 803 |
Table B4.
Correlation of regional distribution of significant atrophy in Lewy body spectrum patients with executive functioning: Average Z-score of category fluency and Trails B time
| Anatomic locus (Brodmann area) | Coordinates | Z-score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left anterior frontal (10) | −8 | 60 | −6 | 3.28 | 940 |
| Left ventral medial prefrontal (11) | −3 | 46 | −12 | 3.48 | 760 |
| Left dorsolateral prefrontal (8) | −22 | 39 | 40 | 3.6 | 405 |
| Left ventral medial prefrontal (11/47) |
−29 |
30 |
−19 |
3.66 |
11741 |
| Left dorsolateral prefrontal (8) | −34 | 26 | 44 | 3.97 | 2213 |
| Left dorsolateral prefrontal (44) | −62 | 3 | 21 | 3.1 | 503 |
| Left middle temporal (21) | −49 | 1 | −23 | 4.1 | 3051 |
| Left middle temporal (21) | −57 | −5 | −14 | 4.42 | 215 |
| Left hippocampus | −33 | −12 | −12 | 4.48 | 8203 |
| Left inferior parietal (40) | −65 | −30 | 29 | 3.18 | 1666 |
| Left inferior temporal (20) | −59 | −36 | −15 | 3.9 | 1263 |
| Left inferior parietal (20) | −44 | −39 | 54 | 3.47 | 443 |
| Left middle temporal (21) | −55 | −54 | 2 | 3.74 | 1531 |
| Left inferior parietal (39) | −56 | −59 | 22 | 4.51 | 16375 |
| Left lingual (17) | −8 | −98 | −1 | 3.25 | 652 |
| Right anterior frontal (10) | 9 | 66 | −9 | 3.6 | 1699 |
| Right anterior frontal (9) | 11 | 58 | 27 | 4.56 | 2878 |
| Right ventral lateral prefrontal (47) | 49 | 35 | −2 | 3.2 | 2288 |
| Right ventral medial prefrontal (11/47) |
31 |
30 |
−19 |
3.19 |
1185 |
| Right dorsolateral prefrontal (9) | 42 | 24 | 34 | 3.32 | 287 |
| Right superior temporal (22) | 57 | −2 | 1 | 3.6 | 4007 |
| Right middle temporal (21) | 49 | −3 | −25 | 3.76 | 1884 |
| Right caudate nucleus | 31 | −37 | 2 | 4.45 | 7941 |
| Right superior parietal (7) | 16 | −53 | 70 | 3.13 | 289 |
| Right fusiform (37) | 38 | −53 | −12 | 3.5 | 629 |
| Right nferior parietal (40) | 42 | −61 | 44 | 3.26 | 4226 |
| Right middle occipital (19) | 27 | −81 | 16 | 4.24 | 7221 |
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
REFERENCES
- Aarsland D, Andersen K, Larsen JP, Lolk A. Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Archives of Neurology. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gross RG, Cook P, Gunawardena D, Morgan B, et al. Impairments of speech fluency in Lewy body spectrum disorder. Brain & Language. doi: 10.1016/j.bandl.2011.09.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gross RG, Cook P, Morgan B, Boller A, et al. The organization of narrative discourse in Lewy body spectrum disorder. Brain & Language. 2011;119(1):30–41. doi: 10.1016/j.bandl.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: Discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Avants B, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape and intensity averaging. Neuroimage. 2004;23:S139–S150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants B, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49(3):2457–2466. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19(12):2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Bastiaanse R, Leenders KL. Language and Parkinson’s disease. Cortex. 2009;45(8):912–914. doi: 10.1016/j.cortex.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Bayles KA. Language and Parkinson disease. Alzheimer Dis Assoc Disord. 1990;4(3):171–180. doi: 10.1097/00002093-199040300-00005. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat, v. 5.1.09. Institute of Phonetic Sciences; University of Amsterdam: 1992-2009. [Google Scholar]
- Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson’s disease. J Neural Transm. 2004;111(10-11):1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. How common is dementia in Parkinson’s disease? Lancet. 1984;2:1262–1265. doi: 10.1016/s0140-6736(84)92807-1. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in Parkinson’s disease: from description to theory. Trends Neurosci. 1990;13(1):21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(Pt 1):195–203. doi: 10.1093/brain/awn298. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127(Pt 4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70(13):1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- Calderon J, Perry RJ, Erzinclioglu S, Berrios GE, Dening TR, Hodges JR. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2001;70:157–164. doi: 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Zientz J, Weiner M, Rosenberg R, Frawley W, Burns MH. Discourse changes in early Alzheimer disease, mild cognitive impairment, and normal aging. Alzheimer’s Disease and Associated Disorders. 2002;16:177–186. doi: 10.1097/00002093-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Chenery HJ, Angwin AJ, Copland DA. The basal ganglia circuits, dopamine, and ambiguous word processing: a neurobiological account of priming studies in Parkinson’s disease. J Int Neuropsychol Soc. 2008;14(3):351–364. doi: 10.1017/S1355617708080491. [DOI] [PubMed] [Google Scholar]
- Coelho CA, Grela B, Corso M, Gamble A, Feinn R. Microlinguistic deficits in the narrative discourse of adults with traumatic brain injury. Brain Inj. 2005;19(13):1139–1145. doi: 10.1080/02699050500110678. [DOI] [PubMed] [Google Scholar]
- Colman KS, Koerts J, van Beilen M, Leenders KL, Post WJ, Bastiaanse R. The impact of executive functions on verb production in patients with Parkinson’s disease. Cortex. 2009;45(8):930–942. doi: 10.1016/j.cortex.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Copland DA. Meaning selection and the subcortex: evidence of reduced lexical ambiguity repetition effects following subcortical lesions. J Psycholinguist Res. 2006;35(1):51–66. doi: 10.1007/s10936-005-9003-6. [DOI] [PubMed] [Google Scholar]
- Copland DA, McMahon KL, Silburn PA, de Zubicaray GI. Dopaminergic neuromodulation of semantic processing: a 4-T FMRI study with levodopa. Cereb Cortex. 2009;19(11):2651–2658. doi: 10.1093/cercor/bhp017. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Chute D, Libon D, Moore P, Grossman M. How does the brain support script comprehension? A study of executive processes and semantic knowledge in dementia. Neuropsychology. 2006;20(3):307–318. doi: 10.1037/0894-4105.20.3.307. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Darkins A, Mendez M, Hill MA, Benson DF. Alzheimer’s disease and Parkinson’s disease: comparison of speech and language alterations. Neurology. 1988;38:680–684. doi: 10.1212/wnl.38.5.680. [DOI] [PubMed] [Google Scholar]
- Double KL, Halliday GM, McRitchie DA, Reid WG, Hely MA, Morris JG. Regional brain atrophy in idiopathic parkinson’s disease and diffuse Lewy body disease. Dementia. 1996;7(6):304–313. doi: 10.1159/000106896. [DOI] [PubMed] [Google Scholar]
- Downes JJ, Priestley NM, Doran M, Ferran J, Ghadiali E, Cooper P. Intellectual, mnemonic, and frontal functions in dementia with Lewy bodies: A comparison with early and advanced Parkinson’s disease. Behav Neurol. 1998;11(3):173–183. [PubMed] [Google Scholar]
- Ebmeier KP, Calder SA, Crawford JR, Stewart L, Cochrane RHB, Besson JAO. Dementia in idiopathic Parkinson’s disease: prevalence and relationship with symptoms and signs of Parkinsonism. Psychological Medicine. 1991;21:69–76. doi: 10.1017/s0033291700014665. [DOI] [PubMed] [Google Scholar]
- Eckert T, Barnes A, Dhawan V, Frucht S, Gordon MF, Feigin AS, et al. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage. 2005;26:912–921. doi: 10.1016/j.neuroimage.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Positron emission tomography studies in Parkinsonism. Neurologic Clinics. 1992;10:421–433. [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Dhawan V, Sidtis JJ, Ginos JZ, Strother SC, et al. The metabolic anatomy of Parkinson’s disease: Complementary [18 F] fluorodeoxyglucose and [18 F]fluorodopa positron emission tomographic studies. Movement Disorders. 1990;5:203–213. doi: 10.1002/mds.870050304. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, et al. The metabolic topography of parkinsonism. Journal of Cerebral Blood Flow and MEtabolism. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R, UPDRS Program Members . Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne D, editors. Recent Developments in Parkinson’s Disease. Macmillan Healthcare Information; Florham Park, NJ: 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Farag C, Troiani V, Bonner M, Powers C, Avants B, Gee J, et al. Hierarchical organization of scripts: Converging evidence from fMRI and frontotemporal dementia. Cerebral Cortex. 2010;20(10):2453–2463. doi: 10.1093/cercor/bhp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl EC, Guthke T, von Cramon DY. Text comprehension after brain injury: left prefrontal lesions affect inference processes. Neuropsychology. 2002;16(3):292–308. doi: 10.1037//0894-4105.16.3.292. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SF, McHugh PR. “Mini Mental State.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Giladi N, Treves TA, Paleacu D, Shabtai H, Orlov Y, Kandinov B, et al. Risk factors for dementia, depression and psychosis in long-standing Parkinson’s disease. J Neural Transm. 2000;107(1):59–71. doi: 10.1007/s007020050005. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Girotti F, Soliveri P, Carella F, Piccolo I, Caffarra P, Musicco M, et al. Dementia and cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(12):1498–1502. doi: 10.1136/jnnp.51.12.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RG, Camp E, Gunawardena D, McMillan C, Cook P, Morgan B, et al. Impairment of script comprehension in Lewy body spectrum disorders. Brain & Language. doi: 10.1016/j.bandl.2013.02.006. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RG, McMillan C, Dreyfuss M, Ash S, Avants B, Cook P, et al. Sentence processing in Lewy body spectrum disorder: The role of working memory. Brain & Cognition. doi: 10.1016/j.bandc.2011.12.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. Sentence processing in Parkinson’s disease. Brain and Cognition. 1999;40:387–413. doi: 10.1006/brcg.1999.1087. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Lee C, Alsop D, Detre J, et al. Grammatical and resource components of sentence processing in Parkinson’s disease: An fMRI study. Neurology. 2003;60:775–781. doi: 10.1212/01.wnl.0000044398.73241.13. [DOI] [PubMed] [Google Scholar]
- Harrington CR, Perry RH, Perry EK, Hurt J, McKeith IG, Roth M, et al. Senile dementia of Lewy body type and Alzheimer type are biochemically distinct in terms of paired helical filaments and hyperphosphorylated tau protein. Dementia. 1994;5(5):215–228. doi: 10.1159/000106727. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. Jama. 2002;287(4):455–463. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- Hochstadt J. Set-shifting and the on-line processing of relative clauses in Parkinson’s disease: results from a novel eye-tracking method. Cortex. 2009;45(8):991–1011. doi: 10.1016/j.cortex.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Hoehn MD, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Huang C, Tang C, Feigin A, Lesser M, Ma Y, Pourfar M, et al. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130(7):1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanette Y, Goulet P, Joanette Y, Brownell HH. Discourse ability and brain damage: Theoretical and empirical perspectives. Springer Verlag; New York: 1990. Narrative discourse in right-brain-damaged right-handers. [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, Bowen JD, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–2073. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labov W, Waletzky J. Narrative analysis: Oral versions of personal experience. In: Helm J, editor. Essays on the Verbal and Visual Arts: Proceedings of the 1966 Annual Spring Meeting of the American Ethnological Society; Seattle: University of Washington Press. 1967.pp. 12–44. [Google Scholar]
- Lambon Ralph MA, Powell J, Howard D, Whitworth AB, Garrard P, Hodges JR. Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer’s type: A comparative neuropsychological study and literature review. Journal of Neurology, Neurosurgery and Psychiatry. 2001;70:149–156. doi: 10.1136/jnnp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Park HJ, Park B, Song SK, Sohn YH, Lee JD, et al. A comparative analysis of cognitive profiles and white-matter alterations using voxel-based diffusion tensor imaging between patients with Parkinson’s disease dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2010;81(3):320–326. doi: 10.1136/jnnp.2009.184747. [DOI] [PubMed] [Google Scholar]
- Lees AJ. Parkinson’s disease and dementia. Lancet. 1985;1(8419):43–44. doi: 10.1016/s0140-6736(85)90986-9. [DOI] [PubMed] [Google Scholar]
- Levin BE, Tomer R, Rey GJ. Cognitive impairments in Parkinson’s disease. Neurol Clin. 1992;10(2):471–485. [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23(15):6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Bogdanoff B, Leopold N, Hurka R, Bonavita J, Skalina S, et al. Neuropsychological profiles associated with subcortical white matter alterations and Parkinson’s disease: implications for the diagnosis of dementia. Arch Clin Neuropsychol. 2001;16(1):19–32. [PubMed] [Google Scholar]
- Lozza C, Baron JC, Eidelberg D, Mentis MJ, Carbon M, Marie RM. Executive processes in Parkinson’s disease: FDG-PET and network analysis. Hum Brain Mapp. 2004;22(3):236–245. doi: 10.1002/hbm.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, et al. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998;20(4):536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Mar RA. The neuropsychology of narrative: Story comprehension, story production, and their interrelation. Neuropsychologia. 2004;42:1414–1434. doi: 10.1016/j.neuropsychologia.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Mattis S, Jurica PJ, Leitten CL. Dementia Rating Scale-2: Professional Manual. Psychological Assessment Resources, Inc.; Lutz, FL: 2001. [Google Scholar]
- Mayer M. Frog, Where Are You? Penguin Books; New York: 1969. [Google Scholar]
- Mayeux R, Stern Y, Rosenstein R, Marder K, Hauser A, Cote L, et al. An estimate of the prevalence of dementia in idiopathic Parkinson’s disease. Archives of Neurology. 1988;45:260–262. doi: 10.1001/archneur.1988.00520270034017. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Miceli G, Mazzucchi A, Menn L, Goodglass H. Contrasting cases of Italian agrammatic aphasia without comprehension disorder. Brain and Language. 1983;19:65–97. doi: 10.1016/0093-934x(83)90056-1. [DOI] [PubMed] [Google Scholar]
- Murray LL, Lenz LP. Productive syntax abilities in Huntington’s and Parkinson’s diseases. Brain Cogn. 2001;46(1-2):213–219. doi: 10.1016/s0278-2626(01)80069-5. [DOI] [PubMed] [Google Scholar]
- Parashos SA, Johnson ML, Erickson-Davis C, Wielinski CL. Assessing cognition in Parkinson disease: use of the cognitive linguistic quick test. J Geriatr Psychiatry Neurol. 2009;22(4):228–234. doi: 10.1177/0891988709342721. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bartres-Faz D, Tolosa E. Structural brain correlates of verbal fluency in Parkinson’s disease. Neuroreport. 2009;20(8):741–744. doi: 10.1097/WNR.0b013e328329370b. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Koller WC, Troster AI. Lexical, semantic, and action verbal fluency in Parkinson’s disease with and without dementia. J Clin Exp Neuropsychol. 1999;21(4):435–443. doi: 10.1076/jcen.21.4.435.885. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews: Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Reilly J, Losh M, Bellugi U, Wulfeck B. “Frog, where are you?” Narratives in children with specific language impairment, early focal brain injury, and Williams syndrome. Brain and Language. 2004;88:229–247. doi: 10.1016/S0093-934X(03)00101-9. [DOI] [PubMed] [Google Scholar]
- Sauer J, ffytche DH, Ballard C, Brown RG, Howard R. Differences between Alzheimer’s disease and dementia with Lewy bodies: an fMRI study of task-related brain activity. Brain. 2006;129:1780–1788. doi: 10.1093/brain/awl102. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131(Pt 5):1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournaux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Tam CW, Burton EJ, McKeith IG, Burn DJ, O’Brien JT. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64(5):861–865. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- Troiani V, Fernandez-Seara MA, Wang Z, Detre JA, Ash S, Grossman M. Narrative speech production: an fMRI study using continuous arterial spin labeling. Neuroimage. 2008;40(2):932–939. doi: 10.1016/j.neuroimage.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130:708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]